Key Teaching Points.
-
•
Cardioneuroablation offers an effective and safe strategy for children and adolescents with significant functional bradycardia or atrioventricular block mediated by elevated vagal tone, potentially averting the need for long-term pacemaker therapy.
-
•
An atropine challenge exceeding a 25% increase in heart rate is essential for identifying purely vagally driven bradyarrhythmias. Post ablation, the loss of chronotropic response to atropine, confirms successful ganglionic denervation.
-
•
The choice of a right atrial, bi-atrial, or left atrial approach depends on the specific ganglionated plexus anatomy and clinical presentation. Long-term monitoring is crucial to detect possible reinnervation or recurrence of bradycardia.
Introduction
Functional sinus node dysfunction and atrioventricular (AV) block in children and adolescents can stem from excessive parasympathetic activation.1 Although most patients respond to conservative measures, such as lifestyle modifications or pharmacotherapy, there is a highly symptomatic subgroup that may ultimately require a more definitive intervention.1,2 Although permanent pacemaker implantation effectively prevents asystolic events and hemodynamic compromise, it involves significant risks in the pediatric population, including infection, thrombosis, lead fracture, and repeated generator replacements over the patient’s lifetime.1,3
Cardioneuroablation (CNA), also referred to as ganglionated plexus (GP) ablation, has emerged as a promising alternative in both adult and pediatric populations by attenuating or abolishing the excessive vagal influence responsible for functional bradycardia and AV block.1,2 Recent reports, including from Choi and colleagues,2 have demonstrated encouraging short-term outcomes in pediatric patients (younger than 21 years), thereby supporting CNA as a feasible strategy to avoid or delay pacemaker implantation in selected young individuals.
Herein, we describe 2 adolescents with severe functional bradycardia successfully treated with CNA, with no immediate complications or medium-term recurrences.
Case reports
A comprehensive evaluation was conducted for each patient, including 24-hour Holter monitoring, an atropine challenge to assess vagal influence, and a detailed electrophysiological study. Both patients underwent CNA under general anesthesia using the CARTO 3 electroanatomic mapping system (Biosense Webster, Diamond Bar, CA). The ablation targets were GP sites in the right atrium: the inferior right atrial GP and the superior right atrial GP. The procedural end point included the resolution of bradycardia and a reduction or abolition of the atropine response. However, the chronotropic response to atropine in the pediatric population can vary by age and sedation conditions, with average increases of up to 31% reported in children younger than 36 months; consequently, a fixed cutoff (eg, 25%) may require further adjustments and investigation.4 Post-ablation follow-up consisted of serial clinical evaluations and Holter monitoring at 3 and 6 months to detect any recurrence of functional bradyarrhythmia.
Case 1
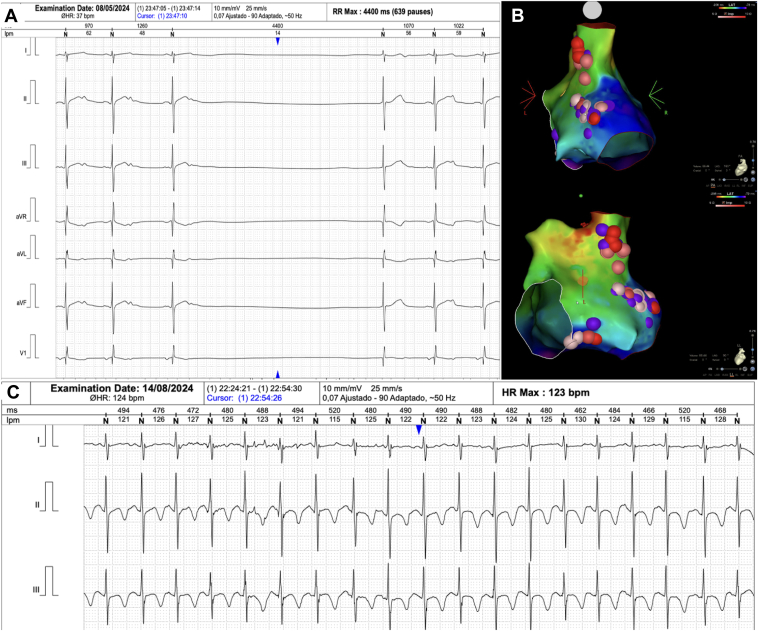
A previously healthy 12-year-old girl presented with recurrent dizziness and syncope. Baseline Holter revealed a mean heart rate (HR) of 60 beats/min, with sinus pauses up to 4.4 seconds. The atropine test showed an increase in HR >25% (final HR of 98 beats/min). Transthoracic echocardiography ruled out structural heart disease. During the electrophysiological study, fractionated potentials were identified in the superior and inferior portions of the right atrium. Twenty-one radiofrequency lesions were delivered (mean power = 30 W, ablation index = 400). On completion, a repeat atropine test showed no significant HR change, indicating adequate vagal denervation. The patient remained asymptomatic, without bradycardia or syncope, at 3- and 6-month follow-up (Figure 1, Table 1, and Supplemental Video 1).
Figure 1.
A: Case 1. Sinus pauses of 4.4 seconds recorded on Holter. B: Three-dimensional CARTO map (Biosense Webster, Diamond Bar, CA) illustrating the 2 ganglionated plexi in the superior and posterior regions of the right atrium in posteroanterior and left lateral views. C: Six-month follow-up Holter showing sinus rhythm with a mean heart rate (HR) of 96 beats/min and pauses reduced to 1.2 seconds.
Table 1.
Clinical and ablation parameters of the 2 adolescents undergoing CNA
| Parameter | Case 1 | Case 2 |
|---|---|---|
| Age, y | 12 | 14 |
| Sex | Female | Female |
| Diagnosis | Sinus pauses (>4.4 s) | Sinus bradycardia with fusion beats and ventricular escape |
| Baseline HR, beats/min | 60 | 40 |
| Syncope history | Yes | Yes |
| Atropine response | >25% HR increase (98 beats/min) | >25% HR increase (128 beats/min) |
| Pre-CNA EP measurements | AH: 80 ms, HV: 42 ms, Wenckebach: 390 | AH: 70 ms, HV: 40 ms, Wenckebach: 360 |
| Post-CNA EP measurements | AH: 72 ms, HV: 42 ms, Wenckebach: 360 | AH: 65 ms, HV: 40 ms, Wenckebach: 350 |
| Targeted GPs | RSGP and RIGP | RSGP and RIGP |
| Total RF lesions | 21 | 25 |
| Ablation Index | 400 | 400 |
| Power, W, mean | 30 | 36 |
| Temperature, °C, mean | 27 | 26 |
| Impedance, Ω, mean | 180 | 122 |
| Impedance drop, Ω, mean | 10 | 16 |
| Total ablation time, min:s | 3:05 | 5:01 |
| Complications | None | None |
| 3-mo follow-up | No syncopal events, mean HR, 110 beats/min | No syncopal events, mean HR, 100 beats/min |
| 6-mo follow-up | No syncopal events, mean HR, 90 beats/min | No syncopal events, mean HR, 105 beats/min |
CNA = cardioneuroablation; EP = electrophysiology; GP = ganglionated plexus; HR = heart rate; RF = radiofrequency; RSGP = superior right atrial ganglionated plexus; RIGP = inferior right atrial ganglionated plexus.
Case 2
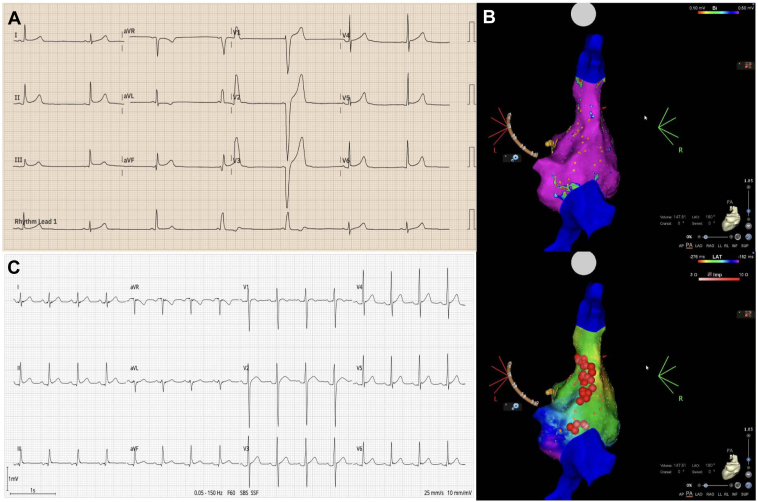
A 14-year-old girl reported occasional dizziness, syncope, and documented sinus bradycardia as low as 40 beats/min. The atropine challenge revealed a marked HR rise to 128 beats/min (>25% from baseline). Similar to case 1, electroanatomic mapping localized the GP in the right atrium, involving the superior and inferior regions. Twenty-five radiofrequency lesions were delivered (mean power = 36 W, ablation index = 400, impedance drop of 16 Ω). Subsequently, atropine produced no chronotropic effect, indicating complete denervation. There were no complications, and the patient remained free of bradycardia or symptoms at 3- and 6-month follow-up (Figure 2, Table 1, Supplemental Video 2).
Figure 2.
A: Case 2. Sinus bradycardia of 40 beats/min with sinus, fusion, and ventricular escape beats. B: Three-dimensional CARTO map (Biosense Webster, Diamond Bar, CA) showing the 2 ganglionated plexi in the right atrium (superior and posterior) in a posteroanterior view. C: Six-month follow-up electrocardiogram demonstrating sinus rhythm at 93 beats/min.
Discussion
CNA is a novel therapeutic option for managing bradycardia and AV block of functional origin in children and adolescents with excessive vagal activity. Unlike permanent pacemaker implantation, which carries the risk of infection; thrombosis; lead malfunction or fracture; and frequent generator replacements, CNA specifically targets the imbalance in parasympathetic tone by ablating the GP located in the epicardial fat.1,3,5 This approach addresses the underlying cause of sinus node dysfunction and/or functional AV block, while preserving the patient’s valvular and vascular anatomy.
In both of our adolescent patients, syncope and marked bradycardia were present: the first had sinus pauses exceeding 4.4 seconds, and the second exhibited rates as low as 40 beats/min with ventricular escape beats. Despite differences in arrhythmic patterns, the atropine test revealed a significant (>25%) increase in HR, ruling out structural conduction system disease and pointing to a functional substrate mediated by excessive vagal tone.1 Post ablation, the absence of chronotropic response on repeated atropine testing confirmed denervation of the implicated GP, consistent with findings from other pediatric series.2,3
Although some protocols advocate for a bi-atrial approach or left-sided access in cases of significant AV block, our patients achieved optimal clinical outcomes by limiting the ablation to the superior and inferior right atrial GP, guided by electroanatomic mapping. However, it has been reported that increasing sinus rates could unmask previously hidden functional AV block, requiring additional ablation in areas such as the posteromedial left atrial plexus.6 Indeed, the literature indicates that in cases of greater nodal involvement or recurrence, a broader approach—encompassing the septum and left atrium— may be necessary.1 This underscores the importance of tailoring the ablation strategy to the anatomic distribution of the GP and the patient’s specific electrophysiological presentation.
Most available data on CNA come from adult studies of vasovagal syncope, suggesting lower clinical success rates when ablation is confined solely to the right atrium. In a recent meta-analysis (n = 465) by Vandenberk and colleagues,7 a 92% syncope-free rate (95% CI, 88.1%–94.6%) was reported; however, only 8.2% of patients received ablation exclusively in the right atrium, showing reduced efficacy compared with those undergoing bi-atrial or left atrial ablation. These findings align with a recent study of 40 patients in which the right atrial–only approach was discontinued after 13 cases (65%) failed to achieve total vagal denervation—requiring a crossover to the left atrium (mainly due to persistent AV block)—compared with only 4 patients (20%) in the left atrium group (P = .0095).8 In the pediatric population, an exclusive right atrial ablation can be sufficient with a lower risk profile, which is particularly desirable in children; however, a greater number of randomized trials with long-term follow-up is needed to confirm the best strategy in this population and to define precisely which patient profile would most benefit from right atrial–only ablation.
In the long term, the potential reinnervation of the ablated GP raises concerns about recurrence of bradycardia or syncope.9 Our follow-up in these cases is limited to 6 months and therefore cannot completely exclude future reinnervation. Moreover, the marked reduction in vagal tone may carry a proarrhythmic risk or diminish parasympathetic protection during myocardial ischemia, particularly in patients with structural heart disease or other risk factors.10 Nonetheless, available data suggest that such complications are uncommon in children and adolescents with structurally normal hearts,1,2 as demonstrated in our 2 patients.
Regarding the use of vagal nerve stimulation to guide the procedure, our current practice relies primarily on abolishing the atropine response and eliminating fractionated potentials as markers of success. We acknowledge that direct vagal stimulation could provide additional information, but there is no consensus on its use in the pediatric population.1,2 Another aspect is the careful planning of lesion sets to avoid creating atrial macroreentrant circuits; we have not observed such arrhythmias in our patients during follow-up.
Despite promising results, multicenter studies with larger pediatric cohorts and longer follow-up periods are essential to optimize candidate selection, standardize ablation parameters (including atropine thresholds), and better understand the implications of parasympathetic suppression in young patients. Future evidence will help determine whether CNA can provide a durable and reliable alternative to permanent pacing in this population, while minimizing the complications associated with conventional pacemaker therapy.
Conclusion
CNA represents an effective and safe option as an alternative to permanent pacing in adolescents with severe functional bradycardia mediated by excessive vagal tone. In these 2 cases, it eliminated bradycardia and syncope without complications at 6-month follow-up. These findings align with other pediatric reports, highlighting the potential of CNA to prevent lifelong pacemaker dependence in selected patients. Nonetheless, additional studies with larger cohorts and longer follow-up are needed to establish standardized CNA protocols, refine selection criteria, and more clearly delineate the procedure’s long-term efficacy and safety in the pediatric population.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2025.04.002.
Appendix. Supplementary Data
Three-dimensional propagation map of the right atrium using CARTO (Biosense Webster) in Case 1.
Three-dimensional propagation map of the right atrium using CARTO (Biosense Webster) in Case 2. Yellow spheres indicate the anatomical locations of the sites where fractionated potentials were identified.
References
- 1.Aksu T., Brignole M., Calo L., et al. Cardioneuroablation for the treatment of reflex syncope and functional bradyarrhythmias: a scientific statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS) and the Latin American Heart Rhythm Society (LAHRS) Europace. 2024;26 doi: 10.1093/europace/euae206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi N.H., Hong J., Moak J.P. Cardioneuroablation for pediatric patients with functional sinus node dysfunction and paroxysmal atrioventricular block. J Cardiovasc Electrophysiol. 2024;35:221–229. doi: 10.1111/jce.16145. [DOI] [PubMed] [Google Scholar]
- 3.Xu X., He S., Liu Q., et al. Cardioneuroablation for successful treatment of symptomatic bradycardia in a 12-year-old child after a 6-month follow-up. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1290482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAuliffe G., Bissonnette B., Cavallé-Garrido T., Boutin C. Heart rate and cardiac output after atropine in anaesthetised infants and children. Can J Anaesth. 1997;44:154–159. doi: 10.1007/BF03013003. [DOI] [PubMed] [Google Scholar]
- 5.Mendoza-Novoa P., Cabrera-Saldaña M., Gonzales-Luna A., et al. A pediatric case of pathologic bradycardia resolved with zero-fluoroscopy cardioneuroablation: clinical and autonomic follow-up. Can J Cardiol. 2024;40:1304–1307. doi: 10.1016/j.cjca.2023.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Ascione C., Benabou L., Hocini M., Jaïs P., Haïssaguerre M., Duchateau J. Cardioneuroablation: don't underestimate the posteromedial left atrial ganglionated plexus. HeartRhythm Case Rep. 2023;9:67–69. doi: 10.1016/j.hrcr.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenberk B., Lei L.Y., Ballantyne B., et al. Cardioneuroablation for vasovagal syncope: a systematic review and meta-analysis. Heart Rhythm. 2022;19:1804–1812. doi: 10.1016/j.hrthm.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Piotrowski R., Baran J., Sikorska A., Niedzwiedz M., Krynski T., Kulakowski P. Cardioneuroablation: comparison of acute effects of the right vs. left atrial approach in patients with reflex syncope: the ROMAN2 study. Europace. 2024;26 doi: 10.1093/europace/euae042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro X., Vivó M., Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Chung W.H., Masuyama K., Challita R., et al. Ischemia-induced ventricular proarrhythmia and cardiovascular autonomic dysreflexia after cardioneuroablation. Heart Rhythm. 2023;20:1534–1545. doi: 10.1016/j.hrthm.2023.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional propagation map of the right atrium using CARTO (Biosense Webster) in Case 1.
Three-dimensional propagation map of the right atrium using CARTO (Biosense Webster) in Case 2. Yellow spheres indicate the anatomical locations of the sites where fractionated potentials were identified.