Abstract
The Internet of Things (IoT) has emerged as a transformative technology in managing Alzheimer’s Disease (AD), offering novel solutions for early diagnosis, continuous patient monitoring, and assistive care. This review presents a comprehensive analysis of IoT-enabled systems tailored to AD care, focusing on wearable biosensors, cognitive monitoring tools, smart home automation, and Artificial Intelligence (AI)-driven analytics. A systematic literature survey was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify, screen, and synthesize 236 relevant studies primarily published between 2020 and 2025 across IEEE Xplore, PubMed, Scopus and Web of Science. The inclusion criteria targeted peer-reviewed articles that proposed or evaluated IoT-based solutions for AD detection, progression monitoring, or patient assistance. Key findings highlight the effectiveness of the IoT in detecting behavioral and cognitive changes, enhancing safety through real-time alerts, and improving patient autonomy. The review also explores integration challenges such as data privacy, system interoperability, and clinical adoption. The study reveals critical gaps in real-world deployment, clinical validation, and ethical integration of IoT-based systems for Alzheimer’s care. This study aims to serve as a definitive reference for researchers, clinicians, and developers working at the intersection of the IoT and neurodegenerative healthcare.
Keywords: Internet of Things, Alzheimer’s disease monitoring, sleep disorder detection, wearable healthcare devices, artificial intelligence in healthcare, Remote Patient Monitoring, machine learning in healthcare, healthcare data privacy and ethics
1. Introduction
Alzheimer’s Disease (AD) is a progressive and irreversible neurodegenerative disorder and a leading cause of dementia worldwide, affecting over 55 million individuals globally. With rising life expectancy, AD prevalence is projected to nearly triple by 2050, significantly burdening global healthcare systems [1]. Current diagnostic methodologies, including neuroimaging (e.g., amyloid Positron Emission Tomography (PET)), cerebrospinal fluid biomarkers, genetic screening (such as APOE genotyping), and tau burden analysis, provide valuable insights into disease progression [2,3]. However, their invasiveness, associated costs, and impracticality for large-scale and early-stage community-based screening limit widespread application. Additionally, genetic and environmental risk factors vary across diverse populations, emphasizing the need for multi-ancestry genetic studies and personalized diagnostic approaches [4,5,6]. Modifiable lifestyle factors, such as diet, physical activity, sleep quality, and cardiovascular health, also critically impact AD risk, accounting for approximately 42.6% of dementia cases [7,8].
The clinical urgency is underscored by alarming epidemiological trends depicted in Figure 1, which project a significant increase in AD cases across elderly age groups from 2020 to 2060, notably among individuals aged 85 and older. Furthermore, Figure 2 highlights an expected increase in late-stage AD diagnoses, surpassing early-stage cases by 2040. This projected imbalance emphasizes the urgent requirement for accessible, proactive healthcare solutions capable of early detection and continuous disease monitoring.
Figure 1.
Worldwide projections of Alzheimer’s prevalence across different age groups (2020–2060) [9].
Figure 2.
Projected numbers of early-stage and late-stage Alzheimer’s disease cases (2005–2050) [10].
In recent years, the Internet of Things (IoT) has emerged as a groundbreaking technology in healthcare, offering promising solutions for real-time monitoring, predictive analytics, and personalized care. These systems include wearable biosensors, ambient environmental sensors, mobile applications, and AI-powered cognitive analytics, enabling continuous, non-invasive monitoring of cognitive, physiological, and behavioral changes in individuals with AD [11,12,13,14,15]. These systems aid in facilitating early identification of cognitive decline, provide continuous health status updates, assist in daily routine management, and improve patient autonomy by alerting caregivers to potential emergencies.
Despite the IoT’s transformative potential, multiple barriers restrict its widespread adoption, including challenges in data security, interoperability among devices, privacy protection, and ethical considerations related to patient consent and equitable access [16,17,18]. Additionally, socioeconomically disadvantaged populations face disparities in healthcare infrastructure, digital literacy, and affordability, significantly limiting IoT system implementation and effectiveness.
This review systematically synthesizes recent advancements in IoT-driven solutions for AD management, focusing explicitly on early detection, continuous monitoring, and assistive care technologies. Adhering to the PRISMA guidelines, we conducted an extensive literature survey of 236 peer-reviewed studies published primarily between 2020 and 2025 from IEEE Xplore, Scopus, PubMed, and Web of Science. Studies were selected based on explicit criteria, prioritizing those evaluating IoT-based diagnostic, monitoring, and supportive care systems specifically designed for AD.
The primary contributions of this review include:
Categorizing IoT interventions according to the stages of AD progression: early-stage cognitive assessments, mid-stage continuous activity monitoring, and late-stage assistive care;
Analyzing technological enablers such as wearable sensors, smart home integration, remote monitoring platforms, and AI-driven cognitive evaluation systems;
Identifying critical research gaps in interoperability, clinical adoption, scalability, data privacy, and the ethical deployment of IoT solutions within real-world clinical settings.
The remainder of this manuscript is organized as follows. Section 2 details the review methodology (search strategy, inclusion/exclusion criteria, study selection, and data abstraction workflow). Section 3 synthesizes IoT-enabled interventions across the Alzheimer’s disease continuum, structuring evidence by stage: (i) preclinical/early detection (digital biomarkers and passive cognitive assessment), (ii) mild stage (remote monitoring and activity and risk detection), and (iii) severe stage (assisted living, wandering prevention, and caregiver support). Section 4 provides a discussion and outlines emerging directions (multimodal digital twins, federated/edge learning, and ethical governance). Finally, Section 5 summarizes key insights and translational implications.
2. Scope of the Review
This review explores the role of the IoT in AD care across different disease stages, examines key enabling technologies, provides comparative analyses of existing solutions, and identifies critical research gaps and future research directions. It systematically investigates how IoT innovations facilitate early diagnosis, disease management, and patient supervision, ultimately aiming to improve care quality and support caregivers.
The paper categorizes IoT applications into three primary intervention stages:
Early detection, utilizing cognitive assessment tools and wearable sensors;
Mild-stage management, including remote monitoring, memory aids, and fall detection systems;
Severe-stage care, involving smart home automation, medication adherence systems, and patient safety through location tracking solutions.
Key enabling IoT technologies analyzed in the review encompass wearable biosensors, ambient intelligent smart home systems, AI-driven predictive analytics, cloud-based healthcare solutions, and existing data security frameworks. Moreover, the effectiveness of monitoring techniques and gaps in the current research landscape are systematically presented and discussed.
Following the PRISMA 2020 guidelines [19], we conducted systematic searches in six databases in May 2025. To balance precision and recall, we queried the following databases:
Scopus, using TITLE-ABS-KEY to restrict hits to titles, abstracts, and author keywords, reducing off-topic records;
IEEE Xplore, Springer, MDPI, Wiley, and PubMed, using All Fields (or equivalent “All Metadata” scopes) to maximize sensitivity given varied indexing practices.
To mitigate potential misses, like synonyms and database-specific indexing gaps, we hand-searched reference lists of key papers and specialized journals post query [19].
The following unified search string was used:
(“Internet of Things” OR IoT) AND (“Alzheimer’s disease” OR dementia) AND (sensor* OR biosensor* OR wearable* OR “smart home” OR “assistive technolog*” OR “video-based monitor*” OR ambient* OR telemonitor*)
A detailed search strategy was employed using consistent and carefully formulated search queries across multiple databases: IEEE Xplore, Scopus, Springer, MDPI, Wiley, and PubMed. Table 1 summarizes the structured search queries used for each database. The selection and consistency of keywords were guided by their comprehensive coverage of the three central domains: IoT technology, AD, and healthcare interventions. Core terms—such as “Internet of Things”, “IoT”, “Alzheimer’s Disease”, “Dementia”, and “Cognitive Impairment”—ensure that the retrieved studies are relevant to both the IoT and neurodegenerative disease management. Additional keywords, including “Sensors”, “Remote Monitoring”, “Smart Home”, “Assistive Technology”, and “Artificial Intelligence”, precisely target intervention methodologies across disease stages, ensuring a thorough examination of relevant literature. Boolean operators (“AND,” “OR”) were uniformly applied across databases to optimize the search precision and recall, promoting reproducibility and rigorous literature coverage. Data from included studies were extracted as reported; no statistical conversions were applied. Incomplete information (e.g., missing device specifications or performance metrics) was noted in the synthesis tables. No imputation methods were used. Heterogeneity was assessed qualitatively by comparing study characteristics, including participant demographics, Alzheimer’s disease stage, IoT device modality, and outcome metrics.
Table 1.
Database-specific search scopes and strings.
| Database | Field Scope | Search String |
|---|---|---|
| Scopus | TITLE-ABS-KEY | As above |
| PubMed | All Fields | As above |
| IEEE Xplore | All Metadata | As above |
| Springer | All Fields | As above |
| MDPI | All Fields | As above |
| Wiley | All Fields | As above |
The systematic literature identification and selection process is visually represented using the PRISMA guidelines, as depicted in Figure 3. The search yielded a total of 1573 records from electronic databases. Before initiating the screening process, 860 records were excluded for various reasons. Specifically, 251 were identified as duplicates and removed through a combination of automated and manual deduplication methods. Another 567 articles were published prior to 2020 and did not meet the temporal inclusion criteria, which focused on capturing recent developments. Additionally, 42 records were excluded for reasons such as being editorials, commentaries, or non-English studies lacking translatable data.
Figure 3.
PRISMA flowchart detailing the systematic literature search and selection process.
This left 713 records that underwent title and abstract screening. During this phase, 286 records were excluded for failing to meet the core eligibility criteria—typically due to an absence of relevance to Alzheimer’s disease or cognitive impairment, a lack of technological intervention (e.g., not using the IoT or AI), or an insufficient empirical basis. Consequently, 427 full-text articles were retrieved for further review. However, 138 of these could not be accessed due to inaccessible links, paywall restrictions, retraction notices, or broken archival sources.
A total of 289 full-text reports were subsequently assessed for eligibility. Of these, 96 were excluded. Specifically, 35 studies did not report relevant outcomes related to the review’s scope, such as early detection, symptom monitoring, or digital health interventions. Another 27 articles focused on non-representative populations or irrelevant conditions, such as studies involving young adults or non-human subjects. The remaining 34 were excluded due to methodological limitations, including a lack of control groups, unclear data sources, or non-reproducible results.
Beyond the initial database search, additional sources were explored to supplement the findings. These included 67 records from institutional websites, 15 from professional organizations, and 91 identified via citation chaining (backward and forward reference checks), amounting to 173 additional records. Of these, 95 could not be retrieved due to factors such as limited public availability, missing full-text versions, or language barriers. The remaining 78 records were screened in full; 46 were excluded due to overlapping data (n = 18), poor methodological quality (n = 16), or insufficient relevance to the research topic (n = 12).
In total, 236 studies were included in this systematic review. These comprised 11 studies retained from a previous version of the review, 193 newly identified and eligible studies from the current database search, and 32 articles sourced from gray literature and manual searches.
This review adhered to core PRISMA 2020 principles. The review protocol was preregistered under the Open Science Framework (OSF) at https://osf.io/uh8mq (accessed on 18 August 2025). No substantial amendments were made to the registered protocol. The protocol is summarized here for transparency: databases searched—IEEE Xplore, PubMed, Scopus, Web of Science, ACM Digital Library, and Google Scholar (covering 1 January 2020–31 May 2025); predefined eligibility based on population—persons at risk of or diagnosed with Alzheimer’s disease; intervention/technology—stage-wise IoT, sensing, monitoring, assistive, or AI-enhanced systems; outcomes—detection, monitoring performance, or functional/assistive impact indicators; study design—original empirical studies, excluding non-English, pre-2020, purely theoretical, and opinion pieces. Screening occurred in two phases (title/abstract, then full text), conducted by two independent reviewers with consensus adjudication. Data extraction captured study metadata (year, setting, and sample size), disease-stage focus, sensor modalities, computational methods, validation design, and reported performance metrics. Formal meta-analysis, risk-of-bias appraisal, certainty assessment (e.g., GRADE), sensitivity analyses, and publication bias tests were not conducted due to heterogeneity in study designs, outcome definitions, and metric reporting; these omissions are acknowledged as limitations rather than selective exclusion criteria. Heterogeneity was assessed qualitatively by comparing study populations, device modalities, and reported performance metrics within each disease-stage category.
Table 2 contrasts five recent IoT–AD review articles (2022–2025) with the present work, illustrating how prior studies either focused narrowly on wearables or provided broad taxonomies without organizing by Alzheimer’s stage. In comparison, our article uniquely delivers a stage-wise synthesis of IoT technologies—from early-detection sensors to severe-stage assistive systems—while also addressing practical implementation challenges such as ethics, interoperability, and deployment in low-resource settings.
Table 2.
Comparison of recent Scopus-indexed reviews on IoT in Alzheimer’s care.
| Article | Scope | IoT Dimensions Covered | Notable Novelty/Gaps |
|---|---|---|---|
| Sheikhtaheri & Sabermahani (2022) [20] | Scoping review of IoT applications in dementia care | Smart home automation and wearable sensors for home monitoring | Broad overview across dementia; lacks AD-stage organization and limited AI focus |
| Thanos et al. (2020) [21] | Review of wearable IoT devices for Alzheimer’s patients | Wearable trackers, smartwatches, mobile-app integration | Detailed wearable-device survey; excludes ambient/nonwearable IoT modalities |
| Esquer-Rochín et al. (2023) [14] | Systematic review and taxonomy of IoT in all dementias | Wearables, ambient sensorsc | Comprehensive IoT taxonomy; not Alzheimer’s-specific or stage-structured |
| Rocha et al. (2024) [22] | Review of wearable monitoring devices for dementia ADLs | Sensorized clothing, wristbands, and ADL task monitoring | In-depth design/usability guidance; limited to wearables and ADL tracking |
| Shaik et al. (2025) [23] | Systematic review of remote monitoring in AD and ADRD | Multimodal IoT: wearables, ambient sensors, and companion robots | Focus on monitoring and AI analytics; lacks early detection tools and stage-wise view. |
| This work (2025) | Stage-wise review of IoT interventions across AD progression | Wearable biosensors, smart home systems, cognitive assessment tools, AI analytics, and LMIC considerations | Holistic coverage of all AD stages; emphasizes real-world deployment, ethics, interoperability, and LMIC scalability |
3. IoT at Different Stages of Alzheimer’s Disease
This section explores the role of IoT technologies in AD across different stages, from early detection to severe-stage care. The content for this section is arranged as shown in Figure 4. It depicts a continuum of IoT-enabled interventions tailored to each stage of AD. In the early stage, wearable sensors, such as wrist-worn accelerometers and sleep trackers, and digital cognitive assessment tools work together to capture subtle changes in gait, tremors, sleep patterns, and response times that may signal the onset of cognitive decline. As patients progress to mild dementia, ambient motion detectors, door-contact sensors, and activity-tracking wearables feed real-time data into anomaly-detection algorithms, while smart pill boxes, voice assistants, and reminder applications support memory and daily routines; concurrently, fall-detection mats and posture sensors automatically alert caregivers or emergency services when needed. Finally, in the severe stage, fully integrated smart home systems adjust lighting, temperature, and door locks to reduce confusion; automated medication dispensers enforce correct dosing schedules; and global positioning system (GPS)-enabled wearables, combined with geofenced home gateways, prevent wandering by notifying caregivers if a patient leaves predefined safe zones. Together, these layered IoT solutions offer a seamless progression of screening, management, and safety support throughout AD.
Figure 4.
Organization of review sections by Alzheimer’s disease stage, encompassing IoT-based early detection tools (cognitive assessments and wearables), mild-stage assistance systems (remote monitoring, cognitive aids, fall alerts, smart home integration, medication reminders, and location tracking), and severe-stage care and supervision.
These heterogeneous IoT-enabled interventions illustrate the adaptability of distributed sensor–actuator networks across the Alzheimer’s disease continuum, while simultaneously emphasizing the need for rigorous quantitative evaluation to ensure clinical reliability. In this work, the performance assessment of IoT-based classifiers for detecting Alzheimer’s-related biomarkers and activities (e.g., agitation episodes and gait anomalies) is grounded in (but not limited to) standard confusion matrix metrics and generic model decision functions.
The confusion matrix (CM) enumerates True Positives (TPs), False Positives (FPs), False Negatives (FNs), and True Negatives (TNs):
| (1) |
From these counts, accuracy is computed (overall proportion correctly classified):
| (2) |
Sensitivity (also termed the True Positive Rate (TPR) or recall) measures the proportion of actual positives detected:
| (3) |
Specificity (also termed the True Negative Rate (TNR)) measures the proportion of actual negatives correctly rejected:
| (4) |
The Area Under the Receiver Operating Characteristic Curve (AUC) is used and defined as the integral of the true positive rate (already introduced as sensitivity) over the false positive rate (FPR), where :
| (5) |
In general, for classification, representative Machine Learning (ML) decision functions are applied. For example, a Support Vector Machine (SVM) with a weight vector (w), bias (b), and input feature vector (x; engineered from multimodal sensor streams) produces the signed decision:
| (6) |
Additionally, an ensemble classifier aggregates M base learners () via non-negative weights (; normalized to sum to one) to yield a weighted vote:
| (7) |
Equations (1)–(5) define the evaluation metrics applied to Alzheimer’s-specific event detection from IoT sensor data, while Equations (6) and (7) specify canonical decision functions (linear Support Vector Machine and weighted ensemble) used to map feature representations to clinically meaningful risk categories [24,25,26].
3.1. Early-Stage Detection and Diagnosis
This section presents two major technology streams that underpin IoT-based early detection: cognitive assessment tools and wearable sensing platforms.
3.1.1. IoT-Based Cognitive Assessment Tools
IoT-enabled digital cognitive assessment tools are emerging as key enablers in the remote and automated detection of early-stage Alzheimer’s. These systems combine AI-driven analytics with real-time/recorded sensor inputs to assess cognitive decline and neurological changes. Figure 5 presents our synthesized illustration of a multi-stage pipeline in which a patient completes a series of cognitive tasks (sentence memory, scene memory, executive function, object differentiation, and fragmented letters) via a mobile or laptop device. Assessment scores are generated by both the AD-Digital Assessment Scale and a computerized cognitive assessment tool and combined with neuroimaging data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). These inputs feed into ML–based classification algorithms to produce diagnostic results for review by a clinician or caregiver.
Figure 5.
Workflow of Web-based and mobile app-based cognitive tasks assessment tools with machine learning enhancements for diagnostic classification (figure is created by authors).
The CogSAS mobile self-assessment scale, developed via a Delphi process and validated in 1272 participants across community and clinical settings, exhibited strong internal consistency (Cronbach’s = 0.81) and test–retest reliability (ICC = 0.82) and high sensitivity (100%) and specificity (78%) in biologically confirmed mild cognitive impairment (MCI)/AD cases (n = 83) [27]. Unsupervised remote digital memory composites implemented on smartphones achieved robust diagnostic accuracy (AUC = 0.83, 95% CI [0.66, 0.99]; sensitivity = 0.82 and specificity = 0.72) in a multicenter memory clinic sample of 199 individuals [28]. Feasibility analyses in the Framingham Heart Study (n = 537) reported high user confidence (76%) and ease-of-use ratings (81%) for longitudinal smartphone-based assessments [29]. Surveys involving 369 participants indicated high willingness (82%) for remote interactive video-based cognitive testing, although access disparities were noted by cognitive status [30]. MRI scans and speech and language processing have also been explored as non-invasive methods for early AD detection. A remote speech-based AI system was developed to screen for AD through smartphone applications, demonstrating its feasibility for large-scale and convenient diagnosis [31,32,33,34]. Despite these advances, challenges remain in scaling studies, ensuring long-term validation, integrating multimodal sensor data, and generalizing across diverse populations.
Digital cognitive tests such as the AD-Digital Assessment Scale (DAS) and the Computerized Cognitive Assessment Tool (cCOG) offer low-burden, at-home alternatives that outperform traditional biomarkers like cortical amyloid-beta (A) and entorhinal tau in specific contexts [32,35,36,37,38]. These systems also reduce rater bias and clinical workload by enabling frequent data collection from natural environments [39,40]. The diagnostic accuracy of these tools is further enhanced by deep learning models such as the Convolutional Learning Attention-Bidirectional Time-Aware Long Short-Term Memory (CL-ATBiLSTM) [41] and Convolutional Encoder–Decoder [42] architectures, which achieve classification accuracies as high as 96.5% and 99.4%, respectively, using datasets like the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
3.1.2. Wearable Sensors for Early Detection
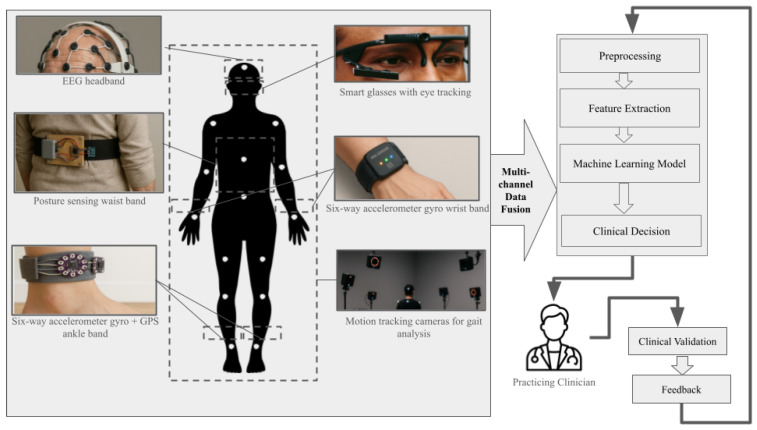
Wearable sensors form the second core pillar of IoT-based early AD detection. Figure 6 offers our consolidated depiction of sensing technologies, such as EEG headbands [43], posture-sensing waist belts [44], eye-tracking smart glasses [45] , multi-axis accelerometer/gyroscope wristbands [46], Inertial Measurement Unit (IMU)+GPS ankle bands/shoes [47], and motion-capture cameras—which are often studied either individually or in combination in the literature. On the right, the typical analytic workflow is illustrated, comprising multichannel data fusion, preprocessing, feature extraction, machine learning classification, and clinical decision support. Multichannel data fusion is an optional process depending on the type of research and sensor used. This consolidated diagram reflects general research practice in developing IoT-based systems for the early detection of AD; however, it is important to have the results validated by the practicing clinician and provide feedback to the system for continuous improvement.
Figure 6.
Schematic of an IoT-enabled multi-sensor system for continuous Alzheimer’s monitoring. Key components include an EEG headband, posture-sensing waist band, six-axis accelerometer/gyro wrist and ankle bands, smart glasses with eye tracking, and motion-capture cameras for gait analysis, all feeding into a multi-channel data fusion and machine learning pipeline for clinical decision support (figure created by authors).
In particular, IMU-based and oculomotor IoT wearables processed via ML classifiers have demonstrated robust performance in detecting early markers of cognitive decline [48,49,50,51]. For example, a waist-mounted inertial sensor system achieved 75.8% accuracy in distinguishing MCI from controls using static postural balance biomarkers [49], while a multi-kinematic gait model in 94 older adults yielded an AUC of 0.96 (95% CI 0.90–0.99), sensitivity = 0.90, specificity = 0.91, and overall accuracy = 0.90 [51]. Integrated eye-tracking networks further identified oculomotor anomalies with 86% accuracy in early-stage Alzheimer’s disease [50]. Similarly, deep learning architectures, such as convolutional encoder–decoders for brain imaging classification and multimodal sensor fusion combining speech, gait, and physiological stress inputs, showed enhancements in telemedicine scalability and patient-centric care [42,52,53,54,55,56].
On the other hand, wearables—such as smart watches, fitness bands, and smart textiles—provide continuous, noninvasive monitoring of heart rate, blood pressure, temperature, and oxygen saturation, facilitating early detection of physiological abnormalities in Alzheimer’s care [57,58,59,60,61]. Devices like the Empatica E4 and Apple Watch have shown high feasibility, acceptability, and adherence—even in rural settings—with users wearing them an average of 11.48 h/day over six months [62,63]. While biocompatibility and sensor drift continue to pose challenges to widespread adoption, advances in IoT frameworks and ML models are helping to overcome these limitations by enabling context-aware feedback and cloud-based analysis [64,65,66].
Current miniaturized electrochemical platforms detect Alzheimer’s biomarkers at ultralow concentrations and transmit results wirelessly. A handheld device achieved laboratory-grade accuracy for amyloid- and tau via Bluetooth [67], while an innovative VG@nanoAu-based immunosensor capable of simultaneously detecting multiple Alzheimer’s biomarkers—A, A, total tau, and p-tau181—with limits of detection as low as 0.07–0.09 pg/mL, and integrating smartphone-based readout for enhanced point-of-care usability [68]. MOF- and FET-based sensors report A and p-tau181 at sub-picogram sensitivity over 72 h operations [69], and a portable micro-workstation multiplexes A, A, and tau isoforms with cloud dashboard transmission [70]. Electrochemical biosensors—integrating electronic transducers with biological recognition elements—enable precise physiological monitoring for diagnostics and digital healthcare applications [71]. Table 3 summarizes five recent IoT-integrated electrochemical biosensors for key Alzheimer’s biomarkers.
Table 3.
Comparative analysis of IoT-based biosensors for Alzheimer’s disease biomarkers (2021–2024).
| Study | Sensor Type | Biomarker(s) | Linear Range | LOD | Key Metrics/IoT Integration | ||
|---|---|---|---|---|---|---|---|
| Liu et al. (2022) [72] | VG@Au aptasensor | Tau | 0.1–1000 pg/mL | 0.034 pg/mL | Bluetooth to smartphone; accuracy comparable to commercial device. | ||
| Li et al. (2023) [73] | Printed VG@nanoAu array |
|
– | 0.072–0.089– 0.071–0.051 pg/mL | Smartphone micro-workstation; high specificity and stability. | ||
| Chakari-Khiavi et al. (2023) [74] | Pt@ZIF-8 immunosensor | Cis-p-tau | 1 fg/mL–10 ng/mL | 1 fg/mL | Validated in patient serum; reproducibility and stability. | ||
| Ciou et al. (2023) [75] | GO/G SGFET | P-tau217 | 10 fg/mL–100 pg/mL | 10 fg/mL | 18.6 mV/dec sensitivity; ≈90% specificity; <2% drift over 7 days. | ||
| Kong et al. (2024) [76] | AuNP-GCE aptasensor | P-tau231 | 10–107 pg/mL | 2.31 pg/mL | Recovery 97.6–103.3%; RSD 3.27%; 7-day stability. |
Alternatively, some wearable chemical sensors sample sweat, saliva, tears, or interstitial fluid to discover noninvasive biomarkers [77]. Building on this approach, computationally optimized molecularly imprinted polymers—designed via density functional theory and molecular docking (QuantumDock)—have enabled sensitive phenylalanine detection in sweat, demonstrating the potential for tailored wearable MIP sensors [78].
Aptamer-based systems further expand the scope of noninvasive detection, yielding rapid and highly sensitive assays for AD biomarkers. Entropy-driven strand displacement assays detect miR-193b at 77 pM and A42 oligomers at 53 pM in exosomes [79], while fluorescent and colorimetric aptasensors measure p-tau231 down to 4.71 pg/mL [80]. Similarly, CNT-FET biosensors functionalized with aptamers achieve sub-femtomolar sensitivity for A and A in serum, offering rapid response, high specificity, and a wide dynamic range [81].
In parallel, microfluidic assay strips integrated with Bluetooth readers and smartphone apps have enabled multiplexed AD biomarker analysis in under 15 min, with automated cloud logging for decentralized screening [82]. Complementing this, an offline-capable deep learning app demonstrated the ability to analyze paper-based electrochemical amyloid- assays, securely transmitting encrypted results upon network reconnection to ensure robustness in intermittent connectivity settings [83].
Continuing the trend toward seamless and user-friendly monitoring, skin-like biosensors provide unobtrusive, high-accuracy tracking of pulse variability and oxygen saturation via optical phenomena, enhancing comfort and portability compared to traditional devices [84]. In addition to physiological sensing, behavioral detection methods—based on body movement analysis, eye movement tracking, and speech behavior quantification—enable objective, quantitative assessments that support early diagnosis in Alzheimer’s research [85]. Complementing these sensing modalities, closed-loop therapeutic systems—capable of adjusting interventions in real time based on biosensor feedback—offer enhanced personalized care [86].
The key challenges of these works include the lack of large, diverse clinical validations; sensor biocompatibility and signal drift under prolonged wear; and the absence of standardized data security frameworks for cloud-aggregated biomarker streams. Future work should prioritize multicenter trials of integrated biosensor networks, interoperability standards across device ecosystems, and real-world assessments of long-term stability, usability, and privacy protection to achieve scalable, connected Alzheimer’s diagnostics.
Recognizing the clinical intersection between AD and diabetes, several studies have explored diabetes-related insights for AD management. Continuous glucose monitoring (CGM) was investigated as a means to address hypoglycemia detection challenges in Alzheimer’s patients with comorbid type 2 diabetes (ADRD-DM)—a population at elevated risk for adverse glycemic events due to cognitive and psychosocial barriers [87,88]. While CGM is known to reduce hypoglycemic episodes in older adults with T2DM, its efficacy in individuals with concurrent dementia remains underexplored.
Beyond glycemic control, metabolic impairments and sleep disturbances were identified as modifiable risk factors in AD, with evidence suggesting that disrupted glucose metabolism impairs sleep quality and accelerates disease progression [89]. Efforts to achieve real-time, non-invasive glucose monitoring using optical absorption techniques have yet to achieve clinical reliability [90]. In contrast, advanced wearable systems—such as electromagnetic sensors inspired by vascular anatomy—have demonstrated strong correlation (>0.9) with blood glucose levels [91]. When paired with ML-based signal processing, these systems achieved 100% diagnostic accuracy in animal models and 99.01% accuracy in human trials [92].
Table 4 provides a concise overview and categorizes wearable biosensors—ranging from smart watches and smart textiles to advanced electrochemical and electromagnetic sensors—by their primary methods, key findings, and evaluation metrics. It highlights diagnostic accuracies (e.g., 86% for eye tracking and 99.01% for non-invasive glucose monitoring) alongside areas lacking quantitative data, thereby providing a clear framework for comparing current technologies and identifying gaps for future large-scale validation.
Table 4.
Summary of wearable biosensors in Alzheimer’s and related healthcare applications.
| Category | Methods | Results | Evaluation Metrics and Performance |
|---|---|---|---|
| Wearable Biosensors for Alzheimer’s Monitoring | Smart watches, fitness bands, and smart textiles with flexible electronics for long-term tracking [57,58,59,60] | High feasibility and adherence; Empatica E4 and Apple Watch widely accepted, including in rural settings [62,63] | High adherence; no specific metrics |
| Digital Biomarkers and AI-Driven Diagnostics | IoT-enabled wearables with cloud-based ML for digital biomarker extraction [50,64] | Eye tracking distinguished AD patients with 86% accuracy [50]; real-time alerts provided [64] | Accuracy: 86% (eye tracking) [50] |
| Advances in Biosensors for Broader Healthcare | Electrochemical and optical biosensors for vital signs and analytes [65,93,94,95] | Enhanced noninvasive monitoring via OLED/OPD sensors [94] | Improved signal quality; no quantitative metrics |
| Behavioral Detection and AI-Enabled Monitoring | Biosensors for movement, eye tracking, and speech analysis [85,86] | Real-time behavioral symptom tracking via sensor fusion | Objective assessments; no metrics provided |
| Continuous Glucose Monitoring in ADRD-DM Patients | IoT-integrated CGM for hypoglycemia detection in ADRD-DM patients [87,88] | Reduced hypoglycemic events; limited ADRD-DM data [88] | Correlation >90% with blood glucose [91] |
| Wearable Glucose Monitoring and Non-Invasive Systems | Flexible electromagnetic sensors with ML-based processing [92,96,97] | 99.01% human and 100% animal accuracy [92] | Accuracy: 99.01% (human), 100% (animal) [92] |
| Multi-Sensor Integration for Personalized Healthcare | Multi-sensor glucose monitoring with Bayesian inference [98] | Explained 40–65% HR variance; 15% glucose variability | Correlation: 40–65% (HR), 15% (glucose) [98] |
| IoT and Smart Clothing for Healthcare | AI-driven IoT models and smart textiles for passive monitoring [99,100,101] | Enhanced adherence and unobtrusive monitoring | No quantitative evaluation |
Table 5 summarizes leading IoT-based early detection technologies. Despite promising outcomes, limitations—including modest sample sizes, cross-sectional designs, sensor-placement variability, and lack of longitudinal or multicenter validation—warrant attention in future large-scale deployments.
Table 5.
Summary of IoT-based early-stage detection technologies.
| Technology and Study | Sample Size (n) | Setting | Performance Metrics | Key Limitations |
|---|---|---|---|---|
| CogSAS mobile self-assessment [27] | 1272 (validation); 83 MCI/AD | Community and clinical | Cronbach’s ; ICC = 0.82; sensitivity = 100%; specificity = 78% | Cross-sectional; limited longitudinal data; clinically confirmed only |
| Remote digital memory composite [28] | 199 (memory clinic) | Multicenter memory clinics | AUC = 0.83 (95% CI [0.66,0.99]); sens = 0.82; spec = 0.72 | No home-use validation; moderate specificity |
| Smartphone-based assessments [29] | 537 (Framingham cohort) | Community (longitudinal study) | User confidence = 76%; ease of use = 81% | Lacks diagnostic accuracy metrics; self-report bias |
| Video-based cognitive testing survey [30] | 369 (participants) | Community (survey) | Willingness = 82% for remote testing | Access disparities by cognitive status; no efficacy data |
| Ambient smart home ADL monitoring [102] | 36 (pilot: healthy, SCD, MCI) | In-home smart apartments | Classification accuracy up to 90% on ADL task durations | Small pilot; single site; task-based only |
| Multi-kinematic gait IMU [51] | 94 (33 CN, 61 aMCI) | Lab-controlled walking tasks | AUC = 0.96; sens = 0.90; spec = 0.91; acc = 0.90 | Controlled setting; sensor placement variability |
| Waist-mounted balance IMU [49] | 60 (30 CN, 30 MCI) | Four static standing tasks | Accuracy = 75.8% after SHAP feature selection | Cross-sectional; no dynamic walking analysis |
| Eye-tracking IoT system [50] | 48 (early-stage AD) | Clinical oculomotor lab | Accuracy = 86% in detecting anomalies | Requires specialized hardware; small cohort |
In summary, early-stage detection of Alzheimer’s leverages two main IoT approaches. First, digital cognitive assessment tools enable remote, automated testing—via mobile apps or smart home sensors—that deliver consistent, reliable scores and strong diagnostic accuracy in community and clinical settings. Second, wearable platforms (e.g., IMU-based gait and balance sensors, eye-tracking glasses, EEG headbands, and emerging skin-like and electrochemical biosensors) provide continuous, noninvasive monitoring of movement, oculomotor function, vital signs, and metabolic markers. Together, these technologies achieve high sensitivity and specificity in detecting subtle neurological and physiological changes, though broader, long-term, multicenter validation is still needed to ensure scalability and generalizability.
3.2. Mild-Stage Management and Assistance
Mild-stage AD introduces functional and cognitive challenges that necessitate continuous yet unobtrusive monitoring, timely interventions, and support systems that promote safety and independence. IoT technologies aid in this stage by enabling remote monitoring, cognitive support, and real-time fall and emergency detection.
3.2.1. Remote Monitoring and Activity Tracking
IoT-enabled wearables and ambient sensors, in combination with AI algorithms, provide real-time, non-intrusive monitoring of activities of daily living (ADLs) to detect subtle behavioral changes [20,103,104]. Feasibility studies, such as the RADAR-AD project, have demonstrated that remote monitoring technologies provide objective, continuous measurements of cognitive and functional status in mild-to-moderate Alzheimer’s populations [105,106].
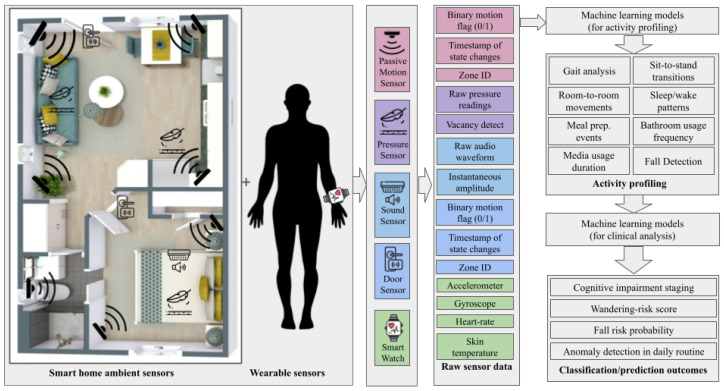
Figure 7 depicts a generic IoT–AD workflow for remote monitoring and activity tracking, showcasing a combination of different popular approaches used across the research. It uses ambient sensors (PIR, pressure, sound, and door contacts) and wearable devices (wrist-worn IMUs and biometric watches), which capture heterogeneous data streams—motion flags, pressure arrays, audio, door state changes, accelerometer/gyroscope readings, heart rate, etc. These feeds undergo ingestion, preprocessing, optional multichannel fusion, and feature extraction before classification models (including CNNs) profile activities (gait, sit to stand, room transitions, sleep/wake cycles, meal prep, and falls) and generate clinical predictions (cognitive staging, wandering risk, fall-risk estimation, and anomaly detection) [107,108].
Figure 7.
Generic consolidated workflow for multimodal IoT-based activity profiling and clinical analysis in Alzheimer’s research. (figure created by authors).
Pilot studies of IoT-based remote monitoring in mild Alzheimer’s populations have demonstrated feasibility and preliminary performance. Ambient and wearable sensors have been used to capture continuous data—e.g., David et al. instrumented 82 older adults (mean age of 80.4 ± 7.8 years), collecting 147,203 measurements over 958,000 participant hours, with engagement stable on 56.2% of days and alert rates of 0.066–0.233 per person day for the detection of infections and bradycardia [109]. Rawtaer et al. tracked daily steps, sleep interruptions, and time away from home in 49 individuals (28 MCI and 21 controls), observing nonsignificant trends toward fewer steps (3407 vs. 4033 steps/day) and more awakenings (2 vs. 1/night) in MCI [110]. Similar pilots achieved ADL classification accuracies up to 88% in 36 participants using motion and door sensors [102] and 85% accuracy for early MCI-related gait and sleep changes with infrared motion and mattress sensors in 30 homes [111]. Complementing ambient systems, Apple Watch-based monitoring in seven cognitively impaired individuals yielded over 700,000 multimodal observations (heart rate, steps, accelerometry, and sleep) with 84.9% daily wear adherence (11.48 h/day) over six months [112]. Reviews of wearable IoT devices for Alzheimer’s care highlight potential to improve patient independence and reduce caregiver burden [113], and prototype systems incorporating CNN-based facial recognition, GPS tracking, and steganography demonstrate secure data transmission despite limited multimodal integration with other diagnostic tools [114].
As discussed previously in Section 3.1.2 and as shown in Figure 6, data from wearable sensors can be used to provide various useful insights at different stages of AD. Wearable IMUs have been extensively validated as noninvasive gait biomarkers for the detection of cognitive impairment. Shank-mounted sensors under single- and dual-task paradigms distinguished MCI from cognitively normal individuals with 71.67% accuracy and 83.33% sensitivity in dual-task walking [115], while center-of-mass IMUs achieved an AUC of 0.811 based on gait speed and variability [116]. Incorporating Timed Up-and-Go (TUG) tasks, generalized models using shank-mounted IMUs reported 86.94% accuracy and 97.40% sensitivity for preclinical screening [117].
Non-contact and vision-based platforms complement wearable sensors by leveraging radar and depth-camera modalities. The STRIDE system integrates millimeter-wave Doppler radar with deep learning and digital-twin simulations to derive personalized gait signatures for Alzheimer’s risk assessment [118]. A deep learning-assisted Azure Kinect setup achieved 93.2% accuracy in gait segmentation, outperforming heuristic approaches [119], and Kinect v2 depth cameras with Random Forest classifiers on 25-joint skeletal features yielded 85.5% accuracy and an 83.9% F1 score in MCI detection during straight and oval walking [120]. Beyond diagnostic classification, specialized gait analysis algorithms enhance fall-risk and anomaly detection. A single waist-worn IMU with deep learning for near-fall detection achieved over 98% accuracy across ADL [121], and wireless pressure-sensor insoles coupled with Random Forest models predicted fall risk with 81% accuracy and 88% specificity [122].
Table 6 compares recent IoT-integrated gait analysis devices, highlighting their protocols and diagnostic performance [51,120,123]. Together with the aforementioned non-contact and near-fall detection studies, these wearable and vision-based platforms offer robust, noninvasive biomarkers for early detection and monitoring of Alzheimer’s disease.
Table 6.
Comparative analysis of sensor-based gait analysis devices for Alzheimer’s and MCI detection (2022–2024).
| Study | Sensor | Protocol | Key Metrics | Performance |
|---|---|---|---|---|
| Li et al. (2023) [123] | MATRIX 2.0 IMU | Single/dual task (walking; minus-seven subtraction) | Pace asymmetry; rhythm; variability | ST: AUC 0.744 (sens 100%, spec 45%); DT: rhythm AUC 0.734 (sens 85.2%, spec 57.5%), variability AUC 0.719 (sens 55.6%, spec 97.5%) |
| Huang et al. (2022) [51] | JiBuEn® inertial-shoe system | Simple gait tasks | TUG time; stride length; joint angles; OLS time; braking force | AUC 0.96; sens 0.90; spec 0.91; acc 0.90 |
| Seifallahi et al. (2024) [120] | Kinect v2 depth camera | Straight and oval-path walking | Skeletal features (25 joints) | RF: acc 85.5%; F1 83.9% (oval) |
Advanced AI and ML techniques enhance activity recognition and anomaly detection in these platforms. Deep neural networks have been applied to food-intake monitoring in AD patients with high predictive accuracy (activity analysis: 78%; object detection: 74%; image classification: 96%) [124], and Naive Bayes classifiers achieve robust ADL recognition (AC: 89.2%) in smart homes despite challenges in unpredictable behavior [125]. Probabilistic model checking using Markov chains enables early anomaly detection in patient behaviors [126], while habit-recognition frameworks track dementia progression over time [127]. Cyber-physical systems leveraging frequency-based locomotion features outperform traditional spatio-temporal methods for movement-pattern recognition in smart home environments [128].
Meanwhile, the integration of robotics and AI-assisted caregiving was explored as a means to enhance home-based elderly care. An Internet of Robotic Things (IoRT) framework was proposed, combining humanoid robots, AI, and sensor networks for continuous health monitoring. This system included an ML model based on the Modified Early Warning Score (MEWS), which delivered real-time health updates to caregivers, thereby improving responsiveness in domestic care environments [129]. However, the study did not assess long-term user acceptance or the practical challenges of real-world deployment.
In parallel, sleep and circadian rhythm monitoring using wearable and contactless sensors has consistently linked disrupted sleep and altered biological rhythms with cognitive decline [130,131,132,133]. While actigraphy-based devices offered a non-invasive means of tracking sleep, they often overestimate total sleep time compared to polysomnography, which is considered the clinical gold standard [134]. Additionally, passive monitoring technologies remain significant barriers to widespread implementation [135]. IoT-based remote health monitoring has advanced with deep learning models analyzing physiological data such as ECG signals, blood oxygen levels, and temperature to detect anomalies. A CNN with an attention layer was used to classify different heartbeat categories and fever conditions, ensuring real-time medical response through automatic doctor connectivity [136,137].
Table 7 summarizes key IoT-enabled remote monitoring studies for mild-stage Alzheimer’s disease, highlighting diverse sensor configurations—from wearable accelerometers and heart-rate monitors to ambient motion and mattress sensors—and their preliminary performance in real-world settings. Despite promising accuracy and engagement metrics, these studies are constrained by small, cross-sectional cohorts (n = 7–82); short follow-up periods, deployment and data-quality challenges; and limited demographic diversity. Future research should focus on larger, multicenter, longitudinal trials with standardized benchmarks, unified metrics, and user-centered design to validate and scale IoT-based monitoring solutions for Alzheimer’s care.
Table 7.
Summary of IoT-Enabled remote monitoring technologies for mild-stage Alzheimer’s disease.
| Technology and Study | Sample Size (n) | Setting | Performance Metrics | Key Limitations |
|---|---|---|---|---|
| [102] | 36 (healthy, SCD, MCI) | Smart home apartment | ADL task duration classification accuracy up to 88% | Pilot scale; single environment; limited ADL scope |
| [109] | 82 | Community-dwelling homes | 147,203 measurements over 958,000 h; 56.2% daily engagement; alert rate of 0.066–0.233/person-day; early detection of acute events | Single-site cohort; no long-term outcome data |
| [110] | 49 (28 MCI, 21 controls) | In-home passive sensors | Steps/day: 3407 vs. 4033; awakenings/night: 2 vs. 1; trends NS | Underpowered; lack of refined biomarkers |
| [112] | 7 | Home (Apple Watch) | >700,000 observations; 84.9% wear adherence (11.48 h/day) over 6 months | Very small sample; no control; limited generalizability |
| [111] | 30 | Home (infrared + mattress sensors) | ∼ 85% accuracy detecting gait and sleep changes | Limited home diversity; short monitoring window; needs larger trials |
3.2.2. IoT-Based Cognitive and Memory Aids
Advances in digital technologies have led to the development of interactive tools aimed at supporting cognition and daily function in older adults. Under this sub-section, interventions using smartphones, VR, voice assistants, and ambient intelligence systems are evaluated for their ability to enhance memory, independence, and engagement.
Smartphone reminder and digital-recorder apps were evaluated in a 4-week randomized controlled trial with 52 older adults, yielding significant improvements in prospective memory performance (baseline: 51.7 ± 27.8%; post intervention: p < 0.001; np2 = 0.285, d = 1.75) and enhanced independence in instrumental ADLs [138]. A pilot fuzzy-logic virtual reality (VR) system integrating six standard cognitive tests (Mini-Cog, SPMSQ, MMSE, SLUMS, CDR, and CASI) was studied in 24 participants aged 50–65 years; the system’s composite score correlated strongly with traditional assessments, and 87.5% of users rated it “good” or better on the System Usability Scale [139]. An e-coaching framework integrated with IoT devices was designed for remote rehabilitation, allowing for real-time measurement of patient performance in directed tasks, demonstrating high accuracy in activity recognition [140]. Voice-assistant cueing (e.g., Alexa) was qualitatively assessed by two family carers and eight professionals via focus groups and case studies, demonstrating feasibility for task reminders and environment control, though formal efficacy data are lacking [141]. The DCARE ambient intelligence framework combines wearable sensors, GPS, and heart-rate variability to generate context-aware prompts; caregiver interviews confirmed its utility, but no clinical outcomes have yet been reported [142]. A proof-of-concept ambient assisted living system with indoor positioning, augmented reality prompts, and a cloud-based fuzzy decision engine demonstrated >90% decision accuracy and sub-second response times in simulated scenarios yet awaits real-world trials [143]. Immersive VR-based cognitive training in a 5-week pilot (n = 31) showed high tolerability (low cybersickness incidence) and preliminary gains in global cognition and mood [144], while a systematic review of 22 VR reminiscence therapy studies reported consistent memory maintenance and emotional benefits but highlighted study heterogeneity and short follow-ups [145]. Finally, a pilot of a smartphone “everyday tasks” assessment (ASSET) in 46 adults demonstrated good test–retest reliability (ICC = 0.87) and construct validity against observer-rated function [146], and an algorithmic spaced retrieval app (n = 61) yielded large memory gains (Cohen’s d = 2.32 for new facts) without adverse effects [147]. Common limitations across these studies include small samples (n = 20–52), short durations (4–5 weeks), lack of long-term follow-up, and variability in outcome measures, underscoring the need for larger, standardized RCTs to establish efficacy and scalability.
3.2.3. Fall Detection and Emergency Alerts
As Alzheimer’s disease progresses, individuals face increasing risks of disorientation, mobility loss, and unattended medical emergencies, making IoT-based fall detection and emergency response systems essential for real-time monitoring and timely intervention. Figure 8 presents our illustration of a consolidated overview of various IMU-based fall detection systems studied during the review process. Raw three-axis accelerometer and gyroscope waveforms—captured during normal gait and during a fall event—are first preprocessed with low-pass and high-pass filters. The continuous stream is then segmented into analysis windows via sliding-window or event-triggered methods. Each window yields temporal features (acceleration magnitude, jerk, mean, variance, skewness, signal magnitude area, and RMS) and spectral features (spectral energy and spectral centroid). The peak impact index () and the post-impact inactivity metric () feed into a simple threshold decision rule:
where triggers a fall alert. This diagram captures the core stages—filtering, segmentation, feature extraction, and decision-making—used in the majority of IMU-only fall detection frameworks [148].
Figure 8.
Generic IMU-only fall detection workflow: raw three-axis accelerometer and gyroscope signals are filtered, segmented, and transformed into temporal and spectral features, then evaluated via a threshold rule on impact magnitude and post-impact inactivity to generate a fall alert (figure is created by authors).
Most existing IoT solutions deploy accelerometers, gyroscopes, and vital sign sensors to detect falls in real time and notify caregivers [149,150]. Several wrist- and chest-worn devices have demonstrated high sensitivity and specificity in simulated and daily-living scenarios [151]. A wrist-worn system with gyroscope/accelerometer sensors achieved 93% sensitivity and 95% specificity in 13 AD patients [152], while a logistic regression model of wrist-strap accelerometer data reported 66% accuracy across 40 simulated falls [153]. Chest-mounted nine-axis IMUs in 30 elderly subjects yielded 97.1% sensitivity and 94.3% specificity [154], and a “CURA” finger-strap platform combining ML inference with IoT alerts exceeded 90% detection accuracy in 12 simulated falls [155]. However, there is scope to improve the accuracy by using more complex algorithms.
Due to the limited compute capacity of offline devices, implementation of complex algorithms is restricted. However, by leveraging the benefits of edge and cloud computing, this challenge can be addressed. Cloud gateways processing three-axis accelerometer features with KNN/ENN achieved >98.5% accuracy on the UniMiB-SHAR dataset [156]. A survey of 65 IoT fall detection studies noted accelerometer wearables’ dominance but highlighted a lack of Alzheimer’s-specific evaluations [157]. A BMC-based smart home nursing pilot (n = 20) integrating waist and chest sensors provided early-warning alerts, though clinical validation remains pending [158].
Beyond direct fall detection, ambient gait analysis improves fall-risk prediction: ML models incorporating gait features achieved an AUROC of 76.2% versus 56.2% without such inputs [159]. AI-powered eHealth systems that trigger health interventions reduced emergency-department visits from 13.4% to 1.5% in a 206-patient trial, demonstrating the impact of real-time monitoring and predictive analytics [160].
As Alzheimer’s disease advances into the severe stage, the focus shifts from monitoring to full-scale supervision and assistive care. While IoT technologies in mild-stage care emphasize cognitive support, activity tracking, and fall prevention, the next section explores how smart home automation, AI-driven interventions, and continuous surveillance systems support patients in the later stages of the disease.
3.3. Severe-Stage Care and Supervision
As AD progresses into its severe stage, the need for intelligent, real-time, and context-aware care becomes paramount. Patients often face challenges such as medication non-adherence and wandering behavior that compromise their safety and increase caregiver burden. IoT-powered technologies play a critical role in mitigating these issues through integrated systems that include smart home automation, automated medication management, and advanced location tracking.
3.3.1. IoT-Enabled Smart Home Systems
Smart homes equipped with IoT sensors can continuously track ADLs and detect deviations characteristic of advanced dementia. Chimamiwa et al. conducted a systematic review of smart home technologies for older adults with dementia, noting that while activity recognition and anomaly detection approaches using motion and contact sensors can identify deviations from habitual routines, they remain insufficient to track habit progression across dementia stages, underscoring the need for longitudinal, real-world validations [127]. Tiersen et al. employed an iterative, user-centered design methodology involving semistructured interviews with 9 people with dementia, 9 caregivers, and 10 health professionals, as well as workshops with 35 patient–caregiver pairs and 12 clinicians, to elicit functional, psychosocial, and environmental requirements for ambient sensing in dementia households, demonstrating high stakeholder engagement but without reporting quantitative accuracy metrics [161].
Ambient IoT systems for severe Alzheimer’s combine environmental (e.g., PIR motion, door, pressure, and temperature) and wearable (e.g., accelerometer, ECG, and EDA) sensosr to continuously monitor patients’ daily behaviors and contextual factors, enabling unobtrusive detection of emergencies and neuropsychiatric symptoms. In a study by Davidoff et al. [162], physiological agitation in dementia patients was assessed through wearable sensors (Chill+), capturing photoplethysmogram (PPG), electrodermal activity (EDA), skin temperature (ST), and accelerometry (ACC) data. Conducted as a cross-sectional repeated-measures study with 30 participants monitored over a week, the system demonstrated strong associations between sensor-derived features and agitation levels, with GLMM models showing statistically significant correlations ( = 0.224 to 0.753). The study revealed that different physiological markers correlate with specific agitation types—motor or verbal—though generalizability remains limited due to the small sample size and the need for personalized modeling. In another work, Javed et al. [163] developed a machine learning-based cognitive health assessment framework in smart homes, integrating ambient and wearable IoT sensors to capture ADLs. Though the study did not provide real-world patient data and primarily focused on simulations, it reported high diagnostic accuracy (94.1%), underscoring the potential of such systems for automated cognitive monitoring. However, its practical applicability is restricted due to a lack of validation in real-life settings and responsiveness analysis. Complementing these findings, a recent pilot study by Grammatikopoulou et al. [102] explored the use of ambient sensor networks in IoT-enabled smart homes to differentiate between healthy individuals, those with MCI, and AD patients. With 37 participants grouped into 11 healthy, 15 MCI, and 11 AD cases, the study identified statistically significant differences in ADL patterns across groups, supporting the clinical utility of activity monitoring for cognitive decline detection. Although the study’s short duration and small cohort size limit its predictive value, it provides compelling evidence for the use of sensor data in cognitive state assessment.
Palermo et al. released the TIHM dataset, aggregating PIR, door, under-mattress, and physiological sensor data from 56 homes over 50 days, facilitating the development and benchmarking of predictive analytics for adverse event detection in people living with dementia [164]. Despite these advances, limitations include small and non-diverse cohorts (–96); brief monitoring durations (4 weeks–12 months); reliance on pilot-scale deployments; and lack of multicenter, longitudinal validation, underscoring the need for large-scale clinical trials with standardized outcome measures to translate ambient IoT monitoring into practice.
Smart home automation for assisted living has been examined in multiple studies. Lee et al. [165] introduced IADLSys, a system using wireless physical tags, wearable sensors, and cloud-based data processing for remote ADL monitoring. Findings showed discrepancies in self-reported ADLs versus objective measurements, reinforcing the necessity of automated tracking. However, the study had a limited sample size, indicating a need for larger-scale research. The authors of [166] explored a personalized home automation intervention for AD caregiving, highlighting benefits such as improved safety and reduced caregiver burden. However, technology unfamiliarity remained a barrier, necessitating early intervention strategies for smoother adoption.
3.3.2. Automated Medication Reminders and Adherence Support
Medication adherence is a critical component of AD management, particularly in the severe stages, where patients are more likely to forget dosages or miss schedules. IoT-enabled medication management systems integrate smart reminders, pill dispensers, and monitoring tools to ensure consistent treatment and reduce caregiver burden.
IoT-enabled medication aids for severe Alzheimer’s disease integrate smart dispensers, ambient alert systems, and AI-driven scheduling to improve on-time dosing and reduce missed doses. Sharanya et al. designed and prototyped an IoT-enabled pill dispenser featuring NFC-based user identification and automated intake logging; technical bench testing confirmed device feasibility, though no pilot data on patient adherence or clinical deployment has been presented [167]. Peddisetti et al. deployed a networked pill dispenser paired with a “smart cup” in a four-week pilot of patients (mean age of 78.2 ± 6.5 years), logging dispensation events via MQTT and reporting adherence improvement from 58% at baseline to 94% (dispensing accuracy = 97%, mean latency = 1.2 s) [168]. Additional systems—for example, cloud-connected voice assistants and wearable alert bands—have demonstrated adherence rates of 85–90% in small trials (n = 12–18) but lack rigorous clinical validation. Common limitations include small, homogeneous samples (n < 30), short intervention periods (<6 weeks), reliance on caregiver setup, and absence of long-term safety or usability data, underscoring the need for multicenter, longitudinal RCTs with standardized adherence metrics to translate these IoT solutions into routine care.
3.3.3. Location Tracking and Wandering Prevention
One of the most critical threats in severe-stage Alzheimer’s is patient wandering. IoT-based location tracking systems—integrated with environmental sensors and wearable devices—offer real-time geolocation, behavioral analysis, and caregiver alerts to prevent risky mobility scenarios. Fine-grained motion analysis via inertial sensors enhances detection of wandering and fall risk. Waist-mounted IMUs in 12 cognitively impaired elders detected wandering with 80% sensitivity and 85% specificity [169], and combined gait and balance sensors in 30 Alzheimer’s patients achieved 89% accuracy for fall-risk and wandering-pattern classification [170]. Chest-worn IMUs enable sub-second turn detection but lack direct wandering metrics [171]. Ethical analyses underscore autonomy and privacy concerns, calling for co-design with persons with dementia [172].
Advanced AI-enhanced IoT frameworks incorporate adaptive learning and robust communication protocols—such as LoRa—for context-aware monitoring and real-time interventions [103,173,174,175]. Specific implementations include wearable GPS/MEMS devices for location and fall detection [176,177]; dorsal belts with GPS, Wi-Fi, and Kalman filtering for improved tracking accuracy [178]; and environmental design frameworks that optimize spatial orientation through layout and color schemes [179]. AI-driven predictive models apply CNN architectures to indoor sensor-derived spatial images for high-precision wandering detection [180]. BLE beacon systems deliver sub-3-second alerts [181], while comprehensive IoT platforms integrate MQTT, HTTP, WebSocket, cloud dashboards, device authentication, and emergency transport integration to address scalability and security [182].
IoT-enabled wandering prevention systems integrate facial recognition, geofencing, reminders, and continuous tracking to support severe-stage Alzheimer’s care. Figure 9 shows our synthesized depiction of a generic wandering system as observed across the literature during review. It shows an IoT-based geofencing and anomaly detection system for Alzheimer’s patients. Using GPS and a mobile phone as an edge node, the system monitors the patient’s location, detects if they exit a predefined safe zone, and checks for unusual movement patterns. If an anomaly is detected, alerts are automatically sent to family members and caregivers to enable timely intervention. Kanchanamala et al. deployed facial authentication, GPS geofencing, and scheduled audio/video reminders in 20 patient–caregiver dyads, achieving 95% on-time medication events and a 40% increase in medication recall (p < 0.01) [183]. Commercial GPS devices used with 45 dyads over six months yielded 93% caregiver satisfaction and uninterrupted logging [184], while reviews report mean localization errors below 15 m and battery life exceeding 8 h [185]. Trajectory-classification algorithms on the Geolife dataset (n = 182) using discrete wavelet transforms and geofencing achieved 83.06% accuracy, 92.62% precision, and an 87.58% F1 score in wandering detection [186]. Similarly, wearable IoT systems have been integrated into long-term care environments to monitor physiological data, prevent wandering through electronic fences, and facilitate remote health management [187].
Figure 9.
IoT system combining GPS, smart home sensors, and edge analytics to monitor geofenced movement and alert caregivers of wandering in Alzheimer’s patients. (figure created by authors).
Collectively, these systems report accuracies between 80% and 94%, demonstrating feasibility in proof-of-concept studies. However, most evaluations rely on small, non-diverse cohorts, short monitoring periods, or simulated or retrospective datasets and lack standardized benchmarks or longitudinal validation, highlighting the need for larger, multicenter, and long-term clinical trials.
Table 8 provides a concise overview of the key IoT-based interventions deployed in severe-stage Alzheimer’s care, including smart home systems, medication support, and location-tracking solutions. Despite the promise of IoT-enabled smart homes, medication supports, and location-tracking systems in severe Alzheimer’s care, several challenges remain. First, most deployments to date involve small, homogeneous cohorts (–96) and short monitoring windows (4 weeks–12 months), limiting statistical power and generalizability. Second, pilot-scale implementations often lack multicenter or cross-cultural validation, raising questions about performance in diverse real-world settings. Third, heterogeneous sensor configurations and proprietary communication protocols impede interoperability and large-scale integration into existing healthcare infrastructure. Fourth, while edge-based analytics (e.g., PRISM) reduce latency, they have exhibited notable performance degradation (e.g., 15% accuracy drop) under cross-home evaluation. Finally, regulatory approval pathways for medical IoT devices remain immature, and long-term usability, data security, and patient privacy considerations have yet to be systematically addressed, underscoring the need for standardized outcome measures, extended longitudinal trials, and robust ethical frameworks.
Table 8.
Summary of IoT-based interventions for severe-stage Alzheimer’s care.
| Study | Domain | Method | Sample (n) | Performance Metrics | Limitations |
|---|---|---|---|---|---|
| [102] | Cognitive Assessment | IoT-based sensor monitoring of ADLs across groups | 37 (11 HC, 15 MCI, 11 AD) | Statistically significant group-wise variation | No prediction modeling; short duration |
| [188] | Behavioral Monitoring | Personalized ML on multimodal wearable sensors (e.g., PPG, EDA) | 28 participants; 16 training, 12 testing | F1 = 0.69; precision = 0.75; recall = 0.65 | Small cohort; requires personalization |
| [189] | Cognitive Detection | Secure Federated Learning on neuroimaging + clinical data | ADNI dataset (n = 1004) | Accuracy = 91.4%, F1 = 91.7% | No real-world IoT sensor integration |
| [190] | Routine Behavior Monitoring | ML on in-home sensor event logs for ADL routines | 35 older adults over 8 weeks | Differentiation of routines and changes | No clinical dementia categorization |
| [191] | Passive Monitoring | Device-free Wi-Fi sensing for motion and ADL tracking | 16 older adults in low-income housing | Feasibility of ADL trend monitoring | No dementia-specific outcomes yet |
| [192] | Biomarker Monitoring | Multimodal federated learning across edge sensors | 91 elderly subjects in 4-week trial | Accuracy = 93.8%; Early AD detection = 88.9% | Complex system; short deployment duration |
| [127] | Smart Home Systems | Systematic review of motion/contact sensors | — | Qualitative insights | No longitudinal validation |
| [161] | Smart Home Systems | Interviews & workshops | 9 PWD, 9 CG, 10 HP; 35 pairs; 12 clinicians | Stakeholder engagement | No quantitative metrics |
| [167] | Smart Home Systems | NFC pill dispenser prototype | — | Bench testing feasibility | No clinical data |
| [162] | Behavioral Monitoring | Wearable sensor profiling (PPG, EDA, ST, and ACC) + GLMM | 30 | Agitation detection ( = 0.224–0.753) | Small cohort; patient-specific tuning |
| [163] | Cognitive Assessment | ML on ambient + wearable sensor data in smart homes | — (Simulated) | Accuracy = 94.1% | No real-world deployment |
| [164] | Smart Home Systems | TIHM dataset (PIR, door, mattress sensors) | 56 homes | — | Dataset only; no evaluation |
| [168] | Medication Support | IoT pill dispenser and smart cup | 33 | Adherence: 58%→94%; accuracy: 97% | Small n, <6-week trial |
| [183] | Medication Support | Face recognition and geofencing reminders | 21 | 95% on time; recall: +40% | Small n |
| [184] | Location Tracking | Commercial GPS trackers | 45 dyads | Satisfaction: 93% | No formal accuracy |
| [186] | Location Tracking | Wavelet and geofencing on GPS data | 182 trajectories | Acc: 83.06%; Prec: 92.62%; F1: 87.58% | No in situ testing |
| [169] | Wandering Prevention | Waist IMU sensors and ML models | 12 | Sensitivity: 80%; specificity: 85% | Small cohort |
| [170] | Wandering Prevention | Gait and balance IMUs | 35 | Accuracy: 89% | No AD-specific validation |
| [171] | Wandering Prevention | Chest-worn IMU turn detection | 23 | Sub-second turn detection | No wandering metrics |
ML underpins modern IoT strategies for AD by translating heterogeneous digital signals—gait kinematics, neuroimaging volumes, multimodal environmental streams, and physiological or behavioral traces—into discriminative biomarkers. Different algorithmic families offer complementary strengths under distinct application umbrellas: (i) Mobility and gait analytics leverage classical and ensemble classifiers on derived pace, rhythm, and variability features from wearable IMUs. (ii) Neuroimaging classification applies deep and hybrid feature–ensemble pipelines to high-dimensional fMRI. (iii) Multimodal ambient and clinical streams exploit recurrent and residual architectures to learn temporal dependencies across audio–video–motion data. (iv) Real-time human activity and fall detection utilize optimized deep or ensemble models for low-latency inference on wearable sensor networks. Representative performance comparisons are summarized in Table 9.
Table 9.
Performance comparison of key ML algorithms in IoT-based Alzheimer’s detection.
| Model | Dataset | Input Modality | Accuracy | AUC | Precision | Recall |
|---|---|---|---|---|---|---|
| SVM [193] | Augmented gait lifelog (wearable IMUs) | Pace, rhythm, and variability features | 87.9% | 76.4% | 100% | 42.9% |
| Random Forest [193] | Augmented gait lifelog (wearable IMUs) | Pace, rhythm, and variability features | 78.8% | 80.8% | 50.0% | 57.1% |
| XGBoost [194] | ADNI fMRI (n ≈ 800) | Cortical ROI features (CNN-extracted + handcrafted) | 98.8% | 98.82% | — | 98.9% |
| CNN [194] | ADNI fMRI (n ≈ 800) | Raw fMRI volumes (3D CNN) | 88% | — | 92% | 89% |
| DRN–LSTM [195] | IoT-assisted hospital testbed | Audio, video, and motion sensors (DRN-LSTM + PI-HHO) | 98% | — | 97% | — |
Ensemble methods (Random Forest and XGBoost) generally surpass linear SVM on heterogeneous sensor features by modeling nonlinear interactions without extensive manual engineering, while XGBoost attains near-perfect discrimination on hybrid neuroimaging feature sets. Deep 3D CNNs deliver strong direct volume classification but slightly lower accuracy than feature–ensemble hybrids. Temporal DRN–LSTM architectures excel on continuous multimodal streams with high precision. In mobility and safety monitoring, real-time human activity recognition reaches 98.28% accuracy across 12 motions [196], and ensemble deep learning for fall detection achieves 98% detection and 96% pre-fall prediction accuracy using wearable accelerometer/gyroscope data [197], underscoring ML’s central role across application domains.
4. Discussion and Future Research Directions
This section provides a structured overview of key areas requiring attention. The first subsection reviews advancements in IoT–enabled early diagnosis, including retinal and other ocular biomarkers, multimodal imaging with deep learning, eye tracking-based digital measures, and emerging molecular and nanosensor approaches. The next subsection addresses the integration of AI with IoT for personalized treatment and remote patient management, covering anomaly detection, prognostic modeling, and supporting platform architectures. Subsequent content examines challenges and ethical considerations encompassing security, privacy, governance, interpretability, regulatory compliance, and equitable deployment across diverse populations. A final thematic area considers cost, scalability, and low- and middle-income country (LMIC) constraints, highlighting economic, infrastructural, and usability factors alongside design adaptations. These elements collectively outline the current translation gap and identify priorities for further investigation.
4.1. Advancements in IoT for Early Diagnosis
Several studies highlight the potential of ocular biomarkers, particularly retinal imaging, for the early detection of Alzheimer’s disease. Noninvasive imaging techniques such as Optical Coherence Tomography (OCT) and OCT Angiography (OCTA) allow researchers to assess retinal thinning, A plaque accumulation, and vascular changes associated with AD pathology [198,199]. These techniques enable early diagnosis before significant cognitive decline occurs, providing a cost-effective alternative to conventional neuroimaging. However, further research is required to validate the robustness of retinal biomarkers and explore their integration into routine clinical screening [200,201].
With the growing need for automated and scalable diagnostic tools, the integration of AI and deep learning (DL) into non-invasive methods, such as retinal imaging and wearable sensors, is gaining significant traction. Recent studies have demonstrated that multimodal fundus imaging, when coupled with deep learning models, can accurately screen for cognitive impairments and serve as a surrogate biomarker for neurodegenerative conditions, including Alzheimer’s disease (AD) [202]. For instance, Shi et al. developed DL models capable of identifying early cognitive decline by extracting features from color and red-free fundus images, achieving promising screening performance in a large-scale evaluation [202]. Similarly, Cheung et al. introduced a robust AI model trained on retinal photographs from diverse populations, showing strong discriminatory power in detecting Alzheimer’s cases across multiple centers and reinforcing the retina’s potential role in oculomics for AD detection [203]. These findings align with prior advancements in deep reinforcement learning (DRL) applied to imaging-based diagnostics, further enhancing automated detection capabilities [204,205]. However, despite these encouraging results, challenges persist in terms of standardizing imaging protocols, acquiring sufficiently annotated datasets, and ensuring generalizability across diverse populations. Future research must prioritize model interpretability, rigorous validation, and regulatory compliance to bridge the translational gap and support real-world clinical adoption [205].
Recent studies suggest that oculomotor behaviors can serve as digital biomarkers for preclinical Alzheimer’s detection. Eye-tracking technology, when combined with memory tasks, can detect subtle changes in cognitive processing, providing a low-cost and accessible diagnostic tool [206]. While promising, further development is needed to create robust and automatic classification models that ensure reliability across diverse populations. Additionally, integrating eye-tracking data with AI models could improve the sensitivity of these digital biomarkers in early AD detection.
Recent advancements in near-infrared fluorescence (NIRF) imaging probes have provided new opportunities for precise AD diagnosis by targeting specific biomarkers such as A plaques and neurofibrillary tangles [207]. These small molecular probes demonstrate strong blood–brain barrier penetration and high sensitivity. Another approach involves nanosensor-driven detection of neuron-derived exosomal A42 using graphene electrolyte-gated transistors, which achieved 100% accuracy in distinguishing AD patients from healthy individuals [208]. Although these technologies hold immense potential, future studies must address scalability, long-term stability, and real-world applicability in clinical settings.
4.2. Integration of AI with IoT for Personalized Treatment
The integration of AI and IoT is playing a transformative role in RPM systems, facilitating real-time patient data collection and analysis. Works such as those by Jyothi et al. [209] and Tsvetanov [210] explore the integration of AI-based Recurrent Neural Networks (RNNs) and IoT sensors for anomaly detection in patient vitals. Similarly, ref. [211] highlights how IoT-based RPM systems combined with AI-powered predictive analytics improve healthcare monitoring in smart city frameworks. Future research should focus on overcoming interoperability and data security challenges in RPM.
Neurodegenerative diseases such as Alzheimer’s disease require continuous monitoring and predictive insights. Ianculescu et al. [212] introduced the NeuroPredict platform, integrating IoT, AI, and cloud computing for personalized patient care. Likewise, ref. [213] explores AI-driven prognostic modeling using neuroimaging techniques. While AI enhances early detection and treatment personalization, ethical and regulatory concerns regarding AI in clinical workflows remain an area for future research.
4.3. Challenges and Ethical Considerations
Machine learning-enabled IoT and IoMT ecosystems for Alzheimer’s and dementia care introduce significant security and privacy challenges that motivate structured protection strategies. A governance-centric taxonomy organized around the “govern, protect, detect” triad underscores the need for stronger end-to-end security frameworks spanning policy, technical safeguards, and continuous monitoring [214,215]. Complementing this architectural view, deep learning-based privacy preservation pipelines separate personally identifiable traits from raw IoT data streams prior to downstream analytics, but unresolved gaps persist in securing latent privacy information within derived features [216]. Redactable (certificateless) signature schemes and related cryptographic primitives offer selective disclosure while maintaining integrity, addressing key-escrow risks and enabling authenticated yet privacy-preserving data exchange [217,218].
AI–IoT convergence in RPM enhances early deterioration detection, chronic disease management, and adaptive intervention delivery, though challenges remain in scalable model deployment, energy constraints, and secure data provenance [219]. The evolution toward Healthcare 5.0 envisions autonomous, context-aware services integrating inter-related patient conditions with personalized, AI-driven decision support, demanding more secure and interoperable IoT frameworks [15]. In the dementia and Alzheimer’s domains, systematic evaluation reveals fragmentation in integration strategies and limited effectiveness evidence, motivating taxonomies to standardize classification and guide interoperability and real-world validation efforts [14]. Multimodal digital biomarkers—spanning wearable neurotechnologies and brain–computer interfaces—promise cognitive support and functional augmentation but elevate privacy, security, and ethical risk profiles that require explicit policy scaffolding [220].
Ethical analyses argue that existing AI health guidelines emphasize transparency, fairness, responsibility, and privacy yet under-represent multi-level sociological impacts; a Multiscale Ethics Framework has been proposed to embed institutional and societal lenses into evaluation [221]. Operationalizing proactive oversight, embedded ethics models integrate ethicists within AI development life cycles to iteratively surface and mitigate normative risks, though empirical pilots in clinical IoT settings remain sparse [222]. Edge–fog–cloud architectures for skeleton-based human interaction recognition demonstrate secure, latency-aware inference through layered pose estimation and encryption while highlighting ongoing needs in pose accuracy scaling across heterogeneous care contexts [223]. Broader aging-care innovation surveys point to AI’s potential for equitable access and enhanced service personalization, calling for deeper investigations into health equity and accessibility outcomes [137].
Robust biomedical data governance across jurisdictions is complicated by evolving national protection statutes and cross-border data flows; proposed legal frameworks advocate for privacy-enhancing technologies, harmonized private law instruments, and the classification of federated analytic approaches outside traditional international transfer triggers to facilitate compliant collaboration [224,225]. Alzheimer’s diagnostic pipelines leveraging multimodal ML (MMML)—for example, eye-tracking node networks in early-stage detection—illustrate high discriminative performance while raising questions about longitudinal generalizability and demographic robustness [50]. Neurotechnology integrations (e.g., BCI) further expand outpatient cognitive and motor monitoring but reinforce the urgency of policy frameworks for data stewardship [220].
Blockchain and distributed ledger technologies (DLTs) have been systematically reviewed with respect to strengthening electronic health record (EHR) security, decentralized consent, and tamper-evident provenance in remote and Alzheimer’s-related care scenarios [226,227,228]. Performance-optimized permissioned architectures combining Hyperledger, attribute-based encryption, and IoT acquisition layers achieve high throughput (1200 tx/s) and low latency (<250 ms) while enabling fine-grained sharing [229]. Context-aware EHR interfaces that adapt dynamically to environmental IoT signals maintain sub-200 ms responsiveness with immutable context logging for auditability [230]. Novel governance constructs using soul-bound tokens (SBTs) extend immutable consent verification across cloud EHR ecosystems [231]. Real-world WBAN deployments integrating encrypted wearable streams over 12-month have been validated for feasibility in Alzheimer’s monitoringyet still require scaling to larger, diverse cohorts and integration with decentralized governance models to meet regulatory thresholds [232].
Global regulatory landscapes (GDPR, CCPA, APPI, and related regimes) remain heterogeneous and often lag technological change; comparative legal analyses underscore the need for adaptive, harmonized, and dynamically updatable frameworks that reconcile rapid AI/IoT innovation with enforceable privacy protections and cross-border interoperability [18]. Future research trajectories include standardized multimodal fusion benchmarks; longitudinal, demographically diverse validation of digital biomarkers; embedded ethics operationalization; cryptographic performance optimization for resource-constrained IoT nodes; federated and privacy-preserving analytics aligned with legal portability principles; and regulatory sandboxes coupling blockchain governance, consent tokenization, and secure edge intelligence within Alzheimer’s care ecosystems.
4.4. Cost, Scalability, and LMIC Considerations
Recent work has begun to quantify the upfront and operational costs associated with IoT-based patient monitoring systems, revealing surprisingly low per patient hardware expenses. For example, Okubanjo et al. reported a total hardware cost of just USD 16.09 per unit for a self-care IoT diabetes monitor built from an Arduino Nano, NodeMCU, and low-cost sensors [233]. Liu et al. surveyed 50 studies employing “low-cost sensors” (under USD 20), demonstrating that components such as MEMS IMUs, and piezoelectric pressure sensors enable continuous health monitoring while keeping unit costs below USD 30 per patient [234].
However, deploying IoT in low- and middle-income countries (LMICs) faces persistent barriers, including unreliable network connectivity, power instability, and low digital literacy [235]. Fixed-broadband penetration remains below 4% in many LMICs, while mobile Internet usage can be under 50% in some regions. To maintain continuous operation, LPWAN protocols such as LoRaWAN have demonstrated over one year of battery life on a single 2400 mAh cell with hourly transmissions [236]. Digital literacy gaps further necessitate community-centered training and highly simplified user interfaces [235].
To enhance affordability and scalability in resource-constrained settings, several strategies have proven effective:
Open-source hardware/software platforms: Leveraging Arduino and NodeMCU ecosystems reduces development costs and encourages local assembly and maintenance [233].
Telecom partnerships: Collaborative agreements with mobile network operators can subsidize IoT data plans or piggyback on existing LPWAN networks, mitigating connectivity expenses [235].
Low-power communication protocols: Adopting LPWAN standards (e.g., LoRaWAN and Sigfox) extends device lifetimes to multiple years on a single battery, drastically cutting maintenance and battery-replacement costs [236].
By pursuing these directions, researchers can generate actionable insights into the affordability, durability, and user acceptance of IoT-enabled Alzheimer’s care solutions in resource-constrained settings.
Table 10 summarizes the principal gaps impeding robust, scalable, and equitable IoT-enabled Alzheimer’s solutions, grouping each challenge with its practical implication and a concise future study direction. The listed items emphasize methodological rigor (larger multicenter, longitudinal cohorts and standardized metrics), technical robustness (interoperability, energy efficiency, and edge–cloud orchestration), trust and fairness (privacy, security, governance, bias mitigation, and explainability), and global translation (cost and regulatory pathways). This structured synthesis is intended to guide prioritization of research investments and to support consistent benchmarking across forthcoming studies.
Table 10.
Concise summary of key research gaps and corresponding future directions.
| Gap/Challenge | Implication | Future Study Direction |
|---|---|---|
| Limited multicenter, longitudinal datasets | Restricts generalizability and external validity | Design coordinated multi-site, year-long cohorts with standardized outcome measures |
| Small sample sizes/modality silos | Inflated performance estimates; weak comparison across approaches | Aggregate multimodal datasets; adopt shared benchmarking protocols |
| Interoperability and data standard gaps | Fragmented pipelines; integration overhead | Implement and evaluate unified data models and open APIs across platforms |
| Privacy, security, and governance weaknesses | Risk of data misuse; reduced stakeholder trust | Embed privacy by design, continuous security monitoring, and clear governance workflows |
| Energy and on-device resource constraints | Limits continuous monitoring and scalability | Optimize models (compression and quantization) and adaptive sensing schedules |
| Bias and demographic under-representation | Potential inequitable performance across groups | Enforce stratified reporting; collect diverse cohorts; apply bias auditing and mitigation |
| Lack of standardized evaluation metrics | Difficult cross-study comparison | Define core metric set (clinical + technical) and publish reporting templates |
| Sparse validation of emerging retinal/nanosensor tools | Uncertain durability and clinical readiness | Conduct stability, calibration, and real-world deployment studies |
| Edge–cloud integration complexity | Latency and reliability variability | Develop adaptive orchestration frameworks with performance monitoring |
| Insufficient explainability and clinician usability evidence | Hinders adoption in clinical workflows | Integrate interpretable outputs and perform user-centered usability trials |
| Cost and scalability barriers in resource-constrained settings | Limits global deployment | Evaluate low-cost architectures, local maintenance models, and implementation economics |
| Regulatory pathway uncertainty | Delays translation to practice | Map compliance requirements and pilot regulatory sandbox evaluations |
5. Conclusions
This PRISMA-guided review (236 studies) presents a stage-wise synthesis of IoT + AI interventions in Alzheimer’s disease (AD)—early detection, mild-stage management, and severe-stage assistance—alongside enabling platforms, barriers, and future directions. Early detection leverages wearable IMUs for gait, smartphone and video cognitive self-assessments, and multimodal deep learning (e.g., CL-ATBiLSTM and CNN encoders), achieving >90% accuracy in controlled settings, but most evaluations are short, single-site, and underpowered. Excluding studies with fewer than 20 participants did not change the overall conclusions regarding the feasibility of wearable IoT systems in early detection. Mild-stage management uses remote monitoring (smartwatches, ambient sensors, and AI home automation) to track ADLs, detect falls/agitation (sensitivities typically >90%), and support memory via voice assistants, VR training, and spaced-retrieval apps—yet requires larger randomized trials and Alzheimer’s-specific benchmarking. Severe-stage care emphasizes continuous supervision: smart home platforms (e.g., integrated ambient + wearable sensing) for anomaly detection, smart pill dispensers and facial recognition schedulers (up to 95% on-time adherence), and GPS/IMU wandering detection (>90% accuracy) that largely remain at pilot/proof-of-concept stages.
Enabling technologies (wearable biosensors; AI gait analytics; sleep/circadian; noninvasive glucose, and other types of biosensing monitoring) show high analytical performance (e.g., >93% gait anomaly detection and >99% glucose accuracy) but face interoperability gaps, battery and durability limits, and limited longitudinal real-world validation. Several device validation studies have reported high diagnostic accuracy without independent replication. This, along with the absence of negative results in many domains, suggests possible selective reporting. The most common sources of bias included small sample sizes, absence of independent validation, and incomplete reporting of device performance metrics. Systemic challenges span privacy/security risk, absence of mature HL7 FHIR-based standardization, unclear regulatory pathways, algorithmic bias, unequal LMIC access, and ethical issues around data sovereignty and consent. The convergence of AI, blockchain, and IoT infrastructure promises secure, equitable, and efficient care if accompanied by human-centered co-design, federated/privacy-preserving analytics, and robust governance frameworks.
In summary, IoT–AI ecosystems offer a powerful toolkit for earlier diagnosis, continuous monitoring, and targeted intervention in AD; however, clinical translation demands multicenter longitudinal trials; interoperability standards; standardized digital biomarker definitions; ethical AI implementation; and scalable, patient-centric design. Addressing these gaps can accelerate the evolution from isolated prototypes to integrated, equitable dementia care infrastructure.
A limitation of the review process was that the protocol was not prospectively registered, and no quantitative synthesis was performed, which may have limited reproducibility and the scope of inferential conclusions. The findings highlight the potential for IoT-based solutions to enhance stage-specific Alzheimer’s care in clinical practice; inform policy frameworks that encourage adoption of such technologies; and guide future research toward large-scale, multi-center trials and standardized evaluation protocols.
Acknowledgments
During the preparation of this manuscript, the author(s) used generative AI (ChatGPT, o4-min-high) to create illustrative visuals of device concepts presented in Figure 6, with the goal of enhancing conceptual clarity and reader comprehension. These illustrations are copyright-free in accordance with OpenAI’s content policies: https://openai.com/policies/row-terms-of-use/?utm_source=chatgpt.com (accessed on 18 July 2025). The authors have thoroughly reviewed and refined the generated content and assume full responsibility for its accuracy and presentation in this publication.
Abbreviations
| Acronym | Full Form |
| IoT | Internet of Things |
| AD | Alzheimer’s Disease |
| AI | Artificial Intelligence |
| RPM | Remote Patient Monitoring |
| ML | Machine Learning |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| IoMT | Internet of Medical Things |
| EEG | Electroencephalogram |
| CL-ATBiLSTM | Convolutional Learning Attention-Bidirectional Time-Aware Long Short-Term Memory |
| CNN | Convolutional Neural Network |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
| A | Amyloid Beta |
| MCI | Mild Cognitive Impairment |
| TUG | Timed Up and Go |
| MMSE | Mini-Mental State Examination |
| MDR | Micro-Doppler Radar |
| DB | Digital Biomarker |
| BCI | Brain–Computer Interface |
| CGM | Continuous Glucose Monitoring |
| T2DM | Type 2 Diabetes Mellitus |
| CNT-FET | Carbon Nanotube Field-Effect Transistor |
| CSF | Cerebrospinal Fluid |
| PET | Positron Emission Tomography |
| SVM | Support Vector Machine |
| RMSE | Root Mean Square Error |
| QMRA | Quantum Magnetic Resonance Analysis |
| WBAN | Wireless Body Area Network |
| IoRT | Internet of Robotic Things |
| DBN | Deep Belief Network |
| MOA | Mayfly Optimization Algorithm |
| BLE | Bluetooth Low Energy |
| LoRa | Long Range (communication protocol) |
| EMBC | Engineering in Medicine and Biology Conference |
| LSTM | Long Short-Term Memory |
| HAR | Human Activity Recognition |
| AUROC | Area Under the Receiver Operating Characteristic Curve |
| NIRF | Near-Infrared Fluorescence |
| LFA | Lateral Flow Assay |
| DBs | Digital Biomarkers |
| OLED | Organic Light-Emitting Diode |
| OPD | Organic Photodiode |
| HRP | Horseradish Peroxidase |
| TDP-43 | TAR DNA-Binding Protein 43 |
| NfL | Neurofilament Light |
| p-Tau | Phosphorylated Tau |
| t-Tau | Total Tau |
| HIPAA | Health Insurance Portability and Accountability Act |
| GDPR | General Data Protection Regulation |
| CCPA | California Consumer Privacy Act |
Author Contributions
Conceptualization, S.S., L.G., and V.G.; methodology, S.S., L.G., and V.G.; formal analysis, S.S.; investigation, S.S.; resources, V.G.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, L.G. and V.G.; visualization, S.S.; supervision, V.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack C.R.J., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack C.R., Therneau T.M., Lundt E.S., Wiste H.J., Mielke M.M., Knopman D.S., Graff-Radford J., Lowe A.J., Vemuri P., Schwarz C.G., et al. Long-term associations between amyloid positron emission tomography, sex, apolipoprotein E and incident dementia and mortality among individuals without dementia: Hazard ratios and absolute risk. Brain Commun. 2022;4:fcac017. doi: 10.1093/braincomms/fcac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horimoto A.R.V.R., Blue E., Grinde K., Nafikov R.A., Sohi H., Nato A.Q., Bis J.C., Brusco L.I., Morelli L., Ramirez A., et al. Admixture mapping implicates 13q33.3 as ancestry-of-origin locus for Alzheimer disease in Hispanic and Latino populations. HGG Adv. 2023;4:100207. doi: 10.1016/j.xhgg.2023.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard H.L. Multi-ancestry meta-analysis and fine-mapping in Alzheimer’s disease. Mol. Psychiatry. 2023;28:3121–3132. doi: 10.1038/s41380-023-02089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manly J.J., Jones R.N., Langa K.M., Ryan L.H., Levine D., McCammon R.J., Heeringa S.G., Weir D. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79:1242. doi: 10.1001/jamaneurol.2022.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J.J., Keohane L.M., Pan X.F., Qu R., Shu X.O., Lipworth L., Braun K., Steinwandel M., Dai Q., Shrubsole M.J., et al. Association of Healthy Lifestyles with Risk of Alzheimer Disease and Related Dementias in Low-Income Black and White Americans. Neurology. 2022;99:e944–e953. doi: 10.1212/WNL.0000000000200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu D., Yang J.J., Keohane L.M., Shu X.O., Lipworth L., Braun K., Dai Q., Steinwandel M., Shrubsole M.J., Zheng W., et al. Healthy Lifestyles and the Risk of Alzheimer’s Disease and Related Dementias among Low-Income Black and White Americans. Curr. Dev. Nutr. 2022;6:967. doi: 10.1093/cdn/nzac067.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 10.Rajan K.B., Weuve J., Barnes L.L., McAninch E.A., Wilson R.S., Evans D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060) Alzheimer’s Dement. 2021;17:1966–1975. doi: 10.1002/alz.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahendran S.R., Priya H.A.G., Mohsen K.S., Khan M.S., Obaid A.J., Shareef A.M. IoT-based Health Monitoring System with AI-Powered Disease Prediction; Proceedings of the 2024 IEEE 13th International Conference on Communication Systems and Network Technologies (CSNT); Jabalpur, India. 6–7 April 2024; [DOI] [Google Scholar]
- 12.Singla D., Kumar S., Dhingra D., Ghandhi A. Blockchain-powered Healthcare: Revolutionizing Security and Privacy in IoT-based Systems; Proceedings of the 2024 International Conference on Computational Intelligence and Computing Applications (ICCICA); Samalkha, India. 23–24 May 2024; pp. 375–380. [DOI] [Google Scholar]
- 13.Conduah A.K., Ofoe S., Siaw-Marfo D. Data privacy in healthcare: Global challenges and solutions. Digit. Health. 2025;11:20552076251343959. doi: 10.1177/20552076251343959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esquer-Rochin M., Rodríguez L.F., Gutiérrez-García J.O. The Internet of Things in Dementia: A Systematic Review. Internet Things. 2023;22:100824. doi: 10.1016/j.iot.2023.100824. [DOI] [Google Scholar]
- 15.Taimoor N., Rehman S. Reliable and Resilient AI and IoT-based Personalised Healthcare Services: A Survey. IEEE Access. 2022;10:535–563. doi: 10.1109/ACCESS.2021.3137364. [DOI] [Google Scholar]
- 16.Williamson S.M., Prybutok V. Balancing Privacy and Progress: A Review of Privacy Challenges, Systemic Oversight, and Patient Perceptions in AI-Driven Healthcare. Appl. Sci. 2024;14:675. doi: 10.3390/app14020675. [DOI] [Google Scholar]
- 17.Hajla S.E., Ennaji E.M., Maleh Y., Mounir S. Security Challenges and Solutions in IoT. IGI Global Scientific Publishing; Hershey, PA, USA: 2024. pp. 25–50. (Advances in Computational Intelligence and Robotics Book Series). [DOI] [Google Scholar]
- 18.Sivan R., Zukarnain Z.A. Security and Privacy in Cloud-Based E-Health System. Symmetry. 2021;13:742. doi: 10.3390/sym13050742. [DOI] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikhtaheri A., Sabermahani F. Applications and Outcomes of Internet of Things for Patients with Alzheimer’s Disease/Dementia: A Scoping Review. Biomed. Res. Int. 2022;2022:6274185. doi: 10.1155/2022/6274185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stavropoulos T.G., Papastergiou A., Mpaltadoros L., Nikolopoulos S., Kompatsiaris I. IoT Wearable Sensors and Devices in Elderly Care: A Literature Review. Sensors. 2020;20:2826. doi: 10.3390/s20102826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote A.C., Phelps R.J., Kabiri N.S., Bhangu J.S., Thomas K.K. Evaluation of Wearable Technology in Dementia: A Systematic Review and Meta-Analysis. Front. Med. 2021;7:501104. doi: 10.3389/fmed.2020.501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaik M.A., Anik F.I., Hasan M.M., Chakravarty S., Ramos M.D., Rahman M.A., Ahamed S.I., Sakib N. Advancing Remote Monitoring for Patients with Alzheimer Disease and Related Dementias: Systematic Review. JMIR Aging. 2025;8:e69175. doi: 10.2196/69175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hastie T., Tibshirani R., Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; New York, NY, USA: 2009. [DOI] [Google Scholar]
- 25.Bishop C.M. Pattern Recognition and Machine Learning. 1st ed. Springer; New York, NY, USA: 2006. p. 778. Information Science and Statistics. [Google Scholar]
- 26.Fawcett T. An Introduction to ROC Analysis. Pattern Recognit. Lett. 2006;27:861–874. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 27.Xie K., Huang J., Chen T., Li D., Xia T., Chu M., Cui Y., Tang M., Peng D., Wang J., et al. A mobile interactive cognitive self-assessment scale for screening cognitive impairment due to Alzheimer’s disease. Age Ageing. 2025;54:afae293. doi: 10.1093/ageing/afae293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berron D., Glanz W., Clark L., Basche K., Grande X., Billette O.V., Teipel S., Jessen F., Düzel E. A remote digital memory composite to detect cognitive impairment in memory clinic samples in unsupervised settings using mobile devices. npj Digit. Med. 2024;7:79. doi: 10.1038/s41746-024-00999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunderaraman P., De Anda-Duran I., Karjadi C., Peterson J., Ding H., Devine S.A., Shih L.C., Popp Z., Low S., Hwang P.H. Design and feasibility analysis of a smartphone-based digital cognitive assessment study in the Framingham Heart Study. J. Am. Heart Assoc. 2024;13:e031348. doi: 10.1161/JAHA.123.031348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs D.M., Peavy G.M., Banks S.J., Gigliotti C., Little E.A., Salmon D.P. A survey of smartphone and interactive video technology use by participants in Alzheimer’s disease research: Implications for remote cognitive assessment. Alzheimer’s Dement. 2021;13:e12188. doi: 10.1002/dad2.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fristed E., Skirrow C., Meszaros M., Lenain R., Meepegama U., Cappa S.F., Aarsland D., Weston J. A remote speech-based AI system to screen for early Alzheimer’s disease via smartphones. Alzheimer’s Dement. 2022;14:e12366. doi: 10.1002/dad2.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva-Spínola A., Baldeiras I., Arrais J.P., Santana I. The Road to Personalized Medicine in Alzheimer’s Disease: The Use of Artificial Intelligence. Biomedicines. 2022;10:315. doi: 10.3390/biomedicines10020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q., Vaci N., Koychev I., Kormilitzin A., Li Z., Cipriani A., Nevado-Holgado A.J. Personalised treatment for cognitive impairment in dementia: Development and validation of an artificial intelligence model. BMC Med. 2022;20:45. doi: 10.1186/s12916-022-02250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziyad S.R., Alharbi M., Altulyan M.S. Artificial Intelligence Model for Alzheimer’s Disease Detection with Convolution Neural Network for Magnetic Resonance Images. J. Alzheimer’s Dis. 2023;93:235–245. doi: 10.3233/JAD-221250. [DOI] [PubMed] [Google Scholar]
- 35.Vanderlip C.R., Stark C.E.L., Initiative A.D.N. Digital cognitive assessments as low-burden markers for predicting future cognitive decline and tau accumulation across the Alzheimer’s spectrum. Alzheimer’s Dement. 2024;20:6881–6895. doi: 10.1002/alz.14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodius-Meester H., Paajanen T., Lötjönen J. cCOG Web-Based Cognitive Assessment Tool. In: Perneczky R., editor. Biomarkers for Alzheimer’s Disease Drug Development. Volume 2785. Humana; New York, NY, USA: 2024. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 37.AlQahtani N.G., Alam S., Aqeel I., Shuaib M., Malibari A. Deep Belief Networks (DBN) with IoT-Based Alzheimer’s Disease Detection and Classification. Appl. Sci. 2023;13:7833. doi: 10.3390/app13137833. [DOI] [Google Scholar]
- 38.Mikolaizak A.S., Rochester L., Maetzler W., Sharrack B., Demeyer H., Mazzà C., Caulfield B., Garcia-Aymerich J., Vereijken B., Arnera V., et al. Connecting real-world digital mobility assessment to clinical outcomes for regulatory and clinical endorsement—The Mobilise-D study protocol. PLoS ONE. 2022;17:e0269615. doi: 10.1371/journal.pone.0269615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perumal T., Wolfer A., Veloso M., Kurniawan I.T., Aponte E.A., Keita G., Hagenbuch N., Shi B., Orfaniotou F., Colell M.G.V., et al. Convergent and clinical validity of a remote smartphone-based self-assessment of cognition, function, and behavior in early Alzheimer’s disease. Alzheimer’s Dement. 2023;19:e077236. doi: 10.1002/alz.077236. [DOI] [Google Scholar]
- 40.Diggin S., Alexander-Sefre A., Murphy B., Nolan H., Rowe J.B., Rueda-Delgado L.M., Buick A.R. A longitudinal real-world study in patients with Alzheimer’s Disease dementia using frequent multi-domain digital measurements performed at-home on the Cumulus Neuroassessment Platform: Usability and feasibility findings. Alzheimer’s Dement. 2024;20:e094325. doi: 10.1002/alz.094325. [DOI] [Google Scholar]
- 41.Khosravi M., Parsaei H., Rezaee K. Fusing convolutional learning and attention-based Bi-LSTM networks for early Alzheimer’s diagnosis from EEG signals towards IoMT. Sci. Rep. 2024;14:26002. doi: 10.1038/s41598-024-77876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonia S.V.E., Mustare N.B., Sailaja V., Sunitha P. IoT-Enabled Alzheimer Disease Detection: A Convolutional Encoder-Decoder Approach Enhanced by Alpine Skiing Optimization; Proceedings of the 2024 Second International Conference on Intelligent Cyber Physical Systems and Internet of Things (ICoICI); Coimbatore, India. 28–30 August 2024; pp. 386–391. [DOI] [Google Scholar]
- 43.Sathish R., Muthukumar R., Dhivya K., Karthikkumar S. Deep Learning and IoT-Enabled Framework for Accurate Classification and Monitoring of Alzheimer’s Disease Based on EEG Signal Analysis; Proceedings of the 2025 Fifth International Conference on Advances in Electrical, Computing, Communication and Sustainable Technologies (ICAECT); Bhilai, India. 9–10 January 2025; pp. 1–8. [DOI] [Google Scholar]
- 44.Tarekegn A.N., Sajjad M., Cheikh F.A., Ullah M., Muhammad K. Efficient Human Gait Activity Recognition Based on Sensor Fusion and Intelligent Stacking Framework. IEEE Sens. J. 2023;23:28355–28369. doi: 10.1109/JSEN.2023.3319353. [DOI] [Google Scholar]
- 45.Greco D., Masulli F., Rovetta S., Cabri A., Daffonchio D. A Cost-Effective Eye-Tracker for Early Detection of Mild Cognitive Impairment; Proceedings of the 2022 IEEE 21st Mediterranean Electrotechnical Conference (MELECON); Palermo, Italy. 14–16 June 2022; pp. 1141–1146. [DOI] [Google Scholar]
- 46.Al-Rowaili B., Al-Obaidli N., Al-Marri D., Abualsaud K., Yaacoub E. Fall Detection Wristband with Optimized Security and Health Monitoring; Proceedings of the 2024 International Wireless Communications and Mobile Computing (IWCMC); Ayia Napa, Cyprus. 27–31 May 2024; pp. 697–702. [DOI] [Google Scholar]
- 47.Chen H. Design of Intelligent Positioning Shoes for Elderly Fall Monitoring Based on GPS and MPU-6000 Acceleration Sensor; Proceedings of the 2022 International Conference on Wearables, Sports and Lifestyle Management (WSLM); Kunming, China. 14–16 January 2022; pp. 43–46. [DOI] [Google Scholar]
- 48.Jeon Y., Kang J., Kim B.C., Lee K.H., Song J.I., Gwak J. Early Alzheimer’s Disease Diagnosis Using Wearable Sensors and Multilevel Gait Assessment: A Machine Learning Ensemble Approach. IEEE Sens. J. 2023;23:10041–10053. doi: 10.1109/JSEN.2023.3259034. [DOI] [Google Scholar]
- 49.Jamshed M., Shahzad A., Riaz F., Kim K. Exploring inertial sensor-based balance biomarkers for early detection of mild cognitive impairment. Sci. Rep. 2024;14:9829. doi: 10.1038/s41598-024-59928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin Y., Wang H., Liu S., Sun J., Jing P., Liu Y. Internet of Things for Diagnosis of Alzheimer’s Disease: A Multimodal Machine Learning Approach Based on Eye Movement Features. IEEE Internet Things J. 2023;10:11476–11485. doi: 10.1109/JIOT.2023.3245067. [DOI] [Google Scholar]
- 51.Huang S., Hou X., Liu Y., Shang P., Luo J., Lv Z., Zhang W., Lin B., Huang Q., Tao S., et al. Diagnostic accuracy of multi-component spatial-temporal gait parameters in older adults with amnestic mild cognitive impairment. Front. Hum. Neurosci. 2022;16:911607. doi: 10.3389/fnhum.2022.911607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dieffenderfer J., Brewer A., Noonan M.A., Smith M., Eichenlaub E., Haley K.L., Jacks A., Lobaton E., Neupert S.D., Hess T.M., et al. A Wearable System for Continuous Monitoring and Assessment of Speech, Gait, and Cognitive Decline for Early Diagnosis of ADRD; Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); Sydney, Australia. 24–27 July 2023; pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 53.Wang W., Chen J., Hu Y., Liu H., Chen J., Gadekallu T.R., Garg L., Guizani M., Hu X. Integration of Artificial Intelligence and Wearable Internet of Things for Mental Health Detection. Int. J. Cogn. Comput. Eng. 2024;5:307–315. doi: 10.1016/j.ijcce.2024.07.002. [DOI] [Google Scholar]
- 54.Massoud M.A., El-Bouridy M.E., Ahmed W.A. Revolutionizing Alzheimer’s detection: An advanced telemedicine system integrating Internet-of-Things and convolutional neural networks. Neural Comput. Appl. 2024;36:16411–16426. doi: 10.1007/s00521-024-09859-9. [DOI] [Google Scholar]
- 55.Kaye J., Marcoe J., Mar A., Hanna E., Pierce A., Silbert L., Shang Y., Schwartz D., Gothard S., Mattek N., et al. DETECT-AD (Digital Evaluations and Technologies Enabling Clinical Translation for Alzheimer’s Disease): A simulated anti-amyloid clinical trial using digital biomarkers as primary outcome measures. Alzheimer’s Dement. 2023;19:e071888. doi: 10.1002/alz.071888. [DOI] [Google Scholar]
- 56.Karri C., Garg L., Prakash V., Pawar B.D. Chapter 9—Healthcare 5.0 opportunities and challenges: A literature review. In: Garg L., Mirajkar G., Misra S., Chattu V.K., editors. Intelligent Biomedical Technologies and Applications for Healthcare 5.0. Volume 16. Academic Press; Cambridge, MA, USA: 2025. pp. 133–146. Advances in Ubiquitous Sensing Applications for Healthcare. [DOI] [Google Scholar]
- 57.Vo D.K., Trinh K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors. 2024;14:560. doi: 10.3390/bios14110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma A., Badea M., Tiwari S., Marty J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules. 2021;26:748. doi: 10.3390/molecules26030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain D.K., Srinivas K., Srinivasu S.V.N., Manikandan R. Machine Learning-Based Monitoring System with IoT Using Wearable Sensors and Pre-Convoluted Fast Recurrent Neural Networks (P-FRNN) IEEE Sens. J. 2021;21:25517–25524. doi: 10.1109/JSEN.2021.3091626. [DOI] [Google Scholar]
- 60.Yang G., Pang G., Pang Z., Gu Y., Mäntysalo M., Yang H. Non-Invasive Flexible and Stretchable Wearable Sensors with Nano-Based Enhancement for Chronic Disease Care. IEEE Rev. Biomed. Eng. 2019;12:34–71. doi: 10.1109/RBME.2018.2887301. [DOI] [PubMed] [Google Scholar]
- 61.Shi Q., Yang Y., Sun Z., Lee C. Progress of Advanced Devices and Internet of Things Systems as Enabling Technologies for Smart Homes and Health Care. ACS Mater. Sci. Au. 2022;2:394–435. doi: 10.1021/acsmaterialsau.2c00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhodus E., Onim M., Roberts C., Kumar S., Burhan A., Thapliyal H. Utilization of Wearable Devices as Means for Remote Digital Biometric Data Collection in a Community-Based, Rural Randomized Controlled Trial among Alzheimer’s Disease Dyads. Alzheimer’s Dement. 2024;20:e090883. doi: 10.1002/alz.090883. [DOI] [Google Scholar]
- 63.Butler P.M., Yang J., Brown R., Hobbs M., Becker A., Penalver-Andres J., Syz P., Muller S., Cosne G., Juraver A., et al. Smartwatch- and smartphone-based remote assessment of brain health and detection of mild cognitive impairment. Nat. Med. 2025;31:829–839. doi: 10.1038/s41591-024-03475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krizea M., Gialelis J., Protopsaltis G., Mountzouris C., Theodorou G. Empowering People with a User-Friendly Wearable Platform for Unobtrusive Monitoring of Vital Physiological Parameters. Sensors. 2022;22:5226. doi: 10.3390/s22145226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duan H., Peng S., He S., Tang S., Goda K., Wang C., Li M. Wearable Electrochemical Biosensors for Advanced Healthcare Monitoring. Adv. Sci. 2024;12:2411433. doi: 10.1002/advs.202411433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erdem A., Eksin E., Senturk H., Yildiz E., Maral M. Recent Developments in Wearable Biosensors for Healthcare and Biomedical Applications. TrAC Trends Anal. Chem. 2024;171:117510. doi: 10.1016/j.trac.2023.117510. [DOI] [Google Scholar]
- 67.Bodily T.A., Ramanathan A., Wei S., Karkisaval A., Bhatt N., Jerez C., Haque M.A., Ramil A., Heda P., Wang Y., et al. In pursuit of degenerative brain disease diagnosis: Dementia biomarkers detected by DNA aptamer-attached portable graphene biosensor. Proc. Natl. Acad. Sci. USA. 2023;120:e2311565120. doi: 10.1073/pnas.2311565120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J., Lu X., He Y. Electrochemical Technology for the Detection of Tau Proteins as a Biomarker of Alzheimer’s Disease in Blood. Biosensors. 2025;15:85. doi: 10.3390/bios15020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phan L.M.T., Vo T.A.T., Pham H.L., Cho S. Nanomaterial-based Optical and Electrochemical Biosensors for Amyloid-β and Tau: Potential for Early Diagnosis of Alzheimer’s Disease. Expert Rev. Mol. Diagn. 2021;21:175–193. doi: 10.1080/14737159.2021.1887732. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y., Huang Z., Xu Q., Zhang L., Liu Q., Xu T. Portable electrochemical micro-workstation platform for simultaneous detection of multiple Alzheimer’s disease biomarkers. Mikrochim. Acta. 2022;189:91. doi: 10.1007/s00604-022-05199-4. [DOI] [PubMed] [Google Scholar]
- 71.Bocu R. Extended Review Concerning the Integration of Electrochemical Biosensors into Modern IoT and Wearable Devices. Biosensors. 2024;14:214. doi: 10.3390/bios14050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y., Liu X., Li M., Liu Q., Xu T. Portable Vertical Graphene@Au-Based Electrochemical Aptasensing Platform for Point-of-Care Testing of Tau Protein in the Blood. Biosensors. 2022;12:564. doi: 10.3390/bios12080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li M., Zeng Y., Huang Z., Zhang L., Liu Y. Vertical Graphene-Based Printed Electrochemical Biosensor for Simultaneous Detection of Four Alzheimer’s Disease Blood Biomarkers. Biosensors. 2023;13:758. doi: 10.3390/bios13080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakari-Khiavi F., Mirzaie A., Khalilzadeh B., Yousefi H., Abolhasan R., Kamrani A., Pourakbari R., Shahpasand K., Yousefi M., Rashidi M.R. Application of Pt@ZIF-8 Nanocomposite-Based Electrochemical Biosensor for Sensitive Diagnosis of Tau Protein in Alzheimer’s Disease Patients. Sci. Rep. 2023;13:16163. doi: 10.1038/s41598-023-43180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciou S.H., Hsieh A.H., Lin Y.X., Sei J.L., Govindasamy M., Kuo C.F., Huang C.H. Sensitive Label-Free Detection of the Biomarker Phosphorylated Tau-217 Protein in Alzheimer’s Disease Using a Graphene-Based Solution-Gated Field Effect Transistor. Biosens. Bioelectron. 2023;228:115174. doi: 10.1016/j.bios.2023.115174. [DOI] [PubMed] [Google Scholar]
- 76.Kong Q., Liu C., Zhang Y., He Y., Zhang R., Wang Y., Zhou Q., Cui F. Nucleic Acid Aptamer-Based Electrochemical Sensor for the Detection of Serum P-tau231 and the Instant Screening Test of Alzheimer’s Disease. Mikrochim. Acta. 2024;191:328. doi: 10.1007/s00604-024-06395-0. [DOI] [PubMed] [Google Scholar]
- 77.Sempionatto J.R., Lasalde-Ramírez J.A., Mahato K., Wang J., Gao W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022;6:899–915. doi: 10.1038/s41570-022-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukasa D., Wang M.Q., Min J., Yang Y., Solomon S.A., Ye C., Gao W. A Computationally Assisted Approach for Designing Wearable Biosensors toward Non-invasive Personalized Molecular Analysis. Adv. Mater. 2023;35:2212161. doi: 10.1002/adma.202212161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J., Sun Y., Zhang J., Luo F., Ma H., Guan M., Feng J., Dong X. Dumbbell Aptamer Sensor Based on Dual Biomarkers for Early Detection of Alzheimer’s Disease. ACS Appl. Mater. Interfaces. 2023;15:16394–16407. doi: 10.1021/acsami.2c21379. [DOI] [PubMed] [Google Scholar]
- 80.Phan L.M.T., Cho S. Fluorescent Aptasensor and Colorimetric Aptablot for p-tau231 Detection: Toward Early Diagnosis of Alzheimer’s Disease. Adv. Cardiovasc. Dis. 2022;10:93. doi: 10.3390/biomedicines10010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H., Xiao M., He J., Zhang Y., Liang Y., Liu H., Zhang Z. Aptamer-Functionalized Carbon Nanotube Field-Effect Transistor Biosensors for Alzheimer’s Disease Serum Biomarker Detection. ACS Sens. 2022;7:2075–2083. doi: 10.1021/acssensors.2c00967. [DOI] [PubMed] [Google Scholar]
- 82.Beduk T., Beduk D., Hasan M.R., Guler Celik E., Kosel J., Narang J., Salama K.N., Timur S. Smartphone-Based Multiplexed Biosensing Tools for Health Monitoring. Biosensors. 2022;12:583. doi: 10.3390/bios12080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duan S., Cai T., Liu F., Li Y., Yuan H., Yuan W., Huang K., Hoettges K., Chen M., Lim E.G., et al. Automatic offline-capable smartphone paper-based microfluidic device for efficient biomarker detection of Alzheimer’s disease. Anal. Chim. Acta. 2024;1308:342575. doi: 10.1016/j.aca.2024.342575. [DOI] [PubMed] [Google Scholar]
- 84.Kazanskiy N.L., Butt M.A., Khonina S.N. Recent Advances in Wearable Optical Sensor Automation Powered by Battery versus Skin-like Battery-Free Devices for Personal Healthcare—A Review. Nanomaterials. 2022;12:334. doi: 10.3390/nano12030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun X., Sun X., Wang Q., Wang X., Feng L., Yang Y., Jing Y., Yang C., Zhang S. Biosensors toward behavior detection in diagnosis of Alzheimer’s disease. Front. Bioeng. Biotechnol. 2022;10:1031833. doi: 10.3389/fbioe.2022.1031833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng Z., Zhu R., Peng I., Xu Z., Jiang Y. Wearable and implantable biosensors: Mechanisms and applications in closed-loop therapeutic systems. J. Mater. Chem. B. 2024;12:8577–8604. doi: 10.1039/D4TB00782D. [DOI] [PubMed] [Google Scholar]
- 87.Savoy A., Holden R.J., Groot N.D., Clark D.O., Sachs G.A., Klonoff D.C., Weiner M.W. Improving Care for People Living with Dementia and Diabetes: Applying the Human-Centered Design Process to Continuous Glucose Monitoring. J. Diabetes Sci. Technol. 2022;18:201–206. doi: 10.1177/19322968221137907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ergin B., Gadsby-Davis K., Mattishent K., Dhatariya K., Garner N., Hornberger M. Continuous Glucose Monitoring in Comorbid Dementia and Diabetes: The Evidence So Far. J. Diabetes Sci. Technol. 2024 doi: 10.1177/19322968241301058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carroll C.M., Constantino N.J., Irmen R.E., Snipes J.A., Benca R.M., Macauley S.L. Peripheral glucose metabolism bidirectionally modulates sleep in a model of Alzheimer’s disease. Sleep. 2023;46:A38–A39. doi: 10.1093/sleep/zsad077.0085. [DOI] [Google Scholar]
- 90.Robert J.J. Continuous monitoring of blood glucose. Horm. Res. Paediatr. 2002;57:81–84. doi: 10.1159/000053321. [DOI] [PubMed] [Google Scholar]
- 91.Hanna J., Bteich M., Tawk Y., Ramadan A., Dia B., Asadallah F.A., Eid A., Kanj R., Costantine J., Eid A.A. Noninvasive, wearable, and tunable electromagnetic multisensing system for continuous glucose monitoring, mimicking vasculature anatomy. Sci. Adv. 2020;6:eaba5320. doi: 10.1126/sciadv.aba5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanna J., Tawk Y., Azar S., Ramadan A.H., Dia B., Shamieh E., Zoghbi S., Kanj R., Costantine J., Eid A.A. Wearable flexible body matched electromagnetic sensors for personalized non-invasive glucose monitoring. Sci. Rep. 2022;12:14885. doi: 10.1038/s41598-022-19251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao J., Zhang S., Sun Y., Zhou N., Yu H., Zhang H., Jia D. Wearable Optical Sensing in the Medical Internet of Things (MIoT) for Pervasive Medicine: Opportunities and Challenges. ACS Photonics. 2022;9:2579–2599. doi: 10.1021/acsphotonics.2c00898. [DOI] [Google Scholar]
- 94.Dcosta J.V., Ochoa D., Sanaur S. Recent Progress in Flexible and Wearable All Organic Photoplethysmography Sensors for SpO2 Monitoring. Adv. Sci. 2023;10:2302752. doi: 10.1002/advs.202302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kulkarni M.B., Rajagopal S., Prieto-Simón B., Pogue B.W. Recent advances in smart wearable sensors for continuous human health monitoring. Talanta. 2024;272:125817. doi: 10.1016/j.talanta.2024.125817. [DOI] [PubMed] [Google Scholar]
- 96.Barata J.J.R., Munoz R., Silva R.D.D.C., Rodrigues J.J.P.C., de Albuquerque V.H.C. Internet of Things Based on Electronic and Mobile Health Systems for Blood Glucose Continuous Monitoring and Management. IEEE Access. 2019;7:175116–175125. doi: 10.1109/ACCESS.2019.2956745. [DOI] [Google Scholar]
- 97.Safarkhani M., Aldhaher A., Heidari G., Zare E.N., Warkiani M.E., Akhavan O., Huh Y., Rabiee N. Nanomaterial-assisted wearable glucose biosensors for noninvasive real-time monitoring: Pioneering point-of-care and beyond. Nano Mater. Sci. 2023;6:263–283. doi: 10.1016/j.nanoms.2023.11.009. [DOI] [Google Scholar]
- 98.Phillips N.E., Collet T.H., Naef F. Uncovering personalized glucose responses and circadian rhythms from multiple wearable biosensors with Bayesian dynamical modeling. Cell Rep. Methods. 2023;3:100545. doi: 10.1016/j.crmeth.2023.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma S., Dudeja R.K., Aujla G.S., Bali R.S., Kumar N. DeTrAs: Deep Learning-based Healthcare Framework for IoT-based Assistance of Alzheimer Patients. Neural Comput. Appl. 2020 doi: 10.1007/s00521-020-05327-2. [DOI] [Google Scholar]
- 100.Popp Z., Low S., Igwe A., Rahman M.S., Kim M., Khan R., Oh E., Kumar A., Anda-Duran I.D., Ding H., et al. Shifting From Active to Passive Monitoring of Alzheimer Disease: The State of the Research. J. Am. Heart Assoc. 2024;13:e031247. doi: 10.1161/JAHA.123.031247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Creese B., Arnold J., McDermid J., Medina-Lara A., Ismail Z., Steer Z. Protocol for an acceptability and feasibility study of sensor-instrumented SmartSocks® for use by people with dementia. Alzheimer’s Dement. 2024;20:e086878. doi: 10.1002/alz.086878. [DOI] [Google Scholar]
- 102.Grammatikopoulou M., Lazarou I., Alepopoulos V., Mpaltadoros L., Oikonomou V., Stavropoulos T.G., Nikolopoulos S., Kompatsiaris I., Tsolaki M. Assessing the cognitive decline of people in the spectrum of AD by monitoring their activities of daily living in an IoT-enabled smart home environment: A cross-sectional pilot study. Front. Aging Neurosci. 2024;16:1375131. doi: 10.3389/fnagi.2024.1375131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Javheri R., Patil D., Kamble A., Yadav A., Kolhe P., Mate G.S. A Review: Smart Wristwear for Alzheimer Patients with an Advanced Tracking System; Proceedings of the 2024 IEEE Region 10 Symposium (TENSYMP); New Delhi, India. 27–29 September 2024; pp. 1–6. [DOI] [Google Scholar]
- 104.Mani K., Singh K.K., Litoriya R. Transformative Potential IoT Sensor Frameworks for Real-Time Alzheimer’s Detection and Monitoring in Elderly Populations; Proceedings of the 2024 International Conference on Advances in Computing Research on Science Engineering and Technology (ACROSET); Indore, India. 27–28 September 2024; pp. 1–8. [DOI] [Google Scholar]
- 105.Muurling M.M., de Boer R., Kozak R., Lucivero F., Lancaster C., Hinds C., Stravopoulos T.G., Nikolopoulos S., Kompatsiaris I., Manyakov N.V., et al. Remote monitoring technologies in Alzheimer’s disease: Design of the RADAR-AD study. Alzheimer’s Res. Ther. 2021;13:89. doi: 10.1186/s13195-021-00825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muurling M., de Boer C., Hinds C., Atreya A., Doherty A., Alepopoulos V., Curcic J., Brem A.-K., Conde P., Kuruppu S., et al. Feasibility and usability of remote monitoring in Alzheimer’s disease. Digit. Health. 2024;10:20552076241238133. doi: 10.1177/20552076241238133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patil V., Chikkoppa B. Advanced Alzheimer’s IoT solution for Real-time Monitoring in Healthcare Services; Proceedings of the 2022 International Conference for Advancement in Technology (ICONAT); Goa, India. 21–22 January 2022; pp. 1–7. [DOI] [Google Scholar]
- 108.Cardinali L., Mariano V., Rodriguez-Duarte D.O., Tobón Vasquez J.A., Scapaticci R., Crocco L., Vipiana F. Early Detection of Alzheimer’s Disease via Machine Learning-Based Microwave Sensing: An Experimental Validation. Sensors. 2025;25:2718. doi: 10.3390/s25092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.David M.C.B., Kolanko M., Del Giovane M., Lai H., True J., Beal E., Li L.M., Nilforooshan R., Barnaghi P., Malhotra P.A., et al. Remote monitoring of physiology in people living with dementia: An observational cohort study. JMIR Aging. 2023;6:e43777. doi: 10.2196/43777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rawtaer I., Mahendran S.R., Kua E.H., Tan H.P., Tan H.X., Lee T.S., Ng T.P. Early Detection of Mild Cognitive Impairment with In-Home Sensors to Monitor Behavior Patterns in Community-Dwelling Senior Citizens in Singapore: Cross-Sectional Feasibility Study. J. Med. Internet Res. 2020;22:e16854. doi: 10.2196/16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Au-Yeung W.T.M., Miller L., Beattie Z., May R., Cray H.V., Kabelac Z., Katabi D., Kaye J.A., Vahia I.V. Feasibility of deploying home-based digital technology, environmental sensors, and web-based surveys for assessing behavioral symptoms and identifying their precipitants in older adults: Longitudinal, observational study. JMIR Form. Res. 2024;8:e53192. doi: 10.2196/53192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ford C.T., Galler J.A., He Y., Young C., Simpson B.G.K., Wu C., Pfaffenroth J., Wah E.S., Arnold S.E., Dodge H.H., et al. Using Apple Watches to Monitor Health and Behaviors of Individuals with Cognitive Impairment: A Case Series Study. J. Gerontol. A Biol. Sci. Med. Sci. 2025;80:glae250. doi: 10.1093/gerona/glae250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salehi W., Gupta G., Bhatia S., Koundal D., Mashat A.A., Belay A. IoT-Based Wearable Devices for Patients Suffering from Alzheimer Disease. Contrast Media Mol. Imaging. 2022;2022:3224939. doi: 10.1155/2022/3224939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chokri R., Hanini W., Daoud W.B., Chelloug S.A., Makhlouf A.M. Secure IoT Assistant-Based System for Alzheimer’s Disease. IEEE Access. 2022;10:44305–44314. doi: 10.1109/ACCESS.2022.3168311. [DOI] [Google Scholar]
- 115.Shahzad A., Dadlani A., Lee H., Kim K. Automated Prescreening of Mild Cognitive Impairment Using Shank-Mounted Inertial Sensors Based Gait Biomarkers. IEEE Access. 2022;10:15835–15844. doi: 10.1109/ACCESS.2022.3149100. [DOI] [Google Scholar]
- 116.Park J., Lee H.J., Park J.S., Won S.H., Bae J.B., Han J.W., Kim K.W. Development of a Gait Feature–Based Model for Classifying Cognitive Disorders Using a Single Wearable Inertial Sensor. Neurology. 2023;101:e12–e19. doi: 10.1212/WNL.0000000000207372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cherachapridi P., Wachiraphan P., Rangpong P., Kiatthaveephong S., Kongwudhikunakorn S., Thanontip K., Piriyajitakonkij M., Chinkamol A., Likitvanichkul C., Dujada P., et al. Prescreening MCI and Dementia Using Shank-Mounted IMU During TUG Task. IEEE Sens. J. 2022;22:24550–24558. doi: 10.1109/JSEN.2022.3220238. [DOI] [Google Scholar]
- 118.Wu T., Lure F., Chen V.C. STRIDE: Systematic Radar Intelligence Analysis for ADRD Risk Evaluation with Gait Signature Simulation and Deep Learning. IEEE Sens. J. 2023;23:10998–11006. doi: 10.1109/JSEN.2023.3263071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jing Y., Qin P., Fan X., Wencheng Z., Sun W., Tian F., Wang D. Deep Learning–Assisted Gait Parameter Assessment for Neurodegenerative Diseases: Model Development and Validation. J. Med. Internet Res. 2023;25:e46427. doi: 10.2196/46427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seifallahi M., Galvin J.E., Ghoraani B. Detection of mild cognitive impairment using various types of gait tests and machine learning. Front. Neurol. 2024;15:1354092. doi: 10.3389/fneur.2024.1354092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi A., Kim T.H., Yuhai O., Jeong S., Kim K., Kim H., Mun J.H. Deep Learning-Based Near-Fall Detection Algorithm for Fall Risk Monitoring System Using a Single Inertial Measurement Unit. IEEE Trans. Neural Syst. Rehabil. Eng. 2022;30:2385–2394. doi: 10.1109/TNSRE.2022.3199068. [DOI] [PubMed] [Google Scholar]
- 122.Agrawal D.K., Usaha W., Pojprapai S., Wattanapan P. Fall Risk Prediction Using Wireless Sensor Insoles with Machine Learning. IEEE Access. 2023;11:23119–23126. doi: 10.1109/ACCESS.2023.3252886. [DOI] [Google Scholar]
- 123.Li Z., Zhu J., Liu J., Shi M., Liu P., Guo J., Hu Z., Liu S., Yang D. Using dual-task gait to recognize Alzheimer’s disease and mild cognitive impairment: A cross-sectional study. Front. Hum. Neurosci. 2023;17:1284805. doi: 10.3389/fnhum.2023.1284805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Munteanu D., Bejan C., Munteanu N.A., Zamfir C., Vasić M., Petrea S.M., Cristea D.S. Deep-Learning-Based System for Assisting People with Alzheimer’s Disease. Electronics. 2022;11:3229. doi: 10.3390/electronics11193229. [DOI] [Google Scholar]
- 125.Onthoni D.D., Sahoo P.K. Artificial-Intelligence-Assisted Activities of Daily Living Recognition for Elderly in Smart Home. Electronics. 2022;11:4129. doi: 10.3390/electronics11244129. [DOI] [Google Scholar]
- 126.Gao H., Zhou L., Kim J.Y., Li Y., Huang W. The Behavior Guidance and Abnormality Detection for A-MCI Patients under Wireless Sensor Network. ACM Trans. Sens. Netw. 2022;19:1–24. doi: 10.1145/3499426. [DOI] [Google Scholar]
- 127.Chimamiwa G., Giaretta A., Alirezaie M., Pecora F., Loutfi A. Are Smart Homes Adequate for Older Adults with Dementia? Sensors. 2022;22:4254. doi: 10.3390/s22114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khodabandehloo E., Alimohammadi A., Riboni D. FreeSia: A Cyber-physical System for Cognitive Assessment through Frequency-domain Indoor Locomotion Analysis. ACM Trans. Cyber-Phys. Syst. 2022;6:1–31. doi: 10.1145/3470454. [DOI] [Google Scholar]
- 129.Cantone A.A., Esposito M., Perillo F.P., Romano M., Sebillo M., Vitiello G. Enhancing Elderly Health Monitoring: Achieving Autonomous and Secure Living through the Integration of Artificial Intelligence, Autonomous Robots, and Sensors. Electronics. 2023;12:3918. doi: 10.3390/electronics12183918. [DOI] [Google Scholar]
- 130.Mathunjwa B.M., Kor R.Y.J., Ngarnkuekool W., Hsu Y.L. A Comprehensive Review of Home Sleep Monitoring Technologies: Smartphone Apps, Smartwatches, and Smart Mattresses. Sensors. 2025;25:1771. doi: 10.3390/s25061771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gamel S.A., Talaat F.M. SleepSmart: An IoT-enabled continual learning algorithm for intelligent sleep enhancement. Neural Comput. Appl. 2024;36:4293–4309. doi: 10.1007/s00521-023-09310-5. [DOI] [Google Scholar]
- 132.Blackman J., Morrison H., Gabb V., Biswas B., Turner N., Jolly A., Trender W., Hampshire A., Whone A., Coulthard E. Remote evaluation of sleep to enhance understanding of early dementia due to Alzheimer’s Disease (RESTED-AD): An observational cohort study protocol. BMC Geriatr. 2023;23:590. doi: 10.1186/s12877-023-04288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kolanko M., Soreq E., Golemme M., Walsh C., Giovane M.D., Sharp D. Digital biomarkers of sleep and nocturnal behaviour derived from contactless longitudinal sleep monitoring predict cognitive decline and deterioration in daily function in people living with dementia. Alzheimer’s Dement. 2024;20:e091826. doi: 10.1002/alz.091826. [DOI] [Google Scholar]
- 134.Griffiths T.L., McCreedy E., Reddy A. Sleep in focus: Evaluating wearable biometric devices for dementia sleep monitoring- a scoping review of efficacy. Innov. Aging. 2024;8:1275. doi: 10.1093/geroni/igae098.4076. [DOI] [Google Scholar]
- 135.Tian Y.J., Felber N.A., Pageau F., Roulet Schwab D., Wangmo T. Benefits and barriers associated with the use of smart home health technologies in the care of older persons: A systematic review. BMC Geriatr. 2024;24:152. doi: 10.1186/s12877-024-04702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Islam M.R., Kabir M.M., Mridha M.F., Alfarhood S., Safran M., Che D. Deep Learning-Based IoT System for Remote Monitoring and Early Detection of Health Issues in Real-Time. Sensors. 2023;23:5204. doi: 10.3390/s23115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang Y., Wang J. Technological Innovations and Data-Driven Support for Older Adults. JMIR Aging. 2023;6:e48547. doi: 10.2196/48547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scullin M.K., Jones W.E., Phenis R., Beevers S., Rosen S., Dinh K., Kiselica A.M., Keefe F.J., Benge J.F. Using smartphone technology to improve prospective memory functioning: A randomized controlled trial. J. Am. Geriatr. Soc. 2022;70:459–469. doi: 10.1111/jgs.17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu C.L., Chuang C.J., Chou C.M. A Pilot Fuzzy System with Virtual Reality for Mild Cognitive Impairment (MCI) Assessment. Healthcare. 2023;11:2503. doi: 10.3390/healthcare11182503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim H.J., Jeong S.Y., Kang S.J. Knowledge-Based Remote E-Coaching Framework Using IoT Devices for In-Home ADL Rehabilitation Treatment of Degenerative Brain Disease Patients. Sensors. 2022;22:7957. doi: 10.3390/s22207957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Salai A.M., Kirton A., Cook G., Holmquist L.E. Views and experiences on the use of voice assistants by family and professionals supporting people with cognitive impairments. Front. Dement. 2022;1:1049464. doi: 10.3389/frdem.2022.1049464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Machado S.D., Tavares J.E.d.R., Martins M.G., Barbosa J.L.V., González G.V., Leithardt V.R.Q. Ambient intelligence based on IoT for assisting people with Alzheimer’s disease through context histories. Electronics. 2021;10:1260. doi: 10.3390/electronics10111260. [DOI] [Google Scholar]
- 143.Ghorbani F., Ahmadi A., Kia M., Rahman Q., Delrobaei M. A Decision-Aware Ambient Assisted Living System with IoT Embedded Device for In-Home Monitoring of Older Adults. Sensors. 2023;23:2673. doi: 10.3390/s23052673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu K., Zhang Q., He B., Huang M., Lin R., Li H. Immersive Virtual Reality-Based Cognitive Intervention for the Improvement of Cognitive Function, Depression, and Perceived Stress in Older Adults with Mild Cognitive Impairment and Mild Dementia: Pilot Pre-Post Study. JMIR Serious Games. 2022;10:e32117. doi: 10.2196/32117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mao Q., Zhao Z., Yu L., Zhao Y., Wang H. The Effects of Virtual Reality–Based Reminiscence Therapies for Older Adults with Cognitive Impairment: Systematic Review. J. Med. Internet Res. 2024;26:e53348. doi: 10.2196/53348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dubbelman M., Hall T.C., Levesque I.M., Mimmack K.J., Sikkes S.A.M., Fischer S.H., Rentz D.M., Sperling R.A., Papp K.V., Amariglio R.E., et al. Using a digital tool to detect early changes in everyday functioning in older adults: A pilot study of the Assessment of Smartphone Everyday Tasks (ASSET) Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023;15:e12506. doi: 10.1002/dad2.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smith A.M., Marin A., DeCaro R.E., Feinn R., Wack A., Hughes G.I., Rivard N., Umashankar A., Turk K.W., Budson A.E. Algorithmic Spaced Retrieval Enhances Long-Term Memory in Alzheimer Disease: Case-Control Pilot Study. JMIR Form. Res. 2024;8:e51943. doi: 10.2196/51943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mahesh G., Kalidas M. A Real-Time IoT Based Fall Detection and Alert System for Elderly; Proceedings of the 2023 International Conference on Advances in Computation, Communication and Information Technology (ICAICCIT); Faridabad, India. 23–24 November 2023; pp. 327–331. [DOI] [Google Scholar]
- 149.Abhishek A.P.N., T A.G.C., V B.H., Kanithan S. Smart Biomedical Assisted System for Alzheimer Patient; Proceedings of the 2023 7th International Conference on Design Innovation for 3 Cs Compute Communicate Control (ICDI3C); Karnataka, India. 2 November 2023; pp. 132–136. [DOI] [Google Scholar]
- 150.Guamán-Egas J., Castro-Martin A.P. Fall Detection with Artificial Intelligence and IoT Health Monitoring System; Proceedings of the 2023 IEEE Seventh Ecuador Technical Chapters Meeting (ECTM); Ambato, Ecuador. 10–13 October 2023; pp. 1–6. [DOI] [Google Scholar]
- 151.Syamlan M.A., Noor R.A.M., S. A.S.F., Syamlan M.A., Arifin A., Pramudijanto J., Arrofiqi F. Fuzzy Logic-Based Fall Detection System for Elderly Using a Single Inertial Measurement Unit; Proceedings of the 2024 International Conference on Computer Engineering, Network, and Intelligent Multimedia (CENIM); Surabaya, Indonesia. 19–20 November 2024; pp. 1–5. [DOI] [Google Scholar]
- 152.Al-Naami B., Abu Owida H., Abu Mallouh M., Al-Naimat F., Agha M., Al-Hinnawi A.R. A New Prototype of Smart Wearable Monitoring System Solution for Alzheimer’s Patients. Med. Devices. 2021;14:423–433. doi: 10.2147/MDER.S339855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tibrewal V. Fall Detection in Alzheimers Disease Patients using Machine Learning, Integrated with a Wrist-Wrap Design and Mobile App. Int. J. Electr. Eng. Technol. 2021;12:32–42. doi: 10.34218/ijeet.12.9.2021.004. [DOI] [Google Scholar]
- 154.Lin H.C., Chen M.J., Lee C.H., Kung L.C., Huang J.T. Fall Recognition Based on an IMU Wearable Device and Fall Verification through a Smart Speaker and the IoT. Sensors. 2023;23:5472. doi: 10.3390/s23125472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mishra S., Ngangbam B., Raj S., Pradhan N.R. CURA: Real Time Artificial Intelligence and IoT based Fall Detection Systems for patients suffering from Dementia. EAI Endorsed Trans. Pervasive Health Technol. 2023;9 doi: 10.4108/eetpht.9.3967. [DOI] [Google Scholar]
- 156.Al-Kababji A., Amira A., Bensaali F., Jarouf A., Shidqi L., Djelouat H. An IoT-based framework for remote fall monitoring. Biomed. Signal Process. Control. 2021;67:102532. doi: 10.1016/j.bspc.2021.102532. [DOI] [Google Scholar]
- 157.Karar M.E., Shehata H.I., Reyad O. A survey of IoT-based fall detection for aiding elderly care: Sensors, methods, challenges and future trends. Appl. Sci. 2022;12:3276. doi: 10.3390/app12073276. [DOI] [Google Scholar]
- 158.Lee C.-W., Chuang H.-M. Design of a seniors and Alzheimer’s disease caring service platform. BMC Med. Inform. Decis. Mak. 2021;21:273. doi: 10.1186/s12911-021-01626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ambient Monitoring of Gait and Machine Learning Models for Dynamic and Short-Term Falls Risk Assessment in People with Dementia. IEEE J. Biomed. Health Inform. 2023;27:3599–3609. doi: 10.1109/JBHI.2023.3267039. [DOI] [PubMed] [Google Scholar]
- 160.Belmin J., Villani P., Gay M., Fabries S., Havreng-Théry C., Malvoisin S., Denis F., Veyron J.H. Real-world Implementation of an eHealth System Based on Artificial Intelligence Designed to Predict and Reduce Emergency Department Visits by Older Adults: Pragmatic Trial. J. Med. Internet Res. 2022;24:e40387. doi: 10.2196/40387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tiersen F., Batey P., Harrison M.J.C., Naar L., Serban A.I., Daniels S.J.C., Calvo R.A. Smart Home Sensing and Monitoring in Households with Dementia: User-Centered Design Approach. JMIR Aging. 2021;4:e27047. doi: 10.2196/27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davidoff H., Kraaij A.V., den Bulcke L.V., Lutin E., Vandenbulcke M., Helleputte N.V., Vos M.D., Hoof C.V., Bossche M.V.D. Physiological Profiling of Agitation in Dementia: Insights From Wearable Sensor Data. Innov. Aging. 2024;8:igae057. doi: 10.1093/geroni/igae057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Javed A.R., Fahad L.G., Farhan A.A., Abbas S., Srivastava G., Parizi R.M., Khan M.S. Automated cognitive health assessment in smart homes using machine learning. Sustain. Cities Soc. 2021;65:102572. doi: 10.1016/j.scs.2020.102572. [DOI] [Google Scholar]
- 164.Palermo F., Chen Y., Capstick A., Fletcher-Loyd N., Walsh C., Kouchaki S., True J., Balazikova O., Soreq E., Scott G., et al. TIHM: An open dataset for remote healthcare monitoring in dementia. Sci. Data. 2023;10:606. doi: 10.1038/s41597-023-02519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee M., Mishra R.K., Momin A., El-Refaei N., Bagheri A.B., York M.K., Kunik M.E., Derhammer M., Fatehi B., Lim J., et al. Smart-Home Concept for Remote Monitoring of Instrumental Activities of Daily Living (IADL) in Older Adults with Cognitive Impairment: A Proof of Concept and Feasibility Study. Sensors. 2022;22:6745. doi: 10.3390/s22186745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arthanat S., Wilcox J., LaRoche D. Smart home automation technology to support caring of individuals with Alzheimer’s disease and related dementia: An early intervention framework. Disabil. Rehabil. Assist. Technol. 2022;19:779–789. doi: 10.1080/17483107.2022.2125088. [DOI] [PubMed] [Google Scholar]
- 167.Sharanya C., S K., T. R R.S. Pill Dispenser for Elderly Care using IOT; Proceedings of the 2024 4th Asian Conference on Innovation in Technology (ASIANCON); Pimpri Chinchwad, India. 23–25 August 2024; pp. 1–4. [DOI] [Google Scholar]
- 168.Peddisetti V., Kandregula P.K., John J.A., Poomdla S., George K., Panangadan A. Smart Medication Management: Enhancing Medication Adherence with an IoT-Based Pill Dispenser and Smart Cup; Proceedings of the 2024 IEEE First International Conference on Artificial Intelligence for Medicine, Health and Care (AIMHC); Laguna Hills, CA, USA. 5–7 February 2024; pp. 137–144. [DOI] [Google Scholar]
- 169.Kamil R.J., Bakar D., Ehrenburg M., Wei E.X., Pletnikova A., Xiao G., Oh E.S., Mancini M., Agrawal Y. Detection of Wandering Behaviors Using a Body-Worn Inertial Sensor in Patients with Cognitive Impairment: A Feasibility Study. Front. Neurol. 2021;12:529661. doi: 10.3389/fneur.2021.529661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hsu Y.L., Chung P.C., Wang W.H., Pai M.C., Wang C.Y., Lin C.W. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014;18:1822–1830. doi: 10.1109/JBHI.2014.2325413. [DOI] [PubMed] [Google Scholar]
- 171.El-Gohary M., Pearson S., McNames J., Mancini M., Horak F. Continuous monitoring of turning in patients with movement disability. Sensors. 2013;14:356–369. doi: 10.3390/s140100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Howes J., Denier Y., Gastmans C. Electronic Tracking Devices for People with Dementia: Content Analysis of Company Websites. JMIR Aging. 2022;5:e38865. doi: 10.2196/38865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.H L G., Flammini F., Srividhya S., M L C., Selvam S. Computer Science Engineering: Proceedings of the 1st International Conference on Computing and Intelligent Information Systems (ICCIIS 2024), Bangalore, India, 19–20 April 2024. 1st ed. Volume 2. CRC Press; Bangalore, India: 2024. [DOI] [Google Scholar]
- 174.Bonilla V., Campoverde B., Yoo S.G. A Systematic Literature Review of LoRaWAN: Sensors and Applications. Sensors. 2023;23:8440. doi: 10.3390/s23208440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ponnuviji N.P., Elangovan G., Sujatha K., Ganesan I., Lalitha S.D. IoT-Enabled Assistive Technologies Approach for Personalized Geriatric Health Monitoring and Safety. In: Khade S.M., Mishra R.G., editors. Future of AI in Biomedicine and Biotechnology. IGI Global; Hershey, PA, USA: 2024. pp. 125–146. [DOI] [Google Scholar]
- 176.Parameshwaran, Khan V., Gopi B., H D.R.C. Intelligent Device for Monitoring the Dementia Patients in Real Time using IoT Technology; Proceedings of the 2022 International Conference on Power, Energy, Control and Transmission Systems (ICPECTS); Chennai, India. 8–9 December 2022; pp. 1–3. [DOI] [Google Scholar]
- 177.Nandini B.P., Reddy P., Mary G.I., Julian A., R. R., Rele M. Smart Watch with Machine Learning Based Fall Detection System; Proceedings of the 2024 IEEE Region 10 Symposium (TENSYMP); New Delhi, India. 27–29 September 2024; pp. 1–7. [DOI] [Google Scholar]
- 178.Adardour H., Hadjila M., Irid S., Baouch T., Belkhiter S.E. Outdoor Alzheimer’s Patients Tracking Using an IoT System and a Kalman Filter Estimator. Wirel. Pers. Commun. 2021;116:249–265. doi: 10.1007/s11277-020-07713-4. [DOI] [Google Scholar]
- 179.Li W., Du Z. Research on spatial design method of rehabilitation environment based on behavioral characteristics of Alzheimer’s disease patients; Proceedings of the 2020 International Conference on Innovation Design and Digital Technology (ICIDDT); Zhenjing, China. 5–6 December 2020; pp. 213–216. [DOI] [Google Scholar]
- 180.Oliveira R., Feres R., Barreto F., Abreu R. CNN for Elderly Wandering Prediction in Indoor Scenarios. SN Comput. Sci. 2022;3:230. doi: 10.1007/s42979-022-01091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Akaike H., Katsuta S.I., Aramaki S., Moshnyaga V., Hashimoto K., Ikeda S. Wandering Notification System for Caregivers of People with Dementia; Proceedings of the 2022 IEEE 65th International Midwest Symposium on Circuits and Systems (MWSCAS); Fukuoka, Japan. 7–10 August 2022; pp. 1–2. [DOI] [Google Scholar]
- 182.Oskouei R.J., MousaviLou Z., Bakhtiari Z., Jalbani K.B. IoT-Based Healthcare Support System for Alzheimer’s Patients. Wirel. Commun. Mob. Comput. 2020;2020:8822598. doi: 10.1155/2020/8822598. [DOI] [Google Scholar]
- 183.Kanchanamala P., Chippada R., Bennabatthula T., Muppidi S. AI-Powered Alzheimer’s Care: Smart Face Recognition, Medicine Reminders, and Location; Proceedings of the 2024 IEEE North Karnataka Subsection Flagship International Conference (NKCon); Bagalkote, India. 21–22 September 2024; pp. 1–8. [DOI] [Google Scholar]
- 184.Doyle M., Nwofe E.S., Rooke C., Seelam K., Porter J., Bishop D. Implementing global positioning system trackers for people with dementia who are at risk of wandering. Dementia. 2024;23:964–980. doi: 10.1177/14713012241248556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cullen A., Mazhar M.K.A., Smith M.D., Lithander F.E., Ó Breasail M., Henderson E.J. Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors. 2022;22:3336. doi: 10.3390/s22093336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jafarpournaser N., Delavar M.R., Noroozian M. A Wandering Detection Method Based on Processing GPS Trajectories Using the Wavelet Packet Decomposition Transform for People with Cognitive Impairment. ISPRS Int. J. Geo-Inf. 2023;12:379. doi: 10.3390/ijgi12090379. [DOI] [Google Scholar]
- 187.Wang W.H., Hsu W.S. Integrating Artificial Intelligence and Wearable IoT System in Long-Term Care Environments. Sensors. 2023;23:5913. doi: 10.3390/s23135913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Iaboni A., Spasojevic S., Newman K., Schindel Martin L., Wang A., Ye B., Mihailidis A., Khan S.S. Wearable multimodal sensors for the detection of behavioral and psychological symptoms of dementia using personalized machine learning models. Alzheimer’s Dement. 2022;14:e12305. doi: 10.1002/dad2.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mitrovska A., Safari P., Ritter K., Shariati B., Fischer J.K. Secure federated learning for Alzheimer’s disease detection. Front. Aging Neurosci. 2024;16:1324032. doi: 10.3389/fnagi.2024.1324032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Timon C.M., Hussey P., Lee H., Murphy C., Rai H.V., Smeaton A.F. Automatically detecting activities of daily living from in-home sensors as indicators of routine behaviour in an older population. Digit. Health. 2023;9:20552076231184084. doi: 10.1177/20552076231184084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chung J., Pretzer-Aboff I., Parsons P., Falls K., Bulut E. Using a Device-Free Wi-Fi Sensing System to Assess Daily Activities and Mobility in Low-Income Older Adults: Protocol for a Feasibility Study. JMIR Res. Protoc. 2024;13:e53447. doi: 10.2196/53447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ouyang X., Shuai X., Li Y., Pan L., Zhang X., Fu H., Cheng S., Wang X., Cao S., Xin J., et al. MobiCom ’24: Proceedings of the 30th Annual International Conference on Mobile Computing and Networking, Washington, DC, USA, 18–22 November 2024. ACM; New York, NY, USA: 2024. ADMarker: A Multi-Modal Federated Learning System for Monitoring Digital Biomarkers of Alzheimer’s Disease; pp. 404–419. [DOI] [Google Scholar]
- 193.Hussain M., Shiren Y. Identifying Alzheimer Disease Dementia Levels Using Machine Learning Methods. Med. Res. Arch. 2023;11 doi: 10.18103/mra.v11i7.1.4039. [DOI] [Google Scholar]
- 194.Alayba A.M., Senan E.M., Alshudukhi J.S. Enhancing Early Detection of Alzheimer’s Disease through Hybrid Models Based on Feature Fusion of Multi-CNN and Handcrafted Features. Sci. Rep. 2024;14:31203. doi: 10.1038/s41598-024-82544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arunachalam R., Sunitha G., Shukla S.K., Nath Pandey S., Urooj S., Rawat S. A smart Alzheimer’s patient monitoring system with IoT-assisted technology through enhanced deep learning approach. Knowl. Inf. Syst. 2023;65:5561–5599. doi: 10.1007/s10115-023-01890-x. [DOI] [Google Scholar]
- 196.Mascret Q., Gagnon-Turcotte G., Bielmann M., Fall C.L., Bouyer L.J., Gosselin B. A Wearable Sensor Network with Embedded Machine Learning for Real-Time Motion Analysis and Complex Posture Detection. IEEE Sens. J. 2022;22:7868–7876. doi: 10.1109/JSEN.2021.3139588. [DOI] [Google Scholar]
- 197.Mohammad Z., Anwary A.R., Mridha M.F., Shovon M.S.H., Vassallo M. An Enhanced Ensemble Deep Neural Network Approach for Elderly Fall Detection System Based on Wearable Sensors. Sensors. 2023;23:4774. doi: 10.3390/s23104774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hussain A., Sheikh Z., Subramanian M.L. The Eye as a Diagnostic Tool for Alzheimer’s Disease. Life. 2023;13:726. doi: 10.3390/life13030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Salobrar-García E. Value of the Retina in the Diagnosis of Alzheimer’s Disease. Acta Ophthalmol. 2022;100:S275. doi: 10.1111/j.1755-3768.2022.15575. [DOI] [Google Scholar]
- 200.Leuenberger R.C., Verbraak F.D., Twisk J.W.R., de Ruyter F.J.H., Hoozemans J.J., de Boer J.F., Bouwman F.H. Development of Retinal Biomarkers for Alzheimer’s Disease. Alzheimer’s Dement. 2022;18:e063836. doi: 10.1002/alz.063836. [DOI] [Google Scholar]
- 201.Hao J., Kwapong W.R., Shen T., Fu H., Xu Y., Lu Q., Liu S., Zhang J., Liu Y., Zhao Y., et al. Early detection of dementia through retinal imaging and trustworthy AI. npj Digit. Med. 2024;7:294. doi: 10.1038/s41746-024-01292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shi X.H., Ju L., Dong L., Zhang R.H., Shao L., Yan Y.N., Wang Y.X., Fu X.F., Chen Y.Z., Ge Z.Y., et al. Deep Learning Models for the Screening of Cognitive Impairment Using Multimodal Fundus Images. Ophthalmol. Retin. 2024;8:666–677. doi: 10.1016/j.oret.2024.01.019. [DOI] [PubMed] [Google Scholar]
- 203.Cheung C.Y., Ran A.R., Wang S., Chan V.T.T., Sham K., Hilal S., Venketasubramanian N., Cheng C.Y., Sabanayagam C., Tham Y.C., et al. A deep learning model for detection of Alzheimer’s disease based on retinal photographs: A retrospective, multicentre case-control study. Lancet Digit. Health. 2022;4:e806–e815. doi: 10.1016/S2589-7500(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 204.Vrahatis A.G., Skolariki K., Krokidis M.G., Exarchos T.P., Vlamos P. Revolutionizing the Early Detection of Alzheimer’s Disease through Non-Invasive Biomarkers: The Role of Artificial Intelligence and Deep Learning. Sensors. 2023;23:4184. doi: 10.3390/s23094184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hui H.Y., Ran A., Cheung C.Y. Deep Reinforcement Learning-Based Retinal Imaging in Alzheimer’s Disease: Potential and Perspectives. J. Alzheimer’s Dis. 2023;94:39–50. doi: 10.3233/JAD-230055. [DOI] [PubMed] [Google Scholar]
- 206.Fernandez G., Mendez L., Lopera F., Parra-Rodriguez M.A. The Eye as a Biomarker for Alzheimer’s Disease: Oculomotor Behaviours Yield a Novel Digital Biomarker for Preclinical Risk Detection. Alzheimer’s Dement. 2022;18:e066784. doi: 10.1002/alz.066784. [DOI] [Google Scholar]
- 207.Liu Y., Zhuang D., Wang J., Huang H., Li R., Wu C., Deng Y., Hu G., Guo B. Recent Advances in Small Molecular Near-Infrared Fluorescence Probes for a Targeted Diagnosis of the Alzheimer Disease. Analyst. 2022;147:4701–4723. doi: 10.1039/D2AN01327D. [DOI] [PubMed] [Google Scholar]
- 208.Li J., Ni W., Jin D., Yu Y., Xiao M., Zhang Z., Zhang G.J. Nanosensor-Driven Detection of Neuron-Derived Exosomal Aβ42 with Graphene Electrolyte-Gated Transistor for Alzheimer’s Disease Diagnosis. Anal. Chem. 2023;95:5719–5728. doi: 10.1021/acs.analchem.2c05751. [DOI] [PubMed] [Google Scholar]
- 209.Jyothi E.V.N., Sailaja S., Reddy M.S.L., Sunitha T. Automated Health Monitoring: Integrating AI and IoT for Continuous Patient Observation; Proceedings of the 2024 International Conference on IoT Based Control Networks and Intelligent Systems (ICICNIS); Bengaluru, India. 17–18 December 2024; pp. 519–524. [DOI] [Google Scholar]
- 210.Tsvetanov F. Integrating AI Technologies into Remote Monitoring Patient Systems. Eng. Proc. 2024;70:54. doi: 10.3390/engproc2024070054. [DOI] [Google Scholar]
- 211.Jameil A.K., Al-Raweshidy H. A Digital Twin Framework for Real-Time Healthcare Monitoring: Leveraging AI and Secure Systems for Enhanced Patient Outcomes. Smart Health. 2025;5:37. doi: 10.1007/s43926-025-00135-3. [DOI] [Google Scholar]
- 212.Ianculescu M., Nicolau D., Petrache C., Vasilevschi A. Advancing Neurodegenerative Disease Management: The NeuroPredict Platform Integrating IoT, AI, and Cloud Technologies; Proceedings of the 2024 15th International Conference on Communications (COMM); Bucharest, Romania. 3–4 October 2024; pp. 1–6. [DOI] [Google Scholar]
- 213.Kale M.B., Wankhede N.L., Pawar R.S., Ballal S., Kumawat R., Goswami M., Khalid M., Taksande B., Upaganlawar A., Umekar M., et al. AI-Driven Innovations in Alzheimer’s Disease: Integrating Early Diagnosis, Personalized Treatment, and Prognostic Modelling. Ageing Res. Rev. 2024;101:102497. doi: 10.1016/j.arr.2024.102497. [DOI] [PubMed] [Google Scholar]
- 214.Alturki B., Abu Al-Haija Q., Alsemmeari R.A., Alsulami A.A., Alqahtani A., Alghamdi B.M., Bakhsh S.T., Shaikh R.A. IoMT landscape: Navigating current challenges and pioneering future research trends. Discov. Appl. Sci. 2025;7:26. doi: 10.1007/s42452-024-06351-w. [DOI] [Google Scholar]
- 215.Alhaj T.A., Abdulla S.M., Iderss M.A.E., Ali A.A.A., Elhaj F.A., Remli M.A., Gabralla L.A. A Survey: To Govern, Protect, and Detect Security Principles on Internet of Medical Things (IoMT) IEEE Access. 2022;10:124777–124791. doi: 10.1109/ACCESS.2022.3225038. [DOI] [Google Scholar]
- 216.Bi H., Liu J., Kato N. Deep Learning-Based Privacy Preservation and Data Analytics for IoT Enabled Healthcare. IEEE Trans. Ind. Inform. 2022;18:4798–4807. doi: 10.1109/TII.2021.3117285. [DOI] [Google Scholar]
- 217.Zhu F., Yi X., Abuadbba A., Khalil I., Nepal S., Huang X. Authenticated Data Sharing with Privacy Protection and Batch Verification for Healthcare IoT. IEEE Trans. Sustain. Comput. 2023;8:32–42. doi: 10.1109/TSUSC.2022.3211298. [DOI] [Google Scholar]
- 218.Negi L., Kumar D. ECC based certificateless aggregate signature scheme for healthcare wireless sensor networks. J. Reliable Intell. Environ. 2024;10:489–500. doi: 10.1007/s40860-024-00236-w. [DOI] [Google Scholar]
- 219.Shaik T., Tao X., Higgins N., Li L., Gururajan R., Zhou X., Acharya U.R. Remote patient monitoring using artificial intelligence: Current state, applications, and challenges. Wiley Interdiscip. Rev.-Data Min. Knowl. Discov. 2023;13:e1485. doi: 10.1002/widm.1485. [DOI] [Google Scholar]
- 220.Moonga J. Digital Biomarkers and Wearable Neurotechnologies: A Cost of Privacy and Security for Alzheimer’s Patients. Alzheimer’s Dement. 2023;19:e065477. doi: 10.1002/alz.065477. [DOI] [Google Scholar]
- 221.Smallman M. Multi Scale Ethics—Why We Need to Consider the Ethics of AI in Healthcare at Different Scales. Sci. Eng. Ethics. 2022;28:63. doi: 10.1007/s11948-022-00396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McLennan S., Fiske A., Tigard D.W., Müller R., Haddadin S., Buyx A. Embedded ethics: A proposal for integrating ethics into the development of medical AI. BMC Med. Ethics. 2022;23:6. doi: 10.1186/s12910-022-00746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang C.L., Tampubolon H., Setyoko A., Hua K.L., Tanveer M., Wei W. Secure and Privacy-Preserving Human Interaction Recognition of Pervasive Healthcare Monitoring. IEEE Trans. Netw. Sci. Eng. 2022;10:2439–2454. doi: 10.1109/TNSE.2022.3223281. [DOI] [Google Scholar]
- 224.Bernier A., Molnár-Gábor F., Knoppers B.M. The international data governance landscape. J. Law Biosci. 2022;9:lsac005. doi: 10.1093/jlb/lsac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bernier A., Molnár-Gábor F., Knoppers B.M., Borry P., Devriendt T., Goisauf M., Murtagh M.J., Jiménez P.N., Recuero M.M., Rial-Sebbag E., et al. Reconciling the biomedical data commons and the GDPR: Three lessons from the EUCAN ELSI collaboratory. Eur. J. Hum. Genet. 2023;32:69–76. doi: 10.1038/s41431-023-01403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rocha P. Digital Innovation on Integrated Care for Ageing: A qualitative case study in South Europe with NASSS Framework. Int. J. Integr. Care. 2022;22:134. doi: 10.5334/ijic.ICIC21076. [DOI] [Google Scholar]
- 227.Garcia R.D., Ramachandran G.S., Jurdak R., Ueyama J. Blockchain-Aided and Privacy-Preserving Data Governance in Multi-Stakeholder Applications. IEEE Trans. Netw. Serv. Manag. 2022;19:3781–3793. doi: 10.1109/TNSM.2022.3225254. [DOI] [Google Scholar]
- 228.Upadrista V., Nazir S., Tianfield H. Secure data sharing with blockchain for remote health monitoring applications: A review. J. Reliab. Intell. Environ. 2023;9:349–368. doi: 10.1007/s40860-023-00204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Velmurugan S., Prakash M., Neelakandan S., Ofori M.E. An Efficient Secure Sharing of Electronic Health Records Using IoT-Based Hyperledger Blockchain. Int. J. Intell. Syst. 2024;2024:6995202. doi: 10.1155/2024/6995202. [DOI] [Google Scholar]
- 230.Guimarães T., Duarte R., Hak F., Santos M. Context-Aware Electronic Health Record—Internet of Things and Blockchain Approach. Informatics. 2024;11:98. doi: 10.3390/informatics11040098. [DOI] [Google Scholar]
- 231.Singh P., Sagar S., Singh S., Alshahrani H.M., Getahun M., Soufiene B.O. Blockchain-enabled verification of medical records using soul-bound tokens and cloud computing. Sci. Rep. 2024;14:24830. doi: 10.1038/s41598-024-75708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zheng M., Wu Y., Weng C. A digital intelligence visualization health monitoring device for Alzheimer’s disease patients based on WBAN technology. Sci. Rep. 2025;15:15997. doi: 10.1038/s41598-025-99637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Okubanjo A.A., Ifeanyi C.A., Ajayi R., Ojo D., Iwuanyanwu H.U. Development of a Low-Cost IoT-Based E-Health Monitoring System for Diabetic Patients. J. Electr. Syst. Inf. Technol. 2024;11:54. doi: 10.1186/s43067-024-00178-6. [DOI] [Google Scholar]
- 234.Liu Z., Cascioli V., McCarthy P.W. Healthcare Monitoring Using Low-Cost Sensors to Supplement and Replace Human Sensation: Does It Have Potential to Increase Independent Living and Prevent Disease? Sensors. 2023;23:2139. doi: 10.3390/s23042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yi S., Yam E.L.Y., Cheruvettolil K., Linos E., Gupta A., Palaniappan L., Rajeshuni N., Vaska K.G., Schulman K., Eggleston K.N. Perspectives of Digital Health Innovations in Low- and Middle-Income Health Care Systems From South and Southeast Asia. J. Med. Internet Res. 2024;26:e57612. doi: 10.2196/57612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Casals L., Mir B., Vidal R., Gomez C. Modeling the Energy Performance of LoRaWAN. Sensors. 2017;17:2364. doi: 10.3390/s17102364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.