Abstract
Several tumor predisposition syndromes have been linked to the development of gliomas and glioneuronal tumors (glioma/GNT). For many pathogenic germline variants, the prevalence and clinical significance remain unclear. Germline variants and copy-number variants affecting 76–90 well-established cancer predisposing genes were identified in 2,187 patients with gliomas/GNT, who underwent prospective sequencing of their tumor and a matched normal sample. A germline pathogenic or likely pathogenic (P/LP) mutation was identified in 11% (250/2187, 95% CI 10.1–12.8%). Affected high- and moderate-penetrance genes included BRCA2 (n = 11; 0.5%), TP53 (n = 8; 0.4%), NF1 (n = 8; 0.4%), CHEK2 (n = 21, 0.9% excluding common variant I157T), and the mismatch repair (MMR) genes (n = 22, 1.0%). Biallelic inactivation was identified in 8/8 tumors with a germline NF1 mutation, 7/8 tumors with a germline TP53 alteration, and 10/19 tumors with a heterozygous germline MMR defect. Gliomas/GNT with biallelic inactivation of an MMR gene were characterized by hypermutation, microsatellite instability, and a distinct clinical phenotype. Assessment of zygosity identifies biallelic inactivation of DNA double-strand break repair alterations in a minority of tumors, including BRCA2-deficient gliomas with increased genomic scarring attributable to homologous recombination deficiency, and refutes the contribution of the most common P/LP germline variants. Irrespective of gene, tumors with biallelic inactivation were diagnosed at a younger age than tumors without a germline variant (p = 3.5 × 10–6) and tumors with a monoallelic alteration (p = 0.00014). In conclusion, germline sequencing identifies a P/LP variant in a high proportion of patients with glioma/GNT. Biallelic inactivation was common in younger patients with germline variants and patients with neurofibromatosis type 1/Li-Fraumeni, but was only present in half of the patients with Lynch syndrome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-025-02935-x.
Keywords: Cancer predisposition syndrome, Germline sequencing, Lynch syndrome, Li-Fraumeni syndrome, Neurofibromatosis type 1
Introduction
Several tumor predisposition syndromes caused by a germline loss-of-function mutation in a tumor suppressor gene are known to predispose individuals to the development of gliomas and glioneuronal tumors (glioma/GNT), such as Lynch Syndrome, Li-Fraumeni Syndrome, and neurofibromatosis type 1. In the pediatric population, it has been reported that tumor predisposition syndromes are identified in 11% of pediatric patients with high-grade gliomas [22].
The available studies are limited by the absence of paired tumor and germline sequencing, which aids in evaluating a germline variant’s contribution to tumor development. It cannot be assumed that a pathogenic or likely pathogenic (P/LP) germline alteration contributed to tumorigenesis, as tumor predisposition syndromes are associated with specific tumor types and may be low penetrance [29]. Given the therapeutic actionability of certain germline mutations, such as DNA double-strand break repair pathway alterations (i.e. BRCA1/2 alterations) [23], mismatch repair (MMR) alterations [18], and some RAS pathway alterations such as NF1 [11, 12], there is considerable interest in assessing the contribution of germline alterations in patients with a glioma/GNT.
In this study, we investigate a prospectively collected cohort of patients of all ages who presented to Memorial Sloan Kettering Cancer Center for clinical care after being diagnosed with a glioma/GNT. Patients in this study consented to sequencing of their tumor and a matched normal sample using the next-generation sequencing (NGS) platform, MSK-IMPACT [5], which includes cancer predisposition genes in the American College of Medical Genetics and Genomics (ACMG) recommendations for reporting of secondary findings [14, 21]. We then interrogated the contribution of these genes in the development of the glioma/GNT by evaluating for the presence of a P/LP germline alteration and biallelic inactivation of that variant. Identification of biallelic inactivation supports the role of the germline variant in tumor pathogenesis, and potentially the therapeutic actionability of that variant in the glioma/GNT.
Materials and methods
Patients
2187 patients with a glioma or a glioneuronal/neuronal tumor (WHO grade 1 to 4) underwent matched tumor and normal sequencing with MSK-IMPACT as part of a sequencing protocol, after providing written informed consent [5]. This protocol (NCT01775072) was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center and conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, as well as federal laws. Patients consented to the identification of somatic alterations and either anonymized analysis of germline alterations or un-anonymized clinical germline testing.
Identification of germline variants and copy-number alterations
For the entire cohort, we investigated the prevalence of germline variants and copy-number variants (CNVs) affecting 76–90 well-established cancer predisposing genes via a clinically validated pipeline within a CLIA-certified laboratory [28]. Common variants exceeding 2% frequency in gnomAD exomes (v2.1.1) [13] were not analyzed. Following ACMG guidelines [26], variants were manually curated if previously classified as P/LP by our expert clinical molecular geneticists, reported as such in ClinVar [17], or predicted by variant effect predictor [20] to significantly impact function in tumor-suppressor genes (e.g., truncations, nonsenses, and splicing mutations). Subsequently, CNVs were determined in all normal samples using a previously described clinically validated pipeline [5]. Only deletions with fold-change below − 1.7 and FDR-corrected p-value less than 0.05 were considered. The deletions were required to span contiguous exons and single-exon deletions were excluded as potential false positives. The penetrance of germline pathogenic variants was based on the relative risk of individuals with the variant developing cancer. Variants were classified as high (relative risk > 4), moderate (relative risk of 2 to 4), low (relative risk < 2), or uncertain penetrance [9, 19, 31]. Variants of uncertain significance were not reported.
Identification of biallelic inactivation and MMR deficiency
We inferred somatic zygosity for all germline variants using locus-specific and allele-specific DNA copy-number inference, considering tumor purity and the observed variant allele frequency (VAF) in the tumor. Each germline variant was categorized as heterozygous, biallelic (loss of the WT allele), or having lost the mutant allele based on a previously described framework [28]. For germline CNVs, only the un-anonymized patients could be examined for biallelic deletions in their matched tumors by reviewing copy number segmentation calls overlapping with the affected gene (with a fold-change below –2, indicating a biallelic loss); tumors in the anonymized cohort with germline CNVs could not be evaluated for biallelic inactivation (affecting only 8 out of 268 germline alterations). For one patient with Lynch syndrome, zygosity was inferred based on immunohistochemistry.
MSI-status was determined using MiMSI (https://github.com/mskcc/mimsi) [34] and somatic mutational data were deconvoluted into mutational signatures (https://github.com/mskcc/mutational-signatures) [1], as previously described.
For tumors with a germline BRCA1 or BRCA2 alteration, we computed an IMPACT-HRD score (HRD score), which quantifies genomic scars associated with homologous recombination deficiency. The HRD score is the unweighted sum of the number of telomeric allelic imbalances, large-scale transitions, and the genome-wide loss of heterozygosity [30], and was performed by analyzing allele-specific copy-number alterations using the FACETS algorithm [27] (version 0.5.14) and computing those genomic scars with the IMPACT-HRD package under R v 4.1.2 (https://github.com/danielmuldoon/impact-hrd/).
Biostatistical analyses
The data were summarized using the frequency and percentages for categorical variables and the median for continuous features. All statistical analyses were performed using R. All P-values were two-sided, and P-values of < 0.05 were considered to indicate statistical significance.
Results
Patient cohort
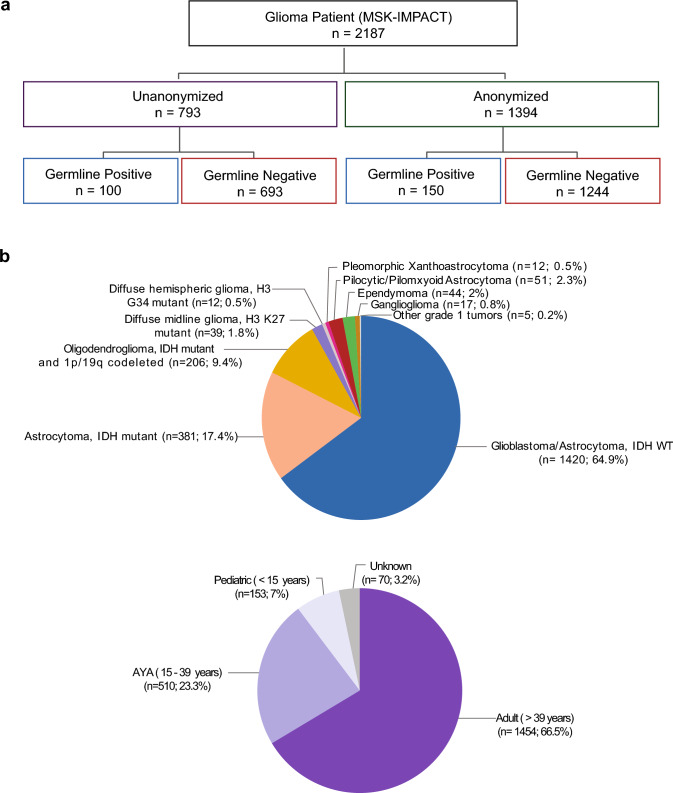
1394 patients underwent sequencing of their tumor and matched normal tissue and were anonymized prior to analysis for a P/LP germline alteration and comprise the “anonymized subset.” An additional 793 patients consented to sequencing of their tumor and clinical review of their germline sequencing data and comprise the “un-anonymized subset” (Fig. 1a).
Fig. 1.
Breakdown of the study cohort. a: Analysis of germline variants was performed in an anonymized and un-anonymized subset. b: Breakdown of cohort by age and tumor type
The combined cohort comprised of 2187 patients between 0.2 and 94 years of age: 1,454 adults (> 39 years, 66.5%), 510 adolescents and young adults (age 15–39 years, 23.3%), and 153 pediatric patients (< 15 years, 7%). 64.9% had glioblastoma, IDH WT, 17.4% had an IDH mutant astrocytoma and 9.4% had an oligodendroglioma, IDH mutant and 1p/19q codeleted (Fig. 1b for lesser represented tumor types).
Frequency of germline alterations
P/LP germline alterations were identified in 11% (150/1394, 95% CI: 9.1%-12.4%) of patients in the unbiased anonymized subset and in 11% (250/2187, 95% CI: 10.1–12.8%) of the entire patient cohort (Fig. 1a, Supplementary Table 1). Of these 250 patients, 8 patients harbored a germline TP53 mutation and had Li-Fraumeni syndrome—a prevalence of 0.2% in the anonymized subset and 0.4% for the entire cohort (Table 1). 22 patients had a germline alteration in MSH6/MSH2/MLH1/PMS2, of which 20 had Lynch Syndrome and 2 had constitutional mismatch repair deficiency (CMMRD)—a prevalence of 0.6% in the anonymized subset and 1% for the entire cohort. 8 patients were found to have a germline alteration in NF1 and have neurofibromatosis type 1—a prevalence of 0.2% in the anonymized subset and 0.4% for the entire cohort. And finally, 1 patient had a germline alteration in PTEN and has PTEN hamartoma tumor syndrome—a prevalence of 0.08% in the anonymized subset and 0.05% for the entire cohort. These 4 genetic predisposition syndromes impacted 1.8% of the patient population; no patients had a TSC1/2 mutation or an APC variant that was not I1307K.
Table 1.
Germline predisposition syndromes and associated glioma/GNT
| Diagnosis | Lynch/CMMRD | Li-Fraumeni | NF1 | PTEN hamartoma tumor syndrome |
|---|---|---|---|---|
| Glioblastoma/astrocytoma, IDH WT | 17 | 7 | 4 | |
| Astrocytoma, IDH mutant | 4 | 1 | ||
| Oligodendroglioma, IDH mutant and 1p/19q codeleted | 1 | |||
| Ependymoma | 1a | |||
| Ganglioglioma | 1b | |||
| Pilocytic/Pilomyxoid astrocytoma | 3 | |||
| Prevalence (%) and n | 1% (22/2187) | 0.36% (8/2187) | 0.36% (8/2187) | 0.05% (1/2187) |
aBiallelic inactivation identified
bBiallelic inactivation was not identified
TP53 mutations and MMR alterations were primarily found in 2 glioma subtypes: glioblastoma, IDH WT, and IDH mutant astrocytoma. As expected, NF1 mutations were primarily associated with IDH WT high-grade gliomas and pilocytic astrocytomas (Table 1, Fig. 2a).
Fig. 2.
Germline alterations and somatic biallelic inactivation in the associated tumor. a Germline alterations by mutation and tumor type. b Breakdown of germline alteration by somatic biallelic inactivation in the associated tumor. c Age of diagnosis in patients with moderate or high penetrance germline alteration with biallelic inactivation (maroon), a monoallelic germline alteration without biallelic inactivation (pink), and no germline alteration (teal)
The remainder of the germline alterations included high and moderate penetrance genes in DNA damage response pathway alterations such as BRCA2 (n = 11; 0.5%), CHEK2 (n = 21, 0.9% excluding common lower penetrance variant I157T), and ATM (n = 8; 0.36%) (Fig. 2a).
Analysis of biallelic inactivation
Our analysis revealed that nearly all patients with Li-Fraumeni and neurofibromatosis type 1 (NF1) had a second hit in their tumor (88% and 100% of tumors, respectively) (Fig. 2b, Supplemental Data Table for patient-level annotation). In patients with NF1, biallelic inactivation was identified in high and low-grade astrocytomas, but also a myxopapillary ependymoma, which is not classically associated with NF1 despite reports of co-occurrence [6, 33].
Surprisingly, in patients with Lynch syndrome and a glioma/GNT, we observed a lower rate of biallelic inactivation (10/19 patients, 53%). Among the patients with biallelic inactivation, 3/10 had high-grade IDH mutant astrocytoma and 7/10 had glioblastoma, IDH WT. Of the remaining patients without biallelic inactivation, 7/9 had glioblastoma, IDH WT; 1/9 had an IDH mutant astrocytoma; and 1/9 had a ganglioglioma.
All tumors with biallelic inactivation of a heterozygous germline mutation (n = 10) and the 2 patients with homozygous germline mutations (CMMRD) were hypermutated with a tumor mutational burden > 20 mutations/mega base. In contrast, tumors without biallelic inactivation, exclusively had a low tumor mutational burden (< 10 mutations/mega base) (Fig. 3a). Ultra-hypermutation (defined as a tumor mutational burden greater than 100 mutations/mega base) [4] exclusively occurred in tumors with a secondary oncogenic/likely oncogenic POLE mutation or occurred in the setting of CMMRD.
Fig. 3.
Patients with a germline mismatch repair defect. a Tumor mutational burden in patients with Lynch without biallelic inactivation (purple), in patients with Lynch with biallelic inactivation without a secondary POLE mutation (red), in patients with Lynch with biallelic inactivation with a secondary POLE mutation (blue), and in patients with constitutional mismatch repair deficiency (green). b MSI status by the MiMSI classifier in glioma and glioneuronal tumors occurring in the setting of constitutional mismatch repair deficiency (CMMRD), biallelic inactivation of an MMR alteration and a secondary POLE mutation (Biallelic MMR + POLE), biallelic inactivation of an MMR alteration without a POLE mutation (Biallelic MMR), and a heterozygous germline MMR alteration without biallelic inactivation (Monoallelic MMR)
MSI status was determined using MiMSI on the 10 patients with Lynch syndrome or CMMRD in the un-anonymized subset. All tumors that were MSI-high (n = 8) had biallelic inactivation of an MMR gene (Fig. 3b). 2 tumors had biallelic inactivation but were microsatellite stable (MSS); these tumors had the SBS15 signature, which is indicative of defective mismatch repair, on mutational signature analysis [1] and had loss of protein expression of the impacted MMR gene on immunohistochemistry.
Biallelic inactivation was least commonly observed in Lynch syndrome patients with P/LP mutations in PMS2—only 1/6 had tumors with biallelic inactivation. In comparison, 4/7 patients with MSH2, 3/4 patients with MSH6, and 2/2 patients with MLH1 mutations had glioma/GNT with biallelic inactivation. Unlike the hypermutated glioblastoma, IDH WT tumors, which never had chromosome 7/10 alterations, EGFR amplification, or TERT promoter mutations (7 out of 7), 7 of 8 patients with an IDH WT glioma without hypermutation had these typical molecular features of glioblastoma, IDH WT. Among patients with Lynch syndrome, the median age of patients with biallelic inactivation of the germline MMR alteration was 32.4 vs a median age of 54.0 for patients with a monoallelic MMR alteration in their tumor.
Biallelic inactivation was least common in patients with germline variants that are not known to be associated with glioma/GNTs. Biallelic inactivation was found in patients with germline BRCA1/BRCA2 alterations in 21.4%, in patients with a low-penetrance germline APC I1307K germline variant in 13.7%, and in patients with MUTYH alterations in 9.5% (Fig. 2b).
Of the 14 patients with germline BRCA1 or BRCA2 alterations, a HRD score could be calculated for 10. 2/10 have tumors with biallelic inactivation of the germline alteration and the 2 highest HRD scores (31 and 49); both these tumors had BRCA2 alterations and were glioblastoma/astrocytoma, IDH WT. For the 8/10 patients with monoallelic BRCA1/BRCA2 alterations, the median HRD score was 9.5, ranging from 6 to 31 (Supplementary Table 2).
APC (specifically, I1307K) and MUTYH variants are common, accounting for 26.5% of identified variants. It has been suggested that these genes are associated with the development of glioma/GNT [2, 3, 15]. The only APC alteration identified, the APC I1307K variant, is present in ~ 6–10% of individuals of Ashkenazi Jewish ancestry and is less frequent in non-Jewish populations. This variant can contribute to colorectal cancer by causing polymerase slippage, resulting in a frameshift mutation. In tumors with this frameshift, a second somatic mutation causing biallelic inactivation is often identifiable [16, 28]. The 4 glioma/GNT identified to be biallelic for APC I1307K had loss of heterozygosity; the absence of an additional somatic mutation decreases the likelihood that this germline variant contributed to tumorigenesis. Of the 42 patients with MUTYH variants, only 4 had biallelic inactivation—1 glioblastoma, IDH WT and 3 oligodendrogliomas. The rate of biallelic inactivation in glioblastoma, IDH WT with MUTYH alteration is 3.8%; the much higher frequency of biallelic inactivation in oligodendroglioma (42.8%) is attributable to the location of MUTYH on the short arm of chromosome 1 (1p34). The 1p arm with the MUTYH mutation was lost (n = 4) or retained (n = 3) to similar extents, which suggests that biallelic inactivation fails to confer a selective advantage.
Germline alteration and clinical characteristics
Patients with a glioma/GNT and biallelic inactivation of a moderate or high penetrance germline alteration were younger (median age at diagnosis: 31.2, Fig. 2c) than the subset of patients with a germline mutation without loss of heterozygosity (median age at diagnosis: 50, p = 0.00014) and were younger than the subset without a germline alteration (median age at diagnosis: 51.4 years, p = 3.5 × 10–6).
Within the pediatric subset (age < 15 years old), 45% of patients (10/22) with germline alterations developed tumors with biallelic inactivation of an affected gene (7 tumors had biallelic inactivation of NF1, a ganglioglioma had biallelic inactivation of SUFU, and individual gliomas had biallelic inactivation of ATM and BRCA2). Compared to the pediatric subset, biallelic inactivation of the germline variant was less frequently identified in AYAs (32%, Fisher’s exact test: p = 0.20) and adults (16%, Fisher’s exact test, p = 0.0017).
Additional clinical data were available for analysis in the un-anonymized subset. Of the 5 patients with Li-Fraumeni who consented to clinical germline testing, 3 had a known germline TP53 variant, while 2 were diagnosed through this testing (a patient with and another without a history suggestive of a tumor predisposition syndrome). Unlike Li-Fraumeni, NF1 has clinical criteria that are highly sensitive and specific for this genetic syndrome. For this reason, all 6 patients with a germline NF1 alteration in the un-anonymized subset previously received a clinical diagnosis.
Among the 10 patients with an MMR-deficient tumor who underwent germline assessment, 2 patients previously tested positive for Lynch syndrome or CMMRD, 3 patients were evaluated due to a suspicious personal or family history, 1 patient underwent evaluation in the absence of clinical suspicion, and 4 patients were tested after somatic hypermutation was identified in the tumor. Of the 5 patients with Lynch syndrome with an MMR proficient tumor, 3 were screened in the absence of clinical suspicion and had low penetrance PMS2 P/LP mutations—the remaining 2 patients were previously diagnosed with Lynch Syndrome due to a personal history of cancer.
The 5 patients with Lynch syndrome with IDH WT gliomas that were MMR proficient succumbed to their tumor in a time frame that is typical for an IDH WT glioblastoma—median survival of 27 months (range of 14 to 40 months). The prognosis of the 7 patients with a germline MMR defect and a hypermutated glioma was more variable. While the median survival of the IDH WT gliomas with MMR deficiency was 30 months, 3 patients have no evidence of active disease at 52, 72, and 207 months of follow-up. Of these 3 long-term survivors, only 1 patient (the individual with the longest follow-up) has a secondary POLE mutation, which has been associated with an improved prognosis [10]. In total, 3 of 7 patients with an IDH WT hypermutated MMR-deficient tumor had a pathogenic or likely pathogenic POLE mutation and harbored the mutational signature associated with these alterations, SBS10a/b [1]. Apart from the long-term survivor, the other patients with POLE mutations succumbed to their tumor at 20 and 21 months. Among the 5 ultra-hypermutated tumors, 3 developed leptomeningeal disease (the 3 ultra-hypermutated tumors with biallelic inactivation of PMS2). None of the 3 patients with IDH mutant, MMR-deficient, high-grade gliomas have progressed after first-line treatment at 29–67 months.
Discussion
This prospective study provides an unbiased snapshot of the frequency of germline alterations in a patient population receiving care at a Comprehensive Cancer Center for the management of a glioma/GNT. In this study, we conducted germline sequencing using a panel, that reflects 76–90 cancer-predisposition genes identified in the ACMG recommendations [14, 21], and found that 1 in 10 patients with a glioma/GNT has a germline alteration. A smaller proportion (39 out of 2187 or 1.8%) were found to have a germline alteration known to be associated with the development of glioma/GNT.
Biallelic inactivation was enriched in tumors that were diagnosed at a younger age and in patients with tumor predisposition syndromes classically associated with glioma/GNTs. Biallelic inactivation was particularly common in tumors occurring in the setting of a germline NF1 or TP53 mutation (100% and 88%, respectively). Surprisingly, only 53% of patients with a heterozygous germline mutation in a mismatch repair gene (Lynch syndrome) had biallelic inactivation in their tumor.
We show that this low rate of biallelic inactivation found by NGS accurately captures the penetrance by correlating the presence of biallelic inactivation with hypermutation and evidence of microsatellite instability/MMR deficiency by immunohistochemistry. MMR proficient high-grade gliomas were common in patients with heterozygous germline alterations in PMS2, MSH2, and MSH6. In a prior study in colorectal cancer (CRC), only 10% of CRC occurring in the context of Lynch Syndrome were MMR proficient, of which 89% had a heterozygous germline variant in MSH6 or PMS2 [24]. 47% of Lynch syndrome patients in our glioma/GNT cohort had an MMR proficient tumor and the patients with the MMR proficient tumors primarily had germline variants in MSH2 and PMS2. Therefore, any patient with Lynch syndrome and a newly diagnosed glioma/GNT, especially patients with these lower penetrance mutations, would benefit from NGS or MMR immunohistochemistry to confirm that the tumor is Lynch syndrome associated.
Patients in our cohort with MMR-deficient gliomas were universally hypermutated and are distinctive in several ways. The molecular hallmarks of glioblastoma, IDH WT were virtually absent in the hypermutated IDH WT tumors and a subset of these tumors have prolonged survival. Ultra-hypermutation (defined as a tumor mutational burden > 100 mutations/mega base) [4] was exclusively found in the context of secondary POLE mutations and CMMRD. The presence of hypermutation in a tumor that has not been exposed to alkylator chemotherapy, should prompt investigation for Lynch syndrome (or CMMRD), recognizing that other genetic alterations can also cause hypermutation, such as somatic MMR mutations, as well as somatic and germline alterations in POLE [10, 32].
MSI-high status by MiMSI on our targeted next-generation sequencing platform was specific and performed well at identifying MMR deficiency. Prior work demonstrated that pediatric brain tumors with MMR deficiency are rarely MSI-high on a PCR-based panel interrogating 5 microsatellite loci [7], and that low-pass whole-genome sequencing, analyzing more than 23,000,000 microsatellites, may be required to reliably test for microsatellite instability [8]. Here, we show that microsatellite instability can be identified in most patients with MMR-deficient gliomas using a targeted next-generation sequencing panel interrogating the protein-coding exons and select introns of 314 to 505 cancer-associated genes [34].
Biallelic inactivation was less commonly identified in patients with germline variants that are not associated with glioma/GNTs. For instance, BRCA1/BRCA2 alterations were found to be biallelic in 21.4%. While biallelic inactivation of MUTYH and APC I1307K appears to be a consequence of chance alone; intriguingly, tumors with biallelic inactivation of BRCA1/2 have the highest HRD scores, demonstrating the functional consequence of the DNA double-strand break repair alteration. A future direction is the further characterization of tumors with biallelic inactivation of BRCA1/2, and other DNA double-strand break repair alterations, by performing deeper sequencing (whole-exome/whole-genome sequencing) and trialing PARP inhibitors on a larger collection of these rare tumors.
Memorial Sloan Kettering Cancer Center is a quaternary care Comprehensive Cancer Center, in which patients self-refer to specialized care for a previously diagnosed tumor. While the patient population seen at MSK reflects the population of a large northeastern United States metropolitan area, referral patterns may influence the prevalence of cancer predisposition syndromes.
We believe that this work provides unique insights into the patient population seen in neuro-oncology clinics, and that the germline analysis performed in this study substantially advances the observations made in prior work. Nevertheless, limitations remain: (I) our platform may miss some P/LP genetic alterations in the genes tested; and (II) MSK-IMPACT does not include SNPs in genes like MGMT that have been associated with gliomagenesis [25]. For both these reasons, there are potentially germline contributors that were not captured.
In conclusion, germline mutations are found in a high proportion of patients, with glioma/GNT. Germline sequencing can identify glioma predisposition syndromes in all age categories (with an enrichment in the pediatric and the AYA categories), which would not be identified without this testing. When a germline alteration is found in a gene that is known to be associated with the development of a glioma/GNT, the likelihood of biallelic inactivation is higher. Even in this situation, particularly in the case of Lynch syndrome, ancillary testing is required to confirm that the germline alteration contributed to the tumor’s pathogenesis and to assess its therapeutic actionability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Miguel Foronda Alvaro for his editorial assistance.
Author Contributions
Manuscript writing: A.L.L. and S.N. with input, edits, and approval from all other authors. Data analysis and interpretation: S.N., A.L.L., Z.K.S., M.M., Y.K., I.K.M., M.B., C.B., D.M., W.K.C., D.M., A.B., P.A., Y.T.L., with input from all other authors. Experimental design: M.B., N.S., Z.K.S., A.L.L., and S.N. Data acquisition: M.K.R., T.B., M.A.K., S.F.S., K.B.E., K.E.T., R.J.Y., B.I., C.B., N.S.M., K.K.H.Y., V.T., S.O., I.T.G., E.P., L.S., J.S., C.N., A.B., C.G., B.D.S., E.L.D., J.W., A.P., T.J.K., L.M.D., I.K.M., and A.L.L.
Funding
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant [P30 CA008748].
Data availability
Deidentified, patient level data, including tumor type, age category, the presence of a pathogenic or likely pathogenic germline alteration, and the presence or absence of biallelic inactivation is included as supplementary data. Deidentified clinical data for the un-anonymized subset will be shared with qualified investigators at reasonable request.
Declarations
Conflict of Interest
R.J. Young reports equity in Agios, and consulting fees from Guerbet, ICON plc, NordicNeuroLab, Olea Medical, RadMD, Servier, and Turing Medical, unrelated to the present work. N.S. Moss has consulted/received speaking fees from AstraZeneca, Daiichi Sankyo and Varian, and received research funding (to institution) from AZ, Amgen and GT. A. Boire is an inventor on the following patents: 62/258,044, 10/413,522, and 63/052,139. C. Grommes reports honoraria from Scripps Health; consulting or advisory roles from BTG, Kite/Gilead, Ono Pharmaceutical, Roche, Curis; speakers’ bureau from Ono Pharmaceutical; travel, accommodations, and expenses from Ono Pharmaceutical, Bayer, Bristol Myers Squibb, Pharmacyclics, Celgene, unrelated to the present work. B.D. Santomasso has received consulting honoraria from Bristol-Myers Squibb, Johnson & Johnson, Janssen, and Gilead and serves on the scientific advisory board of In8bio, all unrelated to the present work. E.L. Diamond discloses unpaid editorial support from Pfizer Inc, unrelated to the present work. T.J. Kaley reports other support from Servier, unrelated to the present work. I.K. Mellinghoff reports researcher fees from Servier, Erasca, Abbvie, Roche, and Global Coalition for Adaptive Research, speaker fees from Baptist Health Care, grants from Indiana University School of Medicine, and advisory board fees from Tango Therapeutics and Pathos Inc, all unrelated to the present work. M. Berger reports personal fees from AstraZeneca and Paige.AI, research support from Boundless Bio, and intellectual property rights from SOPHiA Genetics. Z.K. Stadler has intellectual property rights in SOPHiA Genetics and serves as an Associate Editor for JCO Precision Oncology and as a Section Editor for UpToDate. Z.K. Stadler's immediate family member serves on the Board of Directors for Adverum Biotechnologies, is Co-Founder, CMO and President for Blue Gen Therapeutics Foundation, and serves as a consultant in Ophthalmology for Apellis, Gyroscope, Nanoscope, Novartis, Outlook Therapeutics, and Regeneron unrelated to the present work. A.L. Lin reports funding from Bristol Myers Squibb, unrelated to the present work. All remaining authors have declared no conflicts of interest.
Ethical approval
Patients in this study were enrolled on a sequencing protocol (NCT01775072) that was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center and conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, as well as federal laws, after providing written informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y et al (2020) The repertoire of mutational signatures in human cancer. Nature 578:94–101. 10.1038/s41586-020-1943-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attard TM, Giglio P, Koppula S, Snyder C, Lynch HT (2007) Brain tumors in individuals with familial adenomatous polyposis: a cancer registry experience and pooled case report analysis. Cancer 109:761–766. 10.1002/cncr.22475 [DOI] [PubMed] [Google Scholar]
- 3.Bedics G, Kotmayer L, Zajta E, Hegyi LL, Bruckner EA, Rajnai H et al (2022) Germline MUTYH mutations and high-grade gliomas: novel evidence for a potential association. Genes Chromosomes Cancer 61:622–628. 10.1002/gcc.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell BB, Light N, Fabrizio D, Zatzman M, Fuligni F, de Borja R et al (2017) Comprehensive analysis of hypermutation in human cancer. Cell 171(1042–1056):e1010. 10.1016/j.cell.2017.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A et al (2015) Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251–264. 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng H, Shan M, Feng C, Wang X (2014) Spinal cord ependymoma associated with neurofibromatosis 1: case report and review of the literature. J Korean Neurosurg Soc 55:43–47. 10.3340/jkns.2014.55.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung J, Maruvka YE, Sudhaman S, Kelly J, Haradhvala NJ, Bianchi V et al (2021) DNA polymerase and mismatch repair exert distinct microsatellite instability signatures in normal and malignant human cells. Cancer Discov 11:1176–1191. 10.1158/2159-8290.CD-20-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung J, Negm L, Bianchi V, Stengs L, Das A, Liu ZA et al (2023) Genomic microsatellite signatures identify germline mismatch repair deficiency and risk of cancer onset. J Clin Oncol 41:766–777. 10.1200/JCO.21.02873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL et al (2015) Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 372:2243–2257. 10.1056/NEJMsr1501341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erson-Omay EZ, Caglayan AO, Schultz N, Weinhold N, Omay SB, Ozduman K et al (2015) Somatic POLE mutations cause an ultramutated giant cell high-grade glioma subtype with better prognosis. Neuro Oncol 17:1356–1364. 10.1093/neuonc/nov027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N et al (2019) Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 20:1011–1022. 10.1016/S1470-2045(19)30277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross AM, Wolters PL, Dombi E, Baldwin A, Whitcomb P, Fisher MJ et al (2020) Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382:1430–1442. 10.1056/NEJMoa1912735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudmundsson S, Singer-Berk M, Watts NA, Phu W, Goodrich JK, Solomonson M et al (2022) Variant interpretation using population databases: lessons from gnomAD. Hum Mutat 43:1012–1030. 10.1002/humu.24309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP et al (2017) Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American college of medical genetics and genomics. Genet Med 19:249–255. 10.1038/gim.2016.190 [DOI] [PubMed] [Google Scholar]
- 15.Kline CN, Joseph NM, Grenert JP, van Ziffle J, Yeh I, Bastian BC et al (2016) Inactivating MUTYH germline mutations in pediatric patients with high-grade midline gliomas. Neuro Oncol 18:752–753. 10.1093/neuonc/now013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laken SJ, Petersen GM, Gruber SB, Oddoux C, Ostrer H, Giardiello FM et al (1997) Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nat Genet 17:79–83. 10.1038/ng0997-79 [DOI] [PubMed] [Google Scholar]
- 17.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S et al (2018) Clinvar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res 46:D1062–D1067. 10.1093/nar/gkx1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelker D, Zhang L, Kemel Y, Stadler ZK, Joseph V, Zehir A et al (2017) Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA 318:825–835. 10.1001/jama.2017.11137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F (2010) Deriving the consequences of genomic variants with the Ensembl API and SNP effect predictor. Bioinformatics 26:2069–2070. 10.1093/bioinformatics/btq330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DT, Lee K, Abul-Husn NS, Amendola LM, Brothers K, Chung WK et al (2023) ACMG SF v3.2 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 25:100866. 10.1016/j.gim.2023.100866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muskens IS, de Smith AJ, Zhang C, Hansen HM, Morimoto L, Metayer C et al (2020) Germline cancer predisposition variants and pediatric glioma: a population-based study in California. Neuro Oncol 22:864–874. 10.1093/neuonc/noaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragupathi A, Singh M, Perez AM, Zhang D (2023) Targeting the BRCA1/2 deficient cancer with PARP inhibitors: clinical outcomes and mechanistic insights. Front Cell Dev Biol 11:1133472. 10.3389/fcell.2023.1133472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranganathan M, Sacca RE, Trottier M, Maio A, Kemel Y, Salo-Mullen E et al (2023) Prevalence and clinical implications of mismatch repair-proficient colorectal cancer in patients with Lynch syndrome. JCO Precis Oncol 7:e2200675. 10.1200/PO.22.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapkins RW, Wang F, Nguyen HN, Cloughesy TF, Lai A, Ha W et al (2015) The MGMT promoter SNP rs16906252 is a risk factor for MGMT methylation in glioblastoma and is predictive of response to temozolomide. Neuro Oncol 17:1589–1598. 10.1093/neuonc/nov064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen R, Seshan VE (2016) FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res 44:e131. 10.1093/nar/gkw520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasan P, Bandlamudi C, Jonsson P, Kemel Y, Chavan SS, Richards AL et al (2021) The context-specific role of germline pathogenicity in tumorigenesis. Nat Genet 53:1577–1585. 10.1038/s41588-021-00949-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stadler ZK, Maio A, Chakravarty D, Kemel Y, Sheehan M, Salo-Mullen E et al (2021) Therapeutic implications of germline testing in patients with advanced cancers. J Clin Oncol 39:2698–2709. 10.1200/JCO.20.03661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC et al (2016) Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 22:3764–3773. 10.1158/1078-0432.CCR-15-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tung N, Domchek SM, Stadler Z, Nathanson KL, Couch F, Garber JE et al (2016) Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol 13:581–588. 10.1038/nrclinonc.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber CAM, Kronke N, Volk V, Auber B, Forster A, Trost D et al (2023) Rare germline variants in POLE and POLD1 encoding the catalytic subunits of DNA polymerases epsilon and delta in glioma families. Acta Neuropathol Commun 11:184. 10.1186/s40478-023-01689-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yakar H, Ertugrul B, Kaplan M (2022) A rare tumor case in an adult patient with neurofibromatosis: lumbar ependymoma. Niger J Clin Pract 25:197–199. 10.4103/njcp.njcp_79_21 [DOI] [PubMed] [Google Scholar]
- 34.Ziegler J, Hechtman JF, Rana S, Ptashkin RN, Jayakumaran G, Middha S et al (2025) A deep multiple instance learning framework improves microsatellite instability detection from tumor next generation sequencing. Nat Commun 16:136. 10.1038/s41467-024-54970-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified, patient level data, including tumor type, age category, the presence of a pathogenic or likely pathogenic germline alteration, and the presence or absence of biallelic inactivation is included as supplementary data. Deidentified clinical data for the un-anonymized subset will be shared with qualified investigators at reasonable request.