Abstract
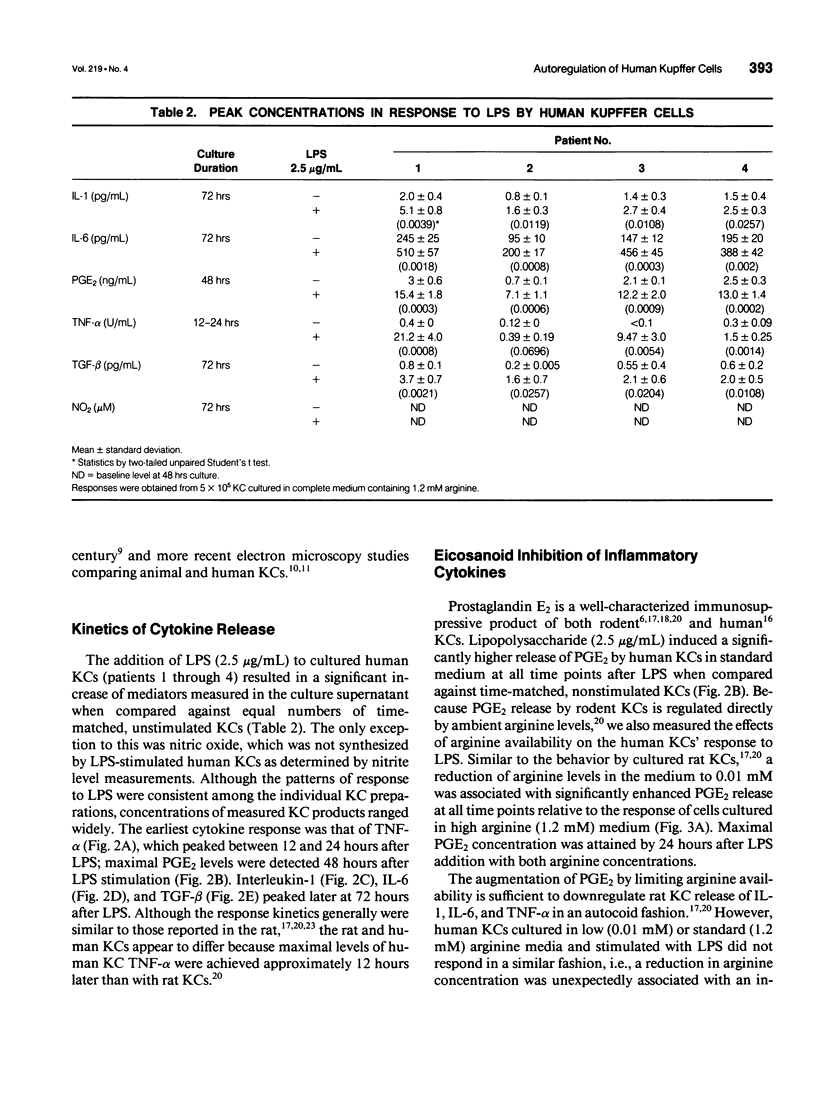
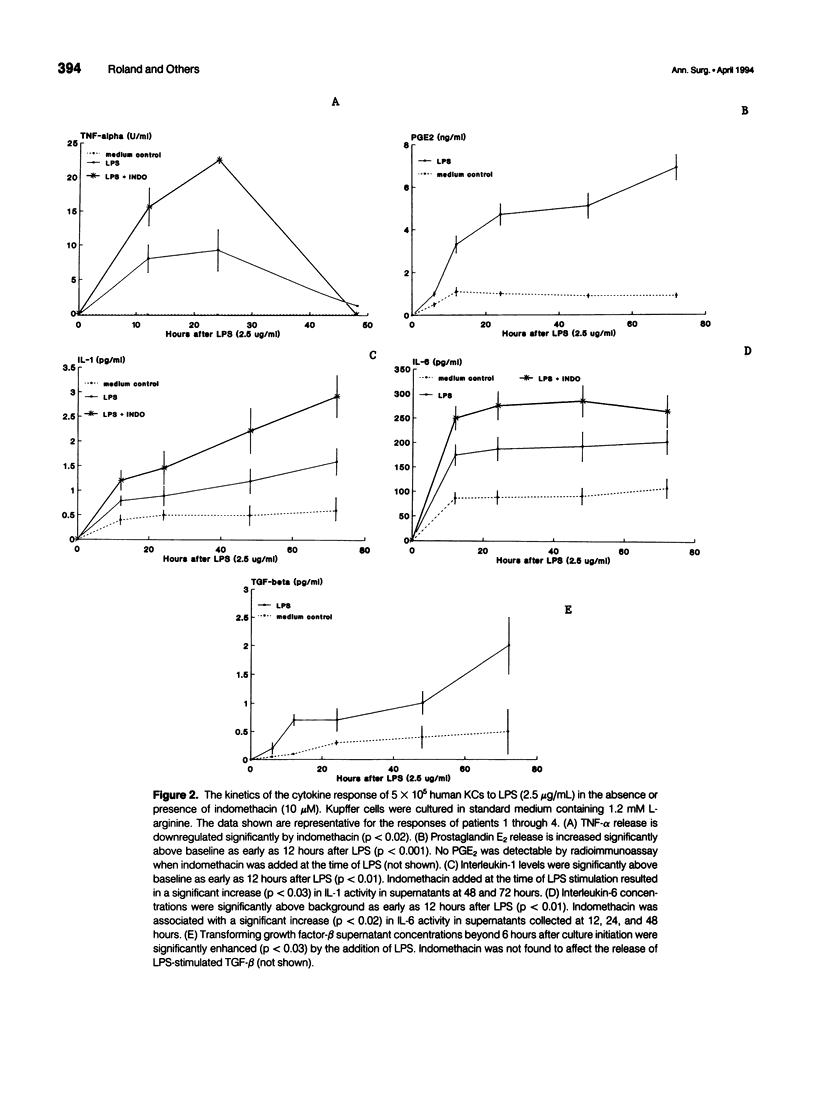
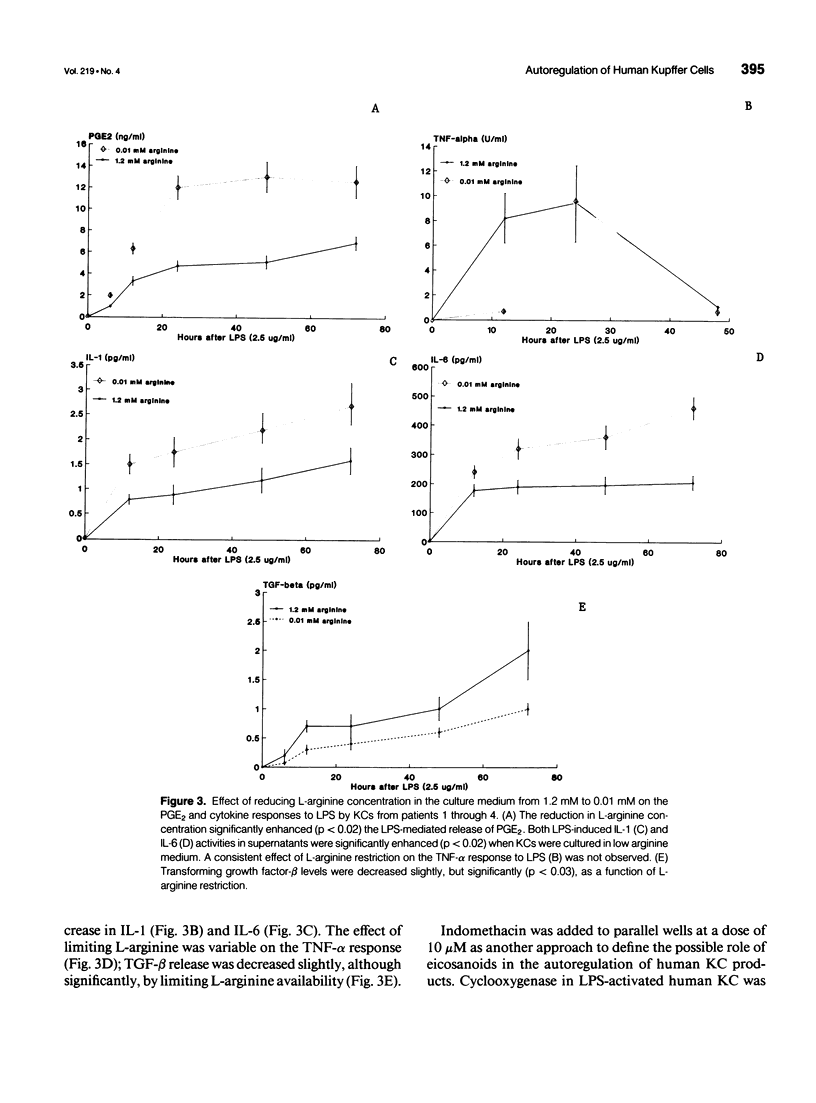
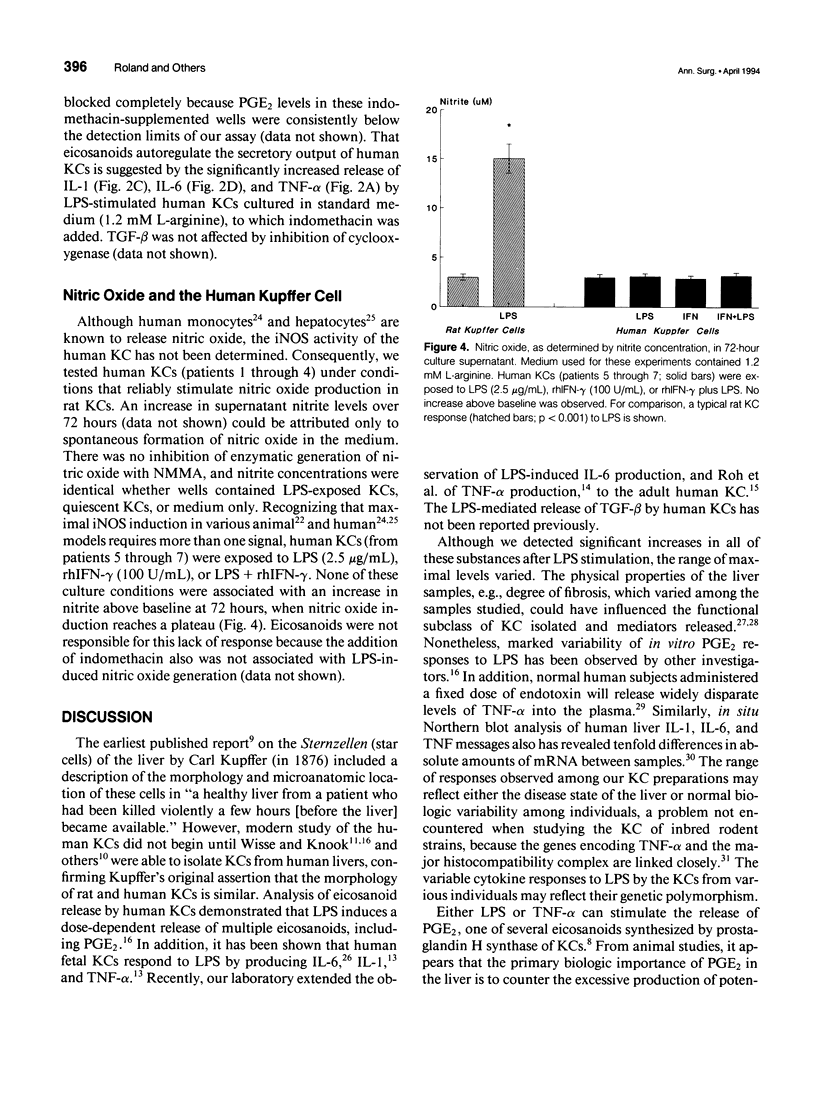
OBJECTIVE: Methods employed previously to analyze the secretory behavior of rodent Kupffer cells (KC) were used to examine the human KC's secretory response to lipopolysaccharide (LPS). SUMMARY BACKGROUND DATA: As the resident hepatic macrophage, the KC resides at the interface between the portal and systemic circulations. Consequently, this cell may play an integral role in the immune response to antigens and bacteria in the sinusoid. Study of cytokine production by the KC has relied predominantly on the rat as the source of these cells. Whether human KCs respond similarly to rat KCs after LPS stimulation has been a matter of speculation. METHODS: Kupffer cells obtained from seven human livers were tested under conditions identical to those used to study rat KCs. Kupffer cells rested for 12 hours after isolation were stimulated with LPS (2.5 micrograms/mL). Arginine concentration in the culture medium varied from 0.01 to 1.2 mM. To examine the role of eicosanoids, parallel culture wells received indomethacin (10 microM). Culture supernatants were assayed for interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-alpha), transforming growth factor-beta (TGF-beta), prostaglandin E2 (PGE2), and nitric oxide. RESULTS: Similar to the rat KC, LPS-stimulated human KCs released IL-1, IL-6, TNF-alpha, TGF-beta, and PGE2. However, unlike rat KCs, nitric oxide could not be detected, regardless of whether the human KCs were exposed to LPS, interferon-gamma (INF-gamma), or LPS + IFN-gamma. Similar to rat KCs, indomethacin prevented PGE2 release while significantly upregulating TNF-alpha, IL-1, and IL-6, but not TGF-beta, consistent with an autoregulatory control of eicosanoids over proinflammatory cytokines. As has been shown in the rat, physiologic levels of L-arginine (0.01 mM) significantly enhanced LPS-induced PGE2 secretion relative to the response in medium containing standard L-arginine concentration (1.2 mM); however, unlike the rat KC, the human's cytokine response to LPS was not downregulated by this enhanced PGE2 release. CONCLUSIONS: Although many functional features are shared by rat and human KCs, significant differences do exist. Such discrepancies reinforce the need to proceed with caution when generalizing from the results obtained in other species to human physiology.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. R., Doherty G. M., Buresh C. M., Venzon D. J., Norton J. A. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med. 1991 Apr 1;173(4):1029–1032. doi: 10.1084/jem.173.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andus T., Bauer J., Gerok W. Effects of cytokines on the liver. Hepatology. 1991 Feb;13(2):364–375. [PubMed] [Google Scholar]
- Baigrie R. J., Lamont P. M., Kwiatkowski D., Dallman M. J., Morris P. J. Systemic cytokine response after major surgery. Br J Surg. 1992 Aug;79(8):757–760. doi: 10.1002/bjs.1800790813. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A., Barelds R. J., de Leeuw A. M., Blauw E., Plas A., Yap S. H., van den Broek A. M., Knook D. L. Isolation and culture of Kupffer cells from human liver. Ultrastructure, endocytosis and prostaglandin synthesis. J Hepatol. 1988 Feb;6(1):36–49. doi: 10.1016/s0168-8278(88)80460-4. [DOI] [PubMed] [Google Scholar]
- Callery M. P., Kamei T., Flye M. W. Endotoxin stimulates interleukin-6 production by human Kupffer cells. Circ Shock. 1992 Jul;37(3):185–188. [PubMed] [Google Scholar]
- Callery M. P., Kamei T., Mangino M. J., Flye M. W. Organ interactions in sepsis. Host defense and the hepatic-pulmonary macrophage axis. Arch Surg. 1991 Jan;126(1):28–32. doi: 10.1001/archsurg.1991.01410250032004. [DOI] [PubMed] [Google Scholar]
- Callery M. P., Mangino M. J., Flye M. W. A biologic basis for limited Kupffer cell reactivity to portal-derived endotoxin. Surgery. 1991 Aug;110(2):221–230. [PubMed] [Google Scholar]
- Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990 Sep 11;192(2):245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Lentz V., Cummings P. J. Transforming growth factor-beta regulation of IL-6 production by unstimulated and IL-1-stimulated human fibroblasts. J Immunol. 1991 May 15;146(10):3437–3443. [PubMed] [Google Scholar]
- Emilie D., Navratil E., Devergne O., Reynes M., Crevon M. C., Samuel D., Galanaud P. Monokine gene expression in normal human liver: selective involvement of the portal compartment. Liver. 1992 Feb;12(1):34–41. doi: 10.1111/j.1600-0676.1992.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Fox E. S., Broitman S. A., Thomas P. Bacterial endotoxins and the liver. Lab Invest. 1990 Dec;63(6):733–741. [PubMed] [Google Scholar]
- Friedman S. L., Rockey D. C., McGuire R. F., Maher J. J., Boyles J. K., Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992 Feb;15(2):234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Nagarajan B. Effect of growth & differentiation on distribution of arginase & arginine in rat tissues. Indian J Biochem Biophys. 1979 Apr;16(2):66–68. [PubMed] [Google Scholar]
- Goretski J., Hollocher T. C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988 Feb 15;263(5):2316–2323. [PubMed] [Google Scholar]
- Goss J. A., Mangino M. J., Callery M. P., Flye M. W. Prostaglandin E2 downregulates Kupffer cell production of IL-1 and IL-6 during hepatic regeneration. Am J Physiol. 1993 Apr;264(4 Pt 1):G601–G608. doi: 10.1152/ajpgi.1993.264.4.G601. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Itoh Y., Okanoue T., Morimoto M., Nagao Y., Mori T., Hori N., Kagawa K., Kashima K. Functional heterogeneity of rat liver macrophages: interleukin-1 secretion and Ia antigen expression in contrast with phagocytic activity. Liver. 1992 Feb;12(1):26–33. doi: 10.1111/j.1600-0676.1992.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Jacob A. I., Goldberg P. K., Bloom N., Degenshein G. A., Kozinn P. J. Endotoxin and bacteria in portal blood. Gastroenterology. 1977 Jun;72(6):1268–1270. [PubMed] [Google Scholar]
- Karck U., Peters T., Decker K. The release of tumor necrosis factor from endotoxin-stimulated rat Kupffer cells is regulated by prostaglandin E2 and dexamethasone. J Hepatol. 1988 Dec;7(3):352–361. doi: 10.1016/s0168-8278(88)80008-4. [DOI] [PubMed] [Google Scholar]
- Kirn A., Bingen A., Steffan A. M., Wild M. T., Keller F., Cinqualbre J. Endocytic capacities of Kupffer cells isolated from the human adult liver. Hepatology. 1982 Mar-Apr;2(2):216–222. doi: 10.1002/hep.1840020205. [DOI] [PubMed] [Google Scholar]
- Kutteh W. H., Rainey W. E., Beutler B., Carr B. R. Tumor necrosis factor-alpha and interleukin-1 beta production by human fetal Kupffer cells. Am J Obstet Gynecol. 1991 Jul;165(1):112–120. doi: 10.1016/0002-9378(91)90237-l. [DOI] [PubMed] [Google Scholar]
- Kutteh W. H., Rainey W. E., Carr B. R. Regulation of interleukin-6 production in human fetal Kupffer cells. Scand J Immunol. 1991 May;33(5):607–613. doi: 10.1111/j.1365-3083.1991.tb02532.x. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Miller-Graziano C. L., Szabo G., Griffey K., Mehta B., Kodys K., Catalano D. Role of elevated monocyte transforming growth factor beta (TGF beta) production in posttrauma immunosuppression. J Clin Immunol. 1991 Mar;11(2):95–102. doi: 10.1007/BF00917745. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991 Aug;21(4):361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Müller U., Jongeneel C. V., Nedospasov S. A., Lindahl K. F., Steinmetz M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature. 1987 Jan 15;325(6101):265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H., Evarts R. P., Hsia C. C., Thorgeirsson S. S. Transforming growth factor-beta 1 and type I procollagen transcripts during regeneration and early fibrosis of rat liver. Lab Invest. 1990 Aug;63(2):171–180. [PubMed] [Google Scholar]
- Nolan J. P. Intestinal endotoxins as mediators of hepatic injury--an idea whose time has come again. Hepatology. 1989 Nov;10(5):887–891. doi: 10.1002/hep.1840100523. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh M. S., Wang L., Oyedeji C., LeRoux M. E., Curley S. A., Pollock R. E., Klostergaard J. Human Kupffer cells are cytotoxic against human colon adenocarcinoma. Surgery. 1990 Aug;108(2):400–405. doi: 10.1002/bjs.1800770937. [DOI] [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Shirahama M., Ishibashi H., Tsuchiya Y., Kurokawa S., Hayashida K., Okumura Y., Niho Y. Kupffer cells may autoregulate interleukin 1 production by producing interleukin 1 inhibitor and prostaglandin E2. Scand J Immunol. 1988 Dec;28(6):719–725. doi: 10.1111/j.1365-3083.1988.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. The transforming growth factor-betas: past, present, and future. Ann N Y Acad Sci. 1990;593:1–6. doi: 10.1111/j.1749-6632.1990.tb16095.x. [DOI] [PubMed] [Google Scholar]
- Stadler J., Curran R. D., Ochoa J. B., Harbrecht B. G., Hoffman R. A., Simmons R. L., Billiar T. R. Effect of endogenous nitric oxide on mitochondrial respiration of rat hepatocytes in vitro and in vivo. Arch Surg. 1991 Feb;126(2):186–191. doi: 10.1001/archsurg.1991.01410260074010. [DOI] [PubMed] [Google Scholar]
- Tamakuma S., Rojas-Corona R., Cuevas P., Fine J. Demonstration of a lethal endotoxemia of intestinal origin in refractory non-septic shock. Ann Surg. 1971 Feb;173(2):219–224. doi: 10.1097/00000658-197102000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Content J., Gresser I., Gugenheim J., Blanchard B., Guymarho J., Poupart P., Gigou M., Shaw A., Fiers W. Genes for IFN-beta-2 (IL-6), tumor necrosis factor, and IL-1 are expressed at high levels in the organs of normal individuals. J Immunol. 1988 Nov 1;141(9):3106–3110. [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Van Bossuyt H., Wisse E. Cultured Kupffer cells, isolated from human and rat liver biopsies, ingest endotoxin. J Hepatol. 1988 Aug;7(1):45–56. doi: 10.1016/s0168-8278(88)80505-1. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Allen J. B., Dougherty E. B., Dougherty S. F. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., ten Cate J. W., Tytgat G. N. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988 Mar;94(3):825–831. doi: 10.1016/0016-5085(88)90261-2. [DOI] [PubMed] [Google Scholar]



