Abstract
Induction of labour and not “expectant management” is often recommended to prevent adverse perinatal outcomes in late-term pregnancies, but no prognostic prediction model exists for an individualized decision-making. The present study used a data-driven approach to predict mode of delivery at or beyond 41 gestational weeks considering the obstetric management. Low-risk nulliparous women were derived from the nationwide Swedish Medical Birth Register (1998–2019). A two-day-wise prediction in four study groups with increasing gestational age (e.g. group 1: induced at 41+0-41+1, expectant management beyond 41+1 gestational weeks) was conducted. Forty-three features available at the time for decision-making on labour induction in each subgroup were used in the models, including the decision on labour induction itself. The subgroups contained 178,932, 129,449, 90,448 and 61,301 pregnancies, respectively, with imbalanced outcome rates (cesarean delivery < 27%, spontaneous birth > 55%, vaginal operative delivery < 18%). Five different classifiers were compared (random forest, mixed naïve bayes, support vector machine, neural network, logistic regression) with the highest value of the area under the curve being 69% in a hold-out sample. Although the considered features lacked predictive power, the study provides valuable methodological information for predicting the timing of labour induction beyond 41 gestational weeks.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-19198-x.
Keywords: Obstetrics, Machine learning, Register data, Late-term pregnancies, Induction of labour, Expectant management
Subject terms: Health care, Medical research
Introduction
Induction of labour (IOL) is worldwide a common medical intervention to prevent adverse maternal and infant outcomes, especially after the estimated delivery date (≥ 40+0 gestational weeks (GW)). For example, the rates of one of the most devastating outcomes for a family, stillbirth, increase significantly beyond 40 GW1. The World Health Organization (WHO) recommends induction of labour for women who are known with certainty to have reached 41 GW2. A later onset of labour, “expectant management” (EM), should not be awaited. Recent systematic reviews with meta-analysis of randomised controlled trials (RCTs) have shown reduced risks of adverse fetal and neonatal outcomes (e.g. stillbirth, perinatal death) without an increase in maternal complications (e.g. cesarean section, vaginal operative delivery, postpartum hemorrhage) when IOL was performed at or beyond 37 GW compared to the respective EM3,4. Conducting IOL and not EM beyond 37 GW even seemed to be beneficial regarding cesarean section (RR 0.90 [95%CI: 0.85, 0.95])3. An individual participant data meta-analysis of RCTs found evidence that nulliparous but not multiparous women have a lower risk for an adverse composite perinatal outcome (perinatal mortality and severe perinatal morbidity) and for perinatal mortality (stillbirth and neonatal death) when induced at 41 weeks compared to EM until 42 weeks5. However, regarding other subgroups or outcomes, no sub-analysis could clearly identify the woman and child pairs who benefit the most from this intervention or narrow the time window at which IOL is indicated after 37 + 0 GW3,5. In contrast, the results of some observational studies, including propensity score matching, showed partly opposite results for selected perinatal outcomes6–8. A prospective cohort study alongside the INDEX RCT9, suggested a non-significant absolute risk reduction for adverse and severe adverse perinatal composite outcomes (including perinatal mortality and morbidity) and a higher risk for cesarean section in nulliparous women when IOL was performed at 41 + 0 compared to EM6. The women in the study were eligible for the trial but did not consent on randomisation and were treated according to their preferences. Studies with a propensity score matching design also indicated higher rates for cesarean section in this timeframe, even in multiparous women7,8.
Prognostic prediction models based on data-driven approaches could support the identification of pregnancies at risk for adverse outcomes and in the future provide more individualized advice for parents-to-be and medical staff. In recent years, several prediction models on adverse pregnancy- or birth-related outcomes were developed, of which some are already available for clinical use (e.g10–16. However, no model on outcomes in prolonged pregnancies exists. The present study therefore aims with its exploratory design to develop and internally validate a model which predicts mode of delivery (i.e. cesarean delivery (CD), vaginal operative delivery (VE) and spontaneous birth (SB)) considering induction of labour at different time thresholds of clinical relevance at or beyond 41+0 GW. For the report of the results we followed the TRIPOD + AI Expanded Checklist instructions for all sections of this publication17,18.
Materials and methods
Data
Data were derived from the Medical Birth Register (MBR) which covers around 98% of all births in Sweden and was established in 197319. Data from ante-, intra- and perinatal care are collected prospectively by the National Board of Health and Welfare (NBHW)19. Through the unique personal identification number (PIN)20 data in the MBR is linked with the Cause of Death Register, the Patient Register for inpatient and outpatient specialized care (main and secondary diagnoses are coded according to the Swedish version of the ICD-system 10th revision since 199819 and the Prescribed Drug Register (established 1952, 1964 and July 2005, respectively)19–23 at the NBHW.
The years included in the respective linked registers are summarized in Table S6 in the supplementary material. Table S7 in the supplement displays the register origin of each variable (outcomes and predictors) considered in the analysis19. Five used variables (mode of delivery, gestational length at birth, preeclampsia, preexisting chronic hypertension and preterm prelabour rupture of the membranes) have already been defined in previous studies24–26 based on existing variables in the MBR (Table S1, S8).”
Participants
The initial study population consisted of nulliparous women across all registered age groups with a low-risk pregnancy who gave birth in Sweden at or beyond 41 + 0 GW between 1998 and 2019. Low-risk was defined as having a singleton fetus in cephalic presentation, no gestational or preexisting diabetes (Type 1 or 2) and an antenatal hospital admission not longer than five days before birth (Fig. 1a, Fig. S1 supplementary material). Pregnancies with known risk-factors are generally induced earlier than 41 + 0 GW.
Fig. 1.

Design of the study population and the study groups 1-4. (a) Inclusion criteria applied to all registered pregnancies between 1992-2019. (b) Design of the study groups based on the study population. 1Induction of labour registered either through checkbox o corresponding ICD-10 code. The gestational age corresponds to the gestational age at the day of admission to the hospital. 2Later onsets of labour than the induced women in the respective group, including later IOL, planned cesarean and spontaneous birth. (c) Construction of study group 1 and the corresponding binary variable “induction of labour”. (d) 1.−4.: The most common diagnosis leading to an induction of labour (“confounding by indication”). Pregnancies with these diagnoses were excluded in the respective induction of labour group, while not in the respective expectant management group. Other onsets of labour (5 and 6) at the same gestational age as the induced women were excluded in respective study group. IOL: induction of labour. EM: expectant management. GW: gestational weeks. HELLP-Syndrom: acronym for hemolysis, elevated liver, low platelet counts. PROM: premature rupture of the membranes.
We modified the inclusion criteria in the initial study population to reflect clinical decision-making and routines as well as for comparability to previous research from RCTs and observational studies7–9,27,28. Obstetricians and women usually decide during a consultation based on the medical findings if the pregnancy should be induced or not (referred to as day of decision-making). Study group 1 included women who were induced at 41+0- 41+1 (referred to as IOL) and women who delivered beyond 41+1 irrespective of onset of labour, i.e. elective cesarean delivery, IOL or spontaneous onset (referred to as EM). Women who delivered spontaneously, including premature rupture of the membranes (PROM) and subsequent induction, or by elective cesarean section at 41+0- 41+1 were excluded for Study group 1. In total, four different study groups (SG) were constructed, depending on gestational length and timing of IOL; SG1: IOL 41+0- 41+1 and EM > 41+1, SG2: IOL 41+2−41+3 and EM > 41+3, SG3: IOL 41+4−41+5 and EM > 41+5; SG4: IOL 41+6−42+0 and EM > 42+0 (Fig. 1b and c, Fig. S6 supplementary material).
The timing of IOL was calculated by using the gestational age at the day of admission to the hospital as a proxy (see section “Data preparation”) since the MBR does not provide the exact day of induction. Under clinical assumptions it is very unlikely that a woman at such a late stage of pregnancy (≥ 41 GW) is admitted to hospital without any intervention or giving birth (Fig. 1c, Fig. S6 supplementary material).
Women in each IOL group who had one of the following diagnoses registered were excluded to ensure that these women were only induced because of gestational age, not because of an underlying pathology: Hypertensive pregnancy disorders (Preeclampsia, eclampsia, HELLP-Syndrome), IUFD (intrauterine fetal death) or any antepartum bleeding (without a diagnosis of placenta previa) (Fig. 1d). Women with these diagnoses were not excluded in the corresponding EM group.
Data preparation
The majority of variables are directly transferred to the NBHW from the standardized clinical records, where the information is generally collected through pre-specified checkboxes or through assigned diagnostic or procedure codes19. At the NBHW, the records are merged, quality checked, and annually released for the use as the MBR19.
The data-preprocessing steps conducted by the authors of the present study were to create the study population (see “Participants”) and the predictors. Variables for “gestational age at day of admission to the hospital”, “length of antenatal stay in hospital” (directly before birth), “onset of labour” and decision on IOL or EM (“induction of labour”) were created.
“Gestational age at the day of admission to the hospital” was calculated based on the existing variables “gestational age at delivery”, “birth date of the infant” and the “day of admission to the hospital”. The difference between the “birth date of the infant” and the “day of admission to the hospital” is the “length of antenatal stay in hospital” in days. In a second step the values for “length of antenatal stay in hospital” were subtracted from “gestational age at delivery” which resulted in the “gestational age at admission to the hospital”.
Onset of labour (elective cesarean, induction of labour or spontaneous onset) was classified hierarchically based on a checkbox for labour onset and a corresponding registered ICD-10 diagnosis (Table S1 Supplementary material). A premature rupture of the membranes with a subsequent induction of labour was classified as a spontaneous onset of labour (Table S1 supplementary material).
The binary variable “induction of labour” in the respective study group (SG1-SG4) was based on “gestational age at day of admission to the hospital” and “onset of labour”. Women classified as IOL in the respective group (Fig. 1b and c, Fig. S6 supplementary material) were the positive class and women who were classified as EM were the negative class.
Dimension reduction was done for smoking and snuff use. The three existing self-reported variables on smoking before, in early and late pregnancy with four categories (unknown, no smoking, 1–9 cigarettes/day, ≥ 10 cigarettes/day) were combined into two variables (smoking before and during pregnancy) with three categories (yes/no/unknown) (Table 1). Those, who smoked at any time during pregnancy were categorized as smoking during pregnancy.
Table 1.
Descriptive analysis of the outcomes and included features in the four different study groups* (SG1-SG4).
| Study Group 1 | Study Group 2 | Study Group 3 | Study Group 4 | |||||
|---|---|---|---|---|---|---|---|---|
| n = 197,567 | n = 142,811 | n = 99,714 | n = 67,699 | |||||
| Outcomes | n | % | n | % | n | % | n | % |
| Cesarean section | 39,239 | 19.9 | 31,196 | 21.8 | 24,111 | 24.2 | 18,009 | 26.6 |
| Vaginal operative delivery | 33,457 | 16.9 | 24,601 | 17.2 | 17,377 | 17.4 | 11,876 | 17.5 |
| Spontaneous vaginal birth | 124,876 | 63.2 | 87,014 | 60.9 | 58,226 | 58.4 | 37,814 | 55.9 |
| Categorical features | ||||||||
| Asthma | 14,835 | 7.5 | 10,770 | 7.5 | 7,584 | 7.6 | 5,104 | 7.5 |
| Antepartum Bleeding | 842 | 0.4 | 595 | 0.4 | 393 | 0.4 | 206 | 0.3 |
| Epilepsy | 977 | 0.5 | 704 | 0.5 | 486 | 0.5 | 313 | 0.5 |
| Antepartum PROM | 168 | < 0.1 | 117 | < 0.1 | 81 | < 0.1 | 58 | < 0.1 |
| IOL at the specific threshold of the respective study group | 6457 | 3.3 | 5,310 | 3.7 | 4,679 | 4.7 | 9,918 | 14.7 |
| Chronic hypertension | 830 | 0.4 | 569 | 0.4 | 399 | 0.4 | 283 | 0.4 |
| Nephrological disease | 768 | 0.4 | 551 | 0.4 | 403 | 0.4 | 269 | 0.4 |
| Assisted reproduction1 | 10,685 | 5.4 | 7,436 | 5.2 | 5,157 | 5.2 | 3,436 | 5.0 |
| Systemic lupus erythematosus | 156 | < 0.1 | 102 | < 0.1 | 64 | < 0.1 | 36 | < 0.1 |
| Inflammatory bowel disease | 1,216 | 0.6 | 841 | 0.6 | 597 | 0.6 | 387 | 0.6 |
| Urinary tract infection | 25,880 | 13.1 | 18,795 | 13.2 | 13,037 | 13.1 | 8,842 | 13.1 |
|
Smoking before pregnancy • unknown |
35,642 17,682 |
18.0 8.9 |
25,840 12,702 |
18.1 8.9 |
18,197 8,745 |
18.2 8.8 |
12,571 5,912 |
18.6 8.7 |
|
Smoking during pregnancy • unknown |
13,204 18,763 |
6.7 9.5 |
9,658 13,446 |
6.8 9.4 |
6,812 9,304 |
6.8 9.3 |
4,762 6,335 |
7.0 9.4 |
|
Snuff use before pregnancy • unknown |
8,190 16,631 |
4.1 8.4 |
5,940 11,931 |
4.2 8.4 |
4,146 8,216 |
4.2 8.2 |
2,810 5,577 |
4.2 8.2 |
|
Snuff use during pregnancy • unknown |
2,417 20,046 |
1.2 10.1 |
1,756 14,382 |
1.2 10.1 |
1,217 9,923 |
1.2 10.0 |
821 6,678 |
1.2 9.9 |
| Mother’s country of birth | ||||||||
| • Sweden | 159,379 | 80.7 | 115,372 | 80.8 | 80,422 | 80.7 | 54,391 | 80.3 |
| • Other Nordic | 2,899 | 1.5 | 2,109 | 1.5 | 1,488 | 1.5 | 1,007 | 1.5 |
| • Europe | 11,718 | 5.9 | 8,339 | 5.8 | 5,796 | 5.8 | 3,963 | 5.9 |
| • Other | 23,306 | 11.8 | 16,803 | 11.8 | 11,869 | 11.9 | 8,246 | 12.2 |
| • Missing | 265 | 0.1 | 188 | 0.1 | 139 | 0.1 | 92 | 0.1 |
| Father’s citizenship | ||||||||
| • Sweden | 165,790 | 83.9 | 120,059 | 84.1 | 83,859 | 84.1 | 56,837 | 84.0 |
| • Other Nordic | 2,538 | 1.3 | 1,837 | 1.3 | 1,296 | 1.3 | 876 | 1.3 |
| • Europe | 7,052 | 3.6 | 5,055 | 3.5 | 3,469 | 3.5 | 2,355 | 3.5 |
| • Other | 13,709 | 6.9 | 9,813 | 6.9 | 6,859 | 6.8 | 4,772 | 7.0 |
| • Missing | 8,478 | 4.3 | 6,047 | 4.2 | 4,231 | 4.2 | 2,859 | 4.2 |
| Family situation | ||||||||
| • living with the infant’s father | 173,720 | 87,9 | 125,698 | 88.0 | 87,867 | 88.1 | 59,725 | 88.2 |
| • Single | 15,111 | 7,6 | 11,024 | 7.7 | 7,756 | 7.8 | 5,276 | 7.8 |
| • Other | 8,736 | 4,4 | 6,089 | 4.3 | 4,091 | 4.1 | 2,698 | 4.0 |
| Numerical features | mean (std) | mean (std) | mean (std) | mean (std) | ||||
|
Antenatal visits • missing |
11.3 (3.2) n = 6,760 (3.4%) |
11.4 (3.2) n = 4,714 (3.3%) |
11.5 (3.2) n = 3,156 (3.2%) |
11.6 (3.3) n = 2,114 (3.1%) |
||||
|
Maternal age at delivery (years) • missing |
28.8 (13–52)4 n = 2 (0.0%) |
28.8 (13–52)4 n = 2 |
28.8 (13–52)4 n = 2 |
28.9 (13–52)4 n = 2 |
||||
|
Mothers height (cm) • missing |
167.0 (6.4) n = 9,167 (4.6%) |
167.1 (6.4) n = 6,490 (4.5%) |
167.0 (6.4) n = 4,394 (4.4%) |
167.0 (6.5) n = 2,961 (4.4%) |
||||
|
Mother’s weight (first antenatal visit) (kg) • missing |
68.8 (13.5) n = 14,825 (7.5%) |
69.0 (13.6) n = 10,575 (7.4%) |
69.3 (13.7) n = 7,296 (7.3%) |
69.5 (13.9) n = 5,001 (7.4%) |
||||
|
Early pregnancy BMI (kg/m2) 2 • missing |
24.6 (4.5) n = 15,452 (7.8%) |
24.7 (4.6) 11.044 (7.7%) |
24.8 (4.6) n = 7,632 (7.7%) |
24.9 (4.6) n = 5.221 (7.7%) |
||||
| Number of previous spontaneous miscarriages3 |
1.3 (0.6) (n = 29,254) |
1.3 (0.6) (n = 21.002) |
1.3 (0.6) n = 14,771 |
1.3 (0.6) n = 10,113 |
||||
| Involuntary childlessness (years)3 |
2.6 (2.0) (n = 23,527) |
2.6 (2.0) (n = 16,773) |
2.6 (2.0) n = 11,773 |
2.6 (2.0) n = 7969 |
||||
1any method (specify), 2imputed with mother’s height, 3self-reported, 4 mean (minimum-maximum).
* SG1: induction of labour (IOL) at 41 + 0–41 + 1 and expectant management (EM) > 41 + 1, SG2: IOL at 41 + 2–41 + 3 and EM > 41 + 3, SG3: IOL 41 + 4–41 + 5 and EM > 41 + 5; SG4: IOL 41 + 6–42 + 0 and EM > 42 + 0.
Outcomes
The main outcomes of the study were mode of delivery categorized into cesarean section, vaginal operative delivery (forceps or ventous) and spontaneous vaginal birth. Each outcome was predicted separately (no composite outcome).
Predictors
To ensure a predictive design with a potential for prospective use, only variables which are known at the day of decision-making regarding IOL in each study group (Fig. 1c) were used as features (Table 1). These features included diagnoses of some diseases which did not meet the exclusion criteria of the study (asthma, antepartum bleeding, epilepsy, chronic hypertension, nephrological disease, systemic lupus erythematosus, inflammatory bowel disease, urinary tract infection), as well as pregnancy-related variables (decision on IOL or EM, antepartum PROM, assisted reproduction, number of antenatal visits in antenatal care, self-reported number of previous spontaneous miscarriages, number of years of involuntary childlessness, smoking/snuff use before or during pregnancy, and mother’s height, weight and BMI at first antenatal visit) and sociodemographic predictors (mother’s country of birth, father’s citizenship, family situation). All non-binary categorical variables were one-hot encoded resulting in n = 43 features used in the prediction models. Diagnoses and clinical characteristics which occurred later (i.e. during expectant management) or during labour were not considered.
Missing data
Missing values for the included variables range over time but rarely exceeds 5–10% with a further decreasing trend since the start of the digitalized report to the NBHW in 200719,29.
In the present study population missing values did not exceed 5% beside body mass index (BMI) and smoking/snuff use (Table 1, Table S2 supplementary material). Missing values were treated according to the mechanism why they were missing (e.g. missing completely at random, missing at random, missing not at random). We classified BMI as missing not at random. It cannot be ruled out that mother’s pre-pregnancy BMI is calculated only when overweight or obesity is obvious30, as well as technical issues29. The rate of missing values of maternal pre-pregnancy height are below 5% (Table 1) and the validity is considered high19. However, the rate of missing values for BMI before imputation in the four study groups ranged between 8.3 and 8.5% (data not shown). BMI was imputed by using mother’s height from a next pregnancy registered in the MBR during the included time-period, which decreased the rate of missingness to 7.7–7.8% (Table 1.)
We also considered the variables smoking and snuff use to be missing not at random. Although the validity of the information on smoking is high, there is evidence on relevant underreporting of active smoking in early and late pregnancy by self-reported quitters31. Assuming that women start smoking (using snuff) during pregnancy very rarely29, missing values were replaced based on the values registered at other time points, i.e. smoking before, in early or late pregnancy (Table S2 supplementary material). The remaining missing values (e.g. values for smoking before pregnancy were not registered before 1999) were summarized in the category “unknown” (Table 1).
Introducing a category “unknown” for categorical variables reflects the nature of register data as well as the clinical setting and is in line with the literature11. This was also applied for the variables for mother’s country of birth and father’s citizenship (Table 1).
An exception in the registers are the diagnoses which are registered by checkboxes. In these cases, there is only a registered value, if the respective diagnosis is present. No missing values can be calculated. The usual procedure for variables based on checkboxes is to replace missing values with zero (no event occurred).
Another exception are the variables “number of previous spontaneous abortions” and “years of involuntary childlessness”, which are self-reported. Zero values (no event occurred) are not registered. For descriptive analysis the missing values were not considered and the mean was only calculated for those women who reported at least one event (Table 1). For the machine learning analyses, the missing values were replaced by zero, meaning that no such event occurred. Similar to the checkboxes, no amount of missingness could be calculated.
For the creation of the study population, observations with missing values in some of the variables had to be excluded. However, missing values for the respective variables did not exceed 1,6% (Fig. S1 supplementary material).
Analytical methods
A complete case analysis was conducted in all models. Observations with at least one missing value in the outcome or predictor variables were excluded (Table 1, Fig. S1-S5 supplementary material). Data preprocessing and handling of missing values were the same for all analyses in each study group.
In each study group, five different classifiers (random forest, support vector machine, neural network, mixed naïve bayes and logistic regression) were compared while predicting every chosen outcome in a binary classification. According to the requirements of the respective classifier, continuous variables were standardized with standard scaler (zero mean and unit variance) for logistic regression, support vector machine and neural network.
Additionally, a multiclass classification analysis was conducted using the best-performing classifier from the separate binary outcome evaluations, in order to account for potential inconsistencies or overlaps in the one-vs-rest approach and to assess performance for the mutually exclusive modes of delivery. This approach ensures a single, unambiguous prediction per observation, which better reflects the clinical decision-making task.
The four study groups were randomly split into a training (70%), a validation (10%), and a test (20%) set with stratification for the outcome rate in each analysis (Table S3 supplementary material). Because of the exploratory approach, initially non-tuned analyses with the default values of each classifier were run and presented to have a benchmark comparison.
All steps of the analyses including data-preparation were processed with Python Version 3.10. The code was written using the scikit-learn machine learning library for the support vector machine (linear kernel), random forest (n_estimators = 100, max_depth = None, min_split = 2 as default values in the library) and logistic regression model (penalty = None). TensorFlow open source machine learning framework was applied to build the neural network. The model was constructed with two hidden layers (64 and 32 neurons respectively) using ReLU activation function and one output layer with a single neuron and a sigmoid activation function for binary classifications. After configuration (optimizer = ‘adam’, loss = ‘binary_crossentropy’) the model was trained for 10 epochs. For the multiclass prediction we used the cross entropy softmax activation function (sparse_categorical_crossentropy) in the output layer, appropriate for mutually exclusive classification tasks. All other characteristics of the neural network architecture and training procedure remained the same as in the binary classification setting. We used the mixed-naïve-bayes package for categorical and Gaussian Naïve Bayes32.
Performance metrics
The performance of the models was evaluated by plotting receiver operating characteristic curves (ROC) and precision recall curves (PR) for each outcome in each group. To quantify the performance, the corresponding areas under the curves (auROC, auPR) were calculated with the roc_auc_score function and average_precision_score from scikit-learn. The respective 95% confidence interval (CI) was assessed by applying the RepeatKFold function with 5 splits and 20 repeats (scikit-learn library).
While auROC is measuring how well the respective algorithm is able to distinguish between the positive and the negative class across different thresholds, auPR provides additional information on performance in case of imbalanced data sets. The auPR is a metric for evaluating the prediction of the positive class only.
Calibration curves were additionally plotted to analyze the reliability of the predicted probabilities for each outcome. The curves were plotted by using CalibrationDisplay.from_predictions (scikit-learn library). The package bins the predicted probabilities into n numbers of bins and calculates both the mean predicted probability and the fraction of true positives in each bin. We chose n = 10 bins to depict every 10% of predicted probabilities. To provide more information of the distribution of the predicted probabilities, histograms were plotted which map how often the respective probabilities were predicted throughout the range (0% − 100%).
Sensitivity, specificity, positive predictive value (PPV) and balanced accuracy were calculated at a 50% threshold (default value).
For consistency, the additional multiclass prediction model was evaluated by calculating the same performance metrices as described for the binary outcomes (sensitivity, specificity, PPV, balanced accuracy, auROC, auPR and calibration curves including histograms for predicted probability).
For the support vector machine classifier, performance metrics on a 50% threshold and not auROC or auPR were evaluated as this classifier does not directly provide probability estimates. The values summarized in the classification report were very low (Table S4 supplementary material).
Results
Study population and study groups
The initial study population comprised 260,718 healthy nulliparous women with a singleton pregnancy in cephalic presentation, a date of admission to the hospital at or beyond 41 + 0 GW and an antenatal stay ≤ 5 days (Fig. S1 supplementary material). The four study groups derived from the population included 197,567 (SG1), 142,811 (SG2), 99,714 (SG3) and 67,699 (SG4) pregnancies, respectively (Fig. S1-S5 supplementary material). The outcome rates were imbalanced and differed between the groups with a trend towards an increase in cesarean Sect. (19.9% (SG1) – 26.6% (SG4)) and vaginal operative delivery (16.9% (SG1) – 17.5% (SG4)), and towards a decrease for spontaneous vaginal birth (63.2% (SG1) – 55.9% (SG4)) (Table 1). The distribution in the selected features were comparable in all study groups (Table 1).
After removing observations with at least one missing value the study groups consisted of 178,932 (SG1), 129,449 (SG2), 90,448 (SG3) and 61,301 complete cases (Fig. 1b).
Model performance
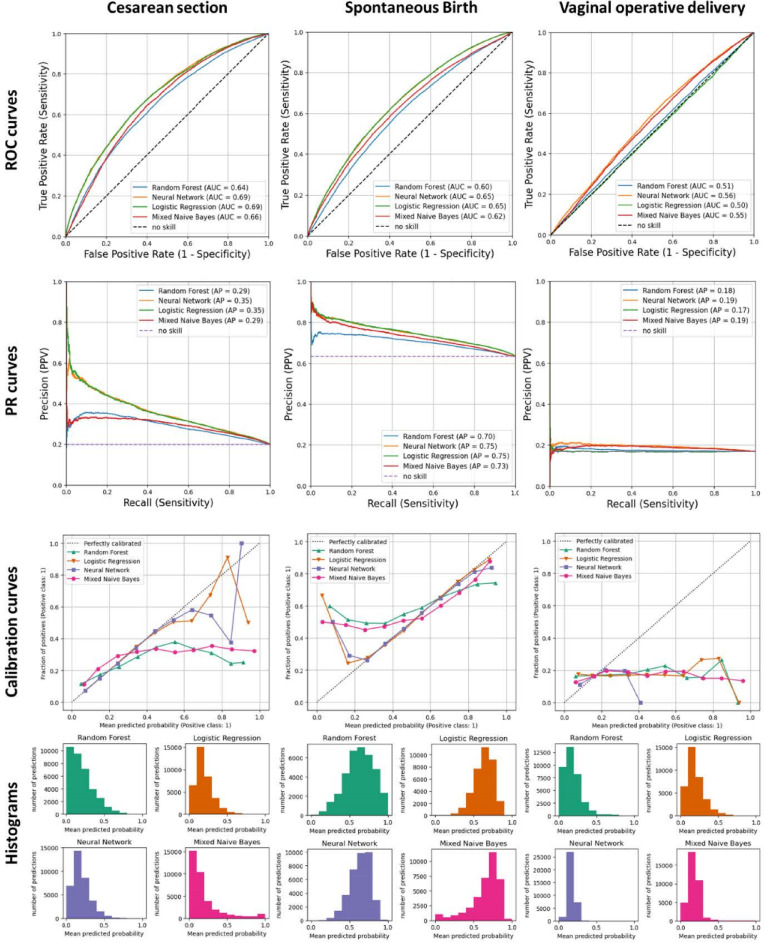
In SG1, logistic regression and neural network had the highest auROC with 69% in predicting cesarean section and 65% in predicting spontaneous vaginal birth. The auROC for vaginal operative delivery reached a maximum of 56% with the neural network, while logistic regression predicted not better than chance (Table 2; Fig. 2).
Table 2.
Performance metrics of the different models in study group 1 for all outcomes.
| CD | SB | VE** | ||
|---|---|---|---|---|
| LR | Sensitivity | 6% | 90% | 0% |
| Specificity | 99% | 24% | 100% | |
| Precision | 53% | 67% | 0% | |
| Balanced accuracy | 52% | 57% | 50% | |
| auROC (95%CI) | 68.70% (68.69–68.70) | 65.15% (65.14–65.15) | 50% | |
| auPR (95%CI) | 35.38% (35.37–35.38) | 74.72% (74.71–74.72) | 17% | |
| MNB | Sensitivity | 15% | 87% | 1% |
| Specificity | 92% | 23% | 99% | |
| Precision | 33% | 66% | 17% | |
| Balanced accuracy | 54% | 55% | 50% | |
| auROC (95%CI) | 65.84% (65.83–65.85) | 62.38% (62.36–62.40) | 55% | |
| auPR (95%CI) | 29.17% (29.16–29.18) | 73.02% (73.00-73.04) | 19% | |
| NN | Sensitivity | 5% | 91% | 0% |
| Specificity | 99% | 22% | 100% | |
| Precision | 53% | 67% | 0% | |
| Balanced accuracy | 52% | 57% | 50% | |
| auROC (95%CI) | 68.77% (68.75–68.79) | 64.84% (64.82–64.86) | 56% | |
| auPR (95%CI) | 35.25% (35.21–35.28) | 74.38% (74.36–74.40) | 19% | |
| RF | Sensitivity | 10% | 80% | 2% |
| Specificity | 96% | 33% | 98% | |
| Precision | 36% | 67% | 20% | |
| Balanced accuracy | 53% | 56% | 50% | |
| auROC (95%CI) | 64.32% (64.29–64.35) | 60.16% (60.13–60.18) | 51% | |
| auPR (95%CI) | 29.02% (28.99–29.04) | 70.40% (70.37–70.43) | 18% | |
| SVM* | Sensitivity | 1% | 100% | 0% |
| Specificity | 100% | 1% | 100% | |
| Precision | 56% | 63% | 0% | |
| Balanced accuracy | 51% | 50% | 50% | |
SG1: induction of labour (IOL) at 41 + 0–41 + 1 and expectant management (EM) > 41 + 1. CD: Cesarean delivery, SB: spontaneous birth, VE: Vaginal operative delivery. Sensitivity, specificity and precision are calculated on a 50% threshold. LR: logistic regression, MNB: mixed naïve bayes, NN: neural network, RF: random forest, SVM: support vector machine. auROC: area under the receiver operating characteristic curve, auPR: area under the precision-recall curve. 95%CI: 95% confidence interval. * no ROC curves or PR curves were calculated for SVM. ** no confidence intervals were calculated for auROC and auPR in VE.
Fig. 2.
Performance metrics (ROC Curves, PR Curves, Calibration Curves) of the models in study group 1 (SG1). ROC curve: receiver operating characteristic curve; PR curve: Precision-Recall Curve; AUC: area under the curve.
The auPR differed between the outcomes with reaching 75% for spontaneous vaginal birth and 36% in cesarean section (Table 2; Fig. 2). Logistic regression and neural network models again performed better than mixed naïve bayes and random forest models. The auPR for vaginal operative delivery was below 20% (Table 2; Fig. 2).
The neural network and the logistic regression model were almost perfectly calibrated for the prediction of spontaneous vaginal birth and well calibrated for the prediction of cesarean section below 60% of predicted probabilities (Fig. 2, Calibration curves). Random forest and mixed naïve bayes underestimated the fraction of positives in lower ranges of predicted probabilities (< 60%) in spontaneous vaginal birth, but overestimated it in the higher ranges of predicted probabilities (> 60%) (Fig. 2, Calibration Curve). The models for vaginal operative delivery underestimated the fraction of positives throughout the range of predicted probabilities (Fig. 2).
A perfect classifier would predict probabilities near 0% (e.g. no cesarean section) and near 100% (e.g. cesarean section is very likely)33. None of the models predicted the outcomes with high certainty, with probabilities either below 50% (cesarean section, vaginal operative delivery), or distributed around 50% (spontaneous vaginal birth) (Fig. 2).
At a 50% threshold, the mixed naïve bayes (study group 1: 15%) and random forest classifier (SG1: 10%) showed the highest sensitivity in predicting cesarean section with an increasing trend along the study groups (Table 2, Table S4 supplementary material). Yet, sensitivity stayed below 20%. In contrast, for spontaneous vaginal birth sensitivity reached 91% using a neural network and 90% with logistic regression with a decreasing trend along the study groups (Table 2, Table S4 supplementary material). However, specificity was under 34% and the PPV only reached a maximum of 67%.
Balanced accuracy ranged between 52% and 57% for spontaneous vaginal birth and cesarean section with all classifiers (Table 2, Table S4 supplementary material). Vaginal operative delivery could not be predicted with any of the algorithms (balanced accuracy = 50%). The support vector machine classifier model also failed to predict any of the outcomes.
The performance metrics did not differ relevantly in the study groups (Table S4, Fig. S7-S9 supplementary material).
The neural network, as being one of the best performing algorithms in the binary outcome prediction, was chosen to predict the multiclass outcome. The overall performance regarding auROC and auPR was comparable to the results obtained from the separate binary classification tasks across all study groups (Fig. S10-S13). While sensitivity, specificity, and positive predictive value differed slightly from those based on the fixed 50% threshold used in the binary classification (Table S5), this is expected in multiclass classification, where a direct threshold is not applied. Instead, the predicted class per observation is determined by selecting the class with the highest predicted probability.
Discussion
To our knowledge, this is the first study predicting mode of delivery in a population of prolonged pregnancies using machine learning methods. Routinely collected prospective data from the population-based Swedish MBR were processed to create different study groups for increasing gestational ages. This reflects the clinical situation when a decision should be made on induction of labour or expectant management in healthy prolonged pregnancies (≥ 41 GW). In this population, different models predicted spontaneous vaginal birth, cesarean section and vaginal operative delivery, respectively. The rates for these outcomes were imbalanced. Variables registered until the day of decision-making were used as predictive features. Even the decision itself (IOL or EM) could be considered in the models by including the corresponding binary variable. However, the predictive power of the features derived was low and the chosen maternal outcomes could not be predicted with high certainty.
A major strength of the study is the use of a large, population-based cohort with a nationwide coverage and a prospective data collection22. In line with clinical management and current research from RCTs9,27,28 and observational studies (e.g7,8.) both women who were induced at a certain threshold (IOL) and women who delivered after the respective threshold (EM) were included in the respective study groups.
However, some misclassification error may have occurred because the actual date of the initiation of IOL is not registered in the MBR and the day of admission to the hospital as the best possible proxy was used instead. Nevertheless, women who were admitted to the hospital and induced more than one day later than the admission could be misclassified in the respective IOL group. To account for this potential misclassification, women with an antenatal stay beyond five days were excluded. This was underpinned by the fact, that 98% of the women in our study population delivered between day 0 and day 5 after the day of admission (data not shown).
Further misclassification could have been possible in the IOL group because the indication for induction is not registered in the MBR. To simulate an RCT and reflect clinical management, women who were grouped into IOL should have been induced due to the gestational age, and not because of other medical reasons. This is why women with e.g. a registered diagnosis of preeclampsia in the IOL group were excluded although this could also be a case of a postpartum preeclampsia and not the reason for the induction.
The lack of indication for induction could have led to a bias towards an IOL group with more pathologies than the EM group. During the study period Swedish Guidelines recommended EM until 42 GW in the absence of any risk factors34. Nevertheless, rates for induction of labour in week 41 increased from 7,7% in 1999 to 43,6% in 2022 with a doubling of the rate after the Swedish RCT on labour induction at 41 weeks (SWEPIS trial28 in 202035. It cannot be completely ruled out that some of the pregnancies classified as IOL at least in study group 1 and 2 before 2020 were induced because of an underlying complication (“confounding by indication”). However, as the rate of IOL was < 5% in study group 1–3 (Table 1) it can be assumed that these cases happened not very frequently.
One of the major technical issues in prediction studies on medical outcomes are the low outcome rates which cause imbalanced data sets. Class imbalance occurs if the number of observations with the outcome is unequal to the number of observations without the outcome36. In this case, the algorithms tend to be biased towards the majority class.
The imbalanced data sets explain in part the pattern of the performance metrics in the present study, including calibration curves and the distribution of the predicted probabilities.
The values for the auROCs reached a maximum of 70%. Hence, the algorithms could not distinguish well between the positive and the negative class. This applies to all models and study groups (Table 2, Table S4 supplementary material).
Precision-recall curves can be more robust compared to ROC curves in imbalanced data sets as they evaluate the fraction of true positives among the positive predictions37. In spontaneous vaginal birth, the auPR more than doubled compared to the auPR of cesarean section. However, it has to be taken into consideration that the rate of spontaneous vaginal birth was approximately 2–3 times higher than the rate for cesarean section (Table 1).
The uncertainty of the prediction models in the present study is supported by the distribution of the predicted probabilities (Fig. 2, histograms). Depending on the chosen algorithm, a perfectly calibrated classifier has two peaks in the distribution for predicted probabilities, one close to 0% and one close to 100%33. For spontaneous vaginal birth the predicted probabilities were grouped around 50%, with a high uncertainty in the prediction of the classes. For cesarean section and vaginal operative delivery, where the outcome rates were similar (between 17% and 27%) (Table 1.), the majority of the probabilities group below 50%. Hence, the algorithms tend to predict the negative class with higher probabilities. This is also reflected by the numbers in the corresponding confusion matrices, where mainly the majority class was predicted (data not shown).
We did not apply methods for correction of the class imbalance in this study. Current research implies, that it should not be recommended to enhance the minority class in prediction models with observational data as the performance might even become worse38.
On the other hand, it can be assumed that the included features provide some predictive information beyond only mapping imbalanced data sets. The rates for vaginal operative delivery and cesarean section did not differ substantially but the auROC (> 60%) and auPR (35%) was higher while predicting cesarean section (Table 2; Fig. 2, Table S4 supplementary material). Future research should investigate the contribution of each feature to the prediction in order to quantify this assumption.
The low performance metrics observed are in line with current research. A recently published study on adverse perinatal outcomes in nulliparous women based on data from a prospective U.S.-cohort study had auROCs with a maximum of 0.673 (95% confidence interval: 0.651–0.694)39. The authors describe the difficulty of predicting adverse perinatal outcomes based on the results from previously published studies with similar auROCs e.g14,40–42.,.
However, in a study by Malacova et al.11 the auROC of ensemble classifiers outperformed other classifiers and reached 84% in predicting stillbirth (outcome rate < 1%). The rates for the PPV was not higher than 5% and much lower than in the present study. Though, these values cannot directly be compared to the values in the present study as the authors did not apply a threshold of 50% but an FPR of 5% and 10%. The authors did not provide a precision-recall curve and corresponding auPR.
Artzi and colleagues12 also described an auROC with a size of 85% from an XGBoost model in a study from 2020. The authors used data from medical records of early pregnancy to predict gestational diabetes (outcome rate 4%) at an earlier stage than the usual 28 GW. In contrast, the auPR did not exceed 32% which was comparable to the auPR of predicting cesarean section in the current study.
As the present study is of exploratory design aiming for high performing prediction models, we did not apply methods of feature ranking or causal inference.
Methods of feature ranking like impurity-based feature importance, permutation importance or Shapley values (SHapley Additive exPlanations) have the potential to generate insights into the algorithms’ decisions by calculating values for the feature importance.
While impurity-based feature importance of random forests can be misleading for high cardinality features43 which are also represented in the data set (e.g. age, height, BMI) permutation importance depends on the chosen classification metric and could be inaccurate in imbalanced data sets44 like in the present study. Moreover, the feature ranking can also differ depending on the performance of the model, which means that one feature can be important in one model, but unimportant in another model44. Given the high clinical impact of the decision on IOL or EM a feature ranking should be based on a high performing model, especially for the implementation in a clinical setting. However, to understand why the model performance in obstetric research is often very low39 and which features are contributing to the prediction, different methods of feature rankings (e.g. permutation importance and Shapley values) should be applied and compared.
The effect of IOL in late pregnancies on perinatal health is still under discussion and there is a strong need to isolate the effect of this intervention in term pregnancies. G-computation is a method for estimating causal effects from observational data by modelling the expected outcome under different exposure scenarios45. It recently has been combined with machine learning methods to quantify the effect of a treatment46. This could also be a promising approach to estimate the effect of IOL in late pregnancies on perinatal health, but requires high performing q-models47.
There is evidence that the performance of prediction studies for adverse perinatal outcomes can be improved by adding more relevant features to the models11,39. In the present study, data from the MBR was used. The available and eligible variables (known on the day of decision-making) include information on diseases, maternal anthropometrics before pregnancy and few socioeconomic factors with assumable limited effect on the outcomes (Table 1). Including more granular data from other registers could enhance predictive power of the models.
Conclusion
We predicted mode of delivery in nulliparous women with a prolonged pregnancy considering the clinically relevant decision-making process on IOL or EM. Predictive power of the included features was low and all models failed to predict the outcomes with high certainty. Including more granular clinical data could potentially address the problem of the lack of information for this prediction.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
SS designed the study together with OS and AS. SS performed the analyses with assistance of MB and CM. SS curated the tables and figures and wrote the manuscript. All authors were involved in the interpretation of the results, contributed to the manuscript, and approved the final version.
Funding
Open access funding provided by Karolinska Institute.
Data availability
The register data analysed during the current study are not publicly available due to the European General Data Protection Regulation 2016/6792 and the Swedish Data Protection Act (2018:218) containing supplementary provisions to the EU General Data Protection Regulation but are available from the corresponding author on reasonable request and with permission of the National Board of Health and Welfare (Socialstyrelsen).
Code availability
The code for data-preprocessing steps only applies to the used, sensitive data and is therefore not published. The code used for the machine learning algorithms is derived from open-source Python packages (e.g. scikit-learn, numpy, pandas, tensorflow, mixed naïve bayes) with the respective documentation and example code.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Swedish Ethical Review Authority (Dnr 2022-04046-01, decision September 5th, 2022). This ethical approval includes the analysis of the data which are collected by the National Board of Health and Welfare. Swedish health data are registered from all persons with a personal identification number in Sweden under Swedish law. Serial numbers have replaced personal identification numbers. All data management and analyses were conducted on de-identified data. Informed consent was waived by the Swedish Ethical Review Authority. All methods were performed following the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muglu, J. et al. Risks of stillbirth and neonatal death with advancing gestation at term: A systematic review and meta-analysis of cohort studies of 15 million pregnancies. PLoS Med.16 (7), e1002838 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Guidelines. Approved by the guidelines review committee, in WHO Recommendations on Induction of Labour at or Beyond Term. World Health Organization: Geneva. (2022).
- 3.Middleton, P. et al. Induction of labour at or beyond 37 weeks’ gestation. Cochrane Database Syst. Rev.7 (7), pCd004945 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeer, B. et al. Perinatal and maternal outcomes according to timing of induction of labour: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reproductive Biology. 288, 175–182 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Alkmark, M. et al. Induction of labour at 41 weeks or expectant management until 42 weeks: A systematic review and an individual participant data meta-analysis of randomised trials. PLoS Med.17 (12), e1003436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruinsma, A. et al. Elective induction of labour and expectant management in late-term pregnancy: A prospective cohort study alongside the INDEX randomised controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. X. 16, 100165 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyykönen, A. et al. Propensity score method for analyzing the effect of labor induction in prolonged pregnancy. Acta Obstet. Gynecol. Scand.97 (4), 445–453 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Ravelli, A. C. J. et al. Does induction of labor at 41 weeks (early, mid or late) improve birth outcomes in low-risk pregnancy? A nationwide propensity score-matched study. Acta Obstet. Gynecol. Scand.102 (5), 612–625 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keulen, J. K. et al. Induction of labour at 41 weeks versus expectant management until 42 weeks (INDEX): multicentre, randomised non-inferiority trial. Bmj364, l344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelovsek, J. E. et al. Predicting risk of pelvic floor disorders 12 and 20 years after delivery. Am. J. Obstet. Gynecol.218 (2), 222e1–222e19 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Malacova, E. et al. Stillbirth risk prediction using machine learning for a large cohort of births from Western australia, 1980–2015. Sci. Rep.10 (1), 5354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artzi, N. S. et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat. Med.26 (1), 71–76 (2020). [DOI] [PubMed] [Google Scholar]
- 13.The fetal medicine foundation. ; (2024). Available from: https://www.fetalmedicine.org/research/assess/preeclampsia/first-trimester
- 14.Trudell, A. S. et al. A stillbirth calculator: development and internal validation of a clinical prediction model to quantify stillbirth risk. PLoS One. 12 (3), e0173461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimovsky, A. C. et al. Pushing the bounds of second stage in term Nulliparas with a predictive model. Am. J. Obstet. Gynecol. MFM. 1 (3), 100028 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Tsur, A. et al. Development and Validation of a machine-learning Model for Prediction of Shoulder Dystocia56 (Ultrasound in Obstetrics & Gynecology, 2020). [DOI] [PubMed]
- 17.TRIPOD + AI Expanded Checklist (Explanation & Elaboration Light). Available from: https://www.tripod-statement.org/wp-content/uploads/2024/04/TRIPODAI-Supplement.pdf
- 18.Collins, G. S. et al. TRIPOD + AI statement: updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ385, e078378 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cnattingius, S. et al. The Swedish medical birth register during five decades: Documentation of the content and quality of the register. Eur. J. Epidemiol.38 (1), 109–120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludvigsson, J. F. et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur. J. Epidemiol.24 (11), 659–667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooke, H. L. et al. The Swedish cause of death register. Eur. J. Epidemiol.32 (9), 765–773 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson, J. F. et al. Registers of the Swedish total population and their use in medical research. Eur. J. Epidemiol.31 (2), 125–136 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Wettermark, B. et al. The new Swedish prescribed drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf.16 (7), 726–735 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Cnattingius, S. et al. Maternal obesity and risk of preterm delivery. Jama309 (22), 2362–2370 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Johansson, K. et al. Risk of pre-eclampsia after gastric bypass: a matched cohort study. Bjog129 (3), 461–471 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Stephansson, O. et al. Delivery outcomes in term births after bariatric surgery: Population-based matched cohort study. PLoS Med.15 (9), e1002656 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grobman, W. A. et al. Labor induction versus expectant management in Low-Risk nulliparous women. N. Engl. J. Med.379 (6), 513–523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennerholm, U. B. et al. Induction of labour at 41 weeks versus expectant management and induction of labour at 42 weeks (SWEdish Post-term induction study, SWEPIS): multicentre, open label, randomised, superiority trial. Bmj367, l6131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Socialstyrelsen Framställning och kvalitet - medicinska födelseregistret (by the Swedish Board of National Health and Welfare). ; (2021). Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2021-9-7547.pdf
- 30.Bhaskaran, K. & Smeeth, L. What is the difference between missing completely at random and missing at random? Int. J. Epidemiol.43 (4), 1336–1339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George, L. et al. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet. Gynecol. Scand.85 (11), 1331–1337 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Mixed Naive Bayes. Available from: https://pypi.org/project/mixed-naive-bayes/
- 33.Niculescu-Mizil, A. & Caruana, R. Predicting good probabilities with supervised learning, in Proceedings of the 22nd international conference on Machine learning. Association for Computing Machinery: Bonn, Germany. pp. 625–632. (2005).
- 34.Svensk Förening för Obstetrik & Gynekologi (SFOG). Available from: https://www.sfog.se/start/kunskapsstoed/obstetrik/foerlossning/
- 35.The National Board of Health and Welfare. Official Statistics of Sweden. Statistics – Health and Medical Care. Pregnancies, Deliveries and Newborn Infants. The Swedish Medical Birth Register 1973–2022. Novemebr 22, 2024]; Available from: https://www.socialstyrelsen.se/statistik-och-data/statistik/alla-statistikamnen/graviditeter-forlossningar-och-nyfodda/
- 36.Megahed, F. M. et al. The class imbalance problem. Nat. Methods. 18 (11), 1270–1272 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Saito, T. & Rehmsmeier, M. The Precision-Recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLOS ONE. 10 (3), e0118432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Goorbergh, R. et al. The harm of class imbalance corrections for risk prediction models: illustration and simulation using logistic regression. J. Am. Med. Inf. Assoc.29 (9), 1525–1534 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, S. J. et al. Interpretable machine learning to predict adverse perinatal outcomes: examining marginal predictive value of risk factors during pregnancy. Am. J. Obstet. Gynecol. MFM. 5 (10), 101096 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Lee, K. S. & Ahn, K. H. Artificial neural network analysis of spontaneous preterm labor and birth and its major determinants. J. Korean Med. Sci.34 (16), e128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, S. et al. Predicting preterm birth through vaginal microbiota, cervical length, and WBC using a machine learning model. Front. Microbiol.13, 912853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yerlikaya, G. et al. Prediction of stillbirth from maternal demographic and pregnancy characteristics. Ultrasound Obstet. Gynecol.48 (5), 607–612 (2016). [DOI] [PubMed] [Google Scholar]
- 43.scikit-learn developers. Feature importances with a forest of trees - scikit-learn 1.70 documentation. [cited 2025 12 June]; (2024). Available from: https://scikit-learn.org/stable/auto_examples/ensemble/plot_forest_importances.html
- 44.scikit-learn developers. 5.2. Permutation feature importance - scikit-learn 1.7.0 documentation. [cited 2025 June 12]; (2024). Available from: https://scikit-learn.org/stable/modules/permutation_importance.html
- 45.Robins, J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math. Modelling. 7 (9–12), 1393–1512 (1986). [Google Scholar]
- 46.Le Borgne, F. et al. G-computation and machine learning for estimating the causal effects of binary exposure statuses on binary outcomes. Sci. Rep.11 (1), 1435 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snowden, J. M., Rose, S. & Mortimer, K. M. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am. J. Epidemiol.173 (7), 731–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The register data analysed during the current study are not publicly available due to the European General Data Protection Regulation 2016/6792 and the Swedish Data Protection Act (2018:218) containing supplementary provisions to the EU General Data Protection Regulation but are available from the corresponding author on reasonable request and with permission of the National Board of Health and Welfare (Socialstyrelsen).
The code for data-preprocessing steps only applies to the used, sensitive data and is therefore not published. The code used for the machine learning algorithms is derived from open-source Python packages (e.g. scikit-learn, numpy, pandas, tensorflow, mixed naïve bayes) with the respective documentation and example code.