Abstract
The clinical application of hepatocyte transplantation has been significantly hindered by the scarcity of primary hepatocytes and the functional immaturity of in vitro–produced hepatocytes. By performing serial allogeneic hepatocyte transplantation in CRISPR/Cas9‐mediated Fah‐knockout pigs, we successfully achieved large‐scale expansion of hepatocytes while maintaining their authentic biological characteristics. Particularly, the established model enables sustained in vivo liver reconstruction, concurrently ameliorating hepatic fibrosis and demonstrating functional microenvironmental remodeling. Moreover, through comprehensive single‐cell transcriptomic profiling of 52 418 hepatocytes across transplant generations (F0–F2), we discovered that the cellular composition of these transplanted hepatocytes is similar to that of wild‐type hepatocytes. The regenerated liver exhibits all six major hepatic cell types identical to the wild‐type counterparts, with the characteristic lobular zonation patterns well preserved. Our research provides valuable insights into the large‐scale expansion of physiologically functional hepatocytes in vivo without compromising their biological properties. This finding holds great promise for advancing the clinical application of human hepatocyte transplantation, potentially offering more effective treatment options for patients with liver diseases.
Keywords: cellular therapy, hepatocyte, large‐scale expansion, regeneration, single‐cell RNA sequencing
This represents the first serial hepatocyte transplantation pig model designed to investigate the massive hepatocyte proliferation in vivo. Leveraging this novel model, we achieved serial hepatocyte transplantation and regeneration in a porcine model, where the transplanted hepatocytes effectively repopulated the livers of two‐generation model pigs in succession. Single‐cell sequencing was carried out at each passage. The results demonstrated that hepatocytes that had undergone extensive in vivo proliferation retained normal physiological functions and cellular composition.
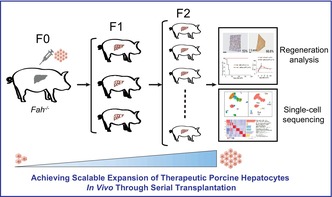
1. INTRODUCTION
Despite liver transplantation being the only curative treatment for end‐stage liver diseases, a significant number of patients die before receiving suitable donor livers. 1 , 2 Hepatocyte transplantation has emerged as a promising alternative therapeutic strategy for liver transplantation, 2 , 3 , 4 yet its clinical application faces major challenges, including the insufficient source of functional hepatocytes and difficulties in the rigorous evaluation of phenotypic and functional fidelity of hepatocytes expanded in vitro. 2 , 5 , 6 , 7 Therefore, it is necessary to investigate and develop strategies for large‐scale expansion of functional hepatocytes, either in vitro or in vivo. Particularly, porcine hepatocytes closely resemble human metabolic pathways, making them a promising alternative model to primary human hepatocytes for studying metabolic diseases, drug metabolism and toxicity, and bioartificial liver devices. 8 , 9 , 10 We achieved large‐scale hepatocyte expansion while preserving their authentic biological characteristics by performing serial allogeneic hepatocyte transplantation in CRISPR/Cas9‐mediated Fah‐knockout pigs. Our work not only establishes a platform for scalable hepatocyte expansion but also provides evidence for the long‐term expansion of hepatocytes with preserved genetic and biological characteristics.
2. METHODS
2.1. Ethics statement
All experimental pigs were reared according to the Guidelines for the Care and Use of Laboratory Animals Committee of the Institute of Zoology. All experiments involving pigs were approved by the Ethical Committee on Animal Experiments of the Institute of Zoology, Chinese Academy of Sciences (approval no. IOZ‐IACUC‐2021‐088). Animals were monitored twice daily for hereditary tyrosinemia type I (HT1)‐associated clinical signs, including lethargy, weight loss, and jaundice. Those exhibiting anorexia, loss of righting reflex, or more than 15% body weight reduction were humanely killed.
2.2. Cell culture and monoclonal cell selection
The fibroblast cells from porcine ear were cultured in Minimum Essential Medium with nucleosides (12 571 063, Gibco, USA) with 1% GlutaMAX (35 050 079, Gibco), 100 U/mL of penicillin–streptomycin (15 140 122, Invitrogen, USA), 1% Non‐Essential Amino Acids Solution (35 050 079, Gibco), and 2.5 ng/mL of basic fibroblast growth factor (233‐FB‐001MG/CF, R&D Systems, USA). All cells were maintained at 37°C under 5% CO2. Subsequently, all plasmids were nucleofected using 4D‐Nucleofector System (Lonza) at 90% confluence, and single clones were sorted into 96‐well plates using a Moflo Astrios EQ cell sorter (Beckman, USA).
2.3. Hematoxylin and eosin staining
The tissue samples were fixed in 10% neutral formalin solution (E672001, Sangon Biotech, China) and trimmed to an appropriate size. The tissues underwent graded ethanol dehydration and then xylene clearing; then they were embedded in paraffin wax. Then the tissues were cut into 5‐μm‐thick slices, dewaxed, dried, and stained with hematoxylin and eosin (H&E). Finally, the sections were scanned using a whole‐slide imaging system (WS‐10, Wisleap, China).
2.4. Immunohistochemistry staining
The dissected tissues were fixed in 10% neutral formalin solution (E672001, Sangon Biotech) for 24–36 h, embedded in paraffin, sectioned, and stained with H&E (Thermo, USA). Liver samples were prepared according to a previously reported protocol, and immunohistochemical analysis was performed using SP Rabbit & Mouse HRP Kits (CW2069S, CWBIO, China).
2.5. Isolation and purification of porcine hepatocytes
The hepatocyte isolation procedure was performed using a two‐step collagenase perfusion technique. The liver lobes were placed in a biosafety cabinet to identify the vein branches and arterial trunk. Perfusion was conducted using a peristaltic pump calibrated to 50–100 rpm as follows: initially, calcium‐free buffer perfusion fluid was used until significant color changes were observed in the liver tissue; subsequently, the enzyme was introduced for perfusion until the liver tissue appeared like jelly. After perfusion, liver tissue was transferred to a sterile 10‐cm cell culture dish and gently dissociated using scalpels to release hepatocytes. Any impurities, such as blood vessels and other fibrous materials, were removed. An appropriate volume of hepatocyte washing solution was added and gently mixed using a 25‐mL pipette; then the mixture was filtered through a 100‐μm nylon mesh into a sterile 50‐mL conical tube. After filtration, the cell suspension was immediately placed on ice and centrifuged at 800 rpm for 3 min. After the supernatant was discarded, the hepatocyte pellet was resuspended in fresh wash buffer. This washing procedure was repeated twice with centrifugation intervals. Cell concentration and viability were counted using a cell counter. Finally, purified hepatocytes were resuspended in sterile physiological saline, with the injection volume typically maintained below 20 mL.
2.6. Transplantation of porcine hepatocyte
A longitudinal incision was made along the midline of the abdomen, approximately 2 cm below the sternum. The portal veins were accessed using a modified butterfly needle, and hepatocytes were injected slowly into the liver at a consistent rate. After a brief interval, the injection was withdrawn, and then pressure was applied immediately on the injection site using gauze for 10 s to stop the bleeding and restore the position of the organ. The procedure was concluded with suturing to close the abdominal wall in the layers. Immediately after hepatocyte transplantation, 2‐(2‐nitro‐4‐trifluoromethylbenzoyl)‐1,3‐cyclohexanedione (NTBC) administration was discontinued. Animals were weighed daily at a fixed time. If body weight loss persisted for four consecutive days, NTBC was reintroduced into the diet for 7 days before subsequent withdrawal. This cycle was repeated until sustained weight gain was achieved, at which point NTBC treatment was permanently discontinued.
2.7. In vivo sampling of pig liver
All experimental pigs received standardized anesthesia: induction with zoletil (4 mg/kg) followed by isoflurane inhalation for maintenance. A longitudinal incision was made along the midline of the abdominal cavity, 2 cm below the sternum of the pig. The target liver lobe was ligated with sutures and resected using surgical scalpel. Hemostasis was achieved through manual compression combined with application of hemostatic sponge. The abdominal incision was subsequently closed in layers with sutures. The postoperative observation was continued for 24 h to prevent any adverse events.
3. RESULTS
3.1. CRISPR/Cas9‐mediated Fah knockout in Bama miniature establishes an efficient liver injury model with impaired hepatocyte regeneration
To investigate the life‐sustaining treatment of hepatocyte transplantation in large animals, a progressive liver failure model in Bama miniature with targeted disruption of the Fah gene was constructed using CRISPR/Cas9 technology. Specifically, the Fah gene was knocked out by introducing indels within its fifth exon, thereby causing translation termination. Subsequently, a clonal cell line with a 1‐bp deletion was selected (Figure 1A,C) for the subsequent somatic cell nuclear transfer (SCNT), by yielding 20 live‐born piglets from 2243 embryos transferred to 9 recipient sows (Figure 1B). One of the piglets was used to assess the expression of Fah in the liver via Western blotting assay. The results clearly demonstrated a successful ablation of Fah in the piglet's liver (Figure 1D). Administration of NTBC was withdrawn from three piglets 1 month after birth. Particularly, 14 days post‐NTBC withdrawal, these pigs exhibited severe symptoms of liver disease, including loss of appetite, growth retardation, and weight loss, resulting in inability to stand. Autopsy examination revealed that the livers of model pigs exhibited an aberrant yellow‐white hue. H&E staining analysis further revealed significant liver damage characterized by hepatic fibrosis, exacerbated inflammation, and ballooning degeneration of hepatocytes with focal necrosis. In accordance with the result of Western blotting assay, the immunohistochemistry (IHC) analysis of Fah also revealed the absence of Fah protein in the model pigs. Additionally, the IHC results of alpha‐smooth muscle actin verified the profound liver fibrosis in the piglets (Figure 1E,F). Furthermore, significant alterations in liver function metrics, such as alanine aminotransferase (ALT)/aspartate aminotransferase (AST), were observed in these pigs but not in age‐matched wild‐type pigs (Figure 1G). Overall, these results indicated the successful establishment of a liver injured pig model with high efficiency.
FIGURE 1.
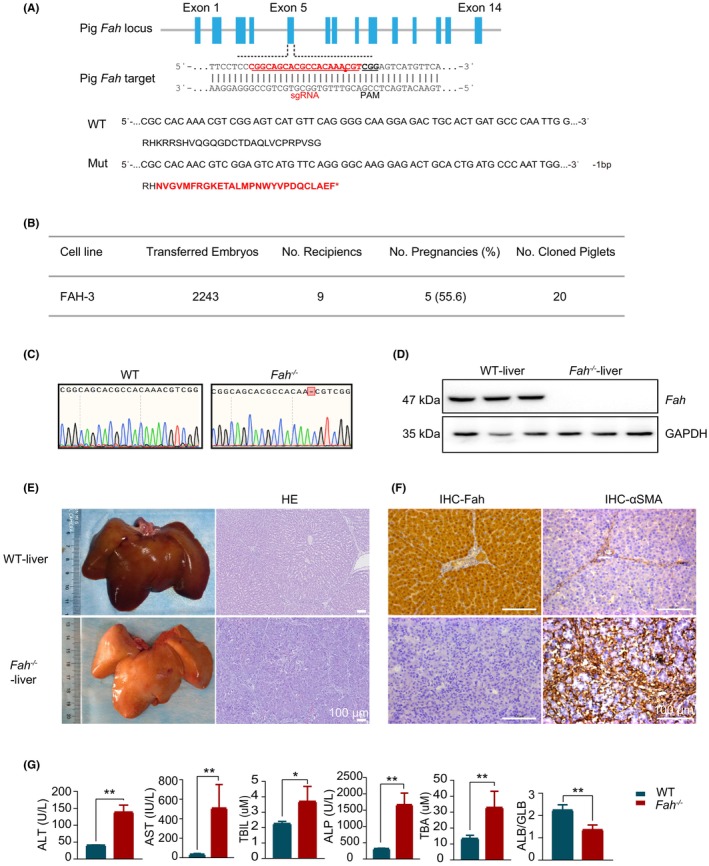
Construction of the Fah −/− pig model, with its associated liver injury phenotype. (A) Pig Fah gene knockout site and prediction of the amino acid sequences of model pig genotypes. Red amino acids signify incorrect residues in the sequence. (B) Fah‐deficient model pig embryo transfer information. (C) Comparative analysis of Sanger sequencing peak maps. (D) Detection of Fah protein in the liver tissue. (E) Liver appearance and pathological detection of liver tissue H&E (hematoxylin and eosin) staining after death. Scale bar: 100 μm. (F) Immunohistochemical analysis of liver Fah and alpha‐smooth muscle actin (α‐SMA). Scale bar: 100 μm. (G) Detection of hepatic function indices in peripheral blood.
3.2. Serial hepatocyte transplantation in isogenic Fah‐knockout pigs achieves high‐efficiency liver reconstruction
Conventional allogeneic liver reconstruction via hepatocyte transplantation requires a combined model of liver injury and severe immunodeficiency. 5 , 10 , 11 , 12 , 13 , 14 , 15 To overcome this limitation and facilitate the in vivo proliferation of porcine hepatocytes, we developed isogenic cell‐derived nonedited and Fah‐knockout pig models using SCNT (Figure 2A). Theoretically, these individuals could circumvent immune rejection between allografts. Hepatocytes from nonedited pigs were transplanted into Fah‐deficient pigs for liver reconstruction, with a subset of the hepatocytes subjected to 10× single‐cell sequencing to characterize F0 primary hepatocyte population. Particularly, NTBC was provided to the pigs during the early stages of liver regeneration. After 10–12 weeks, NTBC was withdrawn, and liver samples from the transplanted group were collected to assess the efficiency of hepatocyte reconstruction; 180 days after transplantation, when the pigs can survive without NTBC, marking complete hepatocyte reconstruction, hepatocytes were isolated and subsequently transplanted into secondary Fah −/− recipients, and simultaneously collected to profile the traits of expanded F1 porcine hepatocytes using 10× single‐cell sequencing in parallel. This serial transplantation protocol was repeated to obtain next‐generation hepatocytes; detailed workflow and sampling time points are shown in Figure 2A and Figure S1A,B. The initial isolated hepatocytes, designated as F0, were transplanted into four F1 pigs. Then, the hepatocytes isolated from F1 pigs were transplanted continuously into three F2 pigs (Figure 2B). A survival curve of Fah −/− pigs with or without hepatocyte transplantation is shown in Figure 2C. The ALT levels in peripheral blood returned to normal in the model pigs after cell transplantation (Figure 2D). To determine the extent of liver reconstruction across various liver lobes, one from each generation was killed and end‐point sampling was conducted at 10–12 weeks posttransplantation. The IHC result of Fah revealed that the ratio of liver reconstruction in F1 pigs ranged from 11.3% to 22.9% at 70 days after cell transplantation. In contrast, F2 pigs exhibited a reconstruction range from 37% to 53% at this time point; at 180 days, the degree of liver reconstruction was 95.8%–98.6% in all transplanted Fah −/− pigs (Figure 2E,F).
FIGURE 2.
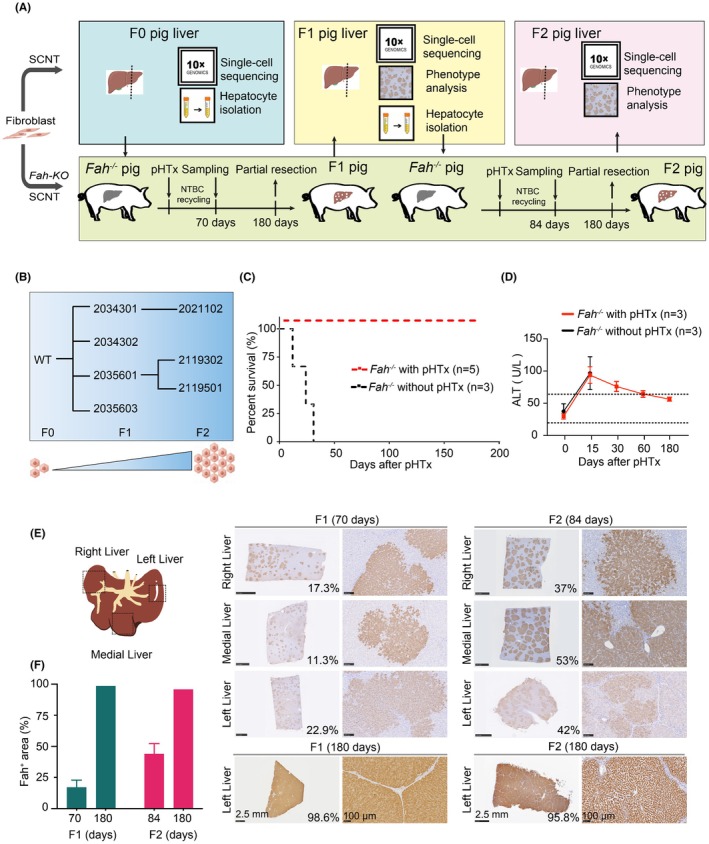
Continuous transplantation of pig hepatocytes into model pigs to reconstruct the liver for two generations. (A) Schematic of the continuous transplantation of porcine hepatocytes for two generations. The pig with a gray‐colored liver represents the Fah knockout pig, whereas the red‐colored liver represents either the liver of an F0 pig or a reconstructed liver. (B) Schematic of the recipients and sources of hepatocytes in a serial transplantation assay involving pig hepatocytes. (C) Survival curves of pigs after experimental hepatocyte transplantation (excluding swine killed during the experiment). (D) Peripheral blood alanine aminotransferase (ALT) in pigs after hepatocyte transplantation. (E) The sampling locations in different liver lobes. The Fah expression in various liver lobes from all reconstructed Fah‐knockout pig models were examined using immunohistochemistry (IHC) during the continuous transplantation of porcine hepatocytes. Scale bar: 2.5 mm or 100 μm. (F) Quantification of Fah immunohistochemical staining in regenerated hepatic tissue post‐reconstruction.
3.3. Single‐cell transcriptomics reveals transplanted hepatocyte‐driven microenvironmental reprogramming for structural and functional liver reconstitution
To delineate the transcriptomic landscape of in vivo–expanded hepatocytes, single‐cell RNA sequencing was performed on parenchymal cells isolated from primary donors (F0), first‐ and second‐generation recipients (F1 and F2), and age‐matched wild‐type controls. Unsupervised graph‐based clustering identified 14 transcriptionally distinct cell populations, visualized using uniform manifold approximation and projection (Figure 3A,B). Cellular composition was dominated by hepatocytes (86.7%), with minor populations of nonparenchymal compartments, such as cholangiocytes (0.39%), endothelial cells (3.87%), hepatic stellate cells (2.49%), Kupffer cells (5.05%) and T cells (1.49%). Gene ontology analysis revealed cell‐type‐specific enrichment patterns aligned with canonical functional annotations (Figure 3C). Longitudinal quantification of lineage‐specific markers across four biological replicates confirmed conserved cellular topology (Figure 3D). Cross‐generational transcriptomic analysis demonstrated significant transcriptional stability in serially reconstituted hepatocytes, with only a small percentage of genes showing differential expression, whereas the total number of transcribed genes remains unchanged (Figure S2A). Particularly, genes maintaining stable transcriptional levels exhibited functional enrichment in core biological processes, including proliferation, gene expression regulation, intracellular signaling, and cellular adhesion (Figure S2B), indicating conservation of fundamental hepatocyte identity across generations. Further characterization of differentially expressed genes demonstrated subtle intersample variations, as systematically characterized in Figure S2C,D.
FIGURE 3.

The classification of cell types and the gene expression profiles of livers that were subjected to the serial transplantation. (A) Uniform manifold approximation and projection (UMAP) plot of the distribution of different cell types in serial hepatocyte transplantation. (B) Dot plots of the expression levels of representative marker genes for hepatocytes in each subtype. Gray to red represents low to high gene expression. The size of dots indicates the percentage of cells with gene expression greater than zero. (C) Heat map (left) showing the differentially expressed genes (DEG) of three hepatocyte subtypes. The DEGs were divided into 14 modules according to the overlap of the three subtypes. Heat map (right) showing the Gene Ontology (GO) terms and pathways of the modules. (D) Sankey diagram showing the number of each cell type and the different cell numbers for consecutive generations of liver transplantation. (E) Hematoxylin and eosin (H&E) and Sirius Red staining of liver after hepatocyte transplantation. Scale bar: 500 μm. (F) The statistics of Sirius Red staining of fibrosis area. (G) Marker staining was conducted on a subset of cells of livers from various experimental groups. Scale bar: 50 μm. (H) Staining of the liver sections from the experimental liver samples. Scale bar: 50 μm.
Furthermore, histopathological evaluation revealed significant attenuation of fibrosis, evidenced by Sirius Red–stained collagen quantification (Figure 3E,F). Immunofluorescence mapping of hepatocytes (HNF4α+), cholangiocytes (CK19+), and vasculature (CD31+) confirmed the preservation of the liver microstructure (Figure 3G), whereas glutamine synthetase and E‐cadherin immunostaining patterns revealed maintained physiological zonation (Figure 3H). Collectively, these findings suggested that the transplanted hepatocytes orchestrate microenvironmental remodeling to achieve both structural and functional liver reconstitution.
4. DISCUSSION
Our study provides a comprehensive investigation of the large‐scale expansion and regeneration of hepatocytes through serial in vivo transplantation in a porcine model. Specifically, we conducted a meticulous characterization of biological and genetic properties of the hepatocytes at each generation. Distinct from previous autologous hepatocyte studies, 16 our methodology integrated allogeneic hepatocyte transplantation and SCNT‐mediated production of donor and recipient pigs. This integration was instrumental in eliminating immune‐related confounding effects, thereby revealing the fundamental aspects of hepatocyte regeneration. The experimental model demonstrated that each founder hepatocyte underwent extensive cell proliferation while maintaining its authentic genetic properties, establishing a robust large‐animal platform for large‐scale expansion of hepatocytes. This innovative platform advances the field of clinical hepatocyte transplantation in two notable dimensions: first as a translational model for optimizing human hepatocyte transplantation protocols and second as a potential bioreactor system for industrial‐scale production of functional human hepatocytes. By addressing critical challenges in cell sourcing and quality control, our model effectively bridges the existing gaps between preclinical research and clinical implementation in the realm of cellular therapy.
AUTHOR CONTRIBUTIONS
Zhiqiang Han: Data curation; formal analysis; methodology. Xin Wang: Data curation; methodology; software; visualization; writing – original draft. Dawei Yu: Data curation; methodology; project administration. Jing Wang: Data curation; methodology. Ke Sun: Methodology. Siqi Wang: Writing – review and editing. Ying Zhang: Project administration; supervision; writing – review and editing. Guihai Feng: Formal analysis; software; writing – original draft. Wei Li: Conceptualization; funding acquisition; project administration; resources; supervision; writing – review and editing. Tang Hai: Conceptualization; funding acquisition; project administration; supervision; writing – review and editing. Jilong Ren: Conceptualization; data curation; methodology; project administration; validation; visualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This study was supported by the National Key Research and Development Program (2021YFA0805905, 2023YFC3404305, 2024YFA1107900), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB1150000), the CAS Project for Young Scientists in Basic Research (YSBR‐012), and the Bingtuan Science and Technology Project (NYHXGG2023AA01).
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflict of interest.
ETHICS STATEMENT
All experimental pigs were reared according to the Guidelines for the Care and Use of Laboratory Animals Committee of the Institute of Zoology. All experiments involving pigs were approved by the Ethical Committee on Animal Experiments of the Institute of Zoology, Chinese Academy of Sciences (approval no. IOZ‐IACUC‐2021‐088).
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Data S1.
ACKNOWLEDGMENTS
We are grateful to W.L. laboratory members for constructive scientific discussions and critical manuscript feedback. We acknowledge Qing Meng (Institute of Zoology, Chinese Academy of Sciences) for technical assistance with fluorescence‐activated cell sorting (FACS). We thank the Beijing Farm Animal Research Center (Institute of Zoology, Chinese Academy of Sciences) staff for expert animal husbandry and porcine model maintenance.
Han Z, Wang X, Yu D, et al. Achieving scalable expansion of therapeutic porcine hepatocytes in vivo through serial transplantation. Anim Models Exp Med. 2025;8:1337‐1344. doi: 10.1002/ame2.70052
Zhiqiang Han, Xin Wang, and Dawei Yu have contributed equally to this work.
Contributor Information
Wei Li, Email: liwei@ioz.ac.cn.
Tang Hai, Email: haitang@ioz.ac.cn.
Jilong Ren, Email: renjilong@ioz.ac.cn.
REFERENCES
- 1. Zhang K, Zhang L, Liu W, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell. 2018;23:806‐819.e804. doi: 10.1016/j.stem.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 2. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288‐298. doi: 10.1038/nrgastro.2010.44 [DOI] [PubMed] [Google Scholar]
- 3. Vons C, Beaudoin S, Helmy N, Dagher I, Weber A, Franco D. First description of the surgical anatomy of the cynomolgus monkey liver. Am J Primatol. 2009;71:400‐408. doi: 10.1002/ajp.20667 [DOI] [PubMed] [Google Scholar]
- 4. Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286‐300. doi: 10.1002/jcp.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azuma H, Paulk N, Ranade A, et al. Robust expansion of human hepatocytes in fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903‐910. doi: 10.1038/nbt1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grompe M, Strom S. Mice with human livers. Gastroenterology. 2013;145:1209‐1214. doi: 10.1053/j.gastro.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 7. Soltys KA, Setoyama K, Tafaleng EN, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017;66:987‐1000. doi: 10.1016/j.jhep.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hannoun Z, Steichen C, Dianat N, Weber A, Dubart‐Kupperschmitt A. The potential of induced pluripotent stem cell derived hepatocytes. J Hepatol. 2016;65:182‐199. doi: 10.1016/j.jhep.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 9. Ren J, Yu D, Wang J, et al. Generation of immunodeficient pig with hereditary tyrosinemia type 1 and their preliminary application for humanized liver. Cell Biosci. 2022;12:26. doi: 10.1186/s13578-022-00760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L, Ge J‐Y, Zheng Y‐W, et al. Survival‐assured liver injury preconditioning (SALIC) enables robust expansion of human hepatocytes in fah(−/−) Rag2(−/−) IL2rg(−/−) rats. Adv Sci. 2021;8:e2101188. doi: 10.1002/advs.202101188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carvalho T. Two US surgical teams transplant functional pig kidneys into humans in xenotransplantation success. Nat Med. 2023;29:2671‐2672. doi: 10.1038/d41591-023-00078-8 [DOI] [PubMed] [Google Scholar]
- 12. Anand RP, Layer JV, Heja D, et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature. 2023;622:393‐401. doi: 10.1038/s41586-023-06594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacParland SA, Liu JC, Ma XZ, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang S, Liu C, Jiang M, et al. A single‐nucleus transcriptomic atlas of primate liver aging uncovers the pro‐senescence role of SREBP2 in hepatocytes. Protein Cell. 2024;15:98‐120. doi: 10.1093/procel/pwad039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michailidis E, Vercauteren K, Mancio‐Silva L, et al. Expansion, in vivo‐ex vivo cycling, and genetic manipulation of primary human hepatocytes. Proc Natl Acad Sci USA. 2020;117:1678‐1688. doi: 10.1073/pnas.1919035117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hickey RD, Mao SA, Glorioso J, et al. Curative ex vivo liver‐directed gene therapy in a pig model of hereditary tyrosinemia type 1. Sci Transl Med. 2016;8:349ra399. doi: 10.1126/scitranslmed.aaf3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


