Summary
Facilitated by the increased frequency of travel and a rise in legal and illegal animal trades, invasive ticks have been inadvertently introduced into the US in recent years. We received ticks removed from human travelers returning to Connecticut, US, identified them to species, and screened for evidence of infection. We report seven nonnative ticks introduced to Connecticut from 2019 to 2023, including two Amblyomma mixtum nymphs from Guatemala and Costa Rica, one Amblyomma coelebs nymph from Belize, one Rhipicephalus pulchellus female from Tanzania, two Ixodes ricinus nymphs from Germany and Poland, and one I. ricinus larva from Scotland. Of these, the two A. mixtum nymphs tested positive for Rickettsia amblyommatis. The importation of exotic tick species and their likelihood to establish populations is a global challenge, and thus the establishment of tick surveillance programs, which include tick species identification and pathogen screening, is important for protecting human and veterinary health.
Subject areas: Parasitology, Public health, Ecology, Zoology, Entomology
Graphical abstract

Highlights
-
•
Emphasize the emergence of invasive ticks as a global public health concern
-
•
Report introduction of seven invasive tick vectors by human travelers into CT, USA
-
•
Document Rickettsia amblyommatis in Amblyomma mixtum from Guatemala and Costa Rica
-
•
Raise public awareness about the risk of invasive ticks and associated pathogens
Parasitology; Public health; Ecology; Zoology; Entomology
Introduction
Tick-borne diseases (TBDs), caused by a wide array of bacterial, protozoan, viral, and filarial pathogens, are a major public health and veterinary concern. More than 90% of all vector-borne disease cases reported annually in the United States (US) are those transmitted by ticks.1 In recent years, the number of reported TBD cases has increased significantly from 22,527 in 2004 to 71,346 cases in 2022.2,3 In addition to a substantial rise in TBD cases, the number of recognized pathogens has also increased in recent years; out of the 15 tick-borne pathogens known to cause human disease in the US, nearly half were discovered in the past two decades.1 The proliferation and expansion of tick species that vector these pathogens, notably Ixodes scapularis, Amblyomma americanum, and Amblyomma maculatum, have placed more people at risk of acquiring TBD in the US. Several factors, including environmental changes, increased human and animal host populations, and frequency of wildlife movement, contribute to the changes in the geographic distribution of tick vectors.
In addition to the ongoing range expansion of endemic ticks, the introduction of invasive ticks into new areas has led to a substantial increase in human-tick encounters. The movement of tick vectors to new areas well beyond their native distribution can be attributed to an uptick in international trade and travel, among other factors. The recent introduction and establishment of the longhorned tick, Haemaphysalis longicornis, is a prime example of the challenges associated with invasive ticks. Native to East Asia and invasive to Australia, New Zealand, and Pacific Islands, this tick was first detected on a sheep in New Jersey, US, in 2017, and due to its parthenogenetic nature, host abundance, and conducive environments, it has now spread to at least 24 mostly eastern states and the District of Columbia with established populations as far north as Connecticut, Rhode Island, and Massachusetts.3,4 In its native and invasive range, H. longicornis is involved in the transmission of nearly 30 pathogens of human and veterinary health concern.3 Though it is not entirely clear how this tick entered into the US, there are several earlier reports of H. longicornis interceptions at ports of entry.5
Numerous studies have reported the interception and removal of ticks from returning travelers throughout the world, including those acquired in Central America, Asia, Africa, and North and South America.6,7,8,9,10,11 In addition to tick species known to be endemic to the US, Americans can also be exposed to exotic ticks in the country and while traveling abroad. During the last half century, more than 140 nonnative tick species have been introduced into the country,12 including a small subset removed from humans.5,9,10,13,14,15,16
The importation of nonnative ticks into the US raises the unsettling possibility that these invasive ticks and associated pathogens become established in the region. Equally as concerning are reports of TBDs in US travelers acquired outside of the country,16,17,18 including a recent Powassan virus disease case and death of a Maryland native after a bite from an infected tick during a trip to Canada.19 Herein, we report the importation of seven nonnative ticks into the US by travelers returning from Central America, Africa, and Europe. We also discuss the public health importance of invasive ticks and pathogens of human and veterinary health concern and emphasize the need for introducing measures to mitigate associated challenges.
Results and discussion
Case description
Cases I and II: Amblyomma mixtum from Guatemala and Costa Rica
A 59-year-old female resident of Connecticut with a recent travel history to Guatemala discovered and removed a fully engorged tick attached to her leg. On 30 April 2019, the tick was sent to the Connecticut Agricultural Experiment Station Tick Testing Laboratory (CAES-TTL) through a local health department for species, life stage, and engorgement status identification and pathogen screening. No additional information regarding the tick encounter was provided with the tick submission.
A 23-year-old female resident of Meriden, New Haven County, CT, discovered a partially engorged tick attached to her right calf on 22 March 2022. The patient noticed the tick the day after her return from a six-day trip to Monteverde, Costa Rica, where she describes participating in outdoor activities including horseback riding in an area covered in tall grasses. Upon removal, the tick was submitted on 7 April 2022 to the CAES-TTL.
Initial examination revealed that the ticks were Amblyomma sp. nymphs. Light microscopy and scanning electron microscopy (SEM) images were taken (Figures 1 and 2). Following further morphological examination, both specimens were identified as Amblyomma mixtum. These findings were corroborated with genetic analysis using the 16S rRNA and mitochondrial cytochrome c oxidase subunit I (COI) genes, in case 1 and case 2, respectively (Table 1), which showed >98% identity with several sequences of the species available through the GenBank database (OP901703.1, KT820359.1, PV033859.1, and PQ373339.1).
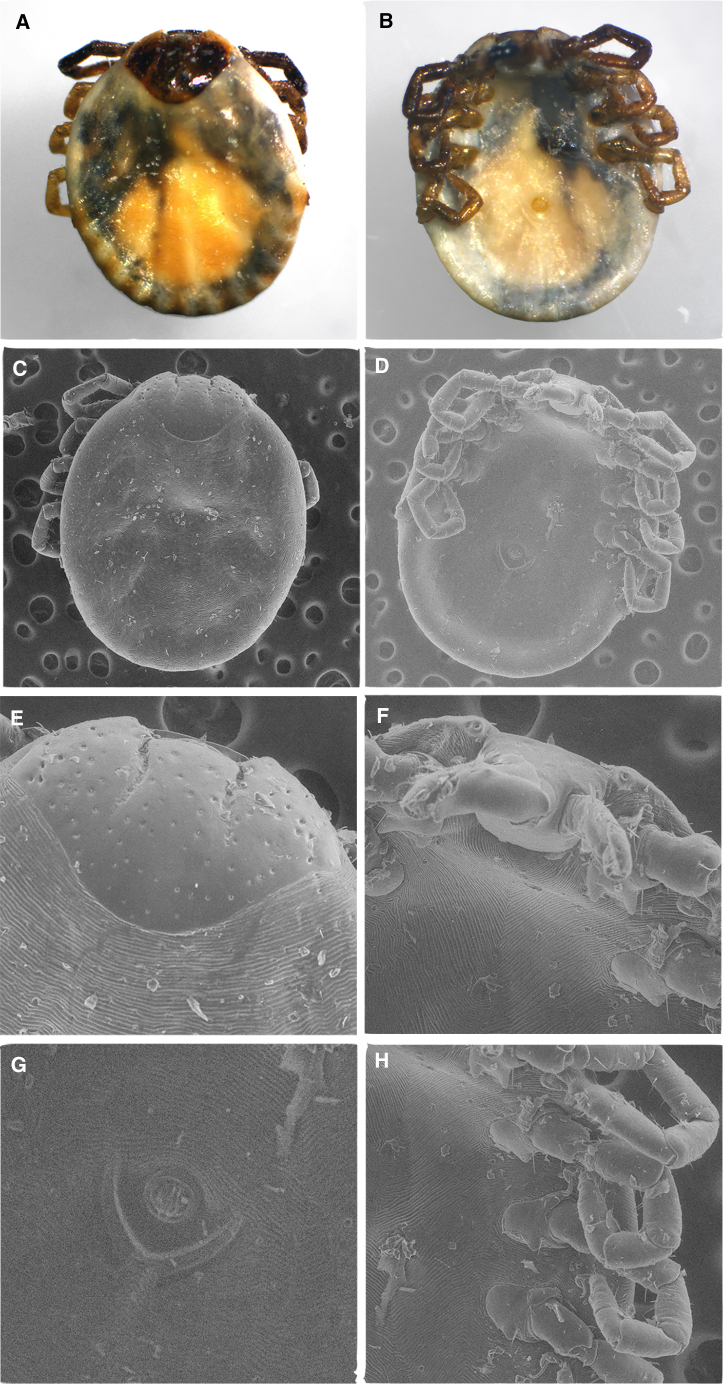
Figure 1.
Light microscopy images of the fully engorged Amblyomma mixtum imported by a US traveler returning from Guatemala
(A) dorsal aspect; (B) ventral aspect; (C) dorsal capitulum; (D) ventral anal groove.
Figure 2.
Light microscopy and scanning electron microscopy images of Amblyomma mixtum imported by a US traveler returning from Costa Rica
Light microscopy (LM) images: (A) dorsal aspect; (B) ventral aspect; scanning electron microscopy (SEM) images: (C) dorsal close-up; (D) ventral close-up; (E) dorsal close-up capitulum-scutellum; (F) ventral close-up capitulum; (G) ventral close-up anal groove; (H) ventral close-up legs, coxae, spurs.
Table 1.
Target genes and primer sequences used for taxonomic identification and pathogen screening of the invasive ticks unintentionally imported to Connecticut from 2019 to 2023
| Taxonomic group/Organism | Gene | Primer sequence (5′ – 3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| Primers of Genetic Identification | ||||
| Eukaryotes | 16S rRNA | CTGCTCAATGATTTTTTAAATTGCTGTGG CCGGTCTGAACTCAGATCAAGTA |
456 | Ushijima et al.20 |
| Invertebrates | CO1 | GGTCAACAAATCATAAAGATATTGG TAAACTTCAGGGTGACCAAAAAATCA |
710 | Folmer et al.21 |
| Hemiptera | 18S rDNA | CTGGTTGATCCTGCCAGTAG CTTCCGCAGGTTCACCTACG |
949 | von Dohlen and Moran22 McDiarmid et al.23 |
| Primers of Pathogen Screening | ||||
| Borrelia burgdorferi | flagellin | ACATATTCAGATGCAGACAGAGGT GCAATCATAGCCATTGCAGATTGT |
664 | Barbour et al.24 |
| 16S rRNA | CTGGCAGTGCGTCTTAAGCA GTATTCACCGTATCATTCTGATATAC |
1488 | Barbour et al.24 | |
| Anaplasma phagocytophilum | 16S rRNA | CACATGCAAGTCGAACGGATTATTC TTCCGTTAAGAAGGATCTAATCTCC |
932 | Massung et al.25 |
| Babesia microti | 18S rRNA | CTTAGTATAAGCTTTTATACAGC ATAGGTCAGAAACTTGAATGATACA |
238 | Persing et al.26 |
| Rickettsia spp. | ompA | ATGGCGAATATTTCTCCAAAA ATTACCTATTGTTCCGTTAATGGCA |
645 | Lee et al.27 |
| Anaplasmataceae | 16S rDNA | GGTACCYACAGAAGAAGTCC TAGCACTCATCGTTTACAGC |
345 | Parola et al.28 |
| Babesia, Theileria, and/or Hepatozoon | 18S rRNA | GTGAAACTGCGAATGGCTCATTAC AAGTGATAAGGTTCACAAAACTTCCC |
1400–1600 | Masatani et al.29 |
Amblyomma mixtum was first described by Koch in 1844 and recently reinstated as a member of the Amblyomma cajennense complex, which also includes A. cajennense sensu stricto, Amblyomma sculptum, Amblyomma interandinum, Amblyomma tonelliae, and Amblyomma patinoi.30 As a neotropical tick species, A. mixtum has the widest distribution among the members of the complex, extending from the extreme southern US to western Ecuador and some Caribbean islands including Cuba.6,31,32 In a recent study investigating the habitat use and niche width of A. mixtum, it was reported that this tick species exhibits a wide tolerance to fluctuations in relative humidity and temperature, a finding corroborated by its geographical range spanning diverse environmental conditions.33 Predictions made by ecological niche modeling suggest that additional areas in Colombia, Mexico, and Central America, notably Belize and Guatemala, could provide suitable environments for A. mixtum in the next 50 years.31
Within the US, A. mixtum is limited to the southern region of the Gulf coastal plains in Texas; however, there have been infrequent reports of its sporadic distribution in other states bordering the Gulf of Mexico. Keirans and Durden12 had described the importation of three A. cajennense ticks into the US prior to 2001: two found on horses from Guatemala and one on a human returning from Jamaica to Connecticut, USA.12 Although the A. cajennense species complex was not yet discovered at the time of the aforementioned publication, A. mixtum was later determined to be the member of the complex present in both Guatemala and Jamaica.30
Amblyomma mixtum is a generalist feeder and parasitizes a wide variety of birds and mammals, including cattle, buffalo, horses, dogs, deer, and rabbits.34,35,36 This tick is an aggressive human-biting species with all life stages frequently feeding on humans as evidenced by numerous reports within the last two decades from all seven Central American countries (Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, and Panama).37 Although there is no evidence of host preference in this tick species, in a recent laboratory study, A. mixtum did not attach to sheep for feeding but did complete its life cycle in 79 and 88 days when infested on cattle and rabbits, respectively.38
The two A. mixtum ticks in this study tested positive for Rickettsia amblyommatis after PCR screening with outer membrane protein A (ompA) primers (Table 1) and verified by comparison of these sequences to those in the NCBI GenBank DNA database (MW384864.1, MZ557071.1, CP003334.1, and CP015012.1) with a >97% similarity. Rickettsia amblyommatis is a member of the spotted fever group Rickettsia, but its public health relevance has yet to be fully elucidated. Amblyomma mixtum ticks have also tested positive for R. amblyommatis in Mexico, Panama, Colombia, Honduras, Costa Rica, Cuba, and El Salvador.39,40,41,42,43,44,45 In addition, a recent study reported the isolation of R. amblyommatis from an A. mixtum tick acquired in Cuba by a German traveler.6 Rickettsia amblyommatis has been isolated from other tick species in the US, Mexico, Australia, and countries in Central and South America.46 In the US, R.amblyommatis is mostly associated with Amblyomma ticks, particularly Amblyomma americanum.46 Although the medical importance of R. amblyommatis remains unclear, there is speculation that some cases of Rocky Mountain spotted fever (RMSF; caused by the more pathogenic Rickettsia rickettsii) in the US could be misdiagnosed and instead due to infection with R. ambylommatis.47 It is noteworthy that the relevance of Amblyomma mixtum to human health is mostly attributed to its role as a vector of R.rickettsii. This tick species is also of great veterinary health concern, particularly in Mexico, where it heavily impacts livestock by producing massive infestations and transmitting Anaplasma marginale, one of the pathogens responsible for bovine anaplasmosis.48
Case III: Amblyomma coelebs from Belize
A 54-year-old female from Willimantic, Windham County, CT, discovered a tick attached to her right arm on the day of her return from a leisure trip to Belize. Her vacation in Central America lasted from 19 to 26 March 2022 and activities included hiking through the woods, touring a national park, and visiting the Community Baboon Sanctuary. Upon removal, the tick was sent to a local health department in Hartford County, CT, before it was submitted to the CAES-TTL on 8 April 2022. The tick was initially determined to be a partially engorged nymph in the genus Amblyomma. Light and SEM images were taken (Figure 3) before the specimen was used for DNA extraction and PCR. The specimen was identified as a nymphal Amblyomma coelebs following sequencing of a portion of the COI gene obtained by PCR (Table 1), which showed a >99% similarity to several sequences belonging to that tick species in GenBank (MH513217.1, MH513216.1, and MH513218.1).
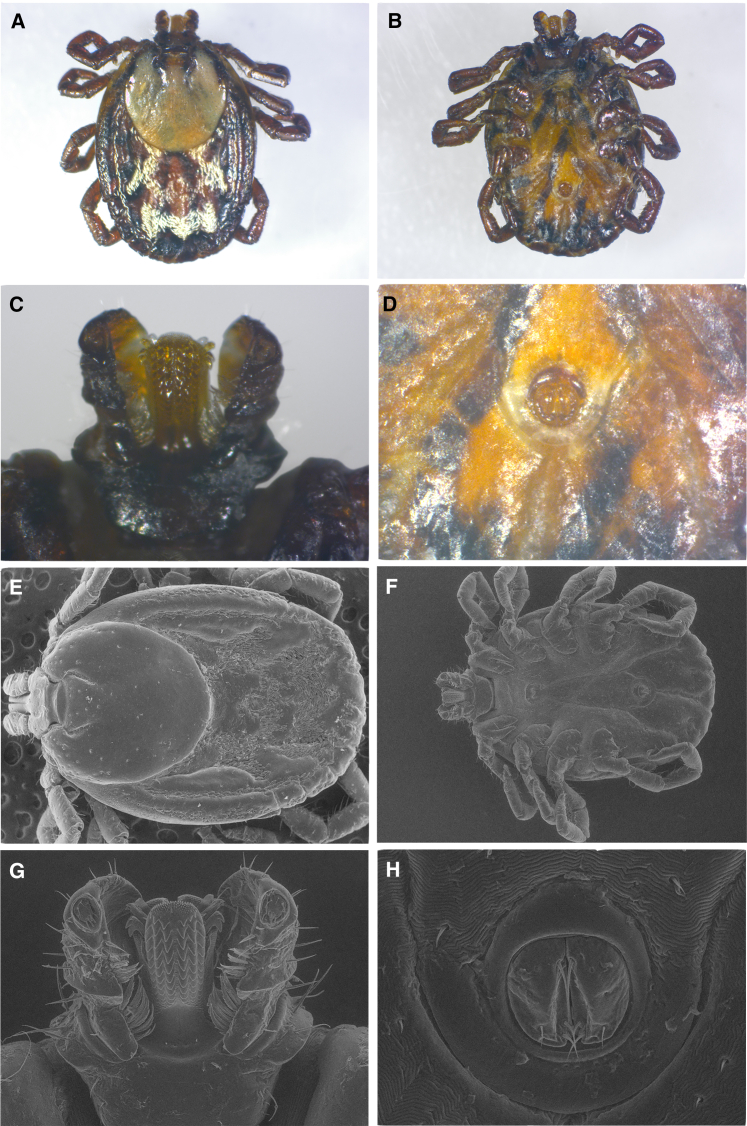
Figure 3.
Light microscopy and scanning electron microscopy images of Amblyomma coelebs imported by a US traveler returning from Belize
Light microscopy (LM) images: (A) dorsal aspect; (B) ventral aspect; scanning electron microscopy (SEM) images: (C) dorsal close-up; (D) ventral close-up; (E) dorsal close-up capitulum-scutellum; (F) ventral close-up capitulum-hypostome; (G) dorsal close-up capitulum-hypostome; (H) ventral close-up legs, coxae, spurs.
Amblyomma coelebs is distributed within the Neotropical region and extends from southern Mexico to as far south as Argentina.49 Although little is known about the suitability of various habitats for A. coelebs, it has been suggested that this tick is often found in forested regions and are not common inhabitants of marshlands.50 Various biomes in South America from which A. coelebs has been reported include the Cerrado and Amazon Rainforests in Brazil,51,52 the Atlantic Forest in northeastern Argentina and Brazil,49,53 and the Chaco region and San Rafael National Park of Paraguay.54,55 This tick appears to be active throughout the year and has been removed from humans in nearly every month of the year in Argentina and Brazil.49,53
The only documented introduction of A. coelebs into the US dates back to 2018, when the CAES-TTL received a specimen of this tick species removed from a 66-year-old Connecticut resident who acquired it during a trip to Costa Rica and Panama.14 An earlier study also reported the identification of this tick species on seeds imported from Costa Rica into Louisiana in 2001.12
Adults of this tick species feed primarily on tapirs but have also been reported parasitizing other hosts, including birds, armadillos, horses, opossums, and peccaries.14,56 Amblyomma coelebs nymphs are considered generalists and exploit a greater variety of hosts, including several species of carnivores, marsupials, rodents, and birds.57 This tick also appears to be an aggressive species and frequently feeds on humans. According to studies conducted on different life stages of several tick species in Argentina and Brazil, A. coelebs nymphs were found overwhelmingly parasitizing humans.49,53
Screening the A. coelebs specimen in this study for evidence of infection with Rickettsia, Babesia, Theileria, and/or Hepatozoon, and Anaplasmataceae pathogens did not identify DNA from these agents. Despite the frequency of A. coelebs feeding on humans, its public health relevance remains to be fully elucidated. Numerous reports have described the infection of A. coelebs with R. amblyommatis in French Guyana and Brazil58,59,60; however, as previously mentioned, the capability of this pathogen to cause human disease is unclear.
Case IV: Rhipicephalus pulchellus from Tanzania
A 67-year-old female resident of Westport, Fairfield County, CT, discovered a tick attached to her navel before removing and submitting it to the CAES-TTL on 17 July 2023. The patient vacationed in Tanzania from 29 June to 12 July 2023, during which she visited the Tarangire National Park and participated in a safari tour. Upon examination at the CAES-TTL using previously published morphological descriptions,61 the specimen was initially determined to be a slightly engorged adult female Rhipicephalus tick. After light and SEM images were taken (Figure 4), DNA was extracted from the specimen. For molecular identification, a portion of 18S rRNA was amplified (Table 1). After the PCR product was purified, sequenced, and compared to available sequences at the NCBI GenBank, the tick was identified as an adult female Rhipicephalus pulchellus, showing >99% similarity to a sequence belonging to that species in GenBank (MW837767.1).
Figure 4.
Light microscopy and scanning electron microscopy images of Rhipicephalus pulchellus imported by a US traveler returning from Tanzania
Light microscopy (LM) images: (A) dorsal aspect; (B) ventral aspect; (C) ventral hypostome; (D) ventral anal groove; scanning electron microscopy (SEM) images: (E) dorsal close-up; (F) ventral close-up; (G) ventral close-up hypostome; (H) ventral close-up anal groove.
Rhipicephalus pulchellus is one of the most common tick species found in countries in the Rift Valley and the Horn of Africa, including Ethiopia, Somalia, Kenya, Uganda, and Tanzania.62,63,64 The vast distribution of this tick species can be attributed to its ability to thrive in arid areas, such as savannas, steppes, and deserts.61 Despite its frequency in dry climates, some studies conducted in Kenya have shown that the abundance and activity of adult R. pulchellus are higher during the rainy season and increase with higher cumulative rainfall in months prior.65,66
According to an earlier report, there are three documented incursions of R. pulchellus into the US found on humans returning from Africa: one by a Michigan resident and two by Connecticut residents (a male specimen discovered on a young girl in 2010, and another male specimen found attached to a woman in 2018, both of which were submitted to the CAES-TTL for identification).5 Since 1958, there have also been numerous interceptions of R. pulchellus found on imported animals, such as zebras, giraffes, and rhinoceroses.5 Regarding all interceptions of this species in the US for which the sex of the tick was reported, significantly more males have been introduced into the country than females; the record presented here is the first of a female attached to a human in the US.
Although it is known as the “zebra tick” due to its frequent feeding on this host species, R. pulchellus infests other herbivores as well, including giraffes and camels.67 In its native region, it is the most abundant tick found on livestock, such as cattle, buffalo, sheep, and goats and has also been found parasitizing dogs.64,67,68,69 Both immature and adult life stages frequently feed on humans, and bites are usually associated with ulcer formation.5,70,71
The R. pulchellus described in this study was screened for evidence of infection with Rickettsia, Babesia, Theileria, and/or Hepatozoon, and Anaplasmataceae pathogens, and no DNA from any of these agents were detected. Rhipicephalus pulchellus has been implicated as a vector of several pathogens of human and veterinary concern. This tick is a confirmed vector of Rickettsia conorii, the causative agent of Mediterranean spotted fever (also known as boutonneuse fever), a rickettsiosis once considered a benign illness; however, following fatal human disease cases associated with septic shock and multiorgan failure, this disease is now regarded as severe as RMSF.72 This tick can also transmit Nairobi sheep disease virus, the causative agent of a disease of the same name that affects sheep and goats.73 Previous studies have identified genetic materials of Anaplasma marginale, Coxiella burnetti, and Crimean-Congo hemorrhagic fever, Dhori, and Dugbe viruses in R. pulchellus; however, the role of this tick species in transmission of these pathogens is unclear.5,65,74,75
Cases V–VII: Ixodes ricinus from Germany, Scotland, and Poland
A 76-year-old male resident of Milford, New Haven County, CT, discovered a slightly engorged tick attached to his right leg on 28 June 2023 after his return from a 10-day vacation in Germany. During his trip, he spent time in Frohnau, a locality in Berlin, where he visited a small park. He recalls that he acquired three ticks during this visit to the park but only submitted the one found on his right leg to the CAES-TTL. After the specimen was received on 13 July 2023, it was morphologically identified as an Ixodes nymph. This specimen along with the two described in the following cases were subjected to genetic identification as explained in the following text.
A 27-year-old female resident of Trumbull, Fairfield County, CT, removed a tick from her body on 21 June 2023, a day after her return from a month-long trip to Scotland, UK. Her course of travel included visiting the Isle of Skye and frequenting areas with tall grasses during nature walks. The tick was received by the CAES-TTL on 25 July 2023 and was determined to be an Ixodes larva.
A 41-year-old male resident of Illinois with a connection to Connecticut submitted a partially engorged tick to the CAES-TTL which he had acquired and removed from his chest after a week-long visit to Poland. He recalls engaging in outdoor activity during his stay, including visiting parks and going on nature walks. On 25 July 2023, the tick was initially identified at the CAES-TTL as an Ixodes nymph.
All three specimens were genetically identified by amplifying a portion of the COI and/or 18S rRNA genes (Table 1). They were identified as Ixodes ricinus with 98%–100% identity to several sequences belonging to this species in GenBank (KF197119.1, KF197127.1, GU074648.1, Z74479.1KF197122.1, and AY945432.1).
Ixodes ricinus, the castor bean tick, is the most common tick species in Europe and one of the most studied in the world.76 Widely distributed throughout the continent, this species spans the Artic circle and extends down to northern Africa and from Portugal to as far east as Azerbaijan and some western parts of Russia.77 Typical habitats where I. ricinus is found include woodlands, heathlands, grasslands, pastures, and forests.78 This species is unable to survive in low humidity, and this intolerance for dry conditions is a major determinant of its southern limit and its absence in the Mediterranean and other parts of Africa.77,78,79 Colder temperatures limit the northern and eastern distribution of this species, although rising temperatures brought about by climate change are expected to contribute to its greater range expansion.77 Climate change is also anticipated to alter precipitation patterns, and as a result, may limit I. ricinus activity in areas with reduced rainfall.76
Records of unintentional importation of I. ricinus into the US are sparse, although this may be due to difficulties in distinguishing this species from the two closely related ticks commonly found in the country: Ixodes scapularis and Ixodes pacificus. From 2008 to 2016, the CAES-TTL received five I. ricinus specimens found on travelers returning from Ireland, Norway, Hungary, England, and Estonia.
Ixodes ricinus is a generalist feeder and has been shown to parasitize more than 300 vertebrate hosts.80 Immature life stages tend to feed on small mammals, reptiles, and birds, while adults feed on medium- to large-sized mammals. This tick species is most frequently reported attached to humans in Europe,81 and all life stages readily feed on these hosts.
The two I. ricinus nymphs from Germany and Poland were screened for Borrelia, Anaplasma, and Babesia pathogens (Table 1), and both ticks were tested negative for these pathogens. In Europe, Ixodes ricinus is a vector of the common tick-borne zoonotic pathogens in the region and can transmit several bacterial, viral, and protozoan agents of medical and veterinary health concern. This tick is involved in the transmission of at least five species of Borrelia (i.e., Borrelia afzelii, Borrelia burgdorferi sensu stricto (s.s.), Borrelia garinii, Borrelia bavariensis, and Borrelia spielmanii), all of which are known to be pathogenic and cause Lyme borreliosis in humans and animals.82 Borrelia miyamotoi and tick-borne encephalitis virus (TBEV) can also be transmitted by I. ricinus. Following Lyme borreliosis, TBEV disease is the second-most burdensome tick-borne illness in European countries.82,83 Other pathogens for which I. ricinus is a known vector include Anaplasma phagocytophilum (anaplasmosis), Babesia microti (babesiosis), Babesia divergens (babesiosis), Rickettsia helvetica (aneruptive fever), Rickettsia monacensis (Mediterranean spotted fever-like illness), and Neoehrlichia mikurensis (human neoehrlichiosis).83,84,85 Some studies have also reported additional pathogens from I. ricinus, albeit not commonly, including Coxiella burnetii (Q fever) and Francisella tularensis (tularemia)82,86,87; nonetheless, it is not clear if this tick species plays a significant role in the eco-epidemiology of the aforementioned pathogens.82,83,88,89
Conclusion
Since 2008, the CAES has documented nearly 20 nonnative ticks found on human travelers returning from Central America, Europe, and Africa. These introductions highlight the risks associated with their establishment and role in pathogen transmission. To restrain their introduction, establishment, and subsequent expansion, prompt interception, and accurate identification of invasive ticks found on humans and wildlife at US ports of entry are of vital importance. It is also imperative that clinicians and public health officials be informed of the various tick species present in their region and how to morphologically distinguish native from nonnative ones. Travelers could also play a crucial role in protecting themselves against ticks encountered abroad and in preventing the importation of these potential vectors into the US. According to widely recognized tick-bite prevention strategies, people should also conduct thorough tick checks while traveling, particularly following outdoor activity in potentially tick-infested areas, and be proactive in monitoring possible symptoms following a tick bite. If a tick is found attached to a returning traveler, they must provide pertinent information to public health officials regarding their recent travel history to aid in tick identification, assess the risk of acquiring tick-borne disease, and make an appropriate diagnosis, particularly in cases of nonspecific symptoms such as fever. In addition, should the situation arise in which exotic ticks establish populations in the US, as was recently the case with H. longicornis, effective eradication measures should be implemented and promptly executed to mitigate ensuing public and veterinary health challenges.
Limitations of the study
Successful morphological identification of invasive ticks depends on the preservation of key taxonomic characters and the availability of regional taxonomic keys. In addition, genetic identification for some tick species might be affected by the availability of genetic markers and the taxonomic relations of different species in a given region. For example, in cases where a single set of primers to a target gene may generate a partial sequence identical to those in multiple closely related tick species, additional target genes may be required to properly distinguish these species. To determine the infection status in invasive ticks, the specimens should be preserved under optimal conditions. This is particularly important in the case of pathogens such as RNA viruses as the storage time and temperature affect screening success. Our determination of the infection status in ticks was achieved by PCR, which confirmed the presence of pathogen DNA/RNA in the specimens, but did not allow for the isolation of the pathogen required for establishing competence in ticks and prompting follow-up investigations. Successful identification of invasive ticks can be further compounded by the fact that human travelers may not have a good recollection of the areas where they may have encountered or been exposed to tick bites, especially when they visited more than one country or region. Nonetheless, we will continue our efforts in documenting the introduction of the invasive ticks into Connecticut (and the US), screening them for evidence of infection, and reporting to the public, public health authorities, and scientific community with the goal of raising awareness and protecting the health and well-being of the communities at risk.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Goudarz Molaei (Goudarz.Molaei@ct.gov).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We express gratitude to Abigail Chang, Katherine Dugas, and Jamie Cantoni for their assistance in creating the figures. We are also grateful to the patients and local health departments for their vigilance and submission of the ticks described here to the CAES-TTL. Funding for this study was provided, in part, by the US Congressionally Directed Spending administered by the USDA/APHIS/Veterinary Services (AP23VSSP0000G001) to the CAES-TTL.
Author contributions
Conceptualization, N.K. and G.M.; data curation, N.K. and G.M.; formal analysis, N.K. and G.M.; funding acquisition, G.M.; investigation, N.K. and G.M.; methodology, N.K., L.S., and G.M.; project administration, G.M.; resources, G.M.; software, N.K. and L.S.; supervision, G.M.; validation, G.M.; visualization, N.K. and L.S.; writing – original draft, N.K. and G.M.; writing – review and editing, N.K., L.S., and G.M.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| DNAzol BD | Molecular Research Center | Cat# DN129 |
| Polyacryl Carrier | Molecular Research Center | Cat# PC152 |
| Critical commercial assays | ||
| Taq PCR Core Kit | Qiagen | Cat# 201225 |
| QIAquick PCR Purification Kit | Qiagen | Cat# 28104 |
| Deposited data | ||
| Publicly available Amblyomma mixtum 16S rRNA, COI, and whole genome sequences | GenBank | GenBank: OP901703.1, KT820359.1, PQ373339.1 |
| Publicly available Rickettsia ambylommatis ompA and whole genome sequences | GenBank | GenBank: MW384864.1, MZ557071.1, CP003334.1, CP015012.1 |
| Publicly available Amblyomma coelebs COI sequences | GenBank | GenBank: MH513217.1, MH513216.1, MH513218.1 |
| Publicly available Rhipicephalus pulchellus 18S rRNA sequence | GenBank | GenBank: MW837767.1 |
| Publicly available Ixodes ricinus COI, 18S rRNA, and whole genome sequences | GenBank | GenBank: KF197119.1, KF197127.1, KF197122.1, AY945432.1, GU074648.1, Z74479.1 |
| Oligonucleotides | ||
| Primers used in this study, see Table 1 | This paper | N/A |
| Software and algorithms | ||
| ChromasPro | Technelysium | https://technelysium.com.au/wp/chromaspro/ |
Method details
Passive tick and tick-borne pathogen surveillance program
The Passive Tick and Tick-borne Pathogen Surveillance Program at the CAES-TTL was established in 1990, following an outbreak of an unknown illness and hospitalization of a large number of children in Lyme, CT, with arthritis and other symptoms in 1977. Ticks removed from humans are submitted by residents, health departments, and healthcare providers for species identification and pathogen testing. The program was established to test ticks primarily for evidence of infection with B. burgdorferi. In recent years, the program was expanded to include testing for two emerging tick-borne pathogens: A. phagocytophilum and B. microti. Ticks are examined under a dissecting microscope and identified to species with standard taxonomic keys,90 and in cases of unusual and unfamiliar ticks, genetic identification is also performed.
For non-Ixodes ticks described in this report, light microscopy images of the dorsal and ventral sides of the specimens were captured using a Zeiss Axio Scope.A1 (Carl Zeiss Microscopy, Göttingen, Germany) with an RT3 camera system (SPOT Imaging Solutions, Sterling Heights, Michigan). In addition, SEM images were taken.
DNA isolation from tick specimens
Invasive ticks DNAs in this report were extracted from whole specimens for molecular identification and pathogen screening using DNAzol BD (Molecular Research Center, Cincinnati, OH, USA), in line with the manufacturer’s recommendation as previously described.91 Briefly, the specimens were individually placed in 1.5-mL tubes filled with 400 μL of DNAzol BD and pulverized using the flattened end of an autoclaved paper clip. The resulting homogenates were incubated at 70°C for 10 min and then centrifuged at 20,817 × g for 10 min. Precipitation of the DNA was carried out by adding 3 μL of Polyacryl Carrier (Molecular Research Center) and 200 μL of EtOH. DNA pellets were washed twice with 75% EtOH and then resuspended in 30 μL of diH2O.
Genetic identification and pathogen screening
Genetic identification of the specimens included amplification of the COI, the 16S rRNA, and/or the 18S rDNA genes (Table 1).20,21,22,23
Ticks were screened for B. burgdorferi using two primer sets targeting the flagellin and 16S rRNA genes (Table 1).24 Screening for A. phagocytophilum and B. microti was performed by using a primer set targeting the 16S rRNA and 18S rDNA genes, respectively (Table 1).25,26 To screen for additional pathogens, the following genes were targeted: ompA for Rickettsia, 16S rDNA for Anaplasmataceae, and 18S rRNA for Babesia, Theileria, and/or Hepatozoon pathogens (Table 1).27,28,29 A detailed description of the screening methods is provided elsewhere.92,93 Screening criteria were based on the known/established role of ticks as vectors of different pathogens, and not necessarily every tick specimen was screened for all pathogens described here.
A Taq PCR Core Kit (Qiagen, Valencia, CA, USA) was used for all PCR reactions. The total reaction volume was 40 μL and consisted of 3 μL of DNA, 4 μL of 10 × Qiagen PCR Buffer (15 mmol/L MgCl2), 0.8 μL of dNTP mix (10 mmol/L), 2 μL each of forward and reverse primers (0.1–0.5 μmol/L), 0.2 μL of Taq DNA polymerase (1.25 U/reaction), and 28 μL of diH2O. The amplified PCR products were purified using a QIAquick PCR Purification Kit (Qiagen) and sequenced at the Keck Sequencing Facility, Yale University (New Haven, CT, USA), with a SeqStudio Genetic Analyzer 3730xl (Applied Biosystems, Foster City, CA, USA). Resulting sequences were annotated using ChromasPro 2.1.8 (Technelysium Pty. Ltd., South Brisbane, Queensland, Australia) and compared to the NCBI GenBank DNA sequence database.
Published: August 8, 2025
References
- 1.Eisen R.J., Kugeler K.J., Eisen L., Beard C.B., Paddock C.D. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017;58:319–335. doi: 10.1093/ilar/ilx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tickborne Disease Surveillance Data Summary. (2024). https://www.cdc.gov/ticks/data-research/facts-stats/tickborne-disease-surveillance-data-summary.html.
- 3.Molaei G., Eisen L.M., Price K.J., Eisen R.J. Range expansion of native and invasive ticks: A looming public health threat. J. Infect. Dis. 2022;226:370–373. doi: 10.1093/infdis/jiac249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monitoring Haemaphysalis longicornis, the Asian longhorned tick, populations in the United States. (2024). https://www.aphis.usda.gov/sites/default/files/monitoring-h-longicornis-plan-final-january-2024.pdf.
- 5.Stafford K.C., Molaei G., Williams S.C., Mertins J.W. Introduction of the ectoparasite Rhipicephalus pulchellus (Ixodida: Ixodidae) into Connecticut with a human traveler from Tanzania, and a review of its importation records into the United States. J. Med. Entomol. 2023;60:1426–1432. doi: 10.1093/jme/tjad109. [DOI] [PubMed] [Google Scholar]
- 6.Chitimia-Dobler L., Schaper S., Mansfeld P., Gonschorrek J., Bröker M., Nava S. Detection of Amblyomma mixtum (Acari: Ixodidae) in Germany on a human traveler returning from Cuba. J. Med. Entomol. 2020;57:962–964. doi: 10.1093/jme/tjz225. [DOI] [PubMed] [Google Scholar]
- 7.Volkow P., Grostieta E., Salceda-Sánchez B., Huerta-Jiménez H., Alcántara-Rodríguez V., Becker I., Sánchez-Montes S. A story of a lone star tick: an imported case of Amblyomma americanum (Linnaeus, 1758) infected with Rickettsia amblyommatis that parasitized a US traveler returning to Mexico. Rev. Inst. Med. Trop. Sao Paulo. 2023;65:e37. doi: 10.1590/s1678-9946202365037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pek C.H., Cheong C.S.J., Yap Y.L., Doggett S., Lim T.C., Ong W.C., Lim J. Rare cause of facial palsy: Case report of tick paralysis by Ixodes holocyclus imported by patient travelling into Singapore from Australia. J. Emerg. Med. 2016;51:e109–e114. doi: 10.1016/j.jemermed.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Mathison B.A., Gerth W.J., Pritt B.S., Baugh S. Introduction of the exotic tick Hyalomma truncatum on a human with travel to Ethiopia: A case report. Ticks Tick. Borne. Dis. 2015;6:152–154. doi: 10.1016/j.ttbdis.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molaei G., Mertins J.W., Stafford K.C. Enduring challenge of invasive ticks: Introduction of Amblyomma oblongoguttatum (Acari: Ixodidae) into the United States on a human traveler returning from Central America. J. Parasitol. 2020;106:670–674. doi: 10.1645/20-85. [DOI] [PubMed] [Google Scholar]
- 11.Xu G., Pearson P., Dykstra E., Andrews E.S., Rich S.M. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 2019;19:106–114. doi: 10.1089/vbz.2018.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keirans J.E., Durden L.A. Invasion: Exotic ticks (Acari: Argasidae, Ixodidae) imported into the United States. A review and new records. J. Med. Entomol. 2001;38:850–861. doi: 10.1603/0022-2585-38.6.850. [DOI] [PubMed] [Google Scholar]
- 13.Stafford K.C., 3rd, Molaei G., Williams S.C., Mertins J.W. Rhipicephalus capensis (Acari: Ixodidae), a geographically restricted South African tick, returning with a human traveler to the United States. Ticks Tick. Borne. Dis. 2022;13 doi: 10.1016/j.ttbdis.2022.101912. [DOI] [PubMed] [Google Scholar]
- 14.Molaei G., Karpathy S.E., Andreadis T.G. First report of the introduction of an exotic tick, Amblyomma coelebs (Acari: Ixodidae), feeding on a human traveler returning to the United States from Central America. J. Parasitol. 2019;105:571–575. [PMC free article] [PubMed] [Google Scholar]
- 15.Burridge M.J., Simmons L.A., Simbi B.H., Mahan S.M., Fournier P.E., Raoult D. Introduction of the exotic tick Amblyomma hebraeum into Florida on a human host. J. Parasitol. 2002;88:800–801. doi: 10.1645/0022-3395(2002)0880800:Ioteta2.0.Co;2. [DOI] [PubMed] [Google Scholar]
- 16.Anderson J.F., Magnarelli L.A., Burgdorfer W., Casper E.A., Philip R.N. Importation into the United States from Africa of Rhipicephalus simus on a boutonneuse fever patient. Am. J. Trop. Med. Hyg. 1981;30:897–899. doi: 10.4269/ajtmh.1981.30.897. [DOI] [PubMed] [Google Scholar]
- 17.Hills S.L., Broussard K.R., Broyhill J.C., Shastry L.G., Cossaboom C.M., White J.L., Machesky K.D., Kosoy O., Girone K., Klena J.D., et al. Tick-borne encephalitis among US travelers, 2010-20. J. Travel. Med. 2022;29 doi: 10.1093/jtm/taab167. [DOI] [PubMed] [Google Scholar]
- 18.Palau L.A., Pankey G.A. Mediterranean spotted fever in travelers from the United States. J. Travel Med. 1997;4:179–182. doi: 10.1111/j.1708-8305.1997.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 19.Maryland Department of Health announces first travel-related case, death due to tickborne illness Powassan. (2023). https://health.maryland.gov/newsroom/Pages/Maryland-Department-of-Health-announces-first-travel-related-case,-death-due-to-tickborne-illness-Powassan.aspx.
- 20.Ushijima Y., Oliver J.H., Jr., Keirans J.E., Tsurumi M., Kawabata H., Watanabe H., Fukunaga M. Mitochondrial sequence variation in Carlos capensis (Neumann), a parasite of seabirds, collected on Torishima Island in Japan. J. Parasitol. 2003;89:196–198. doi: 10.1645/0022-3395(2003)0890196:Msvicc2.0.Co;2. [DOI] [PubMed] [Google Scholar]
- 21.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 22.von Dohlen C.D., Moran N.A. Molecular phylogeny of the Homoptera: a paraphyletic taxon. J. Mol. Evol. 1995;41:211–223. doi: 10.1007/bf00170675. [DOI] [PubMed] [Google Scholar]
- 23.McDiarmid L., Petney T., Dixon B., Andrews R. Range expansion of the tick Amblyomma triguttatum triguttatum, an Australian vector of Q fever. Int. J. Parasitol. 2000;30:791–793. doi: 10.1016/s0020-7519(00)00062-x. [DOI] [PubMed] [Google Scholar]
- 24.Barbour A.G., Maupin G.O., Teltow G.J., Carter C.J., Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 25.Massung R.F., Slater K., Owens J.H., Nicholson W.L., Mather T.N., Solberg V.B., Olson J.G. Nested PCR assay for detection of granulocytic Ehrlichiae. J. Clin. Microbiol. 1998;36:1090–1095. doi: 10.1128/jcm.36.4.1090-1095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persing D.H., Mathiesen D., Marshall W.F., Telford S.R., Spielman A., Thomford J.W., Conrad P.A. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K.M., Choi Y.J., Shin S.H., Choi M.K., Song H.J., Kim H.C., Klein T.A., Richards A.L., Park K.H., Jang W.J. Spotted fever group rickettsia closely related to Rickettsia monacensis isolated from ticks in South Jeolla province, Korea. Microbiol. Immunol. 2013;57:487–495. doi: 10.1111/1348-0421.12062. [DOI] [PubMed] [Google Scholar]
- 28.Parola P., Roux V., Camicas J.L., Baradji I., Brouqui P., Raoult D. Detection of Ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000;94:707–708. doi: 10.1016/s0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- 29.Masatani T., Hayashi K., Andoh M., Tateno M., Endo Y., Asada M., Kusakisako K., Tanaka T., Gokuden M., Hozumi N., et al. Detection and molecular characterization of Babesia, Theileria, and Hepatozoon species in hard ticks collected from Kagoshima, the southern region in Japan. Ticks Tick. Borne. Dis. 2017;8:581–587. doi: 10.1016/j.ttbdis.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Nava S., Beati L., Labruna M.B., Cáceres A.G., Mangold A.J., Guglielmone A.A. Reassessment of the taxonomic status of Amblyomma cajennense with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae) Ticks Tick. Borne. Dis. 2014;5:252–276. doi: 10.1016/j.ttbdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar-Domínguez M., Moo-Llanes D.A., Sánchez-Montes S., Becker I., Feria-Arroyo T.P., de León A.P., Romero-Salas D. Potential distribution of Amblyomma mixtum (Koch, 1844) in climate change scenarios in the Americas. Ticks Tick. Borne. Dis. 2021;12 doi: 10.1016/j.ttbdis.2021.101812. [DOI] [PubMed] [Google Scholar]
- 32.Encinosa P.E., García Y., Lleonart R., Aliaga D., Fernández Y., Bello Y., la Guardia C.d., González Y., Díaz A., Estrada M.P., Rodríguez-Mallon A. Morphological and molecular characterization supporting Amblyomma mixtum presence in Cuba. Ticks Tick. Borne. Dis. 2021;12 doi: 10.1016/j.ttbdis.2020.101602. [DOI] [PubMed] [Google Scholar]
- 33.Forero-Becerra E., Acosta A., Benavides E., Martínez-Díaz H.C., Hidalgo M. Amblyomma mixtum free-living stages: Inferences on dry and wet seasons use, preference, and niche width in an agroecosystem (Yopal, Casanare, Colombia) PLoS One. 2022;17 doi: 10.1371/journal.pone.0245109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guzmán-Cornejo C., Robbins R., Guglielmone A., Montiel G., Pérez T. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa. 2011;2998:16–38. [Google Scholar]
- 35.Rodríguez-Vivas R.I., Apanaskevich D.A., Ojeda-Chi M.M., Trinidad-Martínez I., Reyes-Novelo E., Esteve-Gassent M.D., Pérez de León A.A. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Vet. Parasitol. 2016;215:106–113. doi: 10.1016/j.vetpar.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Aguilar-Domínguez M., Romero-Salas D., Sánchez-Montes S., Barradas-Piña F., Rosas-Saito G., Cruz-Romero A., Ibarra-Priego N., Becker I., Lohmeyer K.H., Pérez de León A. Occurrence of Amblyomma mixtum on the water buffalo (Bubalus bubalis) in Mexico. Int. J. Parasitol. Parasites Wildl. 2018;7:405–408. doi: 10.1016/j.ijppaw.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermúdez C S., Domínguez A L., Troyo A., Montenegro H V.M., Venzal J.M. Ticks infesting humans in Central America: A review of their relevance in public health. Curr. Res. Parasitol. Vector. Borne. Dis. 2022;2 doi: 10.1016/j.crpvbd.2021.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piña F.T.B., da Silva Rodrigues V., de Oliveira Souza Higa L., Garcia M.V., Barros J.C., de León A.A.P., Andreotti R. Life cycle of Amblyomma mixtum (Acari: Ixodidae) parasitizing different hosts under laboratory conditions. Exp. Appl. Acarol. 2017;73:257–267. doi: 10.1007/s10493-017-0178-y. [DOI] [PubMed] [Google Scholar]
- 39.Vázquez-Guerrero E., Reyes-Solís G.C., Cano-Ravell A.E., Machain-Williams C., Leyva-Gastélum M., Estrada-de Los Santos P., Álvarez-Hernández G., Ibarra J.A. Detection of Rickettsia amblyommatis and Rickettsia bellii in ticks collected from pet dogs in peri-urban and rural areas in Yucatan, Mexico. Exp. Appl. Acarol. 2023;90:441–453. doi: 10.1007/s10493-023-00825-z. [DOI] [PubMed] [Google Scholar]
- 40.Bermúdez C S., Zaldívar Y., Domínguez A L., Hernández M., de Antinori M.E.B., Krawczak F.S. Rickettsia amblyommatis isolated from Amblyomma mixtum (Acari: Ixodida) from two sites in Panama. Ticks Tick. Borne. Dis. 2021;12 doi: 10.1016/j.ttbdis.2020.101597. [DOI] [PubMed] [Google Scholar]
- 41.Chaparro-Gutiérrez J.J., Acevedo-Gutiérrez L.Y., Mendell N.L., Robayo-Sánchez L.N., Rodríguez-Durán A., Cortés-Vecino J.A., Fernández D., Ramírez-Hernández A., Bouyer D.H. First isolation of Rickettsia amblyommatis from Amblyomma mixtum in Colombia. Parasit. Vectors. 2023;16:332. doi: 10.1186/s13071-023-05950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novakova M., Literak I., Chevez L., Martins T.F., Ogrzewalska M., Labruna M.B. Rickettsial infections in ticks from reptiles, birds and humans in Honduras. Ticks Tick. Borne. Dis. 2015;6:737–742. doi: 10.1016/j.ttbdis.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Troyo A., Moreira-Soto R.D., Calderon-Arguedas Ó., Mata-Somarribas C., Ortiz-Tello J., Barbieri A.R.M., Avendaño A., Vargas-Castro L.E., Labruna M.B., Hun L., Taylor L. Detection of rickettsiae in fleas and ticks from areas of Costa Rica with history of spotted fever group rickettsioses. Ticks Tick. Borne. Dis. 2016;7:1128–1134. doi: 10.1016/j.ttbdis.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Noda A.A., Rodríguez I., Miranda J., Mattar S., Cabezas-Cruz A. First report of spotted fever group Rickettsia in Cuba. Ticks Tick. Borne. Dis. 2016;7:1057–1058. doi: 10.1016/j.ttbdis.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Romero L., Costa F.B., Labruna M.B. Ticks and tick-borne Rickettsia in El Salvador. Exp. Appl. Acarol. 2021;83:545–554. doi: 10.1007/s10493-021-00610-w. [DOI] [PubMed] [Google Scholar]
- 46.Richardson E.A., Roe R.M., Apperson C.S., Ponnusamy L. Rickettsia amblyommatis in ticks: A review of distribution, pathogenicity, and diversity. Microorganisms. 2023;11 doi: 10.3390/microorganisms11020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apperson C.S., Engber B., Nicholson W.L., Mead D.G., Engel J., Yabsley M.J., Dail K., Johnson J., Watson D.W. Tick-borne diseases in North Carolina: Is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez S.D., García Ortiz M.Á., Jiménez Ocampo R., Vega y Murguía C.A. Molecular epidemiology of bovine anaplasmosis with a particular focus in Mexico. Infect. Genet. Evol. 2009;9:1092–1101. doi: 10.1016/j.meegid.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Lamattina D., Nava S. Ticks infesting humans in northern Misiones, Argentina. Medicina. 2016;76:89–92. [PubMed] [Google Scholar]
- 50.Szabó M.P.J., Castro M.B., Ramos H.G.C., Garcia M.V., Castagnolli K.C., Pinter A., Veronez V.A., Magalhães G.M., Duarte J.M.B., Labruna M.B. Species diversity and seasonality of free-living ticks (Acari: Ixodidae) in the natural habitat of wild Marsh deer (Blastocerus dichotomus) in Southeastern Brazil. Vet. Parasitol. 2007;143:147–154. doi: 10.1016/j.vetpar.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Garcia M.V., Matias J., Aguirre A.D.A.R., Csordas B.G., SzabÓ M.P.J., Andreotti R. Successful feeding of Amblyomma coelebs (Acari: Ixodidae) nymphs on humans in Brazil: Skin reactions to parasitism. J. Med. Entomol. 2015;52:117–119. doi: 10.1093/jme/tju060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labruna M.B., Camargo L.M.A., Terrassini F.A., Ferreira F., Schumaker T.T.S., Camargo E.P. Ticks (Acari: Ixodidae) from the state of Rondonia, western Amazon, Brazil. Syst. Appl. Acarol. 2004;10:17. doi: 10.11158/saa.10.1.4. [DOI] [Google Scholar]
- 53.Suzin A., da Silva M.X., Tognolli M.H., Vogliotti A., Adami S.F., Moraes M.F.D., Nunes P.H., Szabó M.P.J. Ticks on humans in an Atlantic rainforest preserved ecosystem in Brazil: Species, life stages, attachment sites, and temporal pattern of infestation. Ticks Tick. Borne. Dis. 2022;13 doi: 10.1016/j.ttbdis.2021.101862. [DOI] [PubMed] [Google Scholar]
- 54.Nava S., Lareschi M., Rebollo C., Benítez Usher C., Beati L., Robbins R.G., Durden L.A., Mangold A.J., Guglielmone A.A. The ticks (Acari: Ixodida: Argasidae, Ixodidae) of Paraguay. Ann. Trop. Med. Parasitol. 2007;101:255–270. doi: 10.1179/136485907x176319. [DOI] [PubMed] [Google Scholar]
- 55.Ogrzewalska M., Literak I., Martins T.F., Labruna M.B. Rickettsial infections in ticks from wild birds in Paraguay. Ticks Tick. Borne. Dis. 2014;5:83–89. doi: 10.1016/j.ttbdis.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Ogrzewalska M., Pacheco R.C., Uezu A., Richtzenhain L.J., Ferreira F., Labruna M.B. Ticks (Acari: Ixodidae) infesting birds in an Atlantic rain forest region of Brazil. J. Med. Entomol. 2009;46:1225–1229. doi: 10.1603/033.046.0534. [DOI] [PubMed] [Google Scholar]
- 57.Estrada-Peña A., Nava S., Tarragona E., de la Fuente J., Guglielmone A.A. A community approach to the Neotropical ticks-hosts interactions. Sci. Rep. 2020;10:9269. doi: 10.1038/s41598-020-66400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parola P., Matsumoto K., Socolovschi C., Parzy D., Raoult D. A tick-borne Rickettsia of the spotted-fever group, similar to Rickettsia amblyommii, in French Guyana. Ann. Trop. Med. Parasitol. 2007;101:185–188. doi: 10.1179/136485907154557. [DOI] [PubMed] [Google Scholar]
- 59.Souza U.A., Fagundes-Moreira R., Costa F.B., Alievi M.M., Labruna M.B., Soares J.F. Rickettsia amblyommatis-infected Amblyomma coelebs parasitizing a human traveler in Rio Grande do Sul, southern Brazil, after returning from the Amazon. Travel Med. Infect. Dis. 2022;48 doi: 10.1016/j.tmaid.2022.102328. [DOI] [PubMed] [Google Scholar]
- 60.Labruna M.B., Whitworth T., Bouyer D.H., McBride J., Camargo L.M.A., Camargo E.P., Popov V., Walker D.H. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondônia, Western Amazon, Brazil. J. Med. Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- 61.Walker A., Bouattour A., Camicas J.L., Estrada-Peña A., Horak I., Latif A., Pegram R.G., Preston P.M. Bioscience Reports; 2014. Rhipicephalus pulchellus (Gerstäcker, 1873). In Ticks of domestic animals in Africa: A guide to identification of species; pp. 200–201. [Google Scholar]
- 62.Jimale K.A., Wall R., Otranto D. Ticks and tick-borne pathogens of domestic animals in Somalia and neighboring regions of Ethiopia and Kenya. Acta Trop. 2023;243 doi: 10.1016/j.actatropica.2023.106944. [DOI] [PubMed] [Google Scholar]
- 63.Balinandi S., Chitimia-Dobler L., Grandi G., Nakayiki T., Kabasa W., Bbira J., Lutwama J.J., Bakkes D.K., Malmberg M., Mugisha L. Morphological and molecular identification of Ixodid tick species (Acari: Ixodidae) infesting cattle in Uganda. Parasitol. Res. 2020;119:2411–2420. doi: 10.1007/s00436-020-06742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwak Y.S., Kim T.Y., Nam S.H., Lee I.Y., Kim H.P., Mduma S., Keyyu J., Fyumagwa R., Yong T.S. Ixodid tick infestation in cattle and wild animals in Maswa and Iringa, Tanzania. Korean J. Parasitol. 2014;52:565–568. doi: 10.3347/kjp.2014.52.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lutomiah J., Musila L., Makio A., Ochieng C., Koka H., Chepkorir E., Mutisya J., Mulwa F., Khamadi S., Miller B.R., et al. Ticks and tick-borne viruses from livestock hosts in arid and semiarid regions of the eastern and northeastern parts of Kenya. J. Med. Entomol. 2014;51:269–277. doi: 10.1603/me13039. [DOI] [PubMed] [Google Scholar]
- 66.Keesing F., Ostfeld R.S., Young T.P., Allan B.F. Cattle and rainfall affect tick abundance in central Kenya. Parasitology. 2018;145:345–354. doi: 10.1017/s003118201700155x. [DOI] [PubMed] [Google Scholar]
- 67.Jama M.M., Hussein H.A., Abdi S.M., Feyera T. Participatory and conventional investigation of tick infestation in camels and cattle of Somali Pastoral Areas, Eastern Ethiopia. J. Parasitol. Res. 2023;2023 doi: 10.1155/2023/5840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathewos M., Welamu W., Fesseha H., Aliye S., Endale H. Study on prevalence of hard ticks and their associated risk factors in small ruminants of Boloso Sore Districts of Wolaita Zone, Southern Ethiopia. Vet. Med. 2021;12:293–301. doi: 10.2147/vmrr.S336467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumsa B., Abiy Y., Abunna F. Ectoparasites infesting dogs and cats in Bishoftu, central Oromia, Ethiopia. Vet. Parasitol. Reg. Stud. Reports. 2019;15 doi: 10.1016/j.vprsr.2019.100263. [DOI] [PubMed] [Google Scholar]
- 70.Roberts J.I. The ticks of rodents and their nests, and the discovery that Rhipicephalus sanguineus Latr. is the vector of tropical typhus in Kenya. J. Hyg. 1935;35:1–22. doi: 10.1017/S0022172400018933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wanzala W., Ondiaka S.N. Tick-borne lymphadenopathy-like condition in an African woman in Kenya. J. Res. Med. Sci. 2013;18:918–921. [PMC free article] [PubMed] [Google Scholar]
- 72.Rovery C., Brouqui P., Raoult D. Questions on Mediterranean spotted fever a century after its discovery. Emerg. Infect. Dis. 2008;14:1360–1367. doi: 10.3201/eid1409.071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krasteva S., Jara M., Frias-De-Diego A., Machado G. Nairobi Sheep Disease Virus: A historical and epidemiological perspective. Front. Vet. Sci. 2020;7:419. doi: 10.3389/fvets.2020.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumsa B., Socolovschi C., Almeras L., Raoult D., Parola P. Occurrence and genotyping of Coxiella burnetii in Ixodid ticks in Oromia, Ethiopia. Am. J. Trop. Med. Hyg. 2015;93:1074–1081. doi: 10.4269/ajtmh.14-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fyumagwa R.D., Simmler P., Meli M.L., Hoare R., Hofmann-Lehmann R., Lutz H. Prevalence of Anaplasma marginale in different tick species from Ngorongoro Crater, Tanzania. Vet. Parasitol. 2009;161:154–157. doi: 10.1016/j.vetpar.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 76.Gray J., Kahl O., Zintl A. What do we still need to know about Ixodes ricinus? Ticks Tick. Borne. Dis. 2021;12 doi: 10.1016/j.ttbdis.2021.101682. [DOI] [PubMed] [Google Scholar]
- 77.Černý J., Lynn G., Hrnková J., Golovchenko M., Rudenko N., Grubhoffer L. Management options for Ixodes ricinus-associated pathogens: A review of prevention strategies. Int. J. Environ. Res. Public Health. 2020;17:1830. doi: 10.3390/ijerph17061830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-Peña A., George J.C., Golovljova I., Jaenson T.G.T., Jensen J.K., Jensen P.M., et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kahl O., Gray J.S. The biology of Ixodes ricinus with emphasis on its ecology. Ticks Tick. Borne. Dis. 2023;14 doi: 10.1016/j.ttbdis.2022.102114. [DOI] [PubMed] [Google Scholar]
- 80.Gern L., Humair P.-F. In: Lyme Borreliosis: Biology, Epidemiology and Control. Gray J., Kahl O., Lane R., Stanek G., editors. CABI; 2002. Ecology of Borrelia burgdorferi sensu lato in Europe; pp. 149–174. [Google Scholar]
- 81.Gern L. The biology of the Ixodes ricinus tick. Ther. Umsch. 2005;62:707–712. doi: 10.1024/0040-5930.62.11.707. [DOI] [PubMed] [Google Scholar]
- 82.Gray J., Kahl O., Zintl A. Pathogens transmitted by Ixodes ricinus. Ticks Tick. Borne. Dis. 2024;15 doi: 10.1016/j.ttbdis.2024.102402. [DOI] [PubMed] [Google Scholar]
- 83.Rizzoli A., Silaghi C., Obiegala A., Rudolf I., Hubálek Z., Földvári G., Plantard O., Vayssier-Taussat M., Bonnet S., Spitalská E., Kazimírová M. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front. Public Health. 2014;2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stuen S., Granquist E.G., Silaghi C. Anaplasma phagocytophilum--a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gray J.S., Estrada-Peña A., Zintl A. Vectors of babesiosis. Annu. Rev. Entomol. 2019;64:149–165. doi: 10.1146/annurev-ento-011118-111932. [DOI] [PubMed] [Google Scholar]
- 86.Špitalská E., Sparagano O., Stanko M., Schwarzová K., Špitalský Z., Škultéty Ľ., Havlíková S.F. Diversity of Coxiella-like and Francisella-like endosymbionts, and Rickettsia spp., Coxiella burnetii as pathogens in the tick populations of Slovakia, Central Europe. Ticks Tick. Borne. Dis. 2018;9:1207–1211. doi: 10.1016/j.ttbdis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Reye A.L., Stegniy V., Mishaeva N.P., Velhin S., Hübschen J.M., Ignatyev G., Muller C.P. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Körner S., Makert G.R., Ulbert S., Pfeffer M., Mertens-Scholz K. The prevalence of Coxiella burnetii in hard ticks in Europe and their role in Q Fever transmission revisited-a systematic review. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.655715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sormunen J.J., Pakanen V.-M., Elo R., Mäkelä S., Hytönen J. Absence of Francisella tularensis in Finnish Ixodes ricinus and Ixodes persulcatus ticks. Ticks Tick. Borne. Dis. 2021;12 doi: 10.1016/j.ttbdis.2021.101809. [DOI] [PubMed] [Google Scholar]
- 90.Keirans J.E., Litwak T.R. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 1989;26:435–448. doi: 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- 91.Little E.A.H., Molaei G. Passive tick surveillance: exploring spatiotemporal associations of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Babesia microti (Piroplasmida: Babesiidae), and Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) infection in Ixodes scapularis (Acari: Ixodidae) Vector Borne Zoonotic Dis. 2020;20:177–186. doi: 10.1089/vbz.2019.2509. [DOI] [PubMed] [Google Scholar]
- 92.Pokutnaya D., Molaei G., Weinberger D.M., Vossbrinck C.R., Diaz A.J. Prevalence of infection and co-infection and presence of Rickettsial endosymbionts in Ixodes scapularis (Acari: Ixodidae) in Connecticut, USA. J. Parasitol. 2020;106:30–37. [PubMed] [Google Scholar]
- 93.Qiu Y., Kaneko C., Kajihara M., Ngonda S., Simulundu E., Muleya W., Thu M.J., Hang'ombe M.B., Katakura K., Takada A., et al. Tick-borne haemoparasites and Anaplasmataceae in domestic dogs in Zambia. Ticks Tick. Borne. Dis. 2018;9:988–995. doi: 10.1016/j.ttbdis.2018.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.