Abstract
Infection with Shiga toxin (Stx)-producing Escherichia coli O157:H7, which causes diarrhea and hemorrhagic colitis in humans, often results in fatal systemic complications, such as neurological damage and hemolytic–uremic syndrome. Because Stx circulating in the blood is a major causative factor of these complications, the development of a Stx neutralizer that functions in the circulation holds promise as a viable therapy. Here we developed a series of carbosilane dendrimers, in which trisaccharides of globotriaosyl ceramide, a receptor for Stx, were variously oriented at their termini (referred to as SUPER TWIG), and identified a SUPER TWIG with six trisaccharides as a Stx neutralizer functioning in the circulation. This SUPER TWIG specifically bound to Stx with high affinity (Kd = 1.1 × 10−6 M) and inhibited the incorporation of the toxin into target cells. Intravenous administration of the SUPER TWIG along with Stx to mice substantially reduced the fatal brain damage and completely suppressed the lethal effect of Stx. Moreover, the SUPER TWIG protected mice from challenge with a fatal dose of E. coli O157:H7, even when administered after the establishment of the infection. The SUPER TWIG neutralized Stx in vivo by a mechanism in which the accumulation and immediate degradation of Stx by phagocytic macrophages present in the reticuloendothelial system were induced. Taken together, our findings indicate that this SUPER TWIG is therapeutic agent against infections by Stx-producing E. coli.
Shiga toxin (Stx)-producing Escherichia coli (STEC), including O157:H7, causes diarrhea and hemorrhagic colitis in humans. These gastrointestinal diseases are often complicated by potentially fatal systemic sequelae such as neurological damage and hemolytic–uremic syndrome, the leading cause of acute renal failure in children (1–4). Stx produced by STEC in the gut traverses the epithelium and passes into the circulation, where it causes vascular damage in specific target tissues such as brain and kidney, resulting in systemic complications. Therefore, research leading to the development of an effective Stx neutralizer that specifically binds to and inhibits Stx in the circulation would thus be a promising approach as a viable therapy.
Stx, which is classified into two closely related subgroups, Stx1 and Stx2, consists of a catalytic A subunit, which has RNA N-glycosidase activity and inhibits eukaryotic protein synthesis, and a pentameric B subunit, which is responsible for binding to the functional cell-surface receptor, globotriaosyl ceramide [Gb3; Galα(1–4)-Galβ(1–4)-Glcβ1-ceramide] (4–6). Highly selective and potent binding of Stx to Gb3 is mainly attributed to the multiple interaction of the B subunit pentamer with the trisaccharide moiety of Gb3. On the basis of these facts, several Stx neutralizers, in which the trisaccharide moiety of Gb3 is combined with various core structures in multiple ways, have been reported (7–10). However, no Stx neutralizer has been developed that is capable of detoxifying the toxin present in the circulation.
In this study, we used a series of carbosilane dendrimers carrying various numbers of the trisaccharide [referred to as SUPER TWIG (11)] to develop a Stx neutralizer that functions in the circulation, because SUPER TWIGs are unique in that the number of the terminal trisaccharides can be easily changed by regulating the number of silicon atoms present in the core structure and the silicon–carbon bond is generally biologically inert. We found that SUPER TWIG with six trisaccharides effectively neutralized Stx in the circulation. Furthermore, the SUPER TWIG protected mice from challenge with a fatal dose of STEC O157:H7, suggesting that the SUPER TWIG can be a practical therapeutic agent against infections by STEC.
Materials and Methods
Materials.
SUPER TWIGs were synthesized as described (11), and were characterized by NMR and fast-atom bombardment mass spectrometry to confirm their structures. Recombinant Stx1 and -2 were prepared according to published methods (12). Recombinant glutathione S-transferase (GST)-fused Stx1, in which the catalytic A1 subunit of the holotoxin was replaced with GST (referred to as Stx1-A2B5-GST), was prepared as follows. From the pUC118 vector containing the complete coding sequence of Stx1 (construct kindly provided by S. Yamasaki, International Medical Center of Japan, Tokyo) (13), a BamHI–EcoRI fragment was prepared by PCR with the following primers: 5′-AGAGGGATCCTCGCGAGTTGCCAGAATG-3′ and 5′-AGAGGAATTCTCAACGAAAAATAACTTCGC-3′. The fragment obtained was ligated into the BamHI–EcoRI site of the pGEX-2T vector (Amersham Pharmacia). Competent MC1061 E. coli cells (kindly provided by S. Yamasaki) were then transformed with this vector, and the resulting isolated plasmid was designated pStx1-A2B5-GST. pStx1-A2B5-GST-transformed MC1061 E. coli cells were cultured in 1 liter of Luria–Bertani broth (Difco) supplemented with 100 μg/ml ampicillin (Meiji Seika, Tokyo) at 30°C to midexponential phase. The culture was subsequently treated with 0.5 mM isopropyl β-D(−)-thiogalactopyranoside (Wako Pure Industries, Osaka) for 4 h at 30°C. Collected cell pellets were lysed in 10 ml of PBS containing 6,000 units/ml polymyxin B (Sigma). After centrifugation, the resulting supernatants were incubated with 50 μl of glutathione-Sepharose beads (Amersham Pharmacia) for 2 h at 4°C. After extensive washing of the beads, soluble Stx1-A2B5-GST was eluted from the beads by incubating them with elution buffer [75 mM Hepes (pH 7.5)/150 mM NaCl/5 mM DTT/100 mM glutathione; Sigma] for 30 min at 25°C. Hybridoma-13C4, which produces a monoclonal antibody against the Stx1 B subunit, was obtained from the American Type Culture Collection. 125I-labeled Stx1 (125I-Stx1) and Stx2 (125I-Stx2) were prepared by the iodine monochloride method as described (14).
Cells.
Vero cells were maintained in DMEM supplemented with 10% FCS in a 24-well (for binding assay) or 96-well (for cytotoxicity assay) plastic microplate. Mouse peritoneal macrophages were prepared as described (15).
TLC Immunostaining Assay.
The assay for Stx binding to Gb3 was performed as described (16). Porcine erythrocyte Gb3 (500 ng; Wako Pure Industries) was applied to an HPTLC plate (Whatman) and developed in chloroform/methanol/water (60:35:8, vol/vol). After having been blocked with 1% BSA, the plate was incubated with Stx1 (100 ng/ml) in the presence of the desired amount of a given SUPER TWIG. After extensive washing, monoclonal antibody 13C4 was used for the detection of bound Stx1.
Kinetic Analysis of SUPER TWIG Binding to Immobilized Stx1-A2B5-GST.
SUPER TWIG binding to immobilized Stx1-A2B5-GST was quantified by using a BIAcore system instrument (Pharmacia Biosensor, Uppsala, Sweden) (17). Goat anti-GST antibody was immobilized on a CM5 sensor chip. Recombinant GST or Stx1-A2B5-GST (10 μg/ml) was injected into the system to become immobilized on the chip. Various concentrations of SUPER TWIGs were injected (time 0) over the immobilized GST or Stx1-A2B5-GST at a flow rate of 20 μl/ml for at least 8 min to reach plateau at 25°C. The resonance unit is an arbitrary unit used by the BIAcore system. The resonance unit value obtained from immobilized GST was subtracted from the data obtained from immobilized Stx1-A2B5-GST to correct for the background. The binding kinetics were analyzed by Scatchard plot by using the software BIAEVALUATION 3.0.
125I-Stx-Binding Assay.
Vero cells were treated with 125I-Stx1 or 125I-Stx2 (7 × 106 cpm/μg or 2 × 106 cpm/μg of protein, respectively; 1 μg/ml) in the absence or presence of the desired amount of a given SUPER TWIG or with unlabeled Stx1 or Stx2 (50 μg/ml) for 30 min at 4°C. After extensive washing, the cells were dissolved in lysis solution (0.1 M NaOH/0.5% SDS). Recovered radioactivity was measured by a γ-counter (Packard). Specific binding of these radiolabeled Stxs was confirmed by the complete inhibition by unlabeled Stxs (data not shown).
Cytotoxicity Assay.
Subconfluent Vero cells in a 96-well plate were treated with Stx1 or Stx2 (10 pg/ml) in the absence or presence of the desired amount of a given SUPER TWIG for 72 h. Relative cell number was determined by using a WST-1 Cell Counting Kit (Wako Pure Industries).
Intravenous Administration of Stx2 to Mice.
A lethal dose of Stx2 (0.25 ng/g of body weight) was administered to 14–30 female ICR mice (18–20 g, Japan SLC, Shizuoka, Japan) through a tail vein with or without the desired amount of a given SUPER TWIG, and the survival periods of the mice were monitored. The data were analyzed by Kaplan–Meier survival analysis or, when no mice had died by the end of the observation, by Fisher's exact test. For determining the tissue distribution of Stx2, 125I-Stx2 (5 × 106 cpm/μg of protein, 0.25 ng/g of body weight) was administered as described above. After 1 h all mice were killed, and the radioactivity present in individual tissues was measured with a γ-counter. Pieces of the liver were fixed in 10% formalin and used for immunohistochemical examination (see below).
Histology and Immunohistochemistry.
For histologic and immunohistochemical examination of the brain, five mice were injected intravenously with Stx2 (0.25 ng/g of body weight) alone or Stx2 plus SUPER TWIG (1)6 (15 μg/g of body weight). After 48 h the mice were killed, and their brains were immediately fixed in 10% formalin. For histologic examination, some of the paraffin-embedded sections were stained with hematoxylin and eosin; and others, with luxol fast blue and cresyl violet. Immunohistologic localization of Stx2 was detected by a rabbit polyclonal antibody against Stx2, as described (18).
Mouse Infection Protocol.
Specific pathogen-free, 3-week-old female C57BL/6 mice that had been weaned were purchased from Charles River Breeding Laboratories. Animals were fed a low-protein diet (5% protein, wt/wt) for 2 weeks to achieve protein calorie malnutrition (19). Mice at 5 weeks of age were infected intragastrically with 2 × 106 colony-forming units of E. coli O157:H7 strain N-9 as described (19). Mice were fed the low-protein diet even after the start of the infection. Seven infected mice were given intravenously SUPER TWIG (1)6 (50 μg/g of body weight) dissolved in 0.1 ml of saline twice a day; this treatment was initiated on day 3 of infection and continued until day 6. Ten infected mice were treated with 0.1 ml of saline as a vehicle control by using the same protocol as used for the treatment with SUPER TWIG (1)6. Statistical analysis was performed as described above.
Metabolism of 125I-Stx2 in Cells.
Mouse peritoneal macrophages and Vero cells were incubated with 125I-Stx2 (2 × 106 cpm/μg of protein, 1 μg/ml) in the absence or presence of SUPER TWIG (1)6 (10 μg/ml) or a 100-fold excess of nonradioactive Stx2 for the desired periods at 37°C. After extensive washing, cell lysates were separated by electrophoresis on an SDS/15% polyacrylamide gel and visualized by autoradiography. For the degradation experiment of Stx2, macrophages were incubated with 125I-Stx2 (2 × 106 cpm/μg of protein, 1 μg/ml) in the presence of SUPER TWIG (1)6 (10 μg/ml) for 4 h at 37°C. After extensive washing, the culture medium was changed to DMEM without FCS. After the desired periods, the cells were dissolved in lysis solution for SDS/PAGE. The culture medium was recovered, and 100% ice-cold trichloroacetic acid (TCA) was added to a final concentration of 10%. After centrifugation, the precipitated proteins were dissolved in lysis solution for SDS/PAGE. Radioactivity present in each band was quantified by using a bio-imaging analyzer BAS-1000 (Fuji). Radioactivity present in the TCA-soluble supernatants was measured with a γ-counter.
Uptake of 125I-Stx2 by Macrophages.
Mouse peritoneal macrophages were incubated with 125I-Stx2 (2 × 106 cpm/μg of protein, 1 μg/ml) in the absence or presence of the desired amount of a given SUPER TWIG for 30 min at 37°C. After extensive washing, the cells were dissolved in lysis solution. Recovered radioactivity was measured by a γ-counter.
Results and Discussion
SUPER TWIGs (1)6 and (1)12 Directly Bind to the Stx B Subunit with High Affinity.
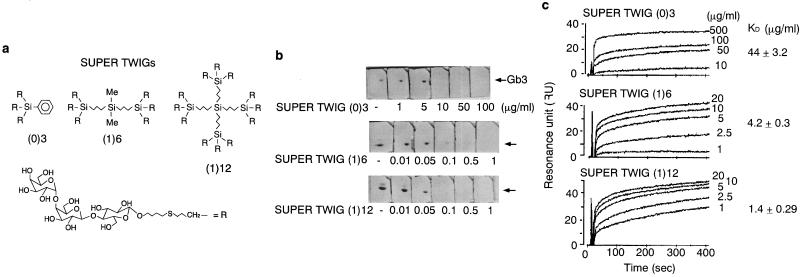
Earlier we had developed three SUPER TWIGs, i.e., SUPER TWIG (0)3, (1)6, and (1)12, carrying 3, 6, and 12 trisaccharides, respectively (11) (Fig. 1a). These SUPER TWIGs have different numbers of the trisaccharides, but the molar content of trisaccharide per weight is almost the same (see the legend for Fig. 1a). Therefore, the concentration of each SUPER TWIG was given as micrograms per milliliter, which enables direct comparison of their activities on a per-trisaccharide basis. Among the SUPER TWIGs, SUPER TWIGs (1)6 and (1)12 at the concentration of 0.5 and 0.1 μg/ml, respectively, completely inhibited the Stx1 binding to Gb3 developed on a TLC plate, both of which concentrations were much lower than the effective concentration observed for SUPER TWIG (0)3 (Fig. 1b). With Stx1-A2B5-GST immobilized on a BIAcore sensor chip, the dissociation constant (Kd) of each SUPER TWIG to the B subunit pentamer was determined by Scatchard plot analysis. SUPER TWIGs (1)6 and (1)12 showed very low Kd values of 4.2 and 1.4 μg/ml, respectively; whereas the Kd value of SUPER TWIG (0)3 was 30 times higher (44 μg/ml) than that of SUPER TWIG (1)12 (Fig. 1c). These results indicate that SUPER TWIGs (1)6 and (1)12 directly bind to the Stx B subunit with high affinity and that six trisaccharides in one molecule are sufficient for the high-affinity binding. Because the terminal trisaccharides of SUPER TWIGs (1)6 and (1)12 were designed to span ≈30 Å from the central silicon atom, which fully embraces the predicted Gb3-binding sites on the B subunit pentamer [both sites 1 and 2 (20)], it is possible that these SUPER TWIGs, but not SUPER TWIG (0)3, effectively occupied the multiple binding sites, resulting in a marked increase in their affinity for the B subunit pentamer. We have already synthesized SUPER TWIGs (1)9, (2)18, and (2)36, carrying 9, 18, and 36 trisaccharides in which all of the —R residues present in SUPER TWIGs (0)3, (1)6, and (1)12, respectively, were replaced with —(CH2)3—SiR3. These SUPER TWIGs had Kd values similar to the B subunit pentamer (1.7, 1.5, and 1.8 μg/ml, respectively; K.N., K.M., K.H., D.T., H.K., and Y.N., unpublished data), further confirming that at least six trisaccharides with appropriate distance in the molecule are sufficient for the high-affinity binding to the B subunit pentamer.
Figure 1.
Structures of SUPER TWIGs and their direct binding to Stx. (a) Structures of SUPER TWIG (0)3 (Mr = 2,006; trisaccharide = 1.50 × 10−3 mol/g), (1)6 (Mr = 4,001; trisaccharide = 1.50 × 10−3 mol/g), and (1)12 (Mr = 7,913; trisaccharide = 1.52 × 10−3 mol/g), carrying 3, 6, and 12 trisaccharides of Gb3, respectively. The numbers in parentheses indicate the generation numbers of the SUPER TWIGs. The zero and the first generation of the SUPER TWIGs have one and three linear silicon atoms, respectively, in their core structures. (b) TLC immunostaining assay. Double bands were detected, and both were confirmed to be Gb3 by anti-Gb3 staining (data not shown). The data are representative of three experiments. (c) Kinetic analysis of SUPER TWIG binding to immobilized Stx1-A2B5-GST by using the BIAcore system. The binding kinetics were analyzed by Scatchard plot to determine Kd and maximum binding values (means ± SE, n = 5). The maximum binding values of SUPER TWIG (0)3, (1)6, and (1)12 were 43 ± 2.9, 63 ± 2.6, and 57 ± 2.0 resonance units (RU), respectively.
Marked Inhibition of the Biological Activities of Stx by SUPER TWIGs (1)6 and (1)12.
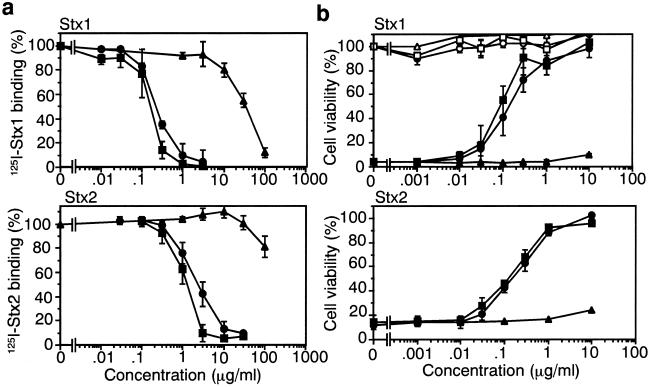
SUPER TWIGs (1)6 and (1)12 markedly inhibited the binding of 125I-labeled Stx1 (125I-Stx1) and Stx2 (125I-Stx2) to Vero cells, one of the cell types most sensitive to Stx. The half-maximal inhibitory concentrations (IC50) of SUPER TWIGs (1)6 and (1)12 for 125I-Stx1 binding were 0.22 and 0.16 μg/ml, respectively; and those for 125I-Stx2 were 2.3 and 1.3 μg/ml, respectively (Fig. 2a). The IC50 value of SUPER TWIG (0)3 for 125I-Stx1 was 270 times higher than that of SUPER TWIG (1)12, and the IC50 value for 125I-Stx2 was more than 100 μg/ml (Fig. 2a). SUPER TWIGs (1)6 and (1)12 markedly inhibited the cytotoxic activity of both Stx1 (IC50 = 0.12 and 0.20 μg/ml, respectively) and Stx2 (IC50 = 0.17 and 0.23 μg/ml, respectively) toward Vero cells (Fig. 2b). In contrast, no inhibitory effect was observed with SUPER TWIG (0)3. Each SUPER TWIG itself did not affect the cell viability. These results indicate that SUPER TWIGs (1)6 and (1)12 markedly inhibited the binding of Stx to the functional cell-surface receptor Gb3 on the target cells, consistent with the direct and high-affinity binding of these SUPER TWIGs to the Stx B subunit as described above.
Figure 2.
Inhibitory effect of SUPER TWIGs on the biological activities of Stx1 (Upper) and Stx2 (Lower) in Vero cells. (a) 125I-Stx-binding assays. ▴, ●, and ■ indicate SUPER TWIG (0)3, (1)6, and (1)12, respectively. The data are presented as the percentage of the activity in the absence of SUPER TWIGs (means ± SE, n = 3). (b) Cytotoxicity assay with Vero cells. Open symbols indicate SUPER TWIGs only. The data are presented as the percentage of the value in the absence of Stxs (means ± SE, n = 3).
Effect of SUPER TWIGs in Vivo.
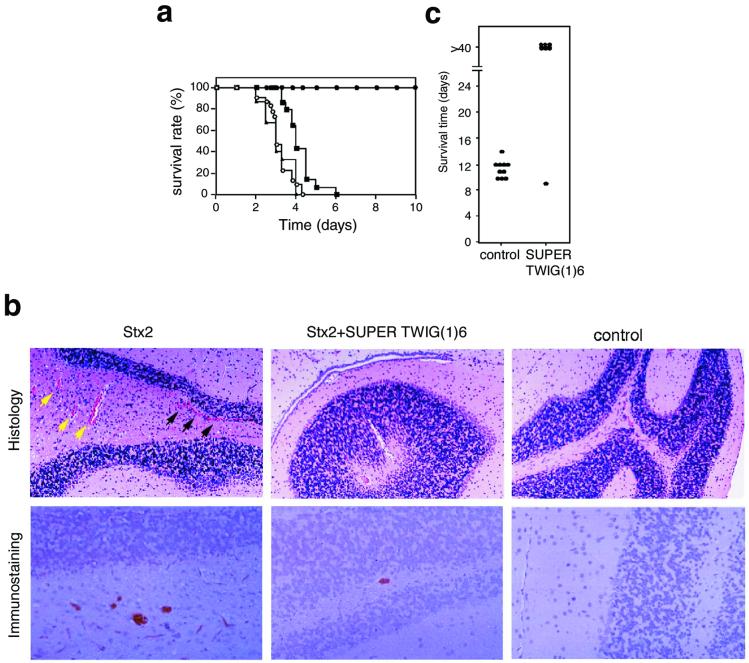
Next, we investigated the inhibitory effects of SUPER TWIGs on the lethality of intravenously administered Stx2 in mice. Stx2 was used in this study because Stx2 is more toxic than Stx1 both in vitro and in vivo, and clinically more significant (6, 21). SUPER TWIG (1)6 completely suppressed the lethal effect of Stx2 when administered along with the toxin; otherwise 100% of the mice died within 5 days (average survival period of mice not given SUPER TWIG (1)6 was 3.2 ± 0.1 days, P < 0.0001; Fig. 3a). The SUPER TWIG (1)6-treated mice survived more than 2 months without any pathological symptoms (data not shown). The dose of SUPER TWIG (1)6 required for the complete suppression could be reduced to 5.0 μg/g of body weight (data not shown). SUPER TWIG (1)12 lengthened the survival period (average survival period was 4.2 ± 0.2 days, P < 0.0001), but none of the SUPER TWIG (1)12-treated mice survived more than 7 days (Fig. 3a). No inhibitory effect was observed with SUPER TWIG (0)3. Each SUPER TWIG itself did not affect the viability (data not shown). Considering the observation that SUPER TWIG (1)12 inhibited Stx more effectively than SUPER TWIG (1)6 in vitro (Fig. 2), these results suggest that some other factor(s), in addition to the high-affinity binding to Stx, might be involved in the potent Stx-neutralizing activity of SUPER TWIG (1)6 in vivo.
Figure 3.
Inhibitory effect of SUPER TWIG (1)6 on the lethality of Stx2 or infection with E. coli O157:H7 in mice. (a) A lethal dose of Stx2 (0.25 ng/g of body weight) was administered to mice without any SUPER TWIG (○; number of mice = 30) or with SUPER TWIG (0)3 (▴; number of mice = 15), (1)6 (●; number of mice = 20), or (1)12 (▴; number of mice = 14) (50 μg/g of body weight). Data represent the survival rate of each group. Data of the first 10 days are shown. (b) Histologic examination and immunostaining of Stx2 in the brain. Sections of cerebellar cortex were used for staining. For histologic examination, the sections were stained with hematoxylin and eosin (Upper). Black and yellow arrowheads indicate perivascular hemorrhage and congestion, respectively. Stx2 present in the sections was detected by using specific antibody against Stx2 (Lower). (×80.) (c) Mice with protein calorie malnutrition were infected intragastrically with E. coli O157:H7 strain N-9 (2 × 106 colony-forming units) on day 0. SUPER TWIG (1)6 (50 μg/g of body weight) or saline alone was administered intravenously to the mice (control, n = 10; SUPER TWIG-treated, n = 7) twice a day from day 3 to day 6. Data represent the survival time of each mouse.
Stx2 causes multifocal vascular damage in the central nervous system, which is closely related to its morbidity and mortality in animal models (3, 18, 19, 22–24). Therefore, pathological changes in cerebral blood vessels, such as congestion and hemorrhage, were investigated after the i.v. administration of mice with Stx2 with or without SUPER TWIG (1)6. Forty-eight hours after the injection of Stx2 alone, congestion was noted in all these brains, most commonly in the cerebellum (Fig. 3b) and hippocampus (data not shown) compared with the brains of normal mice. Multiple parenchymal microhemorrhage was most frequently observed in the cerebellum, whereas neurons and glial cells seemed to be normal. Stx2 was detected by immunohistochemical examination in red blood cells and vascular endothelium in the cerebellum (Fig. 3b), midbrain, and thalamus (data not shown). Neurons, glial cells, and other parenchymal components were negative for Stx2. On the contrary, in SUPER TWIG (1)6-coinjected mice, vascular damage was minimal, where congestion was slight and parenchymal hemorrhage was absent. Concomitantly, Stx2 deposition in the blood vessels was significantly reduced (Fig. 3b). Considering the close relationship between Stx-caused vascular damage in the central nervous system and its mortality in mice (3, 19, 23, 24), these results suggest that SUPER TWIG (1)6 suppressed the lethality of Stx2 by diminishing the deposition of Stx2 in the brain and the subsequent fatal damage.
Next we tested whether SUPER TWIG (1)6 would be effective in protecting mice from death after oral infection with Stx-producing E. coli O157:H7. We used mice with protein calorie malnutrition, which are very susceptible to infection with E. coli O157:H7 (19). In this model the establishment of infection can be diagnosed by the detection of Stx both in stool on day 2 and in serum on day 3 after an intragastric infection with E. coli O157:H7 (19). Using this model, we administered SUPER TWIG (1)6 intravenously twice a day for four consecutive days from day 3 to 6. All of the control animals developed neurologic symptoms after day 5 of infection and succumbed to the infection by day 14 (Fig. 3c). In contrast, six of seven mice treated with SUPER TWIG (1)6 survived (P < 0.01) more than 40 days without any neurologic symptoms. These results clearly indicate that SUPER TWIG (1)6 can protect mice from a challenge with a fatal dose of E. coli O157:H7, even when it is administered after the establishment of an infection.
Mechanism of Stx-Neutralizing Action of SUPER TWIG (1)6.
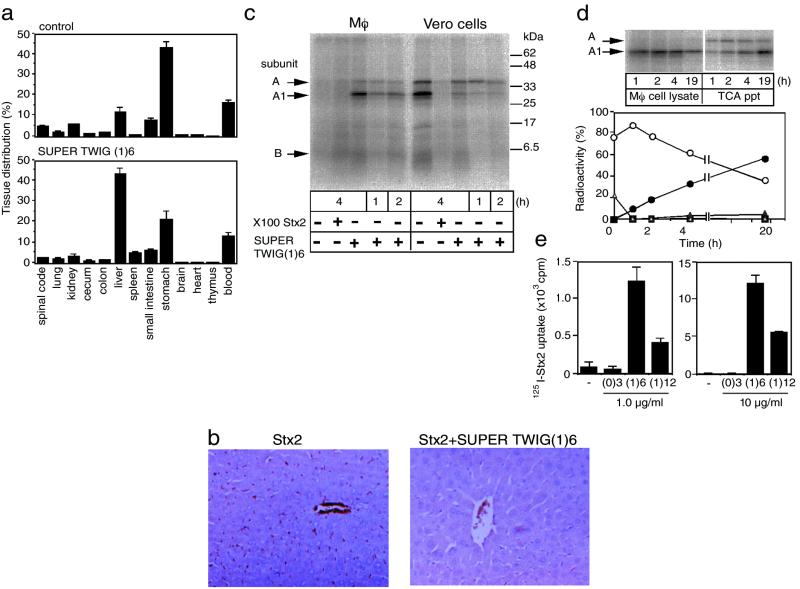
Finally, the possible mechanism of Stx2-neutralizing action of SUPER TWIG (1)6 in vivo was investigated. First, to examine the effect of SUPER TWIG (1)6 on the tissue distribution of Stx2, we injected 125I-Stx2 intravenously into mice with or without SUPER TWIG (1)6. One hour after the injection of 125I-Stx2 alone, 43% of the total radioactivity was recovered from the stomach, and the rest was mostly recovered from the blood, liver, and small intestines (Fig. 4a). Coadministration of SUPER TWIG (1)6 with the toxin dramatically changed the distribution of 125I-Stx2 in that the liver and spleen accumulated the toxin to an extent 3.5 and 5.3 times higher than that observed in the control mice (Fig. 4a). On the contrary, the accumulation of radioactivity in the kidney and brain, well known Stx target organs, was reduced by 1.7 and 3.8 times, respectively.
Figure 4.
SUPER TWIG (1)6-dependent degradation of Stx2 in macrophages. (a) Tissue distribution of 125I-Stx2. The data are presented as the percentage of the total radioactivity present in all of the tissues examined (means ± SE, n = 3). Total radioactivities for control and SUPER TWIG (1)6-coinjected mice were 8,900 ± 400 and 12,200 ± 2,200 cpm (means ± SE, n = 3), respectively. (b) Immunostaining of Stx2 in the liver. Stx2 present in the sections was detected by using specific antibody against Stx2. (c) Metabolism of 125I-Stx2 in cells. Mouse peritoneal macrophages (Mφ) or Vero cells were incubated with 125I-Stx2 (1 μg/ml) in the absence or presence of SUPER TWIG (1)6 (10 μg/ml) or a 100-fold excess of nonradioactive Stx2 for the indicated periods at 37°C. These lysates were separated by electrophoresis on SDS/15% polyacrylamide gel and bands visualized by autoradiography. (d) Degradation of Stx2 in macrophages. Open triangle and open circle indicate A subunit and A1 fragment, respectively, present in the macrophage cell lysate. Filled square and filled triangle indicate A subunit and A1 fragment, respectively, present in the TCA precipitate (ppt) of the culture medium. Because of the low protein content in the TCA ppt fraction, an 8 times larger aliquot of it was used for SDS/PAGE than the amount of the macrophage cell lysate used. Filled circles indicate TCA-soluble supernatants. The data are presented as the percentage of the total radioactivity present in the cell lysate and the culture medium. (e) Effect of SUPER TWIGs on the uptake of 125I-Stx2 by macrophages. Mouse peritoneal macrophages were incubated with 125I-Stx2 (1 μg/ml) in the absence or presence of SUPER TWIG (0)3, (1)6, or (1)12 (1 or 10 μg/ml) for 30 min at 37°C. The data are presented as the total radioactivity (cpm) present in the cell lysate (means ± SE, n = 3).
To confirm the SUPER TWIG (1)6-induced accumulation of Stx2 in the liver, we performed an immunohistochemical analysis. In Stx2-injected mice, Stx2 was found to have been deposited in the wall of sinusoids and in red blood cells (Fig. 4b). Unexpectedly, in SUPER TWIG (1)6-coinjected mice, the amount of Stx2 protein itself detected by the antibody was substantially reduced in the liver (Fig. 4b), although marked accumulation of 125I radioactivity was observed in the same liver (Fig. 4a). Because the liver and spleen are a part of the reticuloendothelial system, it is conceivable that phagocytic macrophages present in these organs had promptly taken up and metabolized Stx2 into small degradation products that could not react with the antibody, when SUPER TWIG (1)6 was administered with the toxin. To test this possibility, we examined SUPER TWIG (1)6-dependent metabolism of Stx2 by using an in vitro culture system. In Vero cells, 125I-Stx2 was effectively incorporated into the cells and further processed to produce its enzymatically active A1 fragment, which causes cell damage, by the action of the membrane-associated protease furin (25) (Fig. 4c). This A1 fragment formation was markedly inhibited by an excess of nonlabeled toxin or by SUPER TWIG (1)6. On the contrary, when macrophages prepared from a mouse peritoneal washing were incubated with 125I-Stx2, 125I-Stx2 was not incorporated at all in the absence of SUPER TWIG (1)6, but the incorporation of 125I-Stx2 and the production of the A1 fragment were dramatically accelerated on incubation with SUPER TWIG (1)6 (Fig. 4c).
Next, degradation of Stx2 incorporated by macrophages was investigated after the incubation with 125I-Stx2 in the presence of SUPER TWIG (1)6 for 4 h. The amount of both Stx2 A subunit and A1 fragment produced in cells decreased quickly, and acid-soluble small degradation products were concomitantly released into the culture medium (Fig. 4d). These results indicate that macrophages actively incorporated and degraded Stx2 in a SUPER TWIG (1)6-dependent manner. Degradation of the A1 fragment produced in Vero cells was much slower than that observed in macrophages (data not shown). It is plausible, from these results, that SUPER TWIG (1)6 decreases the deposition of Stx2 in pathologically significant target organs such as the brain and kidney by binding to the toxin, and furthermore induces the uptake and immediate degradation of the toxin by phagocytic macrophages present in the reticuloendothelial system in the liver and spleen.
SUPER TWIG (1)12, the most effective SUPER TWIG in the in vitro assay, also induced the uptake of 125I-Stx2 by cultured macrophages; but the efficiency was 3.0 or 2.1 times less (1 or 10 μg/ml of SUPER TWIG (1)12, respectively) than that of SUPER TWIG (1)6 (Fig. 4e). No effect was observed with SUPER TWIG (0)3. These observations might explain in part why SUPER TWIG (1)12 has much less suppressive effect than SUPER TWIG (1)6 on the lethality of Stx2 in mice, although the precise reason remains to be clarified.
In this study, we used a series of carbosilane dendrimers and identified SUPER TWIG (1)6 as a Stx neutralizer that is active in the circulation. Because of their unique characteristics, carbosilane dendrimers make it feasible to optimize the number and the position of the functional terminal trisaccharides, which resulted in SUPER TWIG (1)6, having the most compact structure among all of the Stx neutralizers that have ever been developed (7–10). Furthermore, in the physiological scenario SUPER TWIG (1)6 protected mice from challenge with a fatal dose of E. coli O157:H7, even when administered after the establishment of the infection. Therapeutically, this type of neutralizer is expected to have superior advantages to prevent the progression of the life-threatening systemic complications caused by STEC infection, because recent highly sensitive Stx-detection systems make it possible to diagnose the establishment of these infections at a very early stage (4, 19, 24). Also SUPER TWIG (1)6 functions in vivo by a unique dual mechanism: (i) it binds to Stx with high affinity and inhibits its Gb3-dependent incorporation into target cells; (ii) it induces active uptake and subsequent degradation of Stx by macrophages present in the reticuloendothelium. Although the molecular mechanism of the SUPER TWIG-dependent incorporation of Stx by macrophages remains to be elucidated, this type of neutralizer provides a strategy to eliminate harmful materials from the body. The method presented here should be widely applicable for the design of highly selective inhibitors for other bacterial toxins and also for viruses that infect cells by way of carbohydrate receptors.
Acknowledgments
We thank Dr. Shinji Yamasaki for providing us with the pUC118 vector containing the complete coding sequence of Stx1, and Drs. Hiroyuki Arai and Yoshifumi Takeda for their continuous encouragement and fruitful discussion. This work was supported in part by Health Sciences Research Grant on Emerging and Re-emerging Infectious Diseases H12-E-25 and International Health Cooperation Research Grant 11-S-1 from the Ministry of Health, Labor and Welfare, Japan.
Abbreviations
- Stx
Shiga toxin
- STEC
Shiga toxin-producing Escherichia coli
- Gb3
globotriaosyl ceramide
- GST
glutathione S-transferase
- TCA
trichloroacetic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Karmali M A, Steele B T, Petric M, Lim C. Lancet. 1983;i:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 2.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, et al. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien A D, Holmes R K. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paton J C, Paton A W. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 6.Melton-Celsa A R, O'Brien A D. In: Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Kaper J B, O'Brien A D, editors. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 121–128. [Google Scholar]
- 7.Armstrong G D, Fodor E, Vanmaele R. J Infect Dis. 1991;164:1160–1167. doi: 10.1093/infdis/164.6.1160. [DOI] [PubMed] [Google Scholar]
- 8.Kitov P I, Sadowska J M, Mulvey G, Armstrong G D, Ling H, Pannu N S, Read R J, Bundle D R. Nature (London) 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 9.Dohi H, Nishida Y, Mizuno M, Shinkai M, Kobayashi T, Takeda T, Uzawa H, Kobayashi K. Bioorg Med Chem. 1999;7:2053–2062. doi: 10.1016/s0968-0896(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 10.Paton A W, Morona R, Paton J C. Nat Med. 2000;6:265–270. doi: 10.1038/73111. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka K, Terabatake M, Esumi Y, Terunuma D, Kuzuhara H. Tetrahedron Lett. 1999;40:7839–7842. [Google Scholar]
- 12.Noda M, Yutsudo T, Nakabayashi N, Hirayama T, Takeda Y. Microb Pathog. 1987;2:339–349. doi: 10.1016/0882-4010(87)90076-3. [DOI] [PubMed] [Google Scholar]
- 13.Kurazono H, Sasakawa C, Yoshikawa M, Takeda Y. FEMS Microb Lett. 1987;44:23–26. [Google Scholar]
- 14.Goldstein J L, Basu S K, Brown M S. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa K, Arai H, Inoue K. J Biol Chem. 1990;265:5226–5231. [PubMed] [Google Scholar]
- 16.Yamasaki C, Natori Y, Zeng X-T, Ohmura M, Yamasaki S, Takeda Y, Natori Y. FEBS Lett. 1999;442:231–234. doi: 10.1016/s0014-5793(98)01667-6. [DOI] [PubMed] [Google Scholar]
- 17.Plant A L, Brigham-Burke M, Petrella E C, O'Shannessy D J. Anal Biochem. 1995;226:342–348. doi: 10.1006/abio.1995.1234. [DOI] [PubMed] [Google Scholar]
- 18.Mizuguchi M, Tanaka S, Fujii I, Tanizawa H, Suzuki Y, Igarashi T, Yamanaka T, Takeda T, Miwa M. Acta Neuropathol. 1996;91:254–262. doi: 10.1007/s004010050423. [DOI] [PubMed] [Google Scholar]
- 19.Kurioka T, Yunou Y, Kita E. Infect Immun. 1998;66:1726–1734. doi: 10.1128/iai.66.4.1726-1734.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling H, Boodhoo A, Hazes B, Cummings M D, Armstrong G D, Brunton J L, Read R J. Biochemistry. 1998;37:1777–1788. doi: 10.1021/bi971806n. [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y, Kurazono H, Yamasaki S. Microbiol Immunol. 1993;37:591–599. doi: 10.1111/j.1348-0421.1993.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 22.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N F, Karmali M A. Infect Immun. 1992;60:4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii J, Kita T, Yoshida S-I, Takeda T, Kobayashi H, Tanaka N, Ohsato K, Mizuguchi Y. Infect Immun. 1994;62:3447–3453. doi: 10.1128/iai.62.8.3447-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kita E, Yunou Y, Kurioka T, Harada H, Yoshikawa S, Mikasa K, Higashi N. Infect Immun. 2000;68:1207–1214. doi: 10.1128/iai.68.3.1207-1214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garred O, van Deurs B, Sandvig K. J Biol Chem. 1995;270:10817–10821. doi: 10.1074/jbc.270.18.10817. [DOI] [PubMed] [Google Scholar]