Abstract
Background
Progression independent of relapse activity (PIRA) may occur in patients with relapsing multiple sclerosis (MS) and is a major contributor to disability accumulation.
Objectives
We evaluated whether a panel of cerebrospinal fluid (CSF) biomarkers (neurofilament light chain (NfL), glial fibrillary acid protein (GFAP), total-Tau (T-Tau), ubiquitin C-terminal hydrolase 1 (UCHL-1), and the phospho-Tau (P-Tau)181/T-Tau ratio) at diagnosis could discriminate patients who will develop PIRA in early disease phases.
Methods
CSF levels of NfL, GFAP, T-Tau, and UCHL-1 were measured with SIMOA, of P-Tau181, and the ratio P-Tau181/T-Tau with chemiluminescent immunoassay in 80 newly diagnosed MS patients who were followed up for three years with six-month Expanded Disability Status Scale (EDSS) assessments and yearly MRI scans.
Results
Nineteen participants developing PIRA exhibited elevated GFAP and UCHL-1 levels, and reduced P-Tau181/T-Tau ratio. These biomarkers remained significant predictors of the outcome after adjusting for confounders, particularly older age at onset and higher baseline EDSS. Additionally, we identified moderate positive correlations between NfL-GFAP, NfL-UCHL-1, and GFAP-UCHL-1 levels, and moderate negative correlations between P-Tau181/T-Tau ratio and NfL, GFAP, and UCHL-1 levels.
Conclusions
The elevation of GFAP and UCHL-1 likely reflects the role of astrocytic activation, while the reduction of the P-Tau181/T-Tau ratio may indicate disrupted Tau metabolism in individuals at risk of PIRA. Our findings suggest that combining these innovative biomarkers with clinical risk factors could improve early prognostic accuracy.
Keywords: Multiple sclerosis/MS, progression independent of relapse activity/PIRA, fluid biomarkers/CSF, neurofilament light chains/NfL, glial fibrillary acid protein/GFAP, ubiquitin C-terminal hydrolase 1/UCHL-1, phosphor-Tau181/P-Tau181, total-Tau/T-Tau
Introduction
There are two main mechanisms by which patients with multiple sclerosis (MS) acquire disability: relapse-associated worsening (RAW) and progression independent of relapse activity (PIRA). Recent epidemiological studies showed that PIRA events may yet occur in patients with relapsing MS, representing the major contributor (up to 50%) to disability accumulation.1,2 The most specific definition of PIRA was proposed by Lublin et al. 2 , and it is solely based on physical disability: an increase in the Expanded Disability Status Scale (EDSS) 3 either ≥1.5 points for patients with a baseline disability of zero, ≥1.0 points for patients with baseline EDSS 1–5, or 0.5 points for patients with baseline EDSS ≥ 5.5, confirmed by an EDSS assessment at least six months apart from the onset of the worsening with either no relapses 90 days before the onset or in the 30 days after EDSS confirmation. Apparently, 25% of all patients presenting with clinically isolated syndrome (CIS) may develop a first PIRA event within the first 12 years after symptom onset, and almost 10% may do so within the first five years; PIRA was associated with unfavorable long-term outcomes, and presenting PIRA in early disease phase was related with an even worse prognosis.4,5
As increasingly sensitive technological platforms are being developed, considerable effort has been made to identify accurate fluid biomarkers for rapidly detecting disease processes and predicting different MS trajectories. 6 Potential biomarkers of neuroaxonal degeneration, glial activity, and chronic inflammation have been studied to address this need. The present study aimed to evaluate whether cerebrospinal fluid (CSF) levels of four candidate biomarkers at diagnosis could discriminate between MS patients who would experience PIRA within three years of follow-up. Using a single-molecule array (SIMOA) kit, we measured CSF levels of neurofilament light chains (NfL), glial fibrillary acid protein (GFAP), total-Tau (T-Tau), and ubiquitin C-terminal hydrolase 1 (UCHL-1).
NfL is an extensively studied biomarker of axonal injury in a wide range of neurological disorders. In MS, high CSF NfL levels have been consistently associated with gadolinium-enhancing lesions (GdE) and relapses. 7 However, subsequent studies suggested that higher blood NfL levels also predict a greater risk of disability progression not related to relapse activity.8,9
Tau protein stabilizes axonal microtubules, representing another potential biomarker of axonal damage. Previous studies in MS patients found that CSF Tau levels predict a quicker disease progression (as measured by a one-point increase in EDSS score), 10 and correlate with EDSS scores in both CIS and relapsing MS patients. 11 However, another study did not replicate the same findings. 12
GFAP is a biomarker of reactive astrogliosis and astrocytic damage. Previous studies highlighted an association between CSF and serum GFAP and disease severity, brain volume loss, and disease progression in MS.13-17 GFAP levels in blood were also associated with a greater risk of future PIRA after the initiation of B-cell-depleting therapies 18 and, in another study, they were 50.9% higher in patients with worsening progressive disease compared with those with stable MS. 19 Unlike NfL, GFAP levels do not appear to increase during acute inflammation, reflecting only confirmed disability worsening.
UCHL-1 is a multifunctional protein. It acts as a neuron-specific deubiquitinating enzyme, playing a protective role in neurodegenerative disorders, while significantly impacting axon integrity and regulating axonal transport and synaptic function. 20 UCHL-1 also participates in immune reactions; it is upregulated in multipotent mesenchymal stromal cells following stimulation by proinflammatory cytokines, such as IFN-γ and TNF-α, and is involved in oxidative stress by increasing cellular reactive oxygen species. 21 Duan et al. 22 found a notable release of UCHL-1-positive extracellular vesicles (EVs) by microglia in MS. They also showed that the release of UCHL-1-positive EVs was superior in MS compared to other neuroinflammatory diseases (HTLV-1 associated myelopathy), other neurological disorders (Alzheimer’s and Parkinson’s disease), and controls. These data suggest a potential dual role of UCHL-1 as both a biomarker of microglial activity and neuroaxonal damage. Previous studies have shown that UCHL-1 concentrations in CSF 23 and serum 24 were higher in patients with MS than in controls, allowing for the discrimination of the two populations with high accuracy. In Alzheimer’s disease, data suggest that UCHL-1 plays a protective role by inhibiting the formation of phospho-Tau (P-Tau) and neurofibrillary tangles (NFTs). 20 Tau is phosphorylated by various protein kinases, including extracellular signal-regulated kinases (ERKs), which are part of the mitogen-activated protein kinase cascade. It appears that UCHL-1 reduces Tau phosphorylation by inhibiting ERKs and increases NFT degradation in the ubiquitin-proteasome system. 25 NFTs comprise different P-Tau isoforms, and P-Tau181 is the most represented. 26 Therefore, after measuring UCHL-1 levels in CSF, we employed a chemiluminescent immunoassay (CLEIA) to determine CSF levels of P-Tau181 and assess the P-Tau181/T-Tau ratio.
Materials and methods
The study followed the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The local ethics committee at the University Hospital IRCCS San Gerardo Dei Tintori, Monza, Italy, approved it (ID 4666_16.10.2024_Mter). The guidelines for reporting observational studies (STROBE) 27 were followed, and written informed consent was obtained from all participants.
Study design, participants, and measurements
We included patients diagnosed with MS according to the 2017 revision of McDonald’s Criteria 28 who had CSF collection at the time of diagnosis. To avoid selection biases, lumbar puncture was performed for research purposes in all newly diagnosed patients, regardless of whether MRI scans had already demonstrated dissemination in time. Patients had to be at least 18 years old and able to provide informed consent to participate in the study. A diagnosis of CIS, radiologically isolated syndrome, and CNS disorders other than MS were considered exclusion criteria.
Participants were prospectively followed over three years. The EDSS score was assessed at baseline (t0), coinciding with the lumbar puncture, and at each subsequent follow-up visit, held at six-month intervals. MRI scans were obtained at baseline for all participants, and the numbers of brain, spinal cord, and GdE lesions were recorded. Then, every participant underwent subsequent MRI scans annually; the number of new or enlarging brain and spinal cord lesions was recorded. Other MRIs eventually performed during follow-up were not considered. MRI analyses were performed centrally at the Neuroradiology Department of IRCCS San Gerardo Dei Tintori Hospital by experienced raters blinded to any specific clinical information, except for the referral for the diagnosis of MS. Contiguous, axial, and maximal 5-mm-thick slices at a field strength of 1.5 T or 3 T were acquired. The number of brain lesions at baseline and new/enlarging lesions were assessed in T2-weighted scans. The number of spinal cord lesions at baseline and new spinal cord lesions were evaluated in Short-T1 Inversion Recovery (STIR) sequences. The presence of GdE lesions was evidenced in T1-weighted scans after gadolinium injection.
At the end of the follow-up period, participants were divided into two groups: (i) patients who experienced PIRA and (ii) patients who did not experience PIRA. PIRA was identified using the definition proposed by Lublin et al. 2 : we included in the PIRA group those participants who, during the follow-up period, showed an EDSS worsening, either ≥1.5 points for patients with a baseline disability of zero, ≥1.0 points for patients with baseline EDSS 1–5, or 0.5 points for patients with baseline EDSS ≥ 5.5, confirmed in an EDSS assessment at least six months apart from the onset of the worsening with either no clinical and radiological relapse 90 days before the onset of EDSS worsening or in the 30 days after EDSS confirmation.
Further demographic and clinical variables were collected at baseline and the end of the follow-up period. At baseline: gender, age at onset, disease duration, oligoclonal bands (OCBs) profile, and number of relapses before the diagnosis. After three years (t1): yearly EDSS increase, total number of relapses, annualized relapse ratio (ARR), start of disease-modifying therapies (DMTs), number of DMT switches, and reason for DMT switches.
At diagnosis, CSF samples were collected by lumbar puncture in the L3/L4 or L4/L5 interspace. After the puncture, samples were centrifuged at room temperature at 2000g for 10 min. The resulting supernatants were aliquoted in polypropylene tubes and stored at −80°C. For CSF measurement of NFL, GFAP, T-Tau, and UCHL-1, we used the single-molecule array SR-X analyzer (Quanterix, Boston, MA) with the Neurology 4-Plex B Advantage Kit (Quanterix, Boston, MA). CSF concentrations of T-Tau and P-Tau181 were measured using CLEIA technology via a one-step sandwich immunoassay method approved for clinical use in AD. The assays were performed according to Fujirebio Lumipulse G600 detection system protocols.
Statistical analyses
Sample characteristics were summarized using descriptive statistics, including mean ± standard deviation (SD) and median ± interquartile range (IQR) for continuous variables and absolute numbers and percentages for categorical variables. Comparisons between groups (PIRA vs non-PIRA) were performed using, as needed, unpaired Student t-test or Mann–Whitney and Wilcoxon tests for continuous variables; chi-square tests with or without Fisher correction were used, as needed, to compare categorical variables. Spearman coefficients were calculated to assess the correlation between different analytes. Since multiple biomarker comparisons were simultaneously conducted, Bonferroni correction for multiple comparisons was applied.
Logistic regression models were developed to assess the predictive value of CSF biomarkers and other covariates on the outcome variable (PIRA vs non-PIRA), considering covariates asymmetrically distributed across the two groups. To verify the contribution of biomarker levels in predicting the outcome, we employed likelihood ratio (LR) tests to compare nested models; specifically, the reduced models included only the covariates, whereas the full models incorporated biomarker concentrations. To ensure the validity of model coefficients, multicollinearity was assessed among the independent variables using the Variance Inflation Factor (VIF): a threshold of VIF ≤ 5 was considered indicative of no significant multicollinearity. Lastly, the goodness-of-fit of the logistic regression models was evaluated using the Pearson chi-square test.
Statistical significance was determined at a p-value <.05.
Since the amount of missing data was small and missingness was assumed to be random, missing data were addressed using a listwise deletion approach, thus avoiding potential biases introduced by imputing missing values. Having a sample size of 19 patients who experienced PIRA and 61 who did not, a two-sided Mann–Whitney test reached a power of 82% to demonstrate an effect size of 0.8 (delta of means divided by a typical standard deviation) with an alpha (significance level) of 5%.
Statistical computations were performed with STATA software, version 17.0, and GraphPad PRISM, version 8.0.
Results
We included 80 patients diagnosed with MS. During the three-year follow-up period, 19 (23.8%) participants experienced PIRA, whereas the remaining 61 (74.2%) did not.
Demographic and clinical characteristics
The mean age at onset for the whole sample was 38.9 (SD 10.5), and 52 (65%) participants were female. CSF-specific OCBs were detected in 75 (93.7%) participants; 62 (77.5%) had a profile 2, and 13 (16.2%) had a profile 3. At t0, the median EDSS was 2 [IQR 1.5, 2.5], the median number of T2-brain lesions was 6 [IQR 1, 3], and the median number of spinal cord lesions was 2 [IQR 1, 3]. 49 (61.2%) participants had at least one GdE lesion during lumbar puncture.
Table 1 summarizes the baseline demographic and clinical characteristics stratified by patients with PIRA and without PIRA experience. At baseline, patients who experienced PIRA had a greater age (p < .001), EDSS score (p < .001), and presented increased spinal cord lesions (p < .001) in STIR sequences. No differences were found in the two groups regarding sex (p = .196), OCB profile (p = .806), number of T2 brain lesions (p = .241), and GdE lesions (p = .583).
Table 1.
Baseline demographic and clinical characteristics of the two groups in the study: Patients who experienced PIRA during follow-up (PIRA) and patients who did not (non-PIRA).
| Non-PIRA n = 61 |
PIRA n = 19 |
p-value | |
|---|---|---|---|
| Sex - F - M |
42 (69%) 19 (31%) |
10 (53%) 9 (47%) |
p = .196 |
| Age at diagnosis, years | 36 (9) | 47 (9) | p < .001 |
| EDSS score | 1.5 (1, 2) | 3 (2, 4) | p < .001 |
| OCBs profile - 1 - 2 - 3 - 4 |
2 (3.4%) 46 (75.4%) 11 (18%) 2 (3.3%) |
0 16 (84.2%) 2 (10.5%) 1 (5.3%) |
p = .806 |
| T2-brain lesion, number | 5.5 (4, 11) | 6 (5, 11) | p = .241 |
| Spinal cord lesions, number | 1 (1, 2.5) | 3 (2, 4) | p < .001 |
| GdE lesions - Yes - No |
25 (41%) 36 (59%) |
6 (31.6%) 13 (68.4%) |
p = .539 |
Note: F: female; M: male; EDSS: Expanded Disability Status Score; OCBs: oligoclonal bands; GdE: gadolinium-enhancing; PIRA: progression independent of relapse activity.
Data are reported as absolute numbers (percentages %), mean (standard deviation), or median (25th quartile, 75th quartile).
After three years, the median EDSS was 1.5 [IQR 1; 3], the median EDSS variation from baseline was 0 [IQR −0.5, 1], and the median PI (EDSS at t1/disease duration in years) was 0.3 [IQR 0.2, 0.8]. During follow-up, 52 (65%) patients did not experience any further relapse, 19 (23.7%) had one relapse, and 8 (11.3%) had two or more relapses. Median ARR (number of relapses/disease duration in years) was 0.3 [IQR 0.3, 0.6]. Overall, 11 (14%) participants experienced RAW during follow-up. The median number of new/enlarging T2-lesions was 0 [IQR 0, 1]; 65 (81.2%) participants had no new spinal cord lesions, 9 (11.2%) had one new spinal cord lesion, and 6 (7.5%) had two or more new spinal cord lesions. A DMT was initiated in 68 (85%) patients, and 12 (15%) required at least one DMT switch for inefficacy.
Table 2 summarizes the clinical characteristics at the end of the study period, stratified by patients with PIRA and without PIRA experience. As expected, patients who experienced PIRA had a greater EDSS increase (p < .001) by the end of follow-up. No differences were evident in the number of relapses (p = .689), ARR (p = .172), in the number of patients that experienced RAW (p = .514), in the number of new brain (p = .169) and spinal cord (p = .484) lesions between the two groups. No differences were also highlighted in DMT initiation (p = .304) or the number of DMT switches after failure (p = .417).
Table 2.
Clinical characteristics by the end of follow-up of the two groups in the study: Patients who experienced PIRA during follow-up (PIRA) and patients who did not (non-PIRA).
| Non-PIRA n = 61 |
PIRA n = 19 |
p-value | |
|---|---|---|---|
| Follow-up, months | 37.3 (1.7) | 36.7 (1.9) | p = .270 |
| EDSS score | 1 (1, 1.5) | 5 (4, 6) | p < .001 |
| EDSS, variation t1−t0 | 0 (−1, 1.5) | 2 (1, 2.5) | p < .001 |
| EDSS increase/year | 0 (−0.3, 0) | 0.6 (0.3, 0.8) | p < .001 |
| Relapses during follow-up, number - 0 - 1 - ≥2 |
38 (62.3%) 14 (22.9%) 8 (14.8%) |
14 (73.7%) 5 (21.5%) |
p = .689 |
| Annualized relapse ratio | 0.3 (0.3, 0.7) | 0.6 (0.3, 0.9) | p = .172 |
| Relapse associated worsening - Yes - No |
8 (13%) 53 (87%) |
3 (16%) 16 (84%) |
p = .514 |
| New/enlarging T2-brain lesions, number | 0 (0, 2) | 0 (0, 1) | p = .169 |
| New spinal cord lesions, number - 0 - 1 - ≥2 - |
50 (82%) 5 (8.2%) 6 (9.8%) |
15 (78.9%) 4 (22.2%) 0 |
p = .484 |
| DMTs - Yes - No |
53 (86.9%) 8 (13.1%) |
16 (79%) 4 (21%) |
p = .304 |
| DMT switches for failure - Yes - No |
51 (83.6%) 10 (16.4%) |
17 (89.5%) 2 (10.5%) |
p = .417 |
Note: EDSS: Expanded Disability Status Score; DMT: disease-modifying treatment; PIRA: progression independent of relapse activity.
Data are reported as absolute numbers (percentage %), mean (standard deviation), or median (25th quartile, 75th quartile).
CSF biomarkers
For the whole sample, median baseline CSF concentrations, measured with SIMOA, of NfL, GFAP, T-Tau, and UCHL-1, were respectively 339.6 pg/mL [IQR 196.7, 691.4], 1753 pg/mL [IQR 820.8, 3079], 16.4 pg/mL [IQR 4.4, 34.9], and 431.5 pg/mL [IQR 312.2, 673.1]. The median baseline CSF concentration of P-Tau181, measured by CLEIA, was 14.2 pg/mL [IQR 11.9, 17.8]. To assess the ratio P-Tau181/T-Tau, we remeasured T-Tau concentrations with CLEIA. When using CLEIA, T-Tau concentrations were superior, with a median T-Tau of 89 pg/mL [IQR 60, 136]. We found a moderate correlation (r = .5, p < .001) between T-Tau levels measured using SIMOA and CLEIA (Figure 1). Median P-Tau181/Tau ratio, measured with CLEIA, was 0.17 [IQR 0.14, 0.21].
Figure 1.
Scatter plot demonstrating a moderate correlation (r = .5, p < .001) between T-Tau values measured with single-molecule array and T-Tau values measured with chemiluminescent immunoassay.
Note: T-Tau: Total-Tau.
As shown in Table 3, CSF concentrations of NfL (p = .022), GFAP (p = .001), and UCHL-1 (p < .001) differentiated patients who experienced PIRA from patients who did not. The ratio P-Tau181/T-Tau also differed in the two groups (p < .001), whereas T-Tau levels differed in the two groups only when measured with CLEIA (p = .015). Lastly, we found no differences in the absolute concentrations of P-Tau181 between the two groups. After Bonferroni correction for multiple comparisons, only GFAP (p = .007), UCHL-1 (p = .014) concentrations, and the P-Tau181/T-Tau ratio (p < .001) still differentiated the two groups, whereas NfL and T-Tau differences lost statistical significance. Figures 2 and 3 are graph-bar representations of analyte levels in two groups of patients (PIRA vs non-PIRA).
Table 3.
Analyte concentration differences in cerebrospinal fluid (NfL: neurofilament light chains; GFAP: glial fibrillary acid protein; T-Tau: Total-Tau; UCHL-1: ubiquitin C-terminal hydrolase 1; P-Tau181: phosphor-Tau181) in the two groups in the study: Patients who experienced PIRA during follow-up (PIRA) and patients who did not (non-PIRA).
| non-PIRA | PIRA | p-value | p-value corrected | Detection method | |
|---|---|---|---|---|---|
| CSF biomarker | Median (25th, 75th quartile) | Median (25th, 75th quartile) | |||
| NfL (pg/mL) | 312 (188, 495) | 538 (308, 495) | p = .022 | p = .154 | SIMOA |
| GFAP (pg/mL) | 1595 (718, 2649) | 3023 (1717, 5557) | p = .001 | p = .007 | SIMOA |
| T-Tau (pg/mL) | 13 (4, 33) | 23 (7–68) | p = .099 | p = 1.000 | SIMOA |
| UCHL-1 (pg/mL) | 383 (286, 535) | 612 (460, 935) | p = .002 | p = .014 | SIMOA |
| T-Tau (pg/mL) | 80 (58, 117) | 110 (90, 178) | p = .017 | p = .119 | CLEIA |
| P-Tau181 (pg/mL) | 14 (12, 17) | 15 (13, 22) | p = .309 | p = 1.000 | CLEIA |
| P-Tau181/T-Tau | 0.19 (0.15, 0.23) | 0.14 (0.13, 0.15) | p < .001 | p < .001 | CLEIA |
Note: CSF: cerebrospinal fluid; SIMOA: single-molecule array; CLEIA: chemiluminescent immunoassay.
Data are reported as median (25th quartile, 75th quartile), mean [95% confidence interval].
p-values were corrected for multiple comparisons using Bonferroni correction.
Figure 2.
Column bar graphs demonstrating CSF concentrations of biomarkers measured with single molecule array in MS patients who experienced PIRA and MS patients who did not. Higher CSF concentrations of NfL (p = .022), GFAP (p = .001), and UCHL-1 (p < .001) were found in the PIRA group. In contrast, no statistically significant differences were observed in T-Tau levels. After correction for multiple comparisons, significant differences were observed only in GFAP (p = .007) and UCHL-11 (p = .014) concentrations.
Note: NfL: neurofilament light chains; GFAP: glial fibrillary acid protein; T-Tau: Total-Tau; UCHL-1: ubiquitin C-terminal hydrolase 1; CSF: cerebrospinal fluid; MS: multiple sclerosis; PIRA: progression independent of relapse activity.
Figure 3.
Column bar graphs demonstrating CSF concentrations of biomarkers measured with chemiluminescent immunoassay in MS patients who experienced PIRA and MS patients who did not. We found higher CSF concentrations of T-Tau (p = .016) and an inferior P-Tau181/T-Tau ratio (p < .001) in patients who experienced PIRA, whereas no differences were observed in P-Tau181 concentrations. After correction for multiple comparisons, the difference remained significant only for the P-Tau181/T-Tau ratio (p < .001).
Note: T-Tau: Total-Tau; P-Tau181: phosphor-Tau181; CSF: cerebrospinal fluid; MS: multiple sclerosis; PIRA: progression independent of relapse activity.
As shown in Table 4, we found no differences in the concentrations of SIMOA NfL (p = .073), GFAP (p = .175), T-Tau (p = .152), UCHL-1 (p = .542), CLEIA P-Tau181 (p = .084), and CLEIA T-Tau (p = .841) in those participants who presented macroscopic disease activity at the time of lumbar puncture, as identified by the presence of GdE lesion in baseline MRI. Also, the CLEIA P-Tau181/T-Tau ratio did not differ between the two groups (p = .363). We then stratified both the group of patients without PIRA and the group with PIRA based on the presence of RAW during follow-up (Table 5). Biomarker concentrations did not differ significantly between patients who experienced RAW but not PIRA and those who experienced neither RAW nor PIRA (SIMOA NfL p = .895; GFAP p = .958; T-Tau p = .319; UCHL-1 p = .729; CLEIA T-Tau p = .523; P-Tau181 p = .692; P-Tau181/T-Tau p = .475). Similarly, no significant differences in biomarker concentrations were observed between patients who experienced both RAW and PIRA and those who experienced PIRA but not RAW (SIMOA NfL p = 1.000; GFAP p = .109; T-Tau p = .957; UCHL-1 p = .051; CLEIA T-Tau p = .603; P-Tau181 p = .726; P-Tau181/T-Tau p = .941).
Table 4.
Difference in biomarker concentrations in participants who had diseased activity, identified by the presence of GdE lesions, at the time of lumbar puncture, compared to those who did not present evidence of disease activity at the time of lumbar puncture.
| CSF biomarker | Disease activity at baseline | p-value | Detection method | |
|---|---|---|---|---|
| No (n = 49) | Yes (n = 31) | |||
| Median (25th, 75th quartile) | Median (25th, 75th quartile) | |||
| NfL (pg/mL) | 312 (193, 510) | 405.2 (228.4, 1160.1) | p = .073 | SIMOA |
| GFAP (pg/mL) | 2278 (771, 3264) | 1644 (871, 2317) | p = .175 | SIMOA |
| T-Tau (pg/mL) | 13 (4, 29) | 19 (7, 38) | p = .152 | SIMOA |
| UCHL-1 (pg/mL) | 443 (318, 640) | 399 (280, 612) | p = .542 | SIMOA |
| T-Tau (pg/mL) | 85 (60, 133) | 89 (69, 139) | p = .841 | CLEIA |
| P-Tau181 (pg/mL) | 14 (13, 22) | 13 (11, 17) | p = .084 | CLEIA |
| P-Tau181/T-Tau | 0.17 (0.15, 0.21) | 0.16 (0.10, 0.22) | p = .363 | CLEIA |
Note: GFAP: glial fibrillary acid protein; T-Tau: Total-Tau; UCHL-1: ubiquitin C-terminal hydrolase 1; P-Tau181: phosphor-Tau181.
Data are reported as either median (25th, 75th quartile).
Table 5.
The biomarker concentrations in the PIRA and non-PIRA groups are reported according to the experience of RAW during follow-up.
| Non-PIRA | ||||
|---|---|---|---|---|
| CSF biomarker | RAW | p-value | Detection method | |
| No (n = 53) | Yes (n = 8) | |||
| Median (25th, 75th quartile) | Median (25th, 75th quartile) | |||
| NfL (pg/mL) | 308 (188, 495) | 339 (224, 625) | p = .895 | SIMOA |
| GFAP (pg/mL) | 1476 (718, 2649) | 1660 (795, 2529) | p = .958 | SIMOA |
| T-Tau (pg/mL) | 16 (4, 35) | 6 (3, 29) | p = .319 | SIMOA |
| UCHL-1 (pg/mL) | 383 (306, 510) | 410 (214, 631) | p = .729 | SIMOA |
| T-Tau (pg/mL) | 82 (59, 114) | 61 (24, 150) | p = .523 | CLEIA |
| P-Tau181 (pg/mL) | 14 (12, 17) | 13 (8, 25) | p = .692 | CLEIA |
| P-Tau181/T-Tau | 0.19 (0.15, 0.23) | 0.21 (0.17, 0.31) | p = .475 | CLEIA |
| PIRA | ||||
|---|---|---|---|---|
| CSF biomarker | RAW | p-value | Detection method | |
| No (n = 16) | Yes (n = 3) | |||
| Median (25th, 75th quartile) | Median (25th, 75th quartile) | |||
| NfL (pg/mL) | 544 (349, 744) | 538 (235, 1160) | p = 1.000 | SIMOA |
| GFAP (pg/mL) | 3120 (1829, 5987) | 1879 (570, 2317) | p = .109 | SIMOA |
| T-Tau (pg/mL) | 25 (8, 67) | 13 (5, 110) | p = .957 | SIMOA |
| UCHL-1 (pg/mL) | 662 (537, 1069) | 403 (378, 460) | p = .051 | SIMOA |
| T-Tau (pg/mL) | 110 (89, 178) | 146 (90, 203) | p = .603 | CLEIA |
| P-Tau181 (pg/mL) | 15 (13, 24) | 18 (15, 21) | p = .726 | CLEIA |
| P-Tau181/T-Tau | 0.14 (0.13, 0.16) | 0.14 (0.10, 0.17) | p = .941 | CLEIA |
Since both baseline EDSS and age at onset are known risk factors for PIRA and were increased in the PIRA group, we developed logistic regression models including GFAP, UCHL-1 levels, and the P-Tau181/T-Tau ratio, with baseline EDSS and age at onset as control variables to account for potential confounding effects (Table 6). All models were statistically significant (GFAP p < .001; UCHL-1 p < .001; P-Tau181/T-Tau ratio p < .001). All biomarkers significantly contributed to predicting the disease outcome (GFAP: p = .037; UCHL-1: p = .017; P-Tau181/T-Tau p = .012). The biomarker contribution to the outcome prediction was verified using LR tests; the comparison between the reduced models (including only baseline EDSS and age at onset) and the complete model (including CSF biomarker levels in addition to baseline EDSS and age at onset) significantly improved model fit, suggesting that GFAP (LR chi2 5.71, p = .017), UCHL-1 (LRchi2 5.59, p = .018) and P-Tau181/Tau ratio (LR chi2 14.1, p < .001) provided additional explanatory value. No multicollinearity was found between biomarker levels and the clinical variables, indicating that biomarker concentrations were not directly influenced by baseline EDSS or age at onset. The Pearson chi-square test confirmed the goodness of fit for all the above-mentioned regression models (p = .332 for NfL, p = .224 for GFAP, p = .171 for UCHL-1, p = .828 for P-Tau181/T-Tau ratio).
Table 6.
The results of separate regression models evaluating the predictive value of each biomarker (GFAP: glial fibrillary acid protein; UCHL-1: ubiquitin C-terminal hydrolase 1; ratio between P-Tau181: phosphor-Tau181 and T-Tau: Total-Tau) on PIRA after three years of follow-up, adjusted for age at onset and baseline EDSS.
| LRchi2 | p-value | Pseudo-R2 | OR biomarker [95% CI] | p-value | OR baseline EDSS [95% CI] | p-value | OR age of onset [95% CI] | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| GFAP (per 1000 pg/mL) | 50 | <.001 | 0.57 | 1.5 [1.02–2.3] | .037 | 6.6 [2.1–21.1] | .001 | 1.2 [1.1–1.3] | .003 |
| UCHL-1 (per 100 pg/mL) | 50 | <.001 | 0.57 | 1.2 [1.1–1.3] | .017 | 7.1 [2.2–21.8] | .001 | 1.2 [1.1–1.3] | .003 |
| P-Tau181/T-Tau (per 0.01 units) | 50.8 | <.001 | 0.65 | 0.8 [0.6–0.9] | .012 | 8.6 [2.1–35.7] | .003 | 1.2 [1.1–1.4] | .005 |
Note: EDSS: Expanded Disability Status Score; PIRA: progression independent of relapse activity.
For each model, the likelihood chi-square (LRchi2) and the respective p-value are reported, along with the McFadden R2 (Pseudo-R2) for each single regression model. In addition, Odds Ratios (ORs) for covariates and 95% confidence intervals (CI) are listed with respective p-values.
Lastly, we found a moderate positive correlation between NfL and GFAP levels (r = .5, p < .001), NfL and UCHL-1 levels (r = .4, p < .001), and GFAP and UCHL-1 levels (r = .4, p < .001) (Figure 4). T-Tau levels, measured with SIMOA, showed a moderate positive correlation with NfL (r = .4, p < .001) and GFAP (r = .4, p < .001); no correlation was found comparing T-Tau and UCHL-1 levels (Figure 5). The P-Tau181/T-Tau ratio showed a moderate negative correlation with NfL (r = −.5, p < .001), GFAP (r = −.4, p = .002), and UCHL-1 (r = −.3, p = .005) levels (Figure 6).
Figure 4.
Scatter plots demonstrating a moderate positive correlation between CSF GFAP and NfL levels (r = .5, p < .001), UCHL-1 and NfL levels (r = .4, p < .001), UCHL-1 and GFAP levels (r = .4, p < .001).
Note: NfL: neurofilament light chains; GFAP: glial fibrillary acid protein; UCHL-1: ubiquitin C-terminal hydrolase 1.
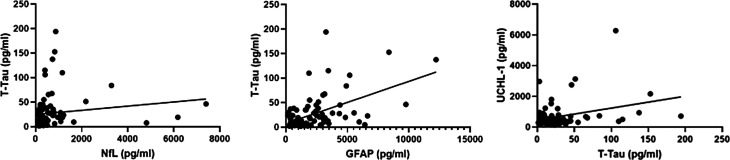
Figure 5.
scatter plot demonstrating a moderate positive correlation between CSF T-Tau levels, measured with single-molecule array and NfL (r = .4, p < .001) and GFAP (r = .4, p < .001); no correlation was found between UCHL-1 and T-Tau levels.
Note: NfL: neurofilament light chains; GFAP: glial fibrillary acid protein; T-Tau: Total-Tau; UCHL-1: ubiquitin C-terminal hydrolase 1; CSF: cerebrospinal fluid.
Figure 6.
Scatter plots demonstrating a moderate negative correlation between the ratio P-Tau181/T-Tau, measured with a chemiluminescent immunoassay, NfL (r = −.5, p < .001), GFAP (r = −.4, p = .002), and UCHL-1 (r = −.3, p = .005) levels, measured with a single-molecule array. Note: NfL: neurofilament light chains; GFAP: glial fibrillary acid protein; T-Tau: Total-Tau; UCHL-1: ubiquitin C-terminal hydrolase 1; P-Tau181: phosphor-Tau181.
Discussion
Clinical outcomes in MS are heterogeneous and largely unpredictable. While the classical phenotypic distinction enhances homogeneity in clinical trials and allows for standardized communication among clinicians, emerging evidence suggests a biological continuum in which pathological mechanisms underlying RAW and PIRA coexist from early disease stages. Since PIRA represents a significant outcome determinant,4,5 understanding mechanisms and identifying biomarkers of such silent deterioration remain essential unmet needs.
Several factors have been associated with a greater risk of PIRA: older age at diagnosis, male sex, longer disease duration, and the presence and number of spinal cord lesions on baseline MRI scans.4,5,29 In our study, we observed that patients who experienced PIRA had an older age at diagnosis, a higher baseline EDSS score, and a greater number of spinal cord lesions; however, the gender distribution did not differ significantly between the two groups. Logistic regression models for biomarkers demonstrated that both baseline EDSS and age at diagnosis significantly impacted the outcome; in all models, unitary increases in both parameters corresponded to marked changes in risk of PIRA. Overall, the sample mean age at baseline was relatively high (38.9 ± 10.5), which may explain why the percentage of patients experiencing PIRA during the early disease course (23%) exceeded the prediction from existing literature (10%).
Neuropathological risk factors for the progressive disease course have also been described 30 : (i) chronic active lesion (CALs), characterized by a rim of activated myeloid phagocytes (microglia and macrophages) with myelin degradation byproducts as an epiphenomenon of continuous lesion activity, (ii) superior rates of brain volume loss, mainly driven by cortical gray matter (GM) lesions that are topographically associated with sizeable immune cell meningeal aggregates of both T- and B-cells.
CALs likely arise from a small proportion of new GdE lesions, predominantly in older patients and advanced disease stages. 31 Histologically, CALs are characterized by intense chronic astrocytic damage and reactive astrogliosis; thus, they are expected to correlate with GFAP release. Consistent with previous studies, our findings revealed significantly higher CSF GFAP concentration in patients who experienced PIRA, even after correcting for multiple comparisons. In our logistic regression model, GFAP levels emerged as a significant predictor, where analyte variation equal to 1.000 pg/mL corresponded to an increase in OR by 1.5 for developing PIRA.
In addition to CALs, cortical GM lesions serve as sites of substantial neurite injury. Neurite injury in both CALs and cortical GM lesions is expected to correlate with the release of biomarkers of neuroaxonal damage, such as NfL and T-Tau. We observed positive correlations between NfL and GFAP levels, as well as between T-Tau and both NfL and GFAP levels, highlighting the interplay between neurodegeneration and astrocytic activation in MS. Although higher NfL were noted in patients with PIRA, this difference lost statistical significance after correction for multiple comparisons. Similarly, T-Tau concentration measured with SIMOA did not differ between groups, and the difference observed using CLEIA was not retained after correction. These findings suggest that T-Tau and NfL are unlikely to reliably predict short-term disability accumulation in the form of PIRA. However, the potential role of repeated measurements of axonal damage biomarkers in predicting the accumulation of long-term disability warrants further investigation.
CSF UCHL-1 levels were increased in the group of patients who experienced PIRA, and these differences remained robust even after correction for multiple comparisons. Logistic regression modeling further revealed that a 100 pg/mL increase in UCHL-1 levels corresponded to a significant 1.2-fold increase in the OR of developing PIRA. The moderate correlation with NfL levels might imply that the rise in CSF UCHL-1 could stem from more pronounced neurodegeneration and axonal damage in PIRA patients. However, recent studies have indicated that UCHL-1 is also expressed and secreted via EVs by activated microglia in MS, 22 and we found a moderate correlation with GFAP levels, in addition to NfL levels. Therefore, the increase in CSF UCHL-1 may also reflect microglial hyperactivation, a known feature of PIRA pathogenesis. This potential dual role positions UCHL-1 as a promising biomarker for MS, with predictive capability for PIRA that may be comparable to GFAP.
Lastly, we evaluated the concentration of P-Tau181 and the P-Tau181/T-Tau ratio. No significant differences were observed in the absolute concentration of P-Tau181 between the two study groups. Considering that P-Tau181 is a product of Tau posttranscriptional modification,25,32 its absolute concentrations were expected to be proportional to T-Tau levels. However, we found a reduced P-Tau181/T-Tau ratio in the PIRA group, and this difference remained significant after correction for multiple comparisons. The logistic regression analysis revealed that increases in the variable equal to 0.01 corresponded to a decrease of 0.2 in the OR for developing PIRA. Moreover, the ratio showed moderate negative correlations with UCHL-1, GFAP, and NfL levels. These findings suggest that specific inflammatory or metabolic processes may influence Tau phosphorylation in patients at risk of PIRA. Given that UCHL-1 is known to have a protective role in neurodegenerative disorders by reducing the accumulation of misfolded proteins33,34,25,32, it is plausible that microglial dysregulation of UCHL-1 in MS patients experiencing PIRA may promote an increased degradation of P-Tau181, thereby altering the P-Tau181/T-Tau ratio. However, as the association between elevated UCHL-1 levels and reduced P-Tau181/T-Tau could also reflect broader neuronal stress, astrocytic activity, or biological variability in early stages of MS, this hypothesis requires further validation. To explore this, we plan to investigate UCHL-1 expression in peripheral blood mononuclear cells derived from patients with and without PIRA, and to assess how UCHL-1 expression is modulated following stimulation with proinflammatory agents, such as lipopolysaccharide (LPS), and its response to LPS inhibitors, such as siRNA.
The present study has limitations worth mentioning. As a pilot, exploratory, and monocentric study, the sample size was relatively small, which limited the possibility of more complex statistical analyses. Analyte concentrations were measured only in CSF and not in blood, which limits the applicability of our results in routine clinical practice. Furthermore, due to the limited availability of commercial kits, we employed various methods to dose the analytes. As demonstrated by the difference in T-Tau concentrations, inter-method reproducibility (SIMOA and CLEIA) remains limited, likely due to the differing degrees of affinity of the antibodies used. Lastly, although normative values for CSF GFAP and NfL exist,35,36 our study did not include a contemporaneously sampled control group. Without direct comparison to healthy subjects processed under identical conditions, it is not possible to precisely estimate if some of the variability in analyte concentrations could have been attributable to assay variability or demographic factors other than age.
In conclusion, fluid biomarkers have the potential to capture various aspects of MS. Currently, only oligoclonal bands and the light chain index are utilized in clinical practice due to their diagnostic relevance. While NfL has been extensively studied as a tool for monitoring disease activity and GFAP for assessing disease progression, our exploratory findings suggest that incorporating innovative biomarkers, as UCHL-1 and the P-Tau181/T-Tau ratio, into routine assessments could significantly enhance the precision of prognostic models, especially when combined with established clinical risk factors, such as EDSS and age. From a broader perspective, the ability to identify robust biomarkers predictive of PIRA could improve disease management by enabling earlier and more targeted adjustments to treatment regimens, potentially delaying or mitigating irreversible disability. Additionally, integrating these biomarkers into clinical practice could offer a valuable tool for evaluating the efficacy of existing therapies in mitigating PIRA progression. However, translating these insights into clinical workflows requires further validation in larger, more diverse cohorts, alongside the standardization of measurement methodologies and the presence of age-matched healthy controls. Expanding biomarker analyses to include blood-based samples could also enhance their accessibility and utility in routine clinical settings, ultimately supporting broader adoption and improved patient outcomes.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Edoardo Dalmato Schilke https://orcid.org/0009-0008-6546-5745
Chiara Paola Zoia https://orcid.org/0000-0003-1654-4996
Guido Cavaletti https://orcid.org/0000-0003-4128-2406
Contributor Information
Edoardo Dalmato Schilke, PhD program in Neuroscience, Department of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Neurology, IRCCS San Gerardo dei Tintori Foundation Hospital, Monza, Italy.
Maria Letizia Fusco, Neurology, IRCCS San Gerardo dei Tintori Foundation Hospital, Monza, Italy.
Adele Cappellani, Clinical Chemistry Laboratory, IRCCS San Gerardo dei Tintori Foundation Hospital, Monza, Italy.
Diletta Cereda, Neurology, IRCCS San Gerardo dei Tintori Foundation Hospital, Monza, Italy.
Chiara Paola Zoia, Laboratory of Neurobiology, Department of Medicine and Surgery, University of Milan-Bicocca, Monza, Italy; Milan Centre for Neuroscience, University of Milan-Bicocca, Milan, Italy.
Guido Cavaletti, Neurology, IRCCS San Gerardo dei Tintori Foundation Hospital, Monza, Italy; Experimental Neurology Unit, Department of Medicine and Surgery, University of Milan-Bicocca, Monza, Italy.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77: 1132–1140. https://pubmed.ncbi.nlm.nih.gov/32511687/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain 2022; 145: 3147–3161. https://pubmed.ncbi.nlm.nih.gov/35104840/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. https://pubmed.ncbi.nlm.nih.gov/6685237/ . [DOI] [PubMed] [Google Scholar]
- 4.Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol 2023; 80: 151–160. https://pubmed.ncbi.nlm.nih.gov/36534392/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portaccio E, Bellinvia A, Fonderico M, et al. Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain 2022; 145: 2796–2805. https://pubmed.ncbi.nlm.nih.gov/35325059/ . [DOI] [PubMed] [Google Scholar]
- 6.Schilke ED, Remoli G, Funelli E, et al. Current use of fluid biomarkers as outcome measures in multiple sclerosis (MS): a review of ongoing pharmacological clinical trials. Neurol Sci 2024; 45: 1931–1944. https://pubmed.ncbi.nlm.nih.gov/38117403/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toscano S, Oteri V, Chisari CG, et al. Cerebrospinal fluid neurofilament light chains predicts early disease-activity in multiple sclerosis. Mult Scler Relat Disord 2023; 80. https://pubmed.ncbi.nlm.nih.gov/37951096/ . [DOI] [PubMed] [Google Scholar]
- 8.Lie IA, Kaçar S, Wesnes K, et al. Serum neurofilament as a predictor of 10-year grey matter atrophy and clinical disability in multiple sclerosis: a longitudinal study. J Neurol Neurosurg Psychiatry 2022; 93: 849–857. https://pubmed.ncbi.nlm.nih.gov/35649699/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelhak A, Benkert P, Schaedelin S, et al. Neurofilament light chain elevation and disability progression in multiple sclerosis. JAMA Neurol 2023; 80: 1317–1325. https://pubmed.ncbi.nlm.nih.gov/37930670/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Yélamos A, Saiz A, Bas J, et al. Tau protein in cerebrospinal fluid: a possible marker of poor outcome in patients with early relapsing-remitting multiple sclerosis. Neurosci Lett 2004; 363: 14–17. https://pubmed.ncbi.nlm.nih.gov/15157986/ . [DOI] [PubMed] [Google Scholar]
- 11.Brettschneider J, Petzold A, Junker A, et al. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult Scler 2006; 12: 143–148. https://pubmed.ncbi.nlm.nih.gov/16629417/ . [DOI] [PubMed] [Google Scholar]
- 12.Guimarães J, Cardoso MJ, Sá MJ. Tau protein seems not to be a useful routine clinical marker of axonal damage in multiple sclerosis. Mult Scler 2006; 12: 354–356. https://pubmed.ncbi.nlm.nih.gov/16764350/ . [DOI] [PubMed] [Google Scholar]
- 13.Abdelhak A, Antweiler K, Kowarik MC, et al. Serum glial fibrillary acidic protein and disability progression in progressive multiple sclerosis. Ann Clin Transl Neurol 2024; 11: 477–485. https://pubmed.ncbi.nlm.nih.gov/38111972/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelhak A, Huss A, Kassubek J, et al. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 2018; 8. https://pubmed.ncbi.nlm.nih.gov/30287870/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saraste M, Bezukladova S, Matilainen M, et al. Increased serum glial fibrillary acidic protein associates with microstructural white matter damage in multiple sclerosis: GFAP and DTI. Mult Scler Relat Disord 2021; 50. https://pubmed.ncbi.nlm.nih.gov/33556656/ . [DOI] [PubMed] [Google Scholar]
- 16.Ayrignac X, Le Bars E, Duflos C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep 2020; 10. https://pubmed.ncbi.nlm.nih.gov/32616916/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barro C, Healy BC, Liu Y, et al. Serum GFAP and NfL levels differentiate subsequent progression and disease activity in patients with progressive multiple sclerosis. Neurology(R) Neuroimmunology & Neuroinflammation 2022; 10. https://pubmed.ncbi.nlm.nih.gov/36376097/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benkert P, Maleska Maceski A, Schaedelin S, et al. Serum glial fibrillary acidic protein and neurofilament light chain levels reflect different mechanisms of disease progression under B-cell depleting treatment in multiple sclerosis. Ann Neurol 2024; 97. https://pubmed.ncbi.nlm.nih.gov/39411917/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier S, Willemse EAJ, Schaedelin S, et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol 2023; 80: 287–297. https://pubmed.ncbi.nlm.nih.gov/36745446/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mi Z, Graham SH. Role of UCHL1 in the pathogenesis of neurodegenerative diseases and brain injury. Ageing Res Rev 2023; 86. https://pubmed.ncbi.nlm.nih.gov/36681249/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matuszczak E, Tylicka M, Komarowska MD, et al. Ubiquitin carboxy-terminal hydrolase L1 – physiology and pathology. Cell Biochem Funct. 2020; 38: 533–540. doi: 10.1002/cbf.3527 [DOI] [PubMed] [Google Scholar]
- 22.Duan J, Lv A, Guo Z, et al. CX3CR1+/UCHL1+ microglial extracellular vesicles in blood: a potential biomarker for multiple sclerosis. J Neuroinflammation 2024; 21: 254. https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-024-03243-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csecsei P, Acs P, Gottschal M, et al. The relevance of combined testing of cerebrospinal fluid glial fibrillary acidic protein and ubiquitin C-terminal hydrolase L1 in multiple sclerosis and peripheral neuropathy. Neurol Sci 2024. https://pubmed.ncbi.nlm.nih.gov/39565457/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Górska E, Tylicka M, Hermanowicz A, et al. UCHL1, besides leptin and fibronectin, also could be a sensitive marker of the relapsing-remitting type of multiple sclerosis. Sci Rep 2023; 13. https://pubmed.ncbi.nlm.nih.gov/36854961/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrer I, Blanco R, Carmona M, et al. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 2001; 11: 144–158. https://pubmed.ncbi.nlm.nih.gov/11303790/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudrabhatla P, Jaffe H, Pant HC. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer’s NFTs. FASEB J 2011; 25: 3896. https://pmc.ncbi.nlm.nih.gov/articles/PMC3205835/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: 1623–1627. https://pubmed.ncbi.nlm.nih.gov/17941714/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. https://pubmed.ncbi.nlm.nih.gov/29275977/ . [DOI] [PubMed] [Google Scholar]
- 29.Prosperini L, Ruggieri S, Haggiag S, et al. Prognostic accuracy of NEDA-3 in long-term outcomes of multiple sclerosis. Neurology(R) Neuroimmunol Neuroinflam 2021; 8. https://pubmed.ncbi.nlm.nih.gov/34373345/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calabrese M, Preziosa P, Scalfari A, et al. Determinants and biomarkers of progression independent of relapses in multiple sclerosis. Ann Neurol 2024; 96: 1–20. https://pubmed.ncbi.nlm.nih.gov/38568026/ . [DOI] [PubMed] [Google Scholar]
- 31.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126: 2597–2609. https://pubmed.ncbi.nlm.nih.gov/27270171/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng J, Lei P. Plasma pTau181 as a biomarker for Alzheimer’s disease. MedComm (Beijing) 2020; 1: 74–76. https://pubmed.ncbi.nlm.nih.gov/34766110/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Liu N, Chen X, et al. UCHL1 regulates inflammation via MAPK and NF-κB pathways in LPS-activated macrophages. Cell Biol Int 2021; 45: 2107–2117. https://pubmed.ncbi.nlm.nih.gov/34288216/ . [DOI] [PubMed] [Google Scholar]
- 34.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014; 23: 37–45. https://pubmed.ncbi.nlm.nih.gov/25130606/ . [DOI] [PubMed] [Google Scholar]
- 35.Vermunt L, Otte M, Verberk IMW, et al. Age- and disease-specific reference values for neurofilament light presented in an online interactive support interface. Ann Clin Transl Neurol 2022; 9: 1832. https://pmc.ncbi.nlm.nih.gov/articles/PMC9639622/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honey MIJ, Wesenhagen KEJ, Willemse EAJ, et al. Comparison between plasma, serum and cerebrospinal fluid glial fibrillary acidic protein in Alzheimer’s disease and dementia with Lewy bodies and the effect of age and sex on diagnostic performance. Alzheimer’s & Dementia. 2022;18:e067313. /doi/pdf/10.1002/alz.067313 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.