Abstract
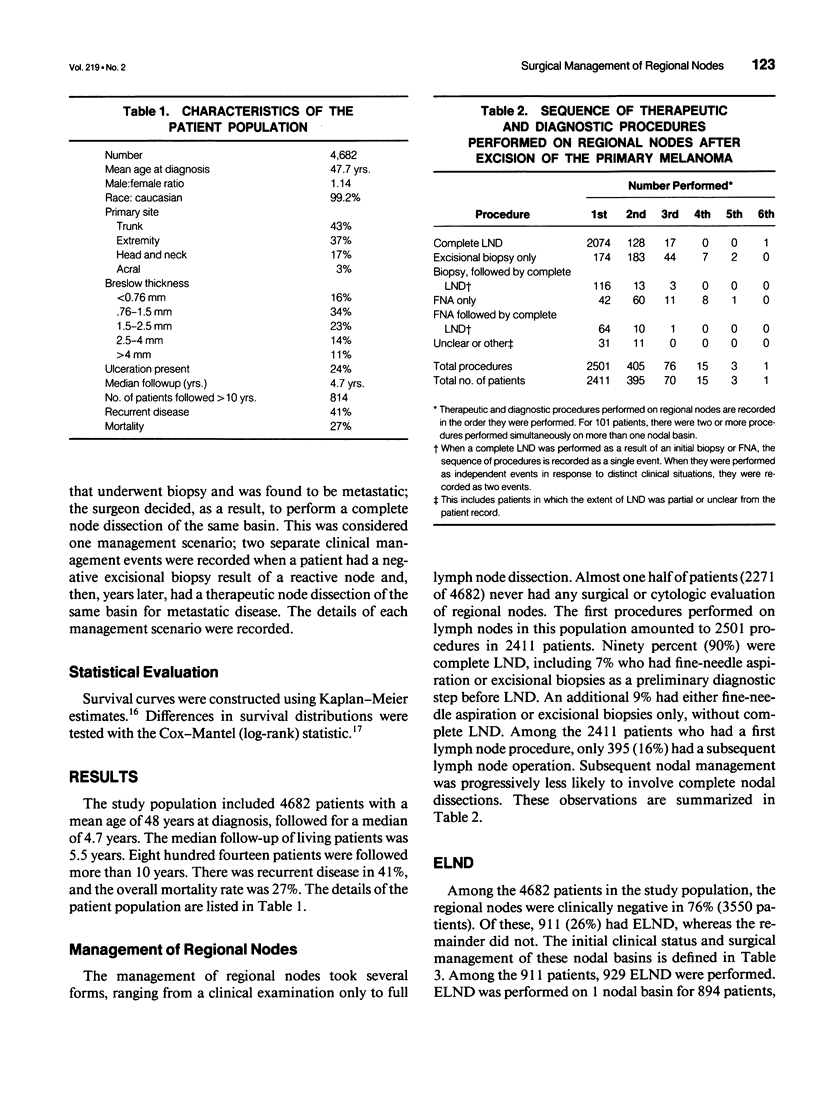
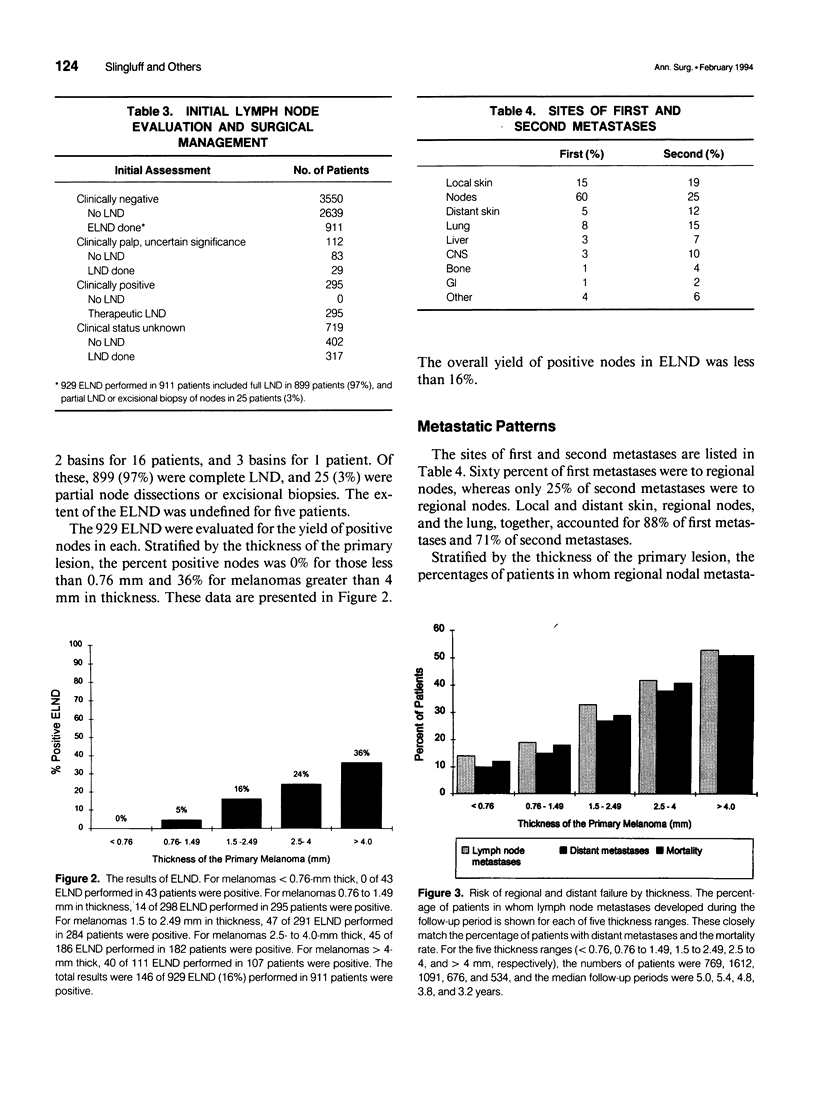
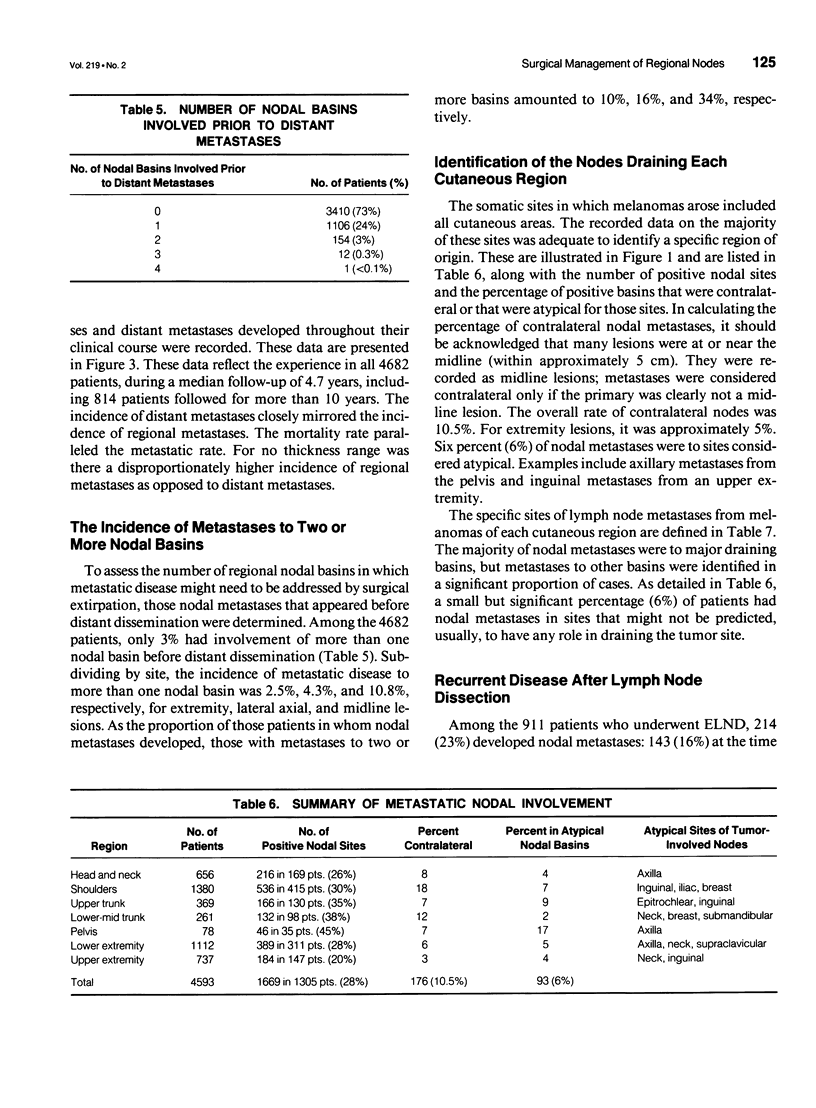
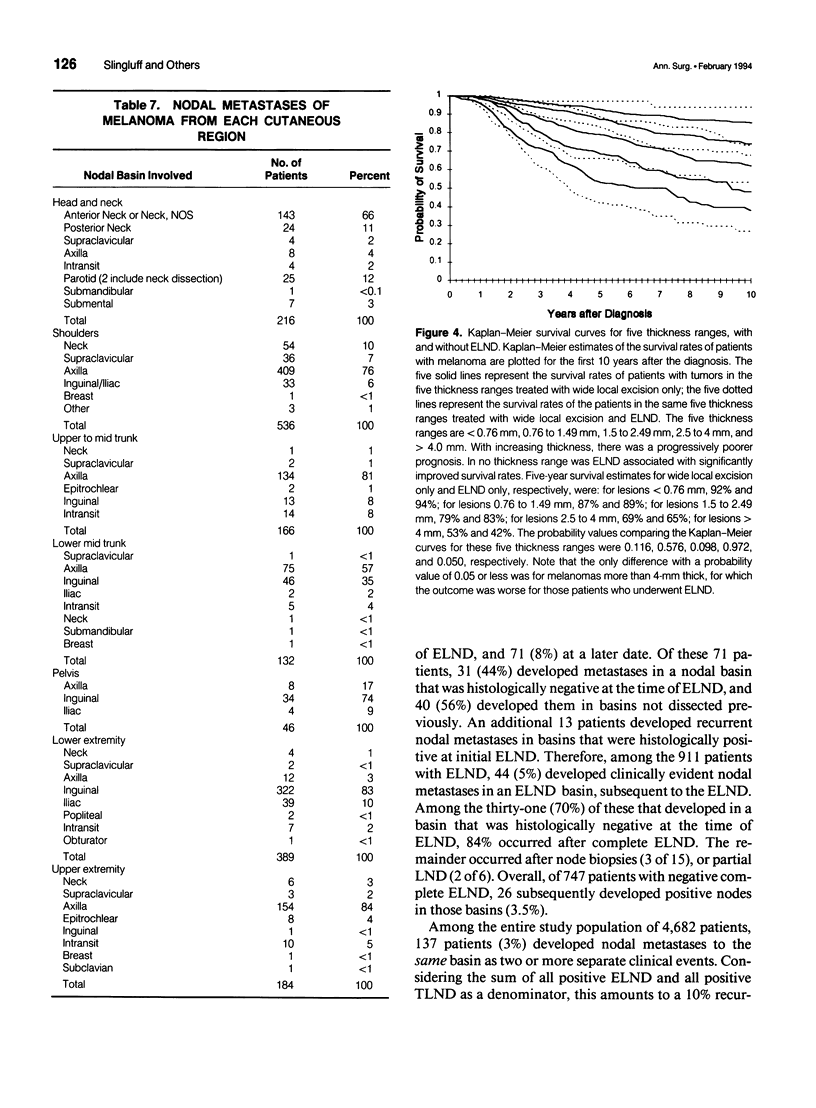
OBJECTIVE: The purpose of this study was to evaluate a large number of patients with cutaneous melanoma who had or who were at risk for lymph node metastases to contribute to the understanding of the behavior of and appropriate management of draining nodes. A major goal of the study was to reassess the clinical impact of elective lymph node dissections (ELND) in a large patient population. SUMMARY BACKGROUND DATA: Large retrospective studies suggest that ELND may improve the prognosis of patients with intermediate thickness melanomas; however, that improvement has not been observed in two randomized prospective controlled trials. METHODS: The charts of 4682 patients treated at a single institution for localized or regional disease were reviewed individually. The median follow-up was 4.7 years, with 814 patients followed more than 10 years. The data were tabulated and evaluated with the aid of a computer data base system. RESULTS: Among patients with nodal metastases, 10% of nodal metastases were to contralateral nodes, and 6% were to nodal basins that would not be predicted by classic models of lymphatic drainage; in 13% of patients, nodal metastases occurred to greater than one nodal basin (3% of the entire study group). For all thickness ranges, the incidence of nodal metastases was comparable to the incidence of distant metastases; intermediate-thickness lesions had no relative predilection for nodal metastases. At the initial evaluation, regional nodal basins were clinically negative in 3550 patients, of whom 911 (25.7%) underwent ELND. Stratified into five thickness groups (< 0.76 mm, 0.76 to 1.5 mm, 1.5 to 2.5 mm, 2.5 to 4 mm, and > 4 mm), pathologically positive nodes were identified in 0%, 5%, 16%, 24%, and 36%, respectively (16% overall). Among the 911 patients who underwent ELND, 214 (23%) had nodal metastases, 143 at the time of ELND and 71 at a later date. Of these 71 patients, 31 (44%) had nodal metastases in a previously dissected nodal basin, and 40 (56%) had them in basins not previously dissected. The survival of patients with clinically negative nodes treated with and without ELND were compared. The two groups were well matched for major prognostic factors. Stratified by Breslow thickness and primary site, no significant improvement in survival was observed with ELND. CONCLUSIONS: Because of the significant incidence of metastases to contralateral and atypical nodal basins, lymphoscintigraphy may be justified for the preoperative evaluation of patients for ELND. However, the therapeutic value of ELND is questionable as a result of (1) the finding that the risk of nodal metastases is not relatively more common than is that of distant metastases among patients with intermediate-thickness melanomas, (2) the fact that only 16% of ELND were positive, (3) the finding that ELND may not prevent recurrent nodal disease in the dissected basin, and (4) the absence of any apparent impact on survival among patients who underwent ELND.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Aukerman S. L., Ogle R. C., Noonan D. M., Fridman R., Martin G. R., Fidler I. J. The in vitro invasiveness and interactions with laminin of K-1735 melanoma cells. Evidence for different laminin-binding affinities in high and low metastatic variants. Clin Exp Metastasis. 1989 Jul-Aug;7(4):437–451. doi: 10.1007/BF01753664. [DOI] [PubMed] [Google Scholar]
- Baas P. C., Schraffordt Koops H., Hoekstra H. J., van Bruggen J. J., van der Weele L. T., Oldhoff J. Groin dissection in the treatment of lower-extremity melanoma. Short-term and long-term morbidity. Arch Surg. 1992 Mar;127(3):281–286. doi: 10.1001/archsurg.1992.01420030043008. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M. Surgical management of regional lymph nodes in cutaneous melanoma. J Am Acad Dermatol. 1980 Nov;3(5):511–524. doi: 10.1016/s0190-9622(80)80118-6. [DOI] [PubMed] [Google Scholar]
- Balch C. M. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol. 1988 Jan;6(1):163–172. doi: 10.1200/JCO.1988.6.1.163. [DOI] [PubMed] [Google Scholar]
- Berman C. G., Norman J., Cruse C. W., Reintgen D. S., Clark R. A. Lymphoscintigraphy in malignant melanoma. Ann Plast Surg. 1992 Jan;28(1):29–32. doi: 10.1097/00000637-199201000-00010. [DOI] [PubMed] [Google Scholar]
- Binder M., Pehamberger H., Steiner A., Wolff K. Elective regional lymph node dissection in malignant melanoma. Eur J Cancer. 1990;26(8):871–873. doi: 10.1016/0277-5379(90)90187-x. [DOI] [PubMed] [Google Scholar]
- Cady B. "Prophylactic" lymph node dissection in melanoma: does it help? J Clin Oncol. 1988 Jan;6(1):2–4. doi: 10.1200/JCO.1988.6.1.2. [DOI] [PubMed] [Google Scholar]
- Crowley N. J., Seigler H. F. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg. 1990 Aug;212(2):173–177. doi: 10.1097/00000658-199008000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Karakousis C. P., Emrich L. J., Driscoll D. L., Rao U. Survival after groin dissection for malignant melanoma. Surgery. 1991 Feb;109(2):119–126. [PubMed] [Google Scholar]
- Karakousis C. P., Goumas W., Rao U., Driscoll D. L. Axillary node dissection in malignant melanoma. Am J Surg. 1991 Sep;162(3):202–207. doi: 10.1016/0002-9610(91)90069-p. [DOI] [PubMed] [Google Scholar]
- Little J. H., Davis N. C. Secondary malignant melanoma in lymph nodes: incidence, time of occurrence, and mortality. Aust N Z J Surg. 1978 Feb;48(1):9–13. doi: 10.1111/j.1445-2197.1978.tb05795.x. [DOI] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., McCarthy W. H., Pearson L., Balch C. M., Soong S. J. Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma: results of surgical treatment in 1319 patients. Br J Surg. 1982 Feb;69(2):108–111. doi: 10.1002/bjs.1800690217. [DOI] [PubMed] [Google Scholar]
- Mukherji B., Nashed A. L., Guha A., Ergin M. T. Regulation of cellular immune response against autologous human melanoma. II. Mechanism of induction and specificity of suppression. J Immunol. 1986 Mar 1;136(5):1893–1898. [PubMed] [Google Scholar]
- Nicolson G. L. Differential organ tissue adhesion, invasion, and growth properties of metastatic rat mammary adenocarcinoma cells. Breast Cancer Res Treat. 1988 Oct;12(2):167–176. doi: 10.1007/BF01805938. [DOI] [PubMed] [Google Scholar]
- Norman J., Cruse C. W., Espinosa C., Cox C., Berman C., Clark R., Saba H., Wells K., Reintgen D. Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma. Am J Surg. 1991 Nov;162(5):432–437. doi: 10.1016/0002-9610(91)90255-c. [DOI] [PubMed] [Google Scholar]
- Pace J. B. Functional overlay. West J Med. 1979 Aug;131(2):157–158. [PMC free article] [PubMed] [Google Scholar]
- Reintgen D. S., Cox E. B., McCarty K. S., Jr, Vollmer R. T., Seigler H. F. Efficacy of elective lymph node dissection in patients with intermediate thickness primary melanoma. Ann Surg. 1983 Sep;198(3):379–385. doi: 10.1097/00000658-198309000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reintgen D. S., McCarty K. S., Woodard B., Cox E., Seigler H. F. Metastatic malignant melanoma with an unknown primary. Surg Gynecol Obstet. 1983 Mar;156(3):335–340. [PubMed] [Google Scholar]
- Seigler H. F., Cox E., Mutzner F., Shepherd L., Nicholson E., Shingleton W. W. Specific active immunotherapy for melanoma. Ann Surg. 1979 Sep;190(3):366–372. doi: 10.1097/00000658-197909000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F. H., Taylor W. F., Pritchard D. J., Soule E. H. Lymphadenectomy in the management of stage I malignant melanoma: a prospective randomized study. Mayo Clin Proc. 1986 Sep;61(9):697–705. doi: 10.1016/s0025-6196(12)62768-2. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Darrow T., Vervaert C., Quinn-Allen M. A., Seigler H. F. Human cytotoxic T cells specific for autologous melanoma cells: successful generation from lymph node cells in seven consecutive cases. J Natl Cancer Inst. 1988 Sep 7;80(13):1016–1026. doi: 10.1093/jnci/80.13.1016. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Dodge R. K., Stanley W. E., Seigler H. F. The annual risk of melanoma progression. Implications for the concept of cure. Cancer. 1992 Oct 1;70(7):1917–1927. doi: 10.1002/1097-0142(19921001)70:7<1917::aid-cncr2820700719>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Vollmer R. T., Seigler H. F. Multiple primary melanoma: incidence and risk factors in 283 patients. Surgery. 1993 Mar;113(3):330–339. [PubMed] [Google Scholar]
- Tukey J. W. Some thoughts on clinical trials, especially problems of multiplicity. Science. 1977 Nov 18;198(4318):679–684. doi: 10.1126/science.333584. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorski V. V., Kirov S. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982 Jun 1;49(11):2420–2430. doi: 10.1002/1097-0142(19820601)49:11<2420::aid-cncr2820491133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wong J. H., Cagle L. A., Morton D. L. Surgical treatment of lymph nodes with metastatic melanoma from unknown primary site. Arch Surg. 1987 Dec;122(12):1380–1383. doi: 10.1001/archsurg.1987.01400240026003. [DOI] [PubMed] [Google Scholar]


