Abstract
Functional neuroimaging in humans with acute brain damage often reveals decreases in blood flow and metabolism in areas unaffected by the lesion. This phenomenon, termed diaschisis, is presumably caused by disruption of afferent excitatory input from the lesioned area to other brain regions. By characterizing its neurophysiological basis, we used cerebellar diaschisis to study the relationship between electrical activity and blood flow during decreased neuronal activity. Here we show that focal cerebral ischemia in rats causes diaschisis in the cerebellar cortex characterized by pronounced decreases in Purkinje cell spiking activity and small decreases in cerebellar blood flow. The findings were explained by decreased excitatory input to the cerebellar cortex, i.e., deactivation, as cerebellar neuronal excitability and vascular reactivity were preserved. Functional ablation of the cerebral cortex by either spreading depression or tetrodotoxin reproduced the changes in cerebellar function with complete recovery of Purkinje cell activity and cerebellar blood flow concomitant with recovery of neocortical function. Decreases of activity involving the contralateral frontal cortex produced the largest decrease in cerebellar electrical activity and blood flow. Our data suggest that deactivation explains the decreases in blood flow and metabolism in cerebellar diaschisis observed in human neuroimaging studies. Decreases in spiking activity were 3–7 times larger than the respective decreases in flow. Therefore, under pathological conditions, neuroimaging methods based on hemodynamic signals may only show small changes, although the underlying decrease in neuronal activity is much larger.
Increases in cerebral blood flow (CBF) and metabolism are used as indicators of cerebral function, because both are coupled to increased neuronal activity (1–3). The influence of decreased neuronal activity on CBF and metabolism is less well understood, but essential for an understanding of the neurophysiological basis of vascular or metabolic signals obtained with functional neuroimaging techniques in humans. Reduced nerve cell activity may be related to increased synaptic inhibition, or to deactivation caused by a decrease of an excitatory synaptic input to a defined neuronal circuit. Deactivation may underlie the phenomenon known as diaschisis in which interruption of the excitatory output from an injured brain region is thought to cause decreases of activity in connecting brain regions (4, 5). We used diaschisis to study the neuronal and vascular mechanisms accompanying deactivation.
Diaschisis is commonly observed in acute brain disorders, such as stroke, causing clinical deficits that exceed the functional consequences expected from the focal lesion with regard to its size and location in the brain. The concept of diaschisis has gained renewed interest from experimental (6, 7) and clinical studies that revealed decreased metabolism and CBF in anatomically intact brain regions remote from a cerebral lesion (8–10), e.g., the cerebellar hemisphere contralateral to a lesion of the cerebral cortex. This crossed cerebellar diaschisis (CCD) is presumably caused by interruption of the cortico-ponto-cerebellar projections (11).
Our goal was to establish CCD in an animal model, to correlate electrophysiological recordings to blood flow changes during CCD, thereby providing an experimental model of deactivation. To this end, we recorded single unit activity (spikes) of Purkinje cells as well as cerebellar blood flow (CeBF) under conditions of unilateral focal cerebral ischemia. In addition, we recorded synaptic field potentials (FP) in the cerebellar cortex, evoked by electrical stimulation of the inferior olivary nucleus to test the excitability of Purkinje cells during cerebellar diaschisis. According to the original theory, diaschisis is based on a decrease in electrical output of a population of cortical neurons, not their anatomical integrity. However, it has been suggested that irreversible cell damage in projecting brain regions may be a condition for CCD (12). This hypothesis was tested in experiments in which we examined whether CCD could be induced by a decrease in cerebral cortical function alone. For this purpose, we applied to the cerebral cortex the sodium channel blocker tetrodotoxin (TTX) that abolishes generation of action potentials, or cortical spreading depression (CSD) that induces a spontaneously reversible suppression of cortical function (13). Finally, we used the sequential blockade of neocortical function produced by the propagation of CSD to identify the neocortical region with most influence on cerebellar hemispheric spontaneous spiking activity and blood flow.
Methods
Experiments were performed in 23 male Wistar rats (250–350 g). All studies were in compliance with the guidelines of the European Community for the Care and Use of Laboratory Animals.
Anesthesia was induced by inhalation of isoflurane (1.5% in 30% O2/70% N2O), and switched to continuous i.v. perfusion of α-chloralose (40 mg/kg body weight) and inhalation of oxygen-enriched air when surgery was completed. The trachea was cannulated for mechanical ventilation. Femoral vessels were continuously perfused with physiological saline through small catheters. Monitoring of blood pressure and hourly blood sample analysis assured maintenance of basic physiological parameters. We used open cranial windows, and superfused the brain with artificial cerebrospinal fluid as described in detail (14). Trepanations were drilled over the cerebellar hemispheres, the left parieto-frontal cortex, or frontal and parietal cortex. For experiments using electrical stimulation of the inferior olive, the cerebellar window was extended caudally for access to the lower part of vermis and the cranial part of spinal cord.
Ischemia.
Focal ischemia in the left cerebral hemisphere was induced by occluding the left distal middle cerebral artery (MCA) plus the common carotid artery (CCA) (15). We accessed the left MCA via a temporal trepanation, and used a micromanipulator to retract a small metal hook to induce permanent MCA occlusion (MCAO). Changes in CBF after occlusion of the MCA, were monitored over the fronto-parietal cortex by using Laser Doppler flowmetry. After 45–60 min of MCAO, we additionally occluded the left CCA (CCAO) with a loop of plastic tubing to monitor the effect of two grades of cerebral ischemia on contralateral cerebellar function.
Functional Ablation.
CSD was elicited in either the parieto-occipital or frontal cortex by a minimally traumatizing needle stab. TTX (Sigma) was dissolved in artificial cerebrospinal fluid (20 μM). The solution was directly applied onto the cortical surface by using a micropipette.
Electrophysiological Recordings.
We used single-barreled glass microelectrodes, filled with 2 M saline (impedance, 2–3 MOhm; tip, 2 μm). Single unit activity (spikes) and synaptic field potentials of Purkinje cells were recorded at a depth of 300–600 μm in Crus II of the right cerebellar hemisphere. Electrocorticograms (ECoGs) were recorded at a depth of 800 μm in the left cerebral cortex with a bipolar montage by using the same type of microelectrode (2-mm tip distance). An Ag/AgCl ground electrode was placed in the neck muscle.
The signal was A/D-converted, amplified and filtered, and digitally sampled by using the 1401-plus hardware (Cambridge Electronic Design) connected to a PC running the SPIKE 2.3 software. Digital sampling rates were at 20 kHz for spikes, and 5 kHz for FP and ECoG.
Inferior Olive Stimulation.
A coated bipolar stainless steel electrode (SNEX 200, RMI, Woodland Hills, CA; 0.25 mm contact separation) was stereotaxically lowered into the caudal part of the inferior olive as described (16). Positioning was optimized by means of the maximal response of FP in the contralateral cerebellar hemisphere to continuous low frequency stimulation (0.25 Hz). Pulses of 200 μs constant current with an intensity of 150 μA were used at frequencies of 1, 5, and 10 Hz. Intervals of 2 min separated stimulations of 30-s duration. A stimulation-free period of 5 min before and after each arterial occlusion allowed undisturbed observation of Purkinje cell spiking activity to show CCD in these animals.
Laser-Doppler Measurements.
Optic probes (410, Perimed, 780 nm wavelength, 250 μm fiber separation) were used for Laser Doppler flowmetry (Periflux 4001 Master, Perimed) of CBF and CeBF. The probes were placed on the cortical surface of a region devoid of large vessels (>100 μm) as close as possible to the microelectrode. The signal was A/D converted and recorded by using the CED 1401-plus interface and the SPIKE 2.3 software (200-Hz digital sampling rate).
Data Analysis and Statistics.
Values are expressed as mean ± SEM. Data were normalized before statistical analysis. Normalized data of ECoG changes are based on the root mean square of the ECoG-amplitude. Levels of significance were determined by paired t test between groups. Changes were considered statistically significant at P < 0.05.
Spikes were identified by shape and amplitude before data acquisition. Automatic spike sorting was used to remove noise contributions to the calculated event rate (SPIKE 2.3 software).
The FPs were averaged and amplitudes were calculated as difference of peak to baseline (mean of the 15 ms before stimulation onset). The mean amplitude was calculated for each frequency in all three circumstances (control, MCAO, and MCA plus CCAO). Linear regression analysis was used to estimate the correlation between the increase in CeBF (area under curve) and the total evoked electrical activity, expressed as the summed field potential amplitude or ΣFP, as described (17).
Results
The effect of unilateral focal cerebral ischemia on electrical activity and blood flow in the contralateral cerebellar hemisphere was detected by extracellular recordings of activity of either individual Purkinje cells (spikes) or groups of neurons (FP), combined with Laser Doppler flowmetry on a real-time basis.
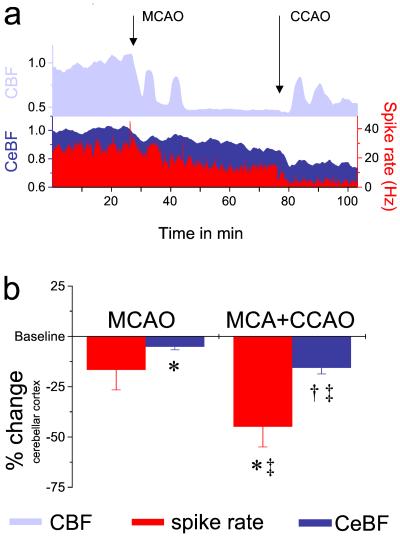
A rapid and pronounced drop in CBF in the fronto-parietal cortex accompanied successful occlusion of the MCA and CCA. This was followed by transient increases in CBF that resulted from peri-infarct depolarizations in the penumbra zone surrounding the lesion (Fig. 1a). Occlusion of the MCA alone was followed by a decrease in spike activity of Purkinje cells in the contralateral cerebellum in 6 of the 10 animals studied (Fig. 1a); in the remaining animals the spike rate either remained constant (n = 2) or increased (n = 2). Thus the decrease in mean spike rate was not statistically significant (16.7 ± 10% decrease compared with preoccluded values in the same animals, Fig. 1b). The changes in electrical activity in the contralateral cerebellar hemisphere were accompanied by a significant, although less pronounced decrease in mean CeBF in all 10 rats (5.2 ± 1.5% decrease compared with preocclusion values, Fig. 1). Simultaneous occlusion of both MCA and CCA significantly decreased the mean spike rate and CeBF compared with preocclusion baselines in all animals studied (by 45 ± 10% and 15.6 ± 3.0%, respectively, Fig. 1). Furthermore, the additional effect of CCAO after MCAO alone was highly significant for both mean spike rate and CeBF (additional decreases of 28.2 ± 8%, and 10 ± 2.7%, respectively). The decrease in relative spike activity was approximately three times larger than the decrease in CeBF (Fig. 1b).
Figure 1.
Occluding the left middle cerebral artery, either alone or in combination with the common carotid artery, reduces spike activity and blood flow in the contralateral cerebellar hemisphere. (a) Representative data from one experiment: The CBF in the left cerebral hemisphere decreased markedly after MCAO. Transient increases in CBF indicated peri-infarct depolarizations (PIDs). Additional CCAO further lowered CBF and induced repetitive PIDs. MCAO and CCAO induced gradual reductions of Purkinje cell spike rate. Decreases in neuronal activity were reflected by moderate decreases in CeBF. The y axis without units indicates normalized data. (b) Statistical analysis for all ischemia experiments presented as normalized data (n = 10). Data significantly different from preocclusion baseline: *, P < 0.01; †, P < 0.001. Data significantly different from levels after MCAO alone: ‡, P < 0.01.
To gain information about the uncrossed component of cerebellar diaschisis, we measured blood flow in the ipsilateral cerebellar cortex in two animals following combined MCA- and CCA-occlusion. Although ipsilateral CeBF decreased in response to focal cerebral ischemia (≈10%), the reduction was less pronounced than that observed in the contralateral cerebellar hemisphere (≈25%).
The effect of the combined arterial occlusion on Purkinje cell spike activity and CeBF in the contralateral cerebellar hemisphere was more pronounced and consistent compared with MCA-occlusion alone. A possible explanation was that infarcts due to combined occlusion cover a larger cortical area that involves regions with strong projections to the cerebellar hemisphere, such as the frontal cortex. This possibility was examined in a series of experiments, in which we first sought to determine whether CCD could be induced by a fully reversible decrease in cortical function and, second, to reveal the specific contribution of topographically distinct regions of the cerebral cortex to CCD.
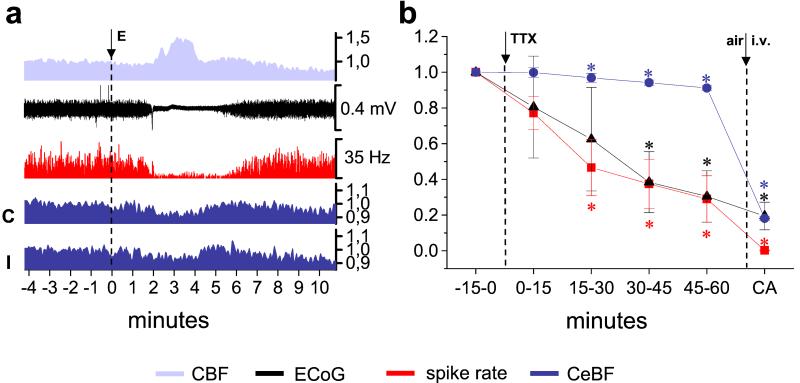
We used the transient unilateral suppression of electrical activity of the cerebral cortex caused by CSD to achieve reversible functional decortication. In four animals we recorded ECoGs and CBF in the fronto-parietal cortex, spike activity, and CeBF in the contralateral and ipsilateral cerebellar hemispheres. CSD was identified by a transient suppression of the ECoG accompanied by a concomitant rise in CBF (Fig. 2a). Spike activity in Purkinje cells decreased concomitantly with the attenuation of the ECoG, remained low throughout the ECoG depression and returned to baseline in parallel with the ECoG signal (Fig. 2a). A simultaneous, transient decrease in CeBF was observed in both cerebellar hemispheres (Fig. 2a, C/I). The mean Purkinje cell spike rate dropped by 56.8 ± 6.7% (P < 0.001) during suppression of the ECoG and returned to 90 ± 4.8% (P < 0.05) of baseline within the following 10 min (measured in a total of 12 episodes of CSD in four animals). Mean CeBF decreased in the contralateral and ipsilateral cerebellar hemisphere (Fig. 2a, C/I), by 8.2 ± 1% (P < 0.01) and 8.3 ± 1% (P < 0.01), respectively. Thus, the decrease in spike rate was approximately 7 times larger than that in CeBF in the cerebellar hemisphere contralateral to CSD. There was no significant difference between the mean CeBF at control conditions and after the ECoG had returned to baseline after CSD. As repeat episodes of CSD do not induce neuronal injury in normal brains (18), these data indicate that suppression of electrical activity in the cortex is sufficient to produce cerebellar diaschisis.
Figure 2.
Unilateral functional ablation of the cerebral cortex induces crossed cerebellar diaschisis. (a) Original recordings from one animal. Cortical spreading depression was elicited (E) in the left parieto-occipital cortex and then reached the site of CBF- and ECoG-recordings in the parieto-frontal, as indicated by the characteristic increase in CBF and the suppression of the ECoG. At the same time, the spontaneous spiking (red) in Crus II of the contralateral cerebellar cortex and both contralateral (C) and ipsilateral CeBF (I) decreased as well. The y axis without units indicates normalized data. (b) Functional ablation of the cerebral cortex with TTX induces crossed cerebellar diaschisis (n = 4). Normalized mean data of 15 min periods of ECoG, as well as spike rate and CeBF in the contralateral cerebellum at baseline and during the 60 min after application of TTX to the left anterior cerebral cortex. The background values obtained 2 min after cardiac arrest (CA) are shown for comparison. *, Values significantly different from baseline (P < 0.05).
To confirm these findings, TTX, which blocks action potential propagation and hence all ongoing neuronal activity, was topically applied to the left anterior cortex of four rats. The ECoG-amplitude (Fig. 2b, black triangle) gradually decreased over time with a slight delay in the first 15 min, which presumably reflects the time taken for TTX to diffuse to deeper cortical layers.
During this initial period after TTX application to the cerebral cortex, the spike rate of Purkinje cells in the contralateral cerebellum (Fig. 2b, red squares) showed a moderate nonsignificant decrease. During the next 60 min, the ECoG-amplitude declined further to a value (69.5 ± 14.4% reduction to pre-TTX level, P < 0.05), which was not significantly different from the noise level observed after cardiac arrest (Fig. 2b, CA), although CBF only decreased by ≈16% (not shown, two animals). The flat ECoG was accompanied by a simultaneous reduction in the spike rate of Purkinje cells in the contralateral cerebellum (71 ± 1.3% reduction to pre-TTX value, P < 0.05). At the same time, CeBF in the contralateral cerebellum (Fig. 2b, dark blue circles) also declined, but by only 8.9 ± 1.2% (P < 0.01) over the whole period. Taken together, these results indicate that tissue damage is not necessary for the development of CCD.
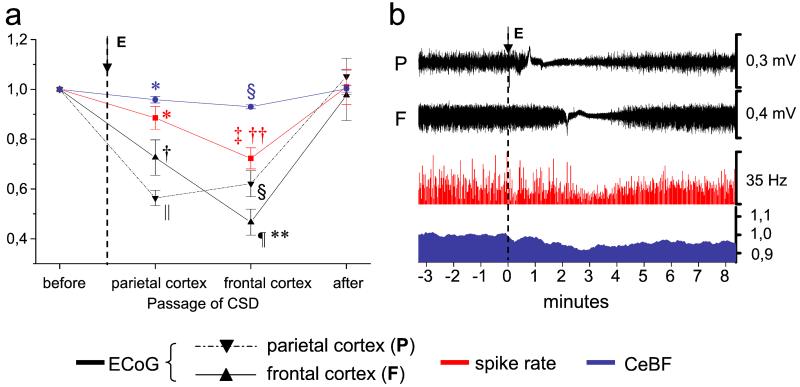
In the next step, we took advantage of the sequential suppression of activity in functionally distinct cortical areas during propagation of cortical spreading depression to study the ability of topographically distinct brain regions to generate CCD. A total of nine CSDs were induced in three additional animals. Four CSDs were elicited in the frontal cortex, and five in the parieto-occipital cortex. Fig. 3b depicts original data from one rat in which a single wave of CSD elicited in the left occipital cortex propagated to the parietal cortex (Fig. 3, P) and, subsequently, to the frontal cortex (Fig. 3, F), as indicated by the suppressed ECoG signal. Mean normalized data of this group are summarized in Fig. 3a; passage of CSD over the parietal cortex had relatively moderate effect (11.5 ± 4.5% decrease, P < 0.05) on the spike rate of Purkinje cells in the contralateral cerebellar hemisphere (Fig. 3, red). The maximum decrease (27.8 ± 4.2%, P < 0.001) occurred when the CSD reached the frontal cortex, at a time when the ECoG in the parietal cortex had already partly recovered (Fig. 3, P). The maximum decrease in contralateral CeBF also occurred when the ECoG in the frontal cortex was maximally suppressed (Fig. 3, dark blue). These data indicate that projections from the frontal cortex have a particularly strong effect on neuronal activity and blood flow in the contralateral cerebellar hemisphere.
Figure 3.
Crossed cerebellar diaschisis is best explained by functional ablation of the frontal cortex. (a) Summary of nine CSDs in three animals: CSD was elicited (E) in the left parieto-occipital (n = 5) or frontal cortex (n = 4), propagated over the hemisphere, and subsequently reached ECoG-recordings, in the left parietal (P) and frontal cortex (F), depending on the point of elicitation. (b) Typical example of one CSD, elicited (E) in the left parieto-occipital cortex: Spike rate and CeBF in the contralateral cerebellar hemisphere reached their minimum when CSD-induced suppression of ECoG-activity involved the frontal cortex (F) and the parietal ECoG (P) had already partly recovered. The y axis without units indicates normalized data. Levels of significance compared with control (baseline): *, P < 0.05; †, P < 0.01; ‡, P < 0.001; §, P < 0.0001; ¶, P < 10−5; ∥, P < 10−6. Levels of significance compared with values at maximal suppression of activity in the parietal cortex: **, P < 0.05; ‡‡, P < 0.01.
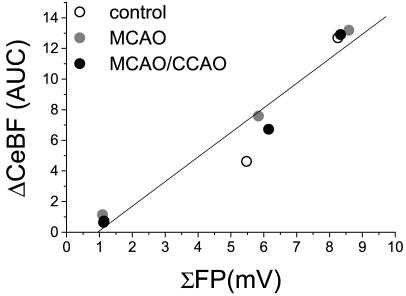
The decrease in baseline Purkinje cell spike rate and CeBF suggested reduced excitatory input from cerebral cortical regions, but may as well be related to decreased cerebellar excitability or vascular reactivity. This possibility was examined in a series of experiments in which we activated Purkinje cells by stimulating the inferior olive, the brainstem nucleus from which climbing fibers originate. Activation of Purkinje cells by means of the monosynaptic excitatory climbing-fiber input evokes synaptic FPs that are coupled to CeBF-increases (16). We used three stimulation trains, each of 30-s duration, at increasing frequencies (1, 5, and 10 Hz) before and after either occlusion of the MCA or occlusion of the MCA plus CCA. The shape and amplitude of FPs, and the increases in CeBF remained unchanged after occlusion of the MCA or MCA plus CCA when compared with control conditions (Table 1). Fig. 4 illustrates the linear relationship between the change in CeBF and the total electrical activity evoked at each stimulation frequency expressed as ΣFP, the summed FP amplitudes (17). The figure shows that the coupling between ΣFP and CeBF was unaffected by unilateral focal cerebral ischemia and hence CCD. These data indicate that Purkinje cell excitability and vascular reactivity in the cerebellum are unchanged during CCD. Our findings support the idea that CCD is explained by deactivation.
Table 1.
Excitability of Purkinje cells and vascular reactivity is preserved during cerebellar diaschisis
| FreqStim | Control | MCAO | MCAO + CCAO | |
|---|---|---|---|---|
| FP, mV | 1 Hz | 1.125 ± 0.221 | 1.092 ± 0.308 | 1.137 ± 0.192 |
| 5 Hz | 1.095 ± 0.101 | 1.168 ± 0.141 | 1.230 ± 0.152 | |
| 10 Hz* | 0.825 ± 0.117 | 0.859 ± 0.104 | 0.834 ± 0.068 | |
| CeBF-increase, AUC | 1 Hz | 0.64 ± 1.00 | 1.14 ± 1.08 | 0.73 ± 1.35 |
| 5 Hz* | 4.62 ± 1.25 | 7.59 ± 3.28 | 6.72 ± 1.98 | |
| 10 Hz* | 12.69 ± 7.63 | 13.19 ± 5.79 | 12.91 ± 5.25 |
FP, mean amplitudes of Purkinje cell field potentials. FreqStim, stimulation frequency. CeBF increase, area under the curve (AUC). At higher frequencies, FPs decrease in amplitude, whereas CeBF responses increase with frequency (16).
, Significance of frequency dependent differences (P < 0.05).
Figure 4.
Coupling between increased electrical activity and blood flow is preserved during cerebellar diaschisis induced by focal cerebral ischemia (n = 3). The graph shows the correlation between the total evoked electrical activity (calculated as the summed field potential; ΣFP) and the coupled CeBF-increase (ΔCeBF; area under curve) evoked by electrical stimulation of the inferior olive at 1, 5, and 10 Hz. The linear correlation (R = 0.97; P < 0.001) was preserved between ΣFP and ΔCeBF before arterial occlusion (control, open circle), after contralateral MCAO (gray circle), and after additional MCAO/CCAO (black circle).
Discussion
Increased excitatory synaptic activity increases energy metabolism and CBF (2, 3). If the relation between neural activity and CBF is linear, then a reduction in nerve cell activity from baseline levels should be associated with decreased CBF. Reduced activity may be a consequence of either deactivation because of decreased excitatory input, or effective synaptic inhibition caused by interaction of γ-aminobutyric acid (GABA) with its receptors that may decrease respectively increase CBF and metabolism (3).
Here we show that reduced neuronal activity in the cerebral cortex leads to a decrease in Purkinje cell spike activity in the contralateral cerebellar hemisphere, which is explained by deactivation. This deactivation is the result of reduced efferent, excitatory activity in crossed projections from the cerebral cortex to brainstem nuclei and relay neurons in the cerebellar cortex to Purkinje cells. This deactivation probably underlies the reduction in CeBF and metabolism in the cerebellum that is commonly observed as crossed cerebellar diaschisis with functional neuroimaging in stroke patients (19).
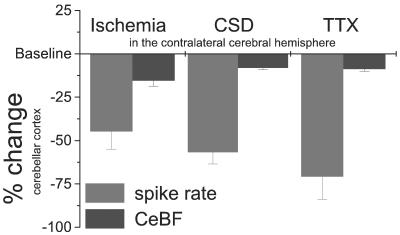
Our data demonstrate that the reduction in CeBF, indicating CCD after permanent, combined occlusion of the contralateral CCA and MCA in rats, occurs in parallel with an immediate decrease in spontaneous activity of Purkinje cells. Although changes in electrical activity and blood flow are coupled in the cerebellar cortex, there are differences in the magnitude of the reduction in CeBF and spike rate of Purkinje cells during CCD; the decreases in Purkinje cell spike rate were ≈3–7 times larger than the decreases in CeBF (Fig. 5). Positron-emission tomography studies of such pathological conditions may therefore underestimate the decrease in neuronal function, because a small relative change in the hemodynamic signal may be based on a much larger decrease in neuronal activity. The small decline in CeBF might be considered in the light of the proportion of the basal blood flow that is influenced by attenuation of spontaneous neuronal activity. Here we show that TTX abolishes activity in the cerebral cortex, whereas it reduces CBF by only ≈15%, which is consistent with previous studies (20). Furthermore, we have shown that direct application of TTX abolishes both spontaneous and evoked electrical activity in the cerebellar cortex and reduces CeBF by ≈10 to 20% in anesthetized rats (21). This finding suggests that the major proportion of the basal CeBF is independent of neuronal activity, which may explain the discrepancy between large activity changes and small blood flow changes during CCD (Fig. 5). Therefore, the maximum decrease in CeBF (16%) that follows combined arterial occlusion is large, considering the dynamic range available for reduction caused by deactivation.
Figure 5.
Spontaneous Purkinje cell spike activity and baseline CeBF in response to impaired contralateral neocortical function caused by focal ischemia (n = 10), CSD (n = 4), and TTX (n = 4). The figure illustrates the discrepancy between the large decreases in spontaneous neuronal activity and the small decreases in baseline blood flow in the cerebellar cortex during deactivation. Relative changes (%) of mean spike rate (black) and CeBF (gray) are shown.
Positron emission tomography studies have revealed a lack of CCD in stroke patients with reduced metabolism and blood flow in the distribution of an occluded internal carotid artery, but without signs of infarction. Therefore, it was argued that for diaschisis to occur, damage to nervous tissue is required (12). To test this hypothesis, we used transient functional ablation of the cerebral cortex by cortical spreading depression, which has been applied previously to study the functional organization of the brain (13, 22, 23). We show that unilateral functional ablation of the cerebral cortex by either CSD or application of TTX reproduced the effects of focal ischemia, which indicates that diaschisis is independent of cell damage. However, our data show immediate changes of cerebellar activity induced by ischemia and functional ablation of the neocortex in an acute time window (1–2 h). The persistence of cerebellar diaschisis observed after stroke might indeed be related to processes subsequent to chronic deafferentiation distinct from decreased neuronal input, as indicated by recent reports of chronic uncoupling of cerebellar oxygen and glucose metabolism in stroke patients (24, 25).
Focal cerebral ischemia and CSD induced bilateral, albeit asymmetrical decreases in CeBF, indicating that diaschisis occurs in both cerebellar hemispheres, but is more pronounced in the contralateral hemisphere, as previously observed in human stroke patients (12, 26). Interestingly, a positron emission tomography study revealed that differences in the metabolic rates and CeBF between both cerebellar hemispheres are only apparent when the infarct involves the frontal lobe (12). Strokes involving other regions produce symmetrical decreases in metabolism and CeBF in both cerebellar hemispheres. Our experiments using CSD revealed that Purkinje cell spiking activity and blood flow in the contralateral cerebellar hemisphere were maximally decreased when suppression of activity mainly involved the frontal cortex. Taken together, these observations reflect the strong functional and anatomical connection between the frontal cerebral cortex and the contralateral cerebellar hemisphere (27, 28). In contrast to focal ischemia, CSD leads to symmetrical CeBF decreases, even though the frontal cortex in one hemisphere was depolarized. Another unresolved issue is the observation that focal ischemia produced much larger CeBF decreases in the contralateral cerebellar hemisphere than functional ablation (Fig. 5). Further experiments are needed to elucidate the mechanisms that account for these differences.
The CeBF decrease and possibly the decrease in electrical activity in the ipsilateral cerebellar hemisphere are yet unexplained, but may involve reduced spiking activity of uncrossed projections of the cortico-ponto-cerebellar pathway (27) or activity decreases in the cerebral hemisphere contralateral to the lesion as a result of transhemispheric diaschisis (5).
The experiments using climbing fiber stimulation indicated that Purkinje cell excitability and vascular reactivity were preserved during CCD. Thus, cerebellar diaschisis is explained by deactivation of Purkinje cells caused by a decrease in the excitatory input to these neurons. Clinical neurophysiological studies (29–34) as well as experimental work (35, 36) indicated increased neuronal excitability in remote areas affected by transcortical or transhemispheric diaschisis, suggesting disinhibition of cortical neurons. However, the normal excitability of Purkinje cells reported here indicates that this is not a factor in CCD.
In conclusion, CCD is characterized by decreases in spontaneous cerebellar electrical activity and hence blood flow. It is an immediate response to reduced afferent input from interconnected brain regions, especially the contralateral frontal cortex. Our data reveal that the neurophysiological basis of acute cerebellar diaschisis is deactivation of Purkinje cells caused by reduced excitatory input from the cerebral cortex. Although structural damage of cortical areas and the projecting fibers might account for chronic effects of CCD and explain its persistence, the acute process is functional, and hence rapidly reversible when the excitatory input to the cerebellum returns. The fact that spontaneous activity of Purkinje cells is decreased without changes in excitability suggests that acute cerebellar diaschisis is an example of reduced nerve cell activity caused by deactivation that does not involve synaptic inhibition. Our data provide evidence, that large decreases of neuronal activity induced by deactivation are represented by very moderate changes of blood flow. This discrepancy should be taken into account in the interpretation of brain images obtained with CBF as indicator of neuronal activity.
Acknowledgments
We thank Lillian Grøndahl for expert technical assistance. This study was supported by NeuroScience PharmaBiotech, the Danish Medical Research Council, the Carlsberg Foundation, the Brødrene Hartmann Foundation, the Lundbeck Foundation, and The NOVO-Nordisk Foundation.
Abbreviations
- CBF
cerebral blood flow
- CCA
common carotid artery
- CCD
crossed cerebellar diaschisis
- CeBF
cerebellar blood flow
- CSD
cortical spreading depression
- ECoG
electrocorticogram
- FP
synaptic field potential
- MCA
middle cerebral artery
- TTX
tetrodotoxin
- CCAO
CCA occlusion
- MCAO
MCA occlusion
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sokoloff L. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 2.Raichle M E. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauritzen M. J Cereb Blood Flow Metab. 2001;21:1367–1383. doi: 10.1097/00004647-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 4.von Monakow C. Die Lokalisation im Grosshirn und der Abbau der Funktion durch kortikale Herde. Wiesbaden, Germany: J. F. Bergmann; 1914. [Google Scholar]
- 5.Andrews R J. Stroke. 1991;22:943–949. doi: 10.1161/01.str.22.7.943. [DOI] [PubMed] [Google Scholar]
- 6.Kempinsky W H. Res Publ Assoc Res Nerv Ment Dis. 1966;41:92–115. [PubMed] [Google Scholar]
- 7.Ginsberg M D, Reivich M, Giandomenico A, Greenberg J H. Neurology. 1977;27:1042–1048. doi: 10.1212/wnl.27.11.1042. [DOI] [PubMed] [Google Scholar]
- 8.Kempinsky W H, Boniface W R, Keating J B A, Morgan P. Circ Res. 1961;9:1051–1058. doi: 10.1161/01.res.9.5.1051. [DOI] [PubMed] [Google Scholar]
- 9.Hoedt-Rasmussen K, Skinhoj E. Acta Neurol Scand. 1964;40:41–46. [Google Scholar]
- 10.Meyer J S, Shinohara Y, Kanda T, Fukuuchi Y, Ericsson A D, Kok N K. Arch Neurol. 1970;23:241–247. doi: 10.1001/archneur.1970.00480270051007. [DOI] [PubMed] [Google Scholar]
- 11.Baron J C, Bousser M G, Comar D, Castaigne P. Trans Am Neurol Assoc. 1980;105:459–461. [PubMed] [Google Scholar]
- 12.Martin W R, Raichle M E. Ann Neurol. 1983;14:168–176. doi: 10.1002/ana.410140203. [DOI] [PubMed] [Google Scholar]
- 13.Bures J, Buresova O, Krivanek J. The Mechanism and Applications of Leão's Spreading Depression of Electroencephalographic Activity. Prague: Academia; 1974. [Google Scholar]
- 14.Caesar K, Akgoren N, Mathiesen C, Lauritzen M. J Physiol (London) 1999;520:281–292. doi: 10.1111/j.1469-7793.1999.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- 16.Mathiesen C, Caesar K, Akgoren N, Lauritzen M. J Physiol (London) 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathiesen C, Caesar K, Lauritzen M. J Physiol (London) 2000;523:235–246. doi: 10.1111/j.1469-7793.2000.t01-1-00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedergaard M, Hansen A J. Brain Res. 1988;449:395–398. doi: 10.1016/0006-8993(88)91062-1. [DOI] [PubMed] [Google Scholar]
- 19.Feeney D M, Baron J C. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- 20.Fabricius M, Lauritzen M. Am J Physiol. 1994;266:H1457–H1464. doi: 10.1152/ajpheart.1994.266.4.H1457. [DOI] [PubMed] [Google Scholar]
- 21.Akgoren N, Fabricius M, Lauritzen M. Proc Natl Acad Sci USA. 1994;91:5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassoni G, Bucherelli C, Bures J. Behav Neurosci. 1992;106:933–939. doi: 10.1037//0735-7044.106.6.933. [DOI] [PubMed] [Google Scholar]
- 23.Rashidy-Pour A, Motaghed-Larijani Z, Bures J. Behav Brain Res. 1995;72:185–188. doi: 10.1016/0166-4328(96)00136-2. [DOI] [PubMed] [Google Scholar]
- 24.Baron J C, Rougemont D, Soussaline F, Bustany P, Crouzel C, Bousser M G, Comar D. J Cereb Blood Flow Metab. 1984;4:140–149. doi: 10.1038/jcbfm.1984.22. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi H, Fukuyama H, Nagahama Y, Nishizawa S, Konishi J. Stroke. 1999;30:1424–1428. doi: 10.1161/01.str.30.7.1424. [DOI] [PubMed] [Google Scholar]
- 26.Pantano P, Baron J C, Samson Y, Bousser M G, Derouesne C, Comar D. Brain. 1986;109:677–694. doi: 10.1093/brain/109.4.677. [DOI] [PubMed] [Google Scholar]
- 27.Brodal P. Brain. 1978;101:251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- 28.Brodal P. Neuroscience. 1979;4:193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- 29.Sakatani K, Iizuka H, Young W. Stroke. 1990;21:124–132. doi: 10.1161/01.str.21.1.124. [DOI] [PubMed] [Google Scholar]
- 30.Matsumiya N, Koehler R C, Traystman R J. Stroke. 1990;21:908–916. doi: 10.1161/01.str.21.6.908. [DOI] [PubMed] [Google Scholar]
- 31.Hossmann K A, Mies G, Paschen W, Csiba L, Bodsch W, Rapin J R, Poncin-Lafitte M, Takahashi K. J Cereb Blood Flow Metab. 1985;5:97–107. doi: 10.1038/jcbfm.1985.13. [DOI] [PubMed] [Google Scholar]
- 32.Graf R, Kataoka K, Rosner G, Heiss W D. J Cereb Blood Flow Metab. 1986;6:566–573. doi: 10.1038/jcbfm.1986.103. [DOI] [PubMed] [Google Scholar]
- 33.Bo P, Cosi V, Introzzi G, Scelsi R, Taccola A, Romani A, Patrucco M, Rozza A, Savoldi F. Ital J Neurol Sci. 1987;8:549–559. doi: 10.1007/BF02333661. [DOI] [PubMed] [Google Scholar]
- 34.Lopes da Silva F H, Van Dieren A, Jonkman J, Tulleken C A. Behav Brain Res. 1985;15:147–157. doi: 10.1016/0166-4328(85)90061-0. [DOI] [PubMed] [Google Scholar]
- 35.Buchkremer-Ratzmann I, Witte O W. Neuroreport. 1997;8:519–522. doi: 10.1097/00001756-199701200-00028. [DOI] [PubMed] [Google Scholar]
- 36.Buchkremer-Ratzmann I, August M, Hagemann G, Witte O W. Stroke. 1996;27:1105–1109. doi: 10.1161/01.str.27.6.1105. [DOI] [PubMed] [Google Scholar]