Abstract
Ebselen [2-phenyl-1,2-benzisoselenazol-3(2H)-one], a seleno-organic compound with glutathione peroxidase-like activity is used in clinical trials against stroke. Human and bovine TrxR catalyzed the reduction of ebselen to ebselen selenol by NADPH with an apparent KM-value of 2.5 μM and a kcat of 588 min−1. The addition of thioredoxin (Trx) stimulated the TrxR-catalyzed reduction of ebselen several-fold. This result was caused by a very fast oxidation of reduced Trx by ebselen with a rate constant in excess of 2 × 107 M−1 s−1. This rate is orders of magnitude faster than the reaction of dithiol Trx with insulin disulfides. Ebselen competed with disulfide substrates for reduction by Trx and, therefore, acted as an inhibitor of protein disulfide reduction by the Trx system. The inherent H2O2 reductase activity of mammalian TrxR dependent on its active-site selenocysteine residue was stimulated 10-fold by 2 μM ebselen and 25-fold in the additional presence of 5 μM Trx. Furthermore, the apparent KM-value of TrxR for H2O2 was lowered 25-fold to about 100 μM. Our results demonstrate that ebselen is a TrxR peroxidase which, in the presence of Trx, acted as a mimic of a peroxiredoxin. The activity with TrxR and oxidation of reduced Trx offer mechanistic explanations for the in vivo effects of ebselen as an antioxidant and anti-inflammatory agent. Our results demonstrate that the mechanism of action of ebselen may be predominantly via the Trx system rather than via glutathione.
Keywords: selenol‖inflammation‖signal transduction‖oxidative stress‖redox regulation
Ebselen [2-phenyl-1,2-benzisoselenazol-3(2H)-one] is a lipid-soluble seleno-organic compound that exhibits a weak glutathione peroxidase (GPx)-like activity in vitro (1–6). The specificity for substrates ranges from H2O2 to smaller organic hydroperoxides and includes membrane-bound phospholipid and cholesterylester hydroperoxides. Because ebselen is effective against membrane hydroperoxides, it inhibits both nonenzymatic and enzymatic lipid peroxidation in cells and has anti-inflammatory activity in various animal models (5, 6). Ebselen also will directly inhibit inflammation-related enzymes such as 5-lipoxygenase, nitric oxide synthases, NADPH oxidase, protein kinase C, and ATPase by chemically modifying an SH-group forming a selenosulfide complex (5, 6).
Because of its anti-inflammatory properties, ebselen has been used in the treatment of patients with acute ischemic stroke (7, 8) or delayed neurological deficits after aneurysmal subarachnoid hemorrhage (9). The results of these clinical trials indicate the benefit of ebselen as a neuroprotective agent. Also, recent animal studies show neuroprotective, antioxidant, and anti-inflammatory actions of ebselen in a rodent model of permanent middle as well as transient cerebral artery occlusion (10–13).
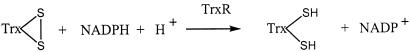
Thioredoxin (Trx) reductase (TrxR) is a dimeric FAD-containing enzyme that catalyzes the NADPH-dependent reduction of the active-site disulfide in oxidized Trx (Trx-S2) to give a dithiol in reduced Trx [Trx-(SH)2] (14, 15). The Trx system (NADPH, Trx, and TrxR) is present in all living organisms (14–17). Trx-(SH)2 is a hydrogen donor for ribonucleotide reductase and a powerful general protein disulfide reductase with a large number of functions in growth and redox regulation by thiol redox control (14–17).
Mammalian TrxRs are particularly interesting because they are large (18) selenoproteins (refs. 19 and 20; Mr 114.000 or larger). Their structures show a close homology to glutathione reductase but with an elongation containing a unique, catalytically active selenolthiol/selenenylsulfide in the conserved C-terminal sequence Gly-Cys-Sec-Gly (20–23). Mammalian TrxRs have a remarkably wide substrate specificity (14–18), reducing not only different Trxs but also, e.g., selenite (24), selenodiglutathione (25), and selenocystine (26). The mammalian enzymes also are NADPH-dependent lipid hydroperoxide reductases (27), a property discovered before it was known that the enzyme contains selenocysteine (Sec).
In this paper, we describe the interaction of ebselen with human TrxR and other mammalian Trx systems. Ebselen was found to be an excellent substrate for mammalian TrxR and a highly efficient oxidant of reduced Trx, and it catalyzed H2O2 reduction. Our results strongly suggest that the antioxidant and anti-inflammatory actions of ebselen are, to a large extent, due to reactions with the Trx system.
Materials and Methods
Materials and Enzymes.
NADPH and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) were obtained from Sigma. Hydrogen peroxide (perhydrol; 30%) and DMSO were obtained from Merck. TrxR from calf thymus or human placenta was purified to homogeneity (25 μmol of NADPH oxidized per min per mg) essentially as described for the rat liver enzyme (16, 18). Trx from Escherichia coli was a homogeneous preparation; recombinant human Trx and the mutant C61S/C72S prepared as described by Ren et al. (28) were obtained from IMCO (Stockholm). The sources of other materials have been described in previous publications (22, 24, 25). Ebselen was a product of Daiichi and was dissolved in fresh DMSO before addition into the aqueous solvents. Concentrations of DMSO were less than 5% of the solvent buffer and were shown to dissolve the drug effectively.
Enzyme Assays.
The activity of enzymes was determined at room temperature by using an Ultrospec 3,000 UV/Visible spectrophotometer (Amersham Pharmacia).
Measurements of TrxR activity were performed in a buffer containing 50 mM Tris⋅Cl, 1 mM EDTA, pH 7.5, generally with 100 μM NADPH and the indicated amounts of ebselen. Reactions were started with the addition of 5 or 10 μl of a stock solution of TrxR in a final total volume of 0.50 ml. Cuvettes, used as the reference, contained the same amount of DMSO as in the samples and also TrxR.
The activity of TrxR was determined in the insulin assay as described (16, 18). The reaction mixture consisted of 100 mM potassium phosphate, pH 7.5, 2 mM EDTA, 0.2 mM NADPH, and 0.16 mM insulin. The reactions were performed by the addition of ebselen, Trx, and finally TrxR, in a total volume of 0.50 ml; the reduction of insulin disulfides was followed in 340 nm. Generated sulfhydryl or selenol groups were observed at 412 nm by the addition of 0.50 ml of 6 M guanidine/HCl, 0.20 M Tris⋅Cl, pH 8.0, 1 mM DTNB using a molar extinction coefficient of 13,600 M−1 cm −1 for the formation of 5-thiol-2-nitrobenzoic acid (TNB).
Activity of TrxR reducing DTNB by NADPH was measured at 412 nm in 100 mM potassium phosphate, pH 7.5/10 mM EDTA/0.2 mM NADPH/5 mM DTNB/0.1 mg per ml of BSA (16).
Fluorescence Measurement.
Protein fluorescence was measured with a thermostatic SPEX-FluoroMax Spectrofluorometer. Trx-(SH)2 was prepared from E. coli Trx-S2, which was incubated at room temperature for 20 min with 10 mM DTT. DTT was subsequently removed by gel chromatography on an NAP-5 column (Amersham Pharmacia) by using N2-equilibrated buffer. Trx-(SH)2 was mixed with ebselen in a total volume of 3 ml of 0.1 M potassium phosphate/1 mM EDTA, pH 7.5 directly at 22°C. Excitation of fluorescence was at 290 nm and emission spectra from 300 to 500 nm were recorded. Emission at 340 nm was followed to record the rate of oxidation of Trx-(SH)2 by ebselen (14, 21).
Results
Reduction of Ebselen by Mammalian TrxR.
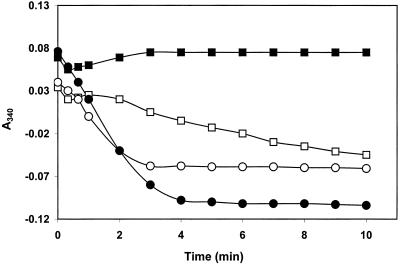
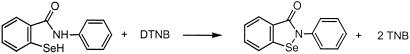
Ebselen is a substrate for mammalian TrxR because the absorption at 340 nm (A340) decreased rapidly when the pure enzyme (50 nM) was added to cuvettes containing 10 μM and 20 μM of the drug and 100 μM of NADPH (Fig. 1). The reactions were complete after 3–4 min and showed no further decrease in A340, demonstrating that the product was not redox cycling with oxygen, in contrast to other selenium compounds like selenite (24) or Sec (26). When one volume of 6 M guanidine hydrochloride containing 1 mM DTNB was added to the cuvettes after 10 min, the absorption of TNB at 412 nm was 0.137 and 0.245, corresponding to concentrations of TNB of ≈10 μM and 20 μM, respectively, which are equal to the complete formation of ebselen selenol (Reactions R1 and R2; considering one equivalent of ebselen gives two equivalents of TNB and the 50% volume dilution). Ebselen itself gave no reaction with DTNB.
Figure 1.
Reduction of ebselen by NADPH catalyzed by human TrxR. Concentrations of 10 μM (○, □) and 20 μM (●, ■) ebselen in 0.5 ml of 50 mM Tris⋅Cl/1 mM EDTA, pH 7.5 containing 100 μM NADPH was mixed with 10 nM (□, ■) and 50 nM (○, ●) human TrxR, and the A340 was followed against identical blank with enzyme but without ebselen.
A number of experiments with lower concentrations of the enzyme showed a complex change in the A340, also seen for 10 nM enzyme in Fig. 1. After an initial decrease, an increase in A340 was observed followed by a decrease to give the same final A340 values after the completion of the reactions. Visible precipitates in the cuvettes were observed with even higher concentrations of ebselen (50 to 100 μM).
Reduction of ebselen by NADPH catalyzed by the enzyme produces the selenol (Reaction R1) presumably via a short-lived isoselenazolone ring opened bound intermediate. Excess of ebselen was found to react with the selenol forming an ebselen diselenide, which absorbs strongly at 340 nm and has a low solubility, giving rise to the precipitate and increase in A340 (Z.R. and A.H., unpublished work).
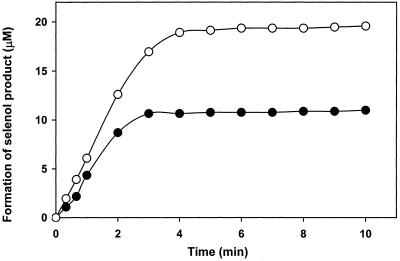
To be able to interpret the kinetic decay curves obtained at 340 nm, the spectra of ebselen with known concentrations were determined in TE (buffer) (10 mM Tris/1 mM EDTA, pH 7.5). The extinction coefficient of ebselen at 340 nm (ɛ340) was calculated to be 5,000 M−1 cm−1. The ɛ340 of the ebselen selenol also was determined to be 2,000 M−1 cm−1 by reducing ebselen with 10× excess of DTT, because both the reduced and oxidized DTT have no measurable absorption at 340 nm. In calculation of the formation of ebselen–selenol product by the enzyme-catalyzed reactions, a change of the molar extinction coefficient of 9,200 M−1 cm−1 was used for the reduction of ebselen, taking Δɛ340 of 6,200 M−1 cm−1 for the oxidation of NADPH to NADP+. Fig. 2 shows the formation of ebselen selenol upon reduction of ebselen by NADPH catalyzed by 50 nM mammalian TrxR. Here, we see again that ebselen is quickly reduced stoichiometrically to form the selenol product after the completion of the reactions.
Figure 2.
Formation of ebselen selenol by reduction of 10 μM (●) and 20 μM (○) ebselen by 100 μM NADPH in 0.5 ml of 50 mM Tris⋅Cl/1 mM EDTA, pH 7.5 with 50 nM human TrxR. The A340 was followed against identical blank without the enzyme.
Steady-State Kinetic Parameters.
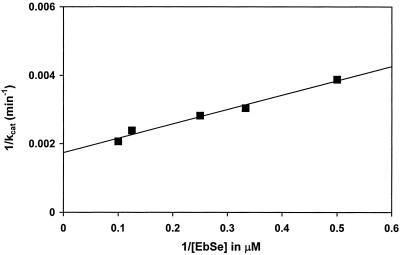
Determination of the steady-state kinetic parameters, KM and kcat value, for the ebselen as a substrate of the enzyme was complicated by the formation of ebselen diselenide that absorbs strongly at 340 nm. From the decrease of A340, a KM-value below 5 μM for TrxR with ebselen can be estimated. Such a low KM-value also makes a direct measurement difficult. Therefore, we have developed a coupled assay using H2O2 as a mediator (Scheme S1). Because ebselen selenol reacts quickly with H2O2 to form the selenenic acid (EbSeOH), which spontaneously eliminates H2O and regenerates the ebselen, H2O2 was chosen to quickly regenerate the ebselen in the solution. An H2O2 concentration of 1 mM was used for its reaction with the selenol to be fast enough to compete with the diselenide formation. Fig. 3 gives a Lineweaver–Burk plot for ebselen (1–10 μM) as a substrate of 5 nM bovine TrxR. The decrease of A340 was measured against an identical blank without ebselen, and the kcat was calculated by using the initial ΔA340 and the Δɛ of 6,200 M−1 cm−1 for the oxidation of NADPH. A KM of 2.5 μM and a kcat of 588 min−1 were derived, and a kcat/KM of 3.9 × 106 M−1 s−1 can be calculated. This rate makes ebselen a substrate of unusual efficiency, because hTrx-S2 has a KM-value of 2.5 μM and a kcat of 3,000 min−1 with TrxR (16–18).
Scheme 1.
Figure 3.
Lineweaver–Burk plot for ebselen as a substrate of mammalian TrxR. To cuvettes containing 0.5 ml of 50 mM Tris⋅Cl, 1 mM EDTA, pH 7.5, 100 μM NADPH, 1 mM H2O2, and 5 nM mammalian TrxR, 1–10 μM of ebselen was added. The A340 was followed against an identical blank without ebselen. The kcat was calculated by using the initial ΔA340 and the Δɛ of 6,200 M−1 cm−1 for the oxidation of NADPH.
Effects of Ebselen on the Enzymatic Activity of the Mammalian Trx System.
To test whether ebselen inhibited TrxR, as has been suggested by others (29), enzyme assays were performed. No inhibition was observed with 5 mM DTNB as substrate using 50 μM ebselen and 10 nM enzyme; only a small effect was seen in insulin disulfide reduction assays using 5 μM Trx and TrxR with excess insulin (data not shown). The later effect should come from competition with Trx in the assay because ebselen did not catalyze insulin disulfide reduction together with the enzyme. Preincubation of enzyme with ebselen in the presence or absence of NADPH did not inhibit the enzyme (data not shown).
Effect of Trx on Ebselen Reduction.
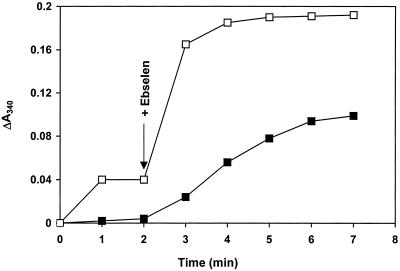
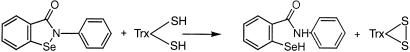
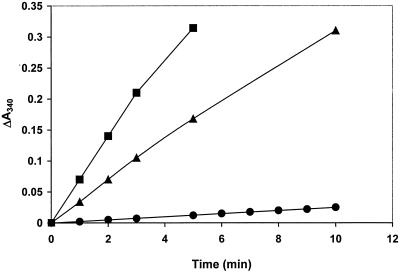
Addition of human Trx to TrxR, NADPH, and ebselen increased the reaction rate (Fig. 4), demonstrating that Trx-(SH)2 is a fast reductant of ebselen, according to Reactions R3 and R4:
Figure 4.
Effect of human Trx on reduction of ebselen by TrxR. The oxidation of NADPH by 20 nM TrxR was recorded in the presence of no (■) or 5 μM (□) of hTrx-S2 in 0.5 ml of 50 mM Tris⋅Cl/1 mM EDTA, pH 7.5/100 μM NADPH. During the first 2 min, Trx-S2 was reduced to Trx-(SH)2. At the arrow, 10 μM ebselen was added to both cuvettes.
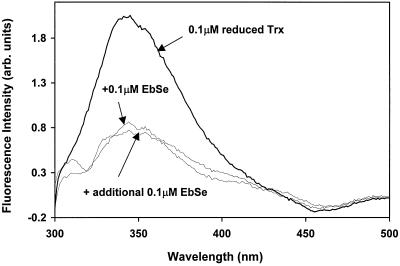
Mammalian and E. coli Trx have the same active site—WCGPC—and reactivity with disulfides (14–18). Because E. coli Trx-(SH)2 has a 3-fold higher tryptophan fluorescence than Trx-S2 (14, 15), this property was used to follow the reaction with ebselen. The spectral changes in 0.1 μM Trx-(SH)2 by mixing with 0.1 μM ebselen showed complete oxidation to Trx-S2 (Fig. 5). The decrease of the fluorescence intensity for the Trx-(SH)2 was very fast, and the reaction was complete in 1.5 s. The estimated half-life of the fluorescence decrease was less than 0.5 s, indicating a rate constant of Reaction R4higher than 2 × 107 M−1 s−1. This rate is by far the fastest known oxidation of reduced Trx by a low molecular weight compound (14, 21, 22, 24, 25).
Figure 5.
Oxidation of E. coli Trx-(SH)2 by ebselen determined by fluorescence spectroscopy. The sample contained 0.1 μM (1.2 μg/ml) of E. coli Trx-(SH)2 in N2-equilibrated 0.1 M potassium phosphate, 1 mM EDTA, pH 7.5. Fluorescence was excited at 290 nm and the emission spectrum from 300 to 500 nm was recorded. Then, 0.1 μM of ebselen was added and a new spectrum was recorded.
Ebselen Strongly Stimulates the H2O2 Reductase Activity of TrxR.
Mammalian TrxR directly reduces H2O2 with an apparent KM value of 2.5 mM and a kcat of 100 min−1 (21). With 17 nM of the human TrxR and 0.50 mM H2O2, a turnover number of 30 min−1 was calculated (Fig. 6). Addition of 2 μM ebselen strongly stimulated the activity of the enzyme, which increased its activity to a turnover of 285 min−1 or ≈10-fold. With 4.5 μM human Trx present, the activity was even higher: 750 min−1 (a 25-fold increase in activity). Thus, ebselen acts to increase dramatically the TrxR H2O2 reductase activity and is also, therefore, a Trx peroxidase or peroxiredoxin mimic.
Figure 6.
Reduction of H2O2 by human TrxR and the effect of ebselen and Trx. To cuvettes containing 50 mM Tris⋅Cl, 1 mM EDTA, pH 7.5, and 100 μM NADPH was added 0.5 mM H2O2 and 17 nM human TrxR (hTrxR) (●), 17 nM hTrxR plus 2 μM ebselen (▴), or 17 nM hTrxR plus 2 μM ebselen and 4.5 μM human Trx (hTrx) (■). The A340 was determined against a blank with 17 nM TrxR but without H2O2.
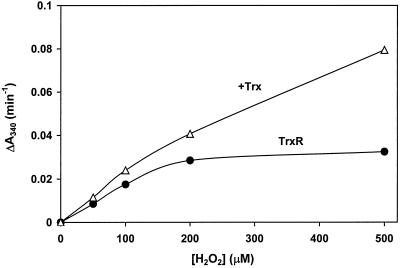
Addition of Trx (4.5 μM) to TrxR (17 nM) stimulated the reduction of H2O2 about 2-fold (Fig. 7). Addition of ebselen at a low concentration (0.5 μM) increased the reaction rate further, and 5.5 μM ebselen stimulated strongly (Fig. 7). With 2 μM ebselen, TrxR (17 nM) showed a high activity with 100 μM H2O2 (Fig. 8). An apparent KM-value of about 100 μM of TrxR for H2O2 was determined. Thus, the effect of ebselen was to increase activity of the enzyme with lower, more physiologically relevant concentrations of H2O2. This increase was about 25-fold of the reaction without ebselen. The Trx-dependent reaction still increased (data not shown). By using 100 μM H2O2, 1, 2, and 5 μM ebselen stimulated the reaction in a similar way, both with and without Trx.
Figure 7.
Effect of Trx and ebselen on reduction of H2O2 by TrxR. The same conditions as in Fig. 6 were used with only 17 nM TrxR (●) and the addition of 4.5 μM Trx (▵), followed by 0.5 μM ebselen (□) and, finally, ebselen to 5.5 μM (■).
Figure 8.
Effect of different H2O2 concentrations on the activity of TrxR with ebselen. The reduction of H2O2 with 17 nM hTrxR and 2 μM ebselen (●) or with 17 nM hTrxR plus 4.5 μM hTrx and 2 μM ebselen (▵) was determined with the indicated concentrations of H2O2.
Discussion
Our results demonstrate that ebselen is an excellent direct substrate for mammalian TrxR, generating the selenol form of the compound. The apparent KM-value of ebselen was determined to be 2.5 μM, which is the same as that of Trx, the natural substrate for TrxR. The kcat was measured to be 588 min−1, which is comparable to the 3,000 min−1 for Trx-S2 (16–18). Thus, the highly efficient reduction of ebselen by TrxR should be a major pathway for ebselen reduction in cells. Ebselen is reported to be able to reach the inside of cells because of its lipid solubility and reactive binding to the intracellular thiol groups, and the neuroprotective effect of ebselen is observed at approximately 10 μM of plasma level. Experimental animal studies show that brain levels of ebselen were about 20% of the plasma level (12, 30). Formation of ebselen diselenide was found to be a common feature when ebselen concentrations are high and enzyme concentrations are low. Our studies of the ebselen diselenide have revealed that it is also a substrate of mammalian TrxR generating the selenol, although with relatively slower turnover rate (Z.R. and A.H., unpublished work). In contrast to other selenium compounds, ebselen was not redox cycling with NADPH in the presence of oxygen, which is consistent with its low toxicity (6). Direct reduction of ebselen also was observed using Trx-(SH)2, and the fluorescence kinetics showed that ebselen is the most efficient oxidant of Trx so far, with a second-order rate constant in excess of 2 × 107 M−1 s−1. Therefore, ebselen will react with both Trx and TrxR for its peroxidase activity (Scheme S2). We found no inhibition of mammalian TrxR by ebselen, in contrast to another report (29). However, high concentrations of ebselen should inhibit protein disulfide reduction by the mammalian Trx system through competition of electrons from Trx and NADPH. This reactivity may explain its inhibition of tumor cell growth, which implies that it is a competitive inhibitor (29) by interference with the synthesis of deoxyribonucleotides by ribonucleotide reductase. In this reaction, reduced Trx acts to reduce a disulfide of ribonucleotide reductase, formed by each turnover of the enzyme, which is essential for DNA synthesis and cell growth (14, 17).
Scheme 2.
Mammalian TrxR is a selenoenzyme containing an essential Sec residue in the unique C-terminal sequence -Gly-Cys-Sec-Gly (19–23). The enzyme is homologous to glutathione reductase, including an identical N-terminal active-site disulfide (20–23). The Sec residue is essential for activity and the site of reduction of Trx (21–23, 31). The substrate specificity is unusually broad and involves selenium compounds, alloxan, and many other compounds (17). Of particular interest has been the observation that the enzyme contains a lipid hydroperoxide reductase activity against hydroperoxyeicosatetraenoic acid (27). In the present study, we could show that H2O2 reduction by the enzyme was strongly stimulated by ebselen with an apparent KM-value of 2.5 μM. Previously, ebselen has been implicated as a GSH-peroxidase mimic, enhancing the spontaneous reaction between GSH and H2O2 up to 5-fold at 20 μM ebselen (1, 2, 6). Our results demonstrate another pathway and suggest that the mechanism of action of ebselen rather is by means of the Trx system, with consequences for redox regulation by Trx.
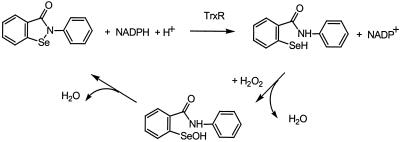
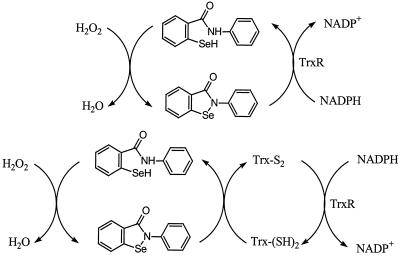
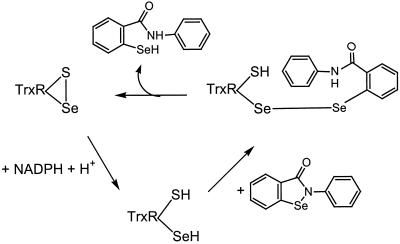
The mechanism of mammalian TrxR acting on ebselen can be illustrated as shown in Scheme S3. The C-terminal active-site selenenylsulfide is reduced by NADPH via the active-site disulfide in the second subunit, forming a thiol and a strong nucleophilic selenolate (22, 23, 31). The two-electron reduction of ebselen by the active site takes place by means of an intermediate, TrxRSe-SeEbSe, presumably short lived. Thus, the ebselen mechanism of action as an H2O2 reductase should start mainly through the formation of the ebselen selenol. The latter will quickly react with H2O2 to form H2O and the ebselen selenenic acid, which spontaneously releases another molecule of H2O and gives back the ebselen, thus finishing a catalytic cycle (Scheme S1). In the presence of Trx, the formation of the ebselen selenol can occur both directly via TrxR and by means of the reduced Trx (Scheme S2). The strong activity of ebselen selenol toward H2O2 should be an effective way of eliminating H2O2 produced in response to growth factors through the action of NADPH oxidase (32). In addition, the reducing activity of Trx in controlling activation of transcription factors also may be affected, eliminating inflammatory responses in cells by means of NFkB, for example (33). The reactions of Scheme S2 also provide a mechanism for ebselen toxicity, because at high doses it will block the action of Trx.
Scheme 3.
In summary, we have found a specific and highly efficient reduction of ebselen by human TrxR and a direct, fast oxidation of Trx. The peroxidase activity of ebselen operating via TrxR should eliminate H2O2 and lipid hydroperoxides, thus giving a mechanistic explanation for the effects in protecting cells from ischemic tissue damage. The clinical trials using ebselen have shown promising effects in stroke, and a defined mechanism of ebselen is essential in understanding its protective effects. TrxR and Trx are present in all cells throughout the body and may be particularly important in cells in the nervous system for protection against oxidative stress (34, 35). In addition, mitochondria have a selenium-dependent TrxR and a Trx of their own (17, 36–38), which also should reduce ebselen. In this way, ebselen may protect the mitochondria from specific damage and affect the signaling pathways from oxidation in cells leading to apoptosis (39). However, high concentrations of ebselen should be toxic by inhibition of the activity of the Trx system. This possibility may explain why 50–100 μM of ebselen induces apoptosis in human hepatoma cells by causing mitochondrial permeability transition (40).
NADPH can either reduce glutathione via glutathione reductase or Trx via TrxR. As shown in this paper, ebselen can thus interact with both systems, but the direct effect of ebselen via glutathione reduction will not be sensed by cells until the large concentrations (in mM) of glutathione become oxidized. In contrast, the reaction of ebselen with Trx and TrxR will be transmitted directly to redox regulation mechanisms in the cell, which have a direct effect upon transcription factors or DNA synthesis. The remarkable reactivity of ebselen at micromolar concentrations with components of the mammalian Trx system will require further studies in relation to selecting Trx targets to explore the mechanisms of this promising clinically used agent.
Acknowledgments
We thank Ms. Marjan H. Amiri for her excellent technical assistance and Dr. Sergei Kuprin for his help with fluorescence measurements. This investigation was supported by grants from the Swedish Cancer Society, Grant 13x 3529 from the Swedish Medical Research Council, and by grants from Daiichi Pharmaceutical Company and the Karolinska Institute.
Abbreviations
- ebselen
2-phenyl-1,2-benzisoselenazol-3(2H)-one
- Trx
thioredoxin
- TrxR
Trx reductase
- Trx-S2, oxidized Trx; Trx-(SH)2, reduced Trx
DTNB, 5,5′-dithiobis(2-nitrobenzoic acid)
- TNB
5-thiol-2-nitrobenzoic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Muller A, Cadenas E, Graf P, Sies H. Biochem Pharmacol. 1984;33:3235–3239. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- 2.Wendel A, Fausel M, Safayhi H, Tiegs G, Otter R. Biochem Pharmacol. 1984;33:3241–3245. doi: 10.1016/0006-2952(84)90084-4. [DOI] [PubMed] [Google Scholar]
- 3.Muller A, Gabriel H, Sies H, Terlinden R, Fischer H, Romer A. Biochem Pharmacol. 1988;37:1103–1109. doi: 10.1016/0006-2952(88)90517-5. [DOI] [PubMed] [Google Scholar]
- 4.Maiorino M, Roveri A, Coassin M, Ursini F. Biochem Pharmacol. 1988;37:2267–2271. doi: 10.1016/0006-2952(88)90591-6. [DOI] [PubMed] [Google Scholar]
- 5.Schewe T. Gen Pharmacol. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 6.Sies H, Masumoto H. Adv Pharmacol. 1997;38:229–246. doi: 10.1016/s1054-3589(08)60986-2. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Stroke (Dallas) 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, Yasuhara H. Cereb Dis. 1999;9:112–118. doi: 10.1159/000015908. [DOI] [PubMed] [Google Scholar]
- 9.Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neurosurgery. 1998;42:269–278. doi: 10.1097/00006123-199802000-00038. [DOI] [PubMed] [Google Scholar]
- 10.Dawson D A, Masayasu H, Graham D I, Macrae I M. Neurosci Lett. 1995;185:65–69. doi: 10.1016/0304-3940(94)11226-9. [DOI] [PubMed] [Google Scholar]
- 11.Takasago T, Peters E E, Graham D I, Masayasu H, Macrae I M. Br J Pharmacol. 1997;122:1251–1256. doi: 10.1038/sj.bjp.0701426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai H, Masayasu H, Dewar D, Graham D I, Macrae I M. Stroke (Dallas) 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 13.Namura S, Nagata I, Takami S, Masayasu H, Kikuchi H. Stroke (Dallas) 2001;32:1906–1911. doi: 10.1161/01.str.32.8.1906. [DOI] [PubMed] [Google Scholar]
- 14.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren A. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 16.Arnér E S, Zhong L, Holmgren A. Methods Enzymol. 1999;300:226–239. doi: 10.1016/s0076-6879(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 17.Arnér E S, Holmgren A. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 18.Luthman M, Holmgren A. Biochemistry. 1982;21:6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Stadtman T C. Proc Natl Acad Sci USA. 1996;93:1006–1011. doi: 10.1073/pnas.93.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong L, Arnér E S, Ljung J, Åslund F, Holmgren A. J Biol Chem. 1998;273:8581–8591. doi: 10.1074/jbc.273.15.8581. [DOI] [PubMed] [Google Scholar]
- 21.Zhong L, Holmgren A. J Biol Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
- 22.Zhong L, Arnér E S, Holmgren A. Proc Natl Acad Sci USA. 2000;97:5854–5859. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandalova T, Zhong L, Lindqvist Y, Holmgren A, Schneider G. Proc Natl Acad Sci USA. 2001;98:9533–9538. doi: 10.1073/pnas.171178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Björnstedt M, Holmgren A. Eur J Biochem. 1992;207:435–439. doi: 10.1111/j.1432-1033.1992.tb17068.x. [DOI] [PubMed] [Google Scholar]
- 25.Björnstedt M, Kumar S, Holmgren A. J Biol Chem. 1992;267:8030–8034. [PubMed] [Google Scholar]
- 26.Björnstedt M, Kumar S, Björkhem L, Spyrou G, Holmgren A. Biomed Environ Sci. 1997;10:271–279. [PubMed] [Google Scholar]
- 27.Björnstedt M, Hamberg M, Kumar S, Xue J, Holmgren A. J Biol Chem. 1995;270:11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- 28.Ren X, Björnstedt M, Shen B, Ericson M L, Holmgren A. Biochemistry. 1993;32:9701–9708. doi: 10.1021/bi00088a023. [DOI] [PubMed] [Google Scholar]
- 29.Powis G, Gasdaska J R, Gasdaska P Y, Berggren M, Kirkpatrick D L, Engman L, Cotgreave I A, Angulo M, Baker A. Oncol Res. 1997;9:303–312. [PubMed] [Google Scholar]
- 30.Ullrich V, Weber P, Meisch F, von Appen F. Biochem Pharmacol. 1996;52:15–19. doi: 10.1016/0006-2952(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 31.Lee S R, Bar-Noy S, Kwon J, Levine R L, Stadtman T C, Rhee S G. Proc Natl Acad Sci USA. 2000;97:2521–2526. doi: 10.1073/pnas.050579797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee S G. Exp Mol Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 33.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 34.Stemme S, Hansson H A, Holmgren A, Rozell B. Brain Res. 1985;359:140–146. doi: 10.1016/0006-8993(85)91421-0. [DOI] [PubMed] [Google Scholar]
- 35.Lippoldt A, Padilla C A, Gerst H, Andbjer B, Richter E, Holmgren A, Fuxe K. J Neurosci. 1995;15:6747–6756. doi: 10.1523/JNEUROSCI.15-10-06747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigobello M P, Callegaro M T, Barzon E, Benetti M, Bindoli A. Free Radical Biol Med. 1998;24:370–376. doi: 10.1016/s0891-5849(97)00216-5. [DOI] [PubMed] [Google Scholar]
- 37.Spyrou G, Enmark E, Miranda-Vizuete A, Gustafsson J A. J Biol Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 38.Lee S R, Kim J R, Kwon K S, Yoon H W, Levine R L, Ginsburg A, Rhee S G. J Biol Chem. 1999;274:4722–4734. doi: 10.1074/jbc.274.8.4722. [DOI] [PubMed] [Google Scholar]
- 39.Maciel E N, Vercesi A E, Castilho R F. J Neurochem. 2001;79:1237–1245. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang C F, Shen H M, Ong C N. Arch Biochem Biophys. 2000;380:319–330. doi: 10.1006/abbi.2000.1939. [DOI] [PubMed] [Google Scholar]