Abstract
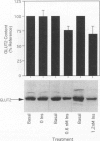
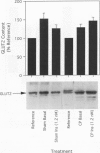
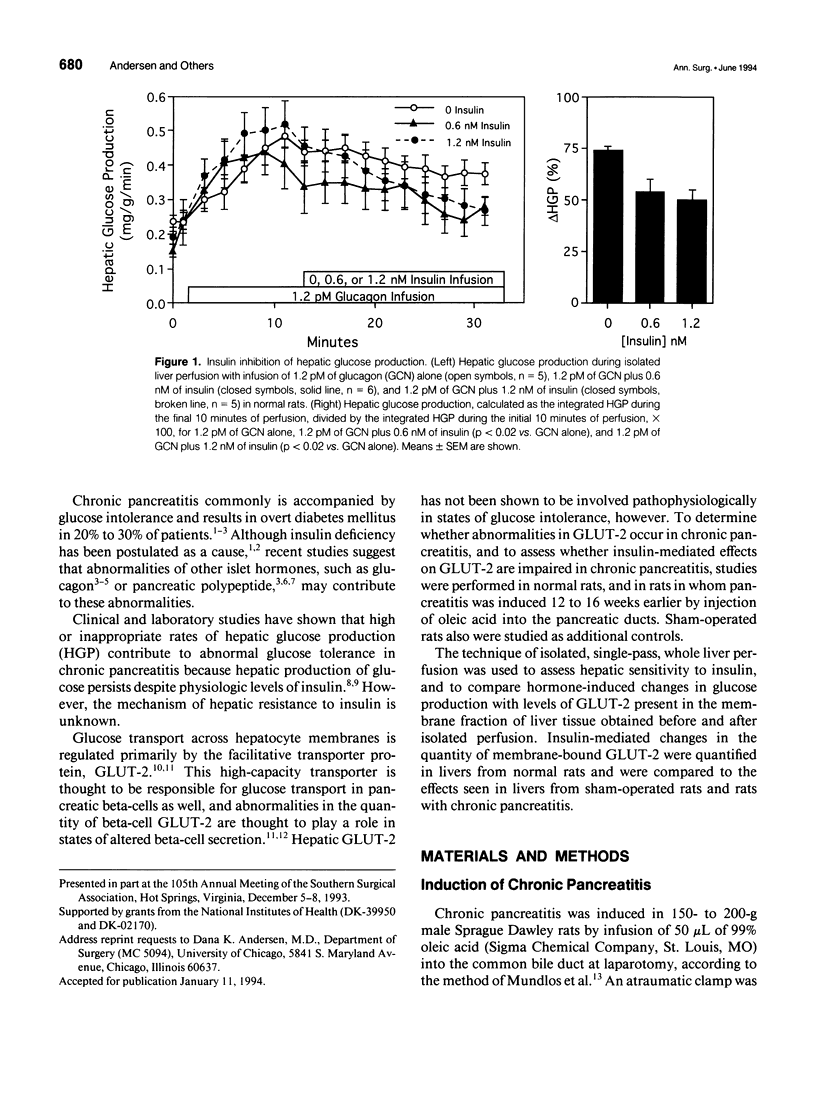
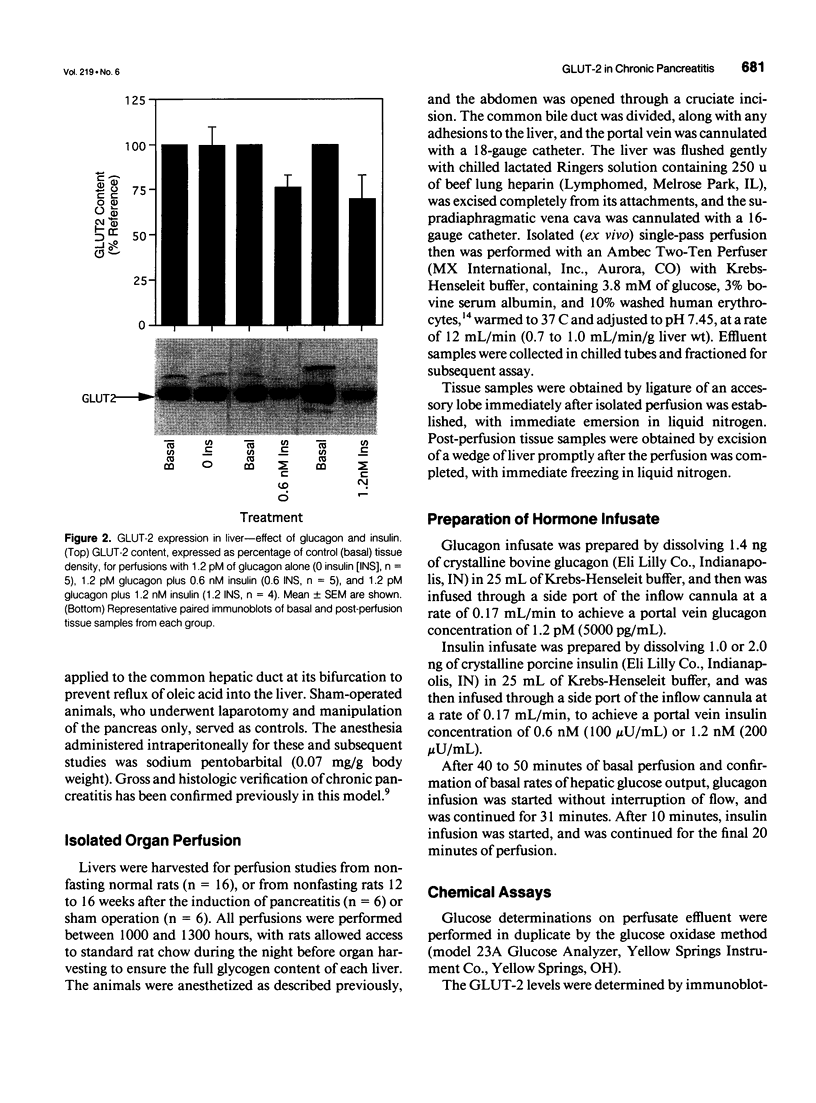
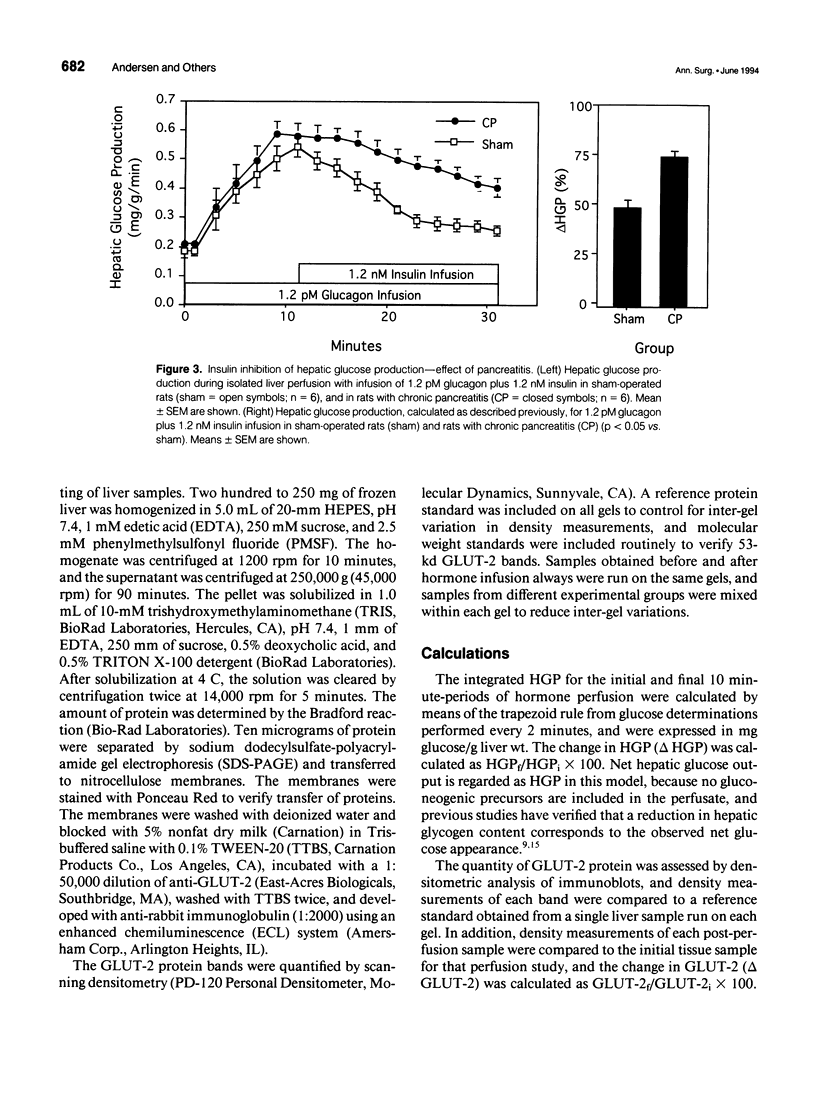
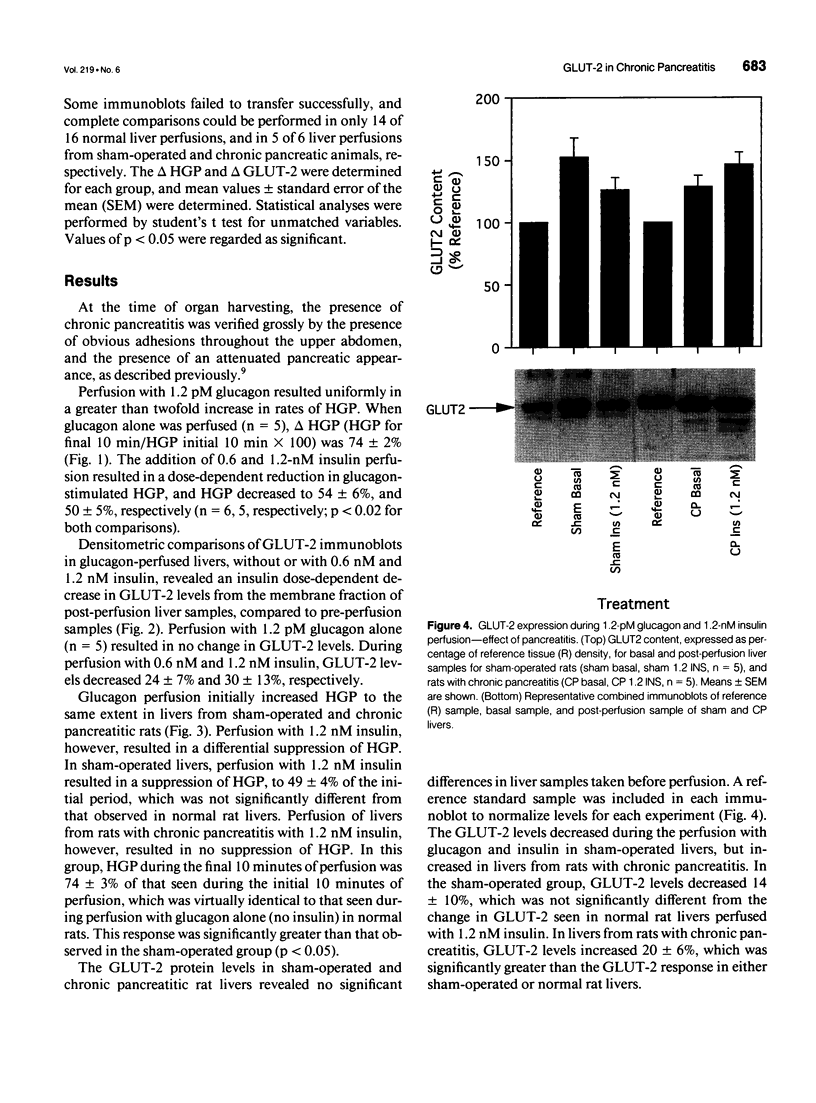
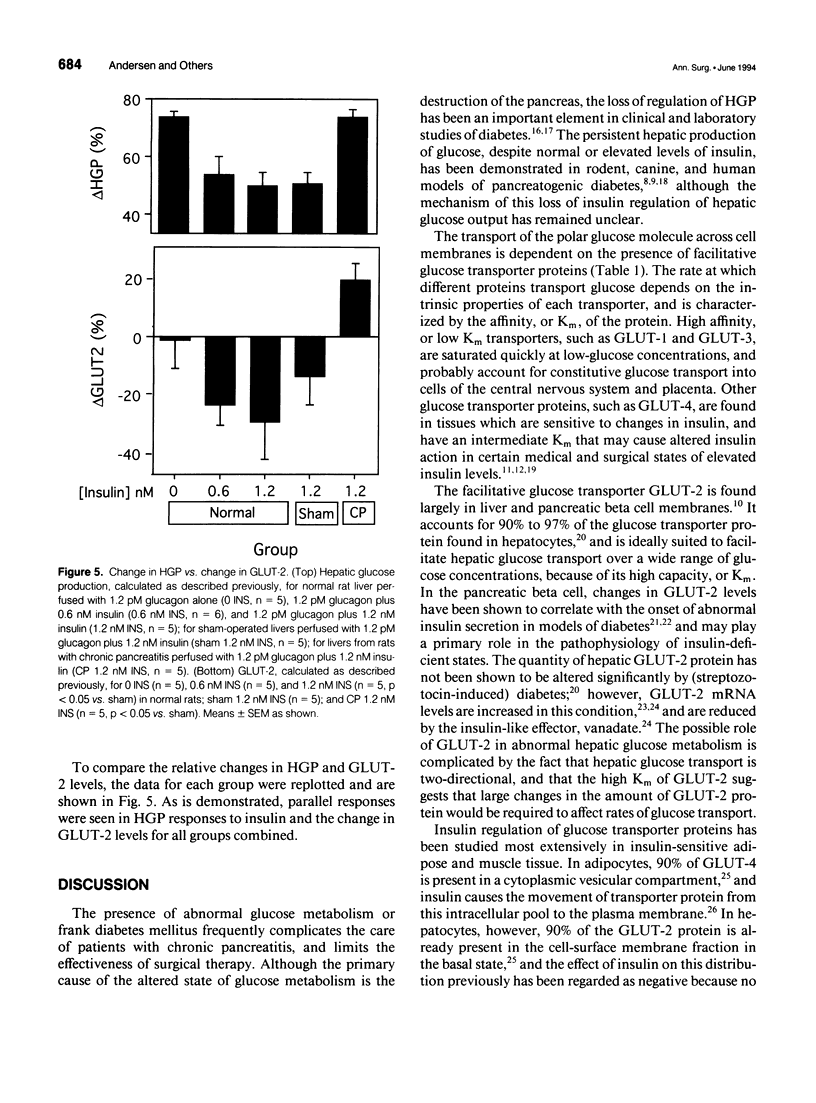
OBJECTIVE: The effect of chronic pancreatitis and insulin on the expression of the hepatic facilitative glucose transporter protein (GLUT-2) was determined in rats. SUMMARY BACKGROUND DATA: Chronic pancreatitis is associated with diabetes mellitus or impaired glucose tolerance. Suppression of hepatic glucose production (HGP) by insulin is impaired, although the mechanism is unknown. METHODS: Normal rats, rats with chronic pancreatitis induced 12 to 16 weeks earlier by oleic acid injection into the pancreatic ducts, and sham-operated rats were studied. Isolated, single-pass liver perfusion was performed, during which glucagon (1.2 pM) was infused, with or without insulin (0.6 or 1.2 nM). The suppression of HGP production by insulin was compared with changes in GLUT-2 in the membrane fraction of liver biopsies obtained before and after hormone perfusion. RESULTS: Glycogen-rich (fed) livers of normal rats (n = 16) demonstrated a dose-dependent suppression of hepatic glucose production by insulin (50 +/- 5% HGP induced by glucagon alone during 1.2-nM insulin perfusion) and a dose-dependent decrease in GLUT-2 (30 +/- 13% of basal level during 1.2-nM insulin perfusion). Sham-operated rats (n = 6) also showed reductions in HGP (51 +/- 4%) and GLUT-2 (14 +/- 10%) during 1.2-nM insulin perfusion. In contrast, rats with chronic pancreatitis (n = 6) showed no suppression of HGP during 1.2-nM insulin perfusion, and an increase in GLUT-2 (+20 +/- 6%) after insulin perfusion (p < 0.02 vs. sham). CONCLUSIONS: Insulin suppresses glucagon-stimulated HGP in normal and sham-operated rats, and this reduction in HGP is associated with a decrease in the membrane-bound quantity of GLUT-2. In chronic pancreatitis, insulin suppression of HGP is absent, and this is accompanied by an increase in GLUT-2 in the hepatocyte membrane. The authors conclude that the insulin-mediated change in the level of hepatocyte GLUT-2 is impaired in chronic pancreatitis, and may contribute to the altered glucose metabolism observed commonly in this disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Burant C. F., Takeda J., Gould G. W. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993 Sep 15;268(26):19161–19164. [PubMed] [Google Scholar]
- Botha J. L., Vinik A. I., Blake K. C., Jackson W. P. Kinetics of insulin secretion in chronic pancreatitis and mild maturity onset diabetes. (Evidence for "gut hormone" action beyond glucoreceptor and cyclic adenosine monophosphate mediated insulin release). Eur J Clin Invest. 1976 Sep 10;6(5):365–372. doi: 10.1111/j.1365-2362.1976.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Ciaraldi T. P., Horuk R., Matthaei S. Biochemical and functional characterization of the rat liver glucose-transport system. Comparisons with the adipocyte glucose-transport system. Biochem J. 1986 Nov 15;240(1):115–123. doi: 10.1042/bj2400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Hendler R., Spiro H. M., Binder H. J., Felig P. Glucagon secretion in acute and chronic pancreatitis. Ann Intern Med. 1975 Dec;83(6):778–781. doi: 10.7326/0003-4819-83-6-778. [DOI] [PubMed] [Google Scholar]
- Elahi D., McAloon-Dyke M., Clark B. A., Kahn B. B., Weinreb J. E., Minaker K. L., Wong G. A., Morse L. A., Brown R. S., Shapiro M. E. Sequential evaluation of islet cell responses to glucose in the transplanted pancreas in humans. Am J Surg. 1993 Jan;165(1):15–22. doi: 10.1016/s0002-9610(05)80398-8. [DOI] [PubMed] [Google Scholar]
- Glaser B., Vinik A. I., Sive A. A., Floyd J. C., Jr Plasma human pancreatic polypeptide responses to administered secretin: effects of surgical vagotomy, cholinergic blockade, and chronic pancreatitis. J Clin Endocrinol Metab. 1980 Jun;50(6):1094–1099. doi: 10.1210/jcem-50-6-1094. [DOI] [PubMed] [Google Scholar]
- Hanks J. B., Meyers W. C., Wellman C. L., Hill R. C., Jones R. S. The effect of cell-free and erythrocyte-containing perfusion in rat livers. J Surg Res. 1980 Aug;29(2):149–160. doi: 10.1016/0022-4804(80)90033-5. [DOI] [PubMed] [Google Scholar]
- Kahn B. B. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992 May;89(5):1367–1374. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk W. J., Vinik A. I., Bank S., Buchanan K. D., Keller P., Jackson W. P. Glucagon responses to arginine in chronic pancreatitis. Possible pathogenic significance in diabetes. Diabetes. 1974 Apr;23(4):257–263. doi: 10.2337/diab.23.4.257. [DOI] [PubMed] [Google Scholar]
- Kalk W. J., Vinik A. I., Bank S., Keller P., Jackson W. P. Selective loss of beta cell response to glucose in chronic pancreatitis. Horm Metab Res. 1974 Mar;6(2):95–98. doi: 10.1055/s-0028-1093889. [DOI] [PubMed] [Google Scholar]
- Mundlos S., Adler G., Schaar M., Koop I., Arnold R. Exocrine pancreatic function in oleic acid-induced pancreatic insufficiency in rats. Pancreas. 1986;1(1):29–36. doi: 10.1097/00006676-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Nealon W. H., Beauchamp R. D., Townsend C. M., Jr, Boyd G., Shabot M., Thompson J. C. Diagnostic role of gastrointestinal hormones in patients with chronic pancreatitis. Ann Surg. 1986 Oct;204(4):430–437. doi: 10.1097/00000658-198610000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A., Johnson J. H., Ohneda M., McAllister C. T., Inman L., Alam T., Unger R. H. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992 Aug;90(2):497–504. doi: 10.1172/JCI115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Akanuma Y., Takaku F. Increased liver glucose-transporter protein and mRNA in streptozocin-induced diabetic rats. Diabetes. 1990 Apr;39(4):441–446. doi: 10.2337/diab.39.4.441. [DOI] [PubMed] [Google Scholar]
- Seymour N. E., Brunicardi F. C., Chaiken R. L., Lebovitz H. E., Chance R. E., Gingerich R. L., Elahi D., Andersen D. K. Reversal of abnormal glucose production after pancreatic resection by pancreatic polypeptide administration in man. Surgery. 1988 Aug;104(2):119–129. [PubMed] [Google Scholar]
- Seymour N. E., Turk J. B., Laster M. K., Tanaka Y., Rosenberg H. E., Rademaker E. A., Pochettino A., Andersen D. K. In vitro hepatic insulin resistance in chronic pancreatitis in the rat. J Surg Res. 1989 May;46(5):450–456. doi: 10.1016/0022-4804(89)90159-5. [DOI] [PubMed] [Google Scholar]
- Sun Y. S., Brunicardi F. C., Druck P., Walfisch S., Berlin S. A., Chance R. E., Gingerich R. L., Elahi D., Andersen D. K. Reversal of abnormal glucose metabolism in chronic pancreatitis by administration of pancreatic polypeptide. Am J Surg. 1986 Jan;151(1):130–140. doi: 10.1016/0002-9610(86)90023-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrettaz J., Jeanrenaud B. In vivo hepatic and peripheral insulin resistance in genetically obese (fa/fa) rats. Endocrinology. 1983 Apr;112(4):1346–1351. doi: 10.1210/endo-112-4-1346. [DOI] [PubMed] [Google Scholar]
- Thorens B., Flier J. S., Lodish H. F., Kahn B. B. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. doi: 10.2337/diab.39.6.712. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Thorens B., Weir G. C., Leahy J. L., Lodish H. F., Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela J. E., Taylor I. L., Walsh J. H. Pancreatic polypeptide response in patients with chronic pancreatitis. Dig Dis Sci. 1979 Nov;24(11):862–864. doi: 10.1007/BF01324903. [DOI] [PubMed] [Google Scholar]
- Valera A., Rodriguez-Gil J. E., Bosch F. Vanadate treatment restores the expression of genes for key enzymes in the glucose and ketone bodies metabolism in the liver of diabetic rats. J Clin Invest. 1993 Jul;92(1):4–11. doi: 10.1172/JCI116580. [DOI] [PMC free article] [PubMed] [Google Scholar]