Abstract
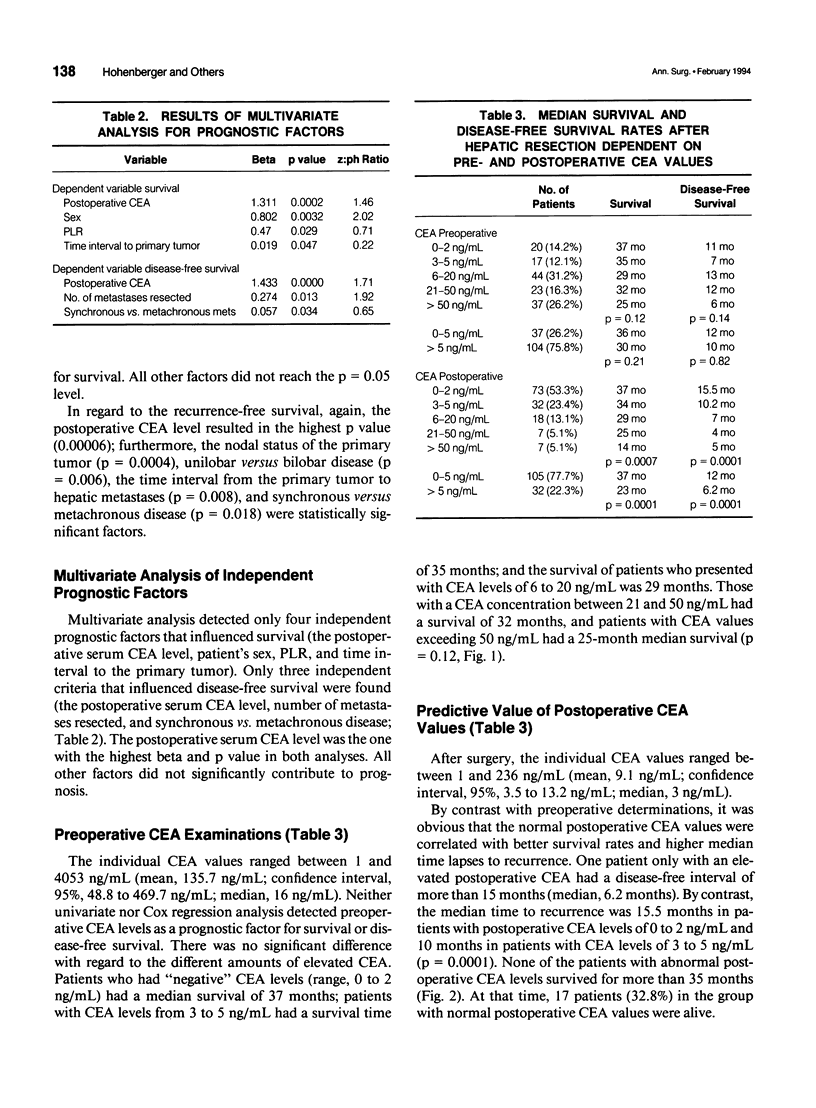
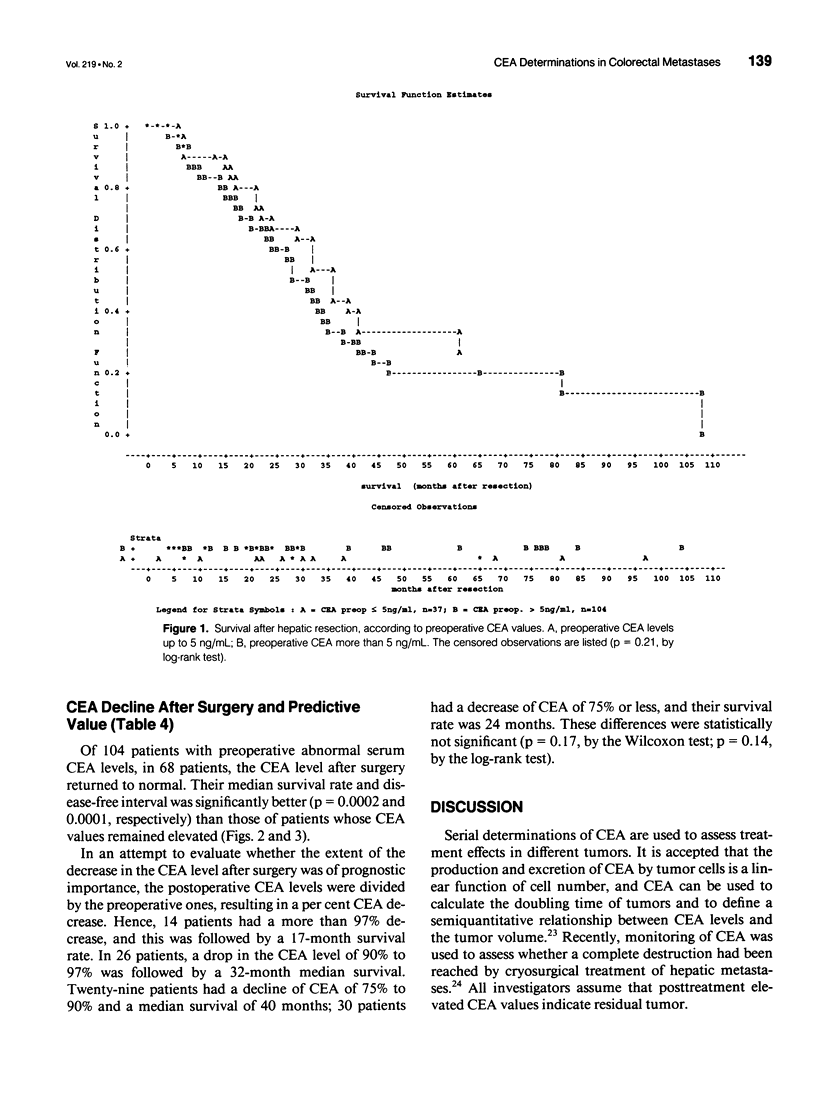
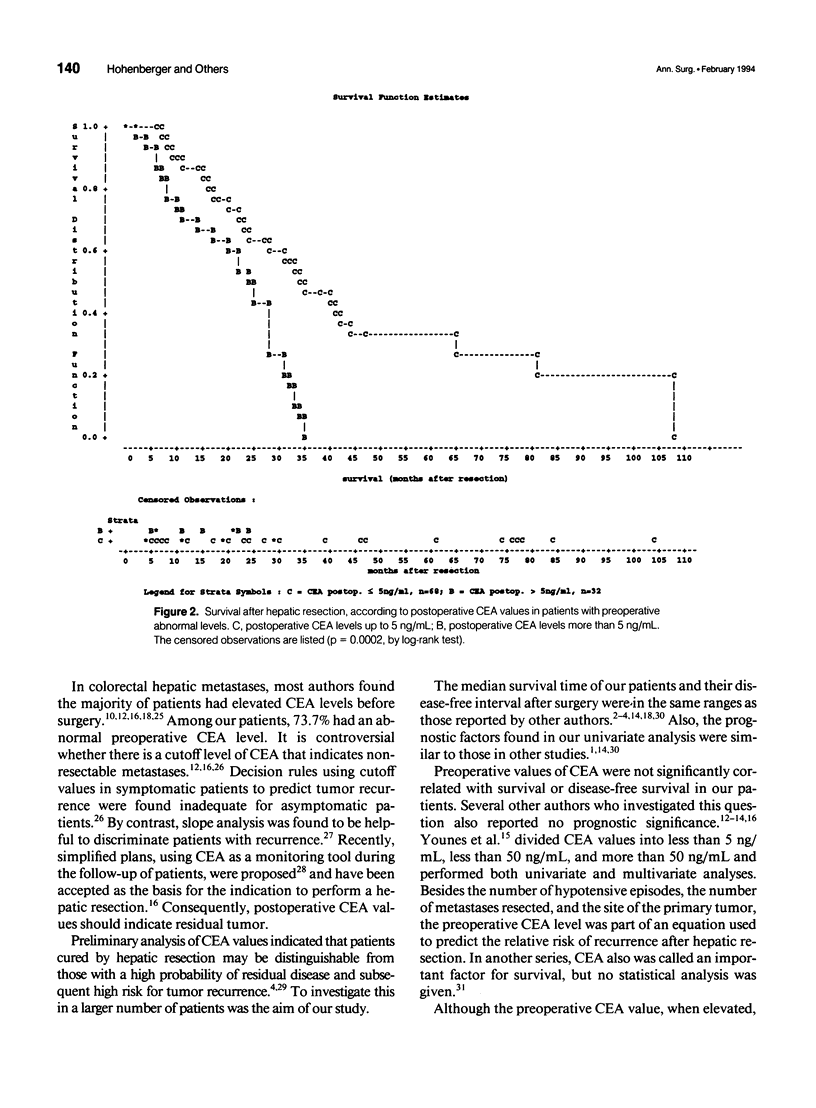
OBJECTIVE: The object of this study was to evaluate the prognostic significance of pre- and postoperative serum carcinoembryonic antigen (CEA) levels in the resectional treatment of colorectal hepatic metastases. The main question was whether postoperative CEA levels correlated with survival and the time to recurrence. SUMMARY BACKGROUND DATA: Despite numerous investigations on prognostic factors in colorectal cancer, only sparse data are available to estimate the patient's individual risk for tumor recurrence postoperatively. It is controversial whether preoperative CEA values are of prognostic significance, and after observing the kinetics of CEA decline, elevated CEA levels postoperatively were found to be an ominous sign. CEA therefore could indicate the presence of a tumor burden after resection. METHODS: One hundred sixty-six patients undergoing hepatic resection for colorectal metastases with curative intent were prospectively documented and underwent multivariate analysis for indicators of prognosis. RESULTS: Abnormal preoperative CEA levels were not of prognostic significance compared with values within the normal range (survival, 36 vs. 30 months; p = 0.12; disease-free survival, 12 vs. 10 months; p = 0.82). The postoperative serum CEA level, however, was the most predictive factor with regard to survival and the disease-free interval. Patients in whom CEA levels were abnormal before surgery and returned into the normal range after resection had significantly better survival times (37 vs. 23 months, p = 0.0001) and disease-free survival times (12 vs. 6.2 months, p = 0.0001) compared with patients with persistently abnormal values. CONCLUSIONS: Pre- and postoperative determination of the serum CEA level is mandatory to judge whether a curative resection has been performed and whether tumor has been left behind after the operation. Postoperative CEA levels also should be used as a stratification criterion in adjuvant treatment studies after hepatic resection to indicate patients with a high risk of tumor recurrence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriole G. L. Serum prostate-specific antigen: the most useful tumor marker. J Clin Oncol. 1992 Aug;10(8):1205–1207. doi: 10.1200/JCO.1992.10.8.1205. [DOI] [PubMed] [Google Scholar]
- August D. A., Sugarbaker P. H., Ottow R. T., Gianola F. J., Schneider P. D. Hepatic resection of colorectal metastases. Influence of clinical factors and adjuvant intraperitoneal 5-fluorouracil via Tenckhoff catheter on survival. Ann Surg. 1985 Feb;201(2):210–218. doi: 10.1097/00000658-198502000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Urist M. M., Soong S. J., McGregor M. A prospective phase II clinical trial of continuous FUDR regional chemotherapy for colorectal metastases to the liver using a totally implantable drug infusion pump. Ann Surg. 1983 Nov;198(5):567–573. doi: 10.1097/00000658-198311000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982 Jan;6(1):3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- Boey J., Cheung H. C., Lai C. K., Wong J. A prospective evaluation of serum carcinoembryonic antigen (CEA) levels in the management of colorectal carcinoma. World J Surg. 1984 Jun;8(3):279–286. doi: 10.1007/BF01655052. [DOI] [PubMed] [Google Scholar]
- Bozzetti F., Doci R., Bignami P., Morabito A., Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg. 1987 Mar;205(3):264–270. doi: 10.1097/00000658-198703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein B. R., Steele G. D., Jr, Ensminger W., Kaplan W. D., Lowenstein M. S., Wilson R. E., Forman J., Zamcheck N. The use and limitations of serial plasma carcinoembryonic antigen (CEA) levels as a monitor of changing metastatic liver tumor volume in patients receiving chemotherapy. Cancer. 1980 Jul 15;46(2):266–272. doi: 10.1002/1097-0142(19800715)46:2<266::aid-cncr2820460208>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Brunt L. M., Wells S. A., Jr Advances in the diagnosis and treatment of medullary thyroid carcinoma. Surg Clin North Am. 1987 Apr;67(2):263–279. doi: 10.1016/s0039-6109(16)44183-6. [DOI] [PubMed] [Google Scholar]
- Chu D. Z., Erickson C. A., Russell M. P., Thompson C., Lang N. P., Broadwater R. J., Westbrook K. C. Prognostic significance of carcinoembryonic antigen in colorectal carcinoma. Serum levels before and after resection and before recurrence. Arch Surg. 1991 Mar;126(3):314–316. doi: 10.1001/archsurg.1991.01410270054010. [DOI] [PubMed] [Google Scholar]
- Denstman F., Rosen L., Khubchandani I. T., Sheets J. A., Stasik J. J., Riether R. D. Comparing predictive decision rules in postoperative CEA monitoring. Cancer. 1986 Nov 1;58(9):2089–2095. doi: 10.1002/1097-0142(19861101)58:9<2089::aid-cncr2820580921>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Doci R., Gennari L., Bignami P., Montalto F., Morabito A., Bozzetti F. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991 Jul;78(7):797–801. doi: 10.1002/bjs.1800780711. [DOI] [PubMed] [Google Scholar]
- Ekberg H., Tranberg K. G., Andersson R., Lundstedt C., Hägerstrand I., Ranstam J., Bengmark S. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg. 1987 Aug;11(4):541–547. doi: 10.1007/BF01655821. [DOI] [PubMed] [Google Scholar]
- Fortner J. G. Recurrence of colorectal cancer after hepatic resection. Am J Surg. 1988 Mar;155(3):378–382. doi: 10.1016/s0002-9610(88)80086-2. [DOI] [PubMed] [Google Scholar]
- Hohenberger P., Schlag P., Schwarz V., Herfarth C. Tumor recurrence and options for further treatment after resection of liver metastases in patients with colorectal cancer. J Surg Oncol. 1990 Aug;44(4):245–251. doi: 10.1002/jso.2930440411. [DOI] [PubMed] [Google Scholar]
- Hughes K. S., Rosenstein R. B., Songhorabodi S., Adson M. A., Ilstrup D. M., Fortner J. G., Maclean B. J., Foster J. H., Daly J. M., Fitzherbert D. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum. 1988 Jan;31(1):1–4. doi: 10.1007/bf02552560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang D. T., Greenberg L. J., Kennedy B. J. Tumor marker kinetics in the monitoring of breast cancer. Cancer. 1990 Jan 15;65(2):193–199. doi: 10.1002/1097-0142(19900115)65:2<193::aid-cncr2820650202>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kortz W. J., Meyers W. C., Hanks J. B., Schirmer B. D., Jones R. S. Hepatic resection for metastatic cancer. Ann Surg. 1984 Feb;199(2):182–186. doi: 10.1097/00000658-198402000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokich J., Ellenberg S., Gerson B., Knox W. E., Zamcheck N. Plasma clearance of carcinoembryonic antigen following hepatic metastatectomy. J Clin Oncol. 1984 May;2(5):462–465. doi: 10.1200/JCO.1984.2.5.462. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Moertel C. G., O'Fallon J. R., Go V. L., O'Connell M. J., Thynne G. S. The preoperative carcinoembryonic antigen test in the diagnosis, staging, and prognosis of colorectal cancer. Cancer. 1986 Aug 1;58(3):603–610. doi: 10.1002/1097-0142(19860801)58:3<603::aid-cncr2820580302>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Onik G., Rubinsky B., Zemel R., Weaver L., Diamond D., Cobb C., Porterfield B. Ultrasound-guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Preliminary results. Cancer. 1991 Feb 15;67(4):901–907. doi: 10.1002/1097-0142(19910215)67:4<901::aid-cncr2820670408>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Quentmeier A., Schlag P., Hohenberger P., Schwarz V., Abel U. Assessment of serial carcinoembryonic antigen: determinations to monitor the therapeutic progress and prognosis of metastatic liver disease treated by regional chemotherapy. J Surg Oncol. 1989 Feb;40(2):112–118. doi: 10.1002/jso.2930400211. [DOI] [PubMed] [Google Scholar]
- Sandler R. S., Freund D. A., Herbst C. A., Jr, Sandler D. P. Cost effectiveness of postoperative carcinoembryonic antigen monitoring in colorectal cancer. Cancer. 1984 Jan 1;53(1):193–198. doi: 10.1002/1097-0142(19840101)53:1<193::aid-cncr2820530134>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Scheele J., Stangl R., Altendorf-Hofmann A., Gall F. P. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991 Jul;110(1):13–29. [PubMed] [Google Scholar]
- Shinkai T., Saijo N., Tominaga K., Eguchi K., Shimizu E., Sasaki Y., Fujita J., Futami H., Ohkura H., Suemasu K. Serial plasma carcinoembryonic antigen measurement for monitoring patients with advanced lung cancer during chemotherapy. Cancer. 1986 Apr 1;57(7):1318–1323. doi: 10.1002/1097-0142(19860401)57:7<1318::aid-cncr2820570711>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Steele G., Jr, Bleday R., Mayer R. J., Lindblad A., Petrelli N., Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol. 1991 Jul;9(7):1105–1112. doi: 10.1200/JCO.1991.9.7.1105. [DOI] [PubMed] [Google Scholar]
- Steele G., Jr, Osteen R. T., Wilson R. E., Brooks D. C., Mayer R. J., Zamcheck N., Ravikumar T. S. Patterns of failure after surgical cure of large liver tumors. A change in the proximate cause of death and a need for effective systemic adjuvant therapy. Am J Surg. 1984 Apr;147(4):554–559. doi: 10.1016/0002-9610(84)90021-7. [DOI] [PubMed] [Google Scholar]
- Sugarbaker P. H., Gianola F. J., Dwyer A., Neuman N. R. A simplified plan for follow-up of patients with colon and rectal cancer supported by prospective studies of laboratory and radiologic test results. Surgery. 1987 Jul;102(1):79–87. [PubMed] [Google Scholar]
- Sugarbaker P. H. Surgical decision making for large bowel cancer metastatic to the liver. Radiology. 1990 Mar;174(3 Pt 1):621–626. doi: 10.1148/radiology.174.3.2406776. [DOI] [PubMed] [Google Scholar]
- Thomas P., Zamcheck N. Role of the liver in clearance and excretion of circulating carcinoembryonic antigen (CEA). Dig Dis Sci. 1983 Mar;28(3):216–224. doi: 10.1007/BF01295116. [DOI] [PubMed] [Google Scholar]
- Tibbetts L. M., Doremus C. M., Tzanakakis G. N., Vezeridis M. P. Liver metastases with 10 human colon carcinoma cell lines in nude mice and association with carcinoembryonic antigen production. Cancer. 1993 Jan 15;71(2):315–321. doi: 10.1002/1097-0142(19930115)71:2<315::aid-cncr2820710208>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Llaneras M., Martin T., Kaiser D. Prospective monitoring trial for carcinoma of colon and rectum after surgical resection. Surg Gynecol Obstet. 1989 Dec;169(6):479–487. [PubMed] [Google Scholar]
- Younes R. N., Rogatko A., Brennan M. F. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991 Aug;214(2):107–113. doi: 10.1097/00000658-199108000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]


