CK-NET Executive Committee
Honorary chairman
Qi-Min Zhan
National Institute of Health Data Science at Peking University, Beijing, China
Chairman
Ming-Hui Zhao
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; Peking-Tsinghua Center for Life Sciences, Beijing, China
Executive chairman
Luxia Zhang
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; National Institute of Health Data Science at Peking University, Beijing, China; Advanced Institute of Information Technology, Peking University, Hangzhou, Zhejiang, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; Center for Digital Health and Artificial Intelligence, Peking University First Hospital, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Vice chairmen
Li Zuo
Department of Nephrology, Peking University People’s Hospital, Beijing, China
Yue Wang
Department of Nephrology, Peking University Third Hospital, Beijing, China
Feng Yu
Department of Nephrology, Peking University International Hospital, Beijing, China
Jie Ding
Department of Pediatrics, Peking University First Hospital, Beijing, China
Haibo Wang
Research Centre of Big Data and Artificial Intelligence for Medicine, First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, China
CK-NET Work Group (Alphabetically)
Hong Chu
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Lanxia Gan
China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Bixia Gao
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Qi Guo
Kidney Disease Center, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
Jianguo Hao
National Institute of Health Data Science at Peking University, Beijing, China
Daijun He
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Shenda Hong
National Institute of Health Data Science at Peking University, Beijing, China
Chenglong Li
National Institute of Health Data Science at Peking University, Beijing, China
Pengfei Li
Advanced Institute of Information Technology, Peking University, Hangzhou, Zhejiang, China
Jianyan Long
Clinical Trial Unit, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
Huijuan Mao
Department of Nephrology, The First Affiliated Hospital of Nanjing Medical University, Nanjing Medical University, Nanjing, Jiangsu, China
Yingying Qin
National Institute of Health Data Science at Peking University, Beijing, China
Ying Shi
China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Xiaoyu Sun
National Institute of Health Data Science at Peking University, Beijing, China
Wen Tang
Department of Nephrology, Peking University Third Hospital, Beijing, China
Fang Wang
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Fulin Wang
National Institute of Health Data Science at Peking University, Beijing, China
Jinwei Wang
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Wanzhou Wang
National Institute of Health Data Science at Peking University, Beijing, China
Shaoqing Wei
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Fengyu Wen
National Institute of Health Data Science at Peking University, Beijing, China
Xingchen Yao
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Chao Yang
Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology, Beijing, China; Advanced Institute of Information Technology, Peking University, Hangzhou, Zhejiang, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; Center for Digital Health and Artificial Intelligence, Peking University First Hospital, Beijing, China; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University, Beijing, China
Guang Yang
Department of Nephrology, The First Affiliated Hospital of Nanjing Medical University, Nanjing Medical University, Nanjing, Jiangsu, China
Ling Yang
National Institute of Health Data Science at Peking University, Beijing, China
Jianhua Ye
Department of Nephrology, General Hospital of Ningxia Medical University, Yinchuang, Ningxia, China
Qiongjing Yuan
Department of Nephrology, Xiangya Hospital, Central South University, Changsha, Hunan, China
Dongliang Zhang
Department of Nephrology, Beijing Jishuitan Hospital, Beijing, China
Feifei Zhang
Center for Digital Health and Artificial Intelligence, Peking University First Hospital, Beijing, China; National Institute of Health Data Science at Peking University, Beijing, China
Ping Zhang
Kidney Disease Center, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
Zhilong Zhang
National Institute of Health Data Science at Peking University, Beijing, China
Xinju Zhao
Department of Nephrology, Peking University People's Hospital, Beijing, China
Zhiye Zhou
China Standard Medical Information Research Center, Shenzhen, Guangdong, China
| CK-NET International Advisory Committee (Alphabetically) | |
| Joseph Coresh | Adeera Levin |
| Harold Feldman | Vlado Perkovic |
| David Jayn | Pierre Ronco |
| Vivekanand Jha | Rajiv Saran |
| Andrew Levey | Sydney Tang |
| CK-NET Domestic Advisory Committee (Alphabetically) | |
| Chairman: Jianghua Chen | |
| Menghua Chen | Wenke Wang |
| Ping Fu | Xiaoqin Wang |
| Detian Li | Changying Xing |
| Guisen Li | Zuying Xiong |
| Shaomei Li | Dongmei Xu |
| Xinling Liang | Hui Xu |
| Yunhua Liao | Xudong Xu |
| Hongli Lin | Xiangdong Yang |
| Jian Liu | Xiaoping Yang |
| Zhangsuo Liu | Fan Yi |
| Yingchun Ma | Yan Zha |
| Yonghui Mao | Aihua Zhang |
| Luying Sun | Chun Zhang |
| Caili Wang | Jinghong Zhao |
| Rong Wang | Qiaoling Zhou |
| Weiming Wang | |
| CK-NET Technical Advisory Committee (Alphabetically) | |
| Jennifer Bragg-Gresham | Guilan Kong |
| Zhihong Deng | Dawei Xie |
| Kevin He | Xiaohua Zhou |
Detailed contents
| e7 | Abbreviations |
| e8 | Preface |
| e10 | Analytical methods |
| e13 | Chapter 1: Identification and characteristics of hospitalized patients with chronic kidney disease |
| e14 | 1.1 Prevalence of CKD among different types of underlying disease |
| e17 | 1.2 Staging of CKD |
| e18 | 1.3 Demographic characteristics of CKD |
| e20 | 1.4 Cause of CKD |
| e23 | 1.5 Mobility pattern of hospitalized patients with CKD |
| e24 | Chapter 2: Cardiovascular disease in hospitalized patients with chronic kidney disease |
| e25 | 2.1 Prevalence of CVD, stratified by patient group |
| e26 | 2.1.1 Prevalence of CHD |
| e28 | 2.1.2 Prevalence of stroke |
| e30 | 2.1.3 Prevalence of heart failure |
| e32 | 2.1.4 Prevalence of atrial fibrillation |
| e34 | 2.2 Prevalence of CVD among patients with CKD |
| e35 | 2.2.1 Prevalence of CHD among patients with CKD |
| e37 | 2.2.2 Prevalence of stroke among patients with CKD |
| e39 | 2.2.3 Prevalence of heart failure among patients with CKD |
| e41 | 2.2.4 Prevalence of atrial fibrillation among patients with CKD |
| e43 | 2.3 Cardiovascular procedures stratified by patient group |
| e44 | 2.3.1 Cardiovascular procedure: coronarography |
| e46 | 2.3.2 Cardiovascular procedure: percutaneous coronary intervention |
| e48 | 2.3.3 Cardiovascular procedure: coronary artery bypass grafting |
| e50 | 2.3.4 Cardiovascular procedure: pacemaker |
| e52 | 2.4 Cardiovascular procedures in patients with CKD |
| e53 | Chapter 3: Health care resource utilization in hospitalized patients with chronic kidney disease |
| e53 | 3.1 Costs |
| e53 | 3.1.1 Overall medical costs stratified by CKD, diabetes, and heart failure |
| e54 | 3.1.2 Costs stratified by types of health insurance |
| e55 | 3.1.3 Costs stratified by sex |
| e56 | 3.1.4 Costs stratified by age |
| e57 | 3.2 LOS |
| e57 | 3.2.1 Overall LOS stratified by CKD, diabetes, and heart failure |
| e58 | 3.2.2 LOS stratified by types of health insurance |
| e59 | 3.2.3 LOS stratified by sex |
| e60 | 3.2.4 LOS stratified by age |
| e61 | Chapter 4: In-hospital mortality in patients with chronic kidney disease |
| e61 | 4.1 In-hospital mortality stratified by CKD, diabetes alone, and heart failure alone |
| e62 | 4.2 In-hospital mortality stratified by types of health insurance |
| e63 | 4.3 In-hospital mortality stratified by sex |
| e64 | 4.4 In-hospital mortality stratified by age |
| e65 | Chapter 5: Acute kidney injury |
| e66 | 5.1 Percentage of AKI |
| e68 | 5.2 Characteristics of AKI |
| e68 | 5.2.1 Age distribution of AKI, stratified by sex |
| e69 | 5.2.2 Sex distribution of AKI, stratified by age |
| e70 | 5.3 Percentages of CKD and diabetes among patients with AKI |
| e72 | Chapter 6: Identification and characteristics of patients on dialysis |
| e74 | Chapter 7: Examinations and treatments of patients on dialysis |
| e77 | Chapter 8: Vascular access |
| e79 | Chapter 9: Cardiovascular disease and diabetes in patients on dialysis |
| e82 | Chapter 10: Hospitalization among patients on dialysis |
| e86 | Chapter 11: Medical expenditures for patients on dialysis |
| e88 | Chapter 12: Regional data from the dialysis registry system |
| e93 | Chapter 13: Kidney transplantation |
| e94 | Chapter 14: Environmental pollution and kidney disease |
| e96 | Chapter 15: Future perspectives |
| e98 | Chapter 16: Discussion |
| e100 | References |
| e101 | Appendix I: Definitions of International Classification of Diseases coding |
| e109 | Appendix II: Appendix tables for Chapters 1–5 |
Abbreviations
| AI | artificial intelligence |
| AKI | acute kidney injury |
| AVF | arteriovenous fistula |
| AVG | arteriovenous graft |
| CHD | coronary heart disease |
| CHIRA | China Health Insurance Research Association |
| CKD | chronic kidney disease |
| CK-NET | China Kidney Disease Network |
| COTRS | China Organ Transplant Response System |
| CVD | cardiovascular disease |
| HD | hemodialysis |
| HQMS | Hospital Quality Monitoring System |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| LLM | large language model |
| LOS | length of stay |
| PD | peritoneal dialysis |
| PM2.5 | particulate matter with an aerodynamic diameter of 2.5 μm or less |
| PMP | per million population |
| RMB | renminbi |
Preface
Chronic kidney disease (CKD) has become a public health issue because of its high prevalence and mortality.1 The burden of CKD is substantial, affecting millions of individuals globally and imposing significant economic and societal costs.2,3 The transition from CKD to kidney failure, requiring kidney replacement therapy such as dialysis or kidney transplantation, marks a critical juncture where the disease burden intensifies exponentially.4 The coronavirus disease 2019 pandemic has further complicated this landscape, casting a shadow over the management and outcomes of patients with CKD and kidney failure.5 Moreover, it has underscored the need for innovative strategies to mitigate the impact of kidney disease.
In line with the national directive aimed at advancing the utilization of big data, the China Kidney Disease Network (CK-NET) initiative was launched in 2014. Since its inception, more than 60 prominent renal centers and numerous regional medical data hubs across China have embraced this collaborative network. By amalgamating diverse data streams and harnessing cutting-edge technologies, CK-NET aspires to evolve into a comprehensive surveillance framework for kidney diseases in the country, furnishing invaluable insights into the epidemiology of CKD and fostering efficient disease management strategies.6, 7, 8 The website of CK-NET is https://www.chinakidney.net/en/.
The CK-NET 2017–2018 Annual Data Report delves into the intricate landscape of kidney disease, offering a nuanced and data-driven examination of its burden and evolving context in China. This report is the fourth nationwide report produced by CK-NET, and it stands as a testament to the relentless pursuit of knowledge within the medical and public health sectors, particularly as it pertains to the complex challenges posed by kidney diseases. By doing so, we hope to contribute to the ongoing dialogue surrounding the prevention, diagnosis, and management of these conditions.
By analyzing comprehensive data spanning the 2017–2018 period, the new report aims to provide a robust foundation for understanding the current state of kidney health in China. This report endeavors to shed light on these challenges, presenting data that illuminate the prevalence, incidence, and trends associated with CKD and kidney failure. We have diligently integrated a comprehensive array of data sources, encompassing health regulatory information, medical claims records, and data from external reports while fostering collaborations with a broadened network of regional dialysis quality control centers. Moreover, the report recognizes the multifaceted nature of kidney disease, acknowledging the pivotal role that environmental and societal factors play in contributing to its development and progression.
However, when interpreting the results presented in this report, the following limitations should be considered: First, the potential for selection bias persists because of inherent constraints in data sampling methodologies, which cannot be definitively dismissed. Despite covering a large and geographically diverse population, our analysis, performed using the available national databases, may still be subject to selection biases such as underreporting, coding variations, and differences in patient populations served. Second, the utilization of International Classification of Diseases, Tenth Revision codes for defining CKD and related diseases may entail lower sensitivity but heightened specificity, necessitating a nuanced understanding of their diagnostic implications. Third, our report’s comprehensive depiction of CKD prevalence, hospitalization rates, and diagnostic frequencies emphasizes the need for meticulous examination when interpreting these figures and epidemiological definitions. Last, the present analysis is solely grounded on cross-sectional data, posing challenges for establishing causal relationships.
We hope that this report will serve as a valuable resource for researchers, policymakers, and health care professionals alike, inspiring new ideas, fostering collaborations, and ultimately leading to improved outcomes for those affected by kidney diseases. As we present the CK-NET 2017–2018 Annual Data Report, we do so with the understanding that data alone cannot solve the challenges facing kidney health. Rather, it is a starting point, a catalyst for action, and a call to arms for the global community to unite in our efforts to improve kidney health outcomes for all.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This article is published as a supplement supported by Peking University.
We thank the National Health Commission of China, the Ministry of Science and Technology of China, the National Natural Science Foundation of China, the China Health Insurance Research Association, the China Organ Transplantation Development Foundation, Peking University, the Chinese Preventive Medicine Association for Kidney Disease, the China Standard Medical Information Research Center, and dialysis quality control centers in Jiangsu, Ningxia, Zhejiang, and Hunan for the support of this study. We gratefully acknowledge the significant contributions of the China Kidney Disease Network collaborating centers, members, and volunteers for their diligent work and efforts. We also express our appreciation to all participants who have provided essential data to support this research endeavor.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (72125009), the National Key Research and Development Program of China (2022YFF1203001 and 2019YFC2005000), the Young Elite Scientists Sponsorship Program by China Association for Science and Technology (2022QNRC001), the Chinese Scientific and Technical Innovation Project 2030 (2018AAA0102100), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5–046), the National High Level Hospital Clinical Research Funding (24QZ007, State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University), and the China–World Health Organization Biennial Collaborative Projects 2018–2019 (2019/892000-0).
Publication Information
Copyright © 2025, International Society of Nephrology. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Analytical methods
This chapter describes data sources, definitions, and analytical methods of the China Kidney Disease Network 2017 to 2018 Annual Data Report. The analyses were performed using 3 nationwide databases including Hospital Quality Monitoring System (HQMS) database, China Health Insurance Research Association (CHIRA) database, and China Organ Transplant Response System (COTRS) database. In addition, regional data from 4 provincial dialysis quality control centers were provided: Jiangsu, Ningxia, Zhejiang, and Hunan. For this report, the main data period we analyzed was from January 2017 to December 2018. However, owing to limitations in the accessibility of the CHIRA database, we provided results only for the year 2017 with regard to the burden on dialysis.
The ethics committee of Peking University First Hospital approved this study. The contents of this report have been internally and externally reviewed. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc.).
Data sources
HQMS database
The HQMS database is a mandatory national inpatient database system under the authority of the National Health Commission of the People’s Republic of China. All tertiary hospitals in China have been requested to submit standardized discharge records to the HQMS on a daily basis since 2013. In contrast to tertiary hospitals within the Western medical system, Chinese tertiary hospitals deliver an extensive spectrum of primary, secondary, tertiary, and specialized health care services, accommodating patients from across the nation. Conversely, primary hospitals in China are established as community-oriented facilities offering fundamental health services with a bed capacity below 100 whereas secondary hospitals function as localized medical hubs providing comprehensive health care with a bed range of 100 to 499. Nephrology units are specialized hospital departments focusing on kidney disease diagnosis, treatment, and management. By the end of 2018, the HQMS had covered more than 80% of tertiary hospitals in 31 provinces (excluding Hong Kong, Macao, and Taiwan) in China.
Patient-level data were collected from the uniform front page of hospitalization medical records. A total of 353 variables were gathered, including demographic characteristics, diagnoses in the form of International Classification of Diseases, Tenth Revision (ICD-10) codes, procedures and operations, financial breakdowns, and information on affiliated hospitals or divisions.9 As an integral part of China’s rigorous standard practice, the front page holds legal validity and must be completed by the attending physicians who have the most accurate and comprehensive understanding of the patient’s medical condition. Subsequently, certified professional medical coders encode the diagnoses according to the ICD-10 coding system. The HQMS data reporting system performs automated daily data quality control during submission to ensure data completeness, consistency, and accuracy.10 In case of any detected inconsistencies, the entire daily data package from the hospital is rejected, necessitating a review and resubmission of data.
CHIRA database
The most common medical insurance scheme in China’s urban areas is called Urban Basic Medical Insurance, which is available in 31 provinces and municipalities (with the exception of Hong Kong, Macao, and Taiwan). The Urban Resident Basic Medical Insurance and Urban Employee Basic Medical Insurance are the 2 components of the Urban Basic Medical Insurance.9 By the end of 2018, the number of participants covered by the Urban Basic Medical Insurance reached 1.214 billion, with a stable coverage of more than 95%.
The CHIRA database is a nationwide claims database that was established in 2007. It contains data on diagnosis, demographic characteristics, laboratory test frequency, prescription medication use, operation procedures, and medical costs for both inpatients and outpatients at all hospital levels (primary, secondary, and tertiary). A national sample of individuals insured by the Urban Employee Basic Medical Insurance and Urban Resident Basic Medical Insurance was extracted using a 2-stage sampling design. This encompassed 22 provinces, 5 autonomous regions, and 4 municipalities directly under the central government in mainland China, excluding Hong Kong, Macao, and Taiwan. In the first stage, convenience sampling was performed across 4 municipalities directly under the central government (Beijing, Shanghai, Tianjin, and Chongqing), 27 provincial capital cities, and a certain number of prefecture-level cities. In the second stage, a systematic random sampling approach sorted by age was used to extract approximately 2% of the insured population from the municipalities/provincial capital cities and approximately 5% from the chosen prefecture-level cities.4,9 For the purpose of privacy protection, all personal information, such as name, identity card number, medical insurance number, telephone number, and home address, underwent anonymization and de-identification before analysis. In 2017, there were 9,765,615 sampled beneficiaries in the CHIRA database and their full year of claims information was documented.
COTRS database
Since September 2013, the allocation of organs in China has become mandatory through the utilization of COTRS, a national open and transparent computer system for organ allocation. The impartial maintenance of the COTRS database is entrusted to a third-party entity. The process of matching donor organs with recipients takes into account factors such as medical emergencies, waiting list duration, and histocompatibility.9 The chapter pertaining to the kidney transplantation waiting list in China was based on an analysis performed using data from the COTRS database. The data on the waiting list for kidney transplantation were provided by the Report on Organ Transplantation Development in China (2015–2018)11; hence, this year’s report did not present detailed data.
Database definitions
Identifying patients with CKD
Three sets of ICD-10 disease codes were used to identify adult patients (age ≥ 18 years) with chronic kidney disease (CKD) in tertiary hospitals in China by using the HQMS database: Beijing version 4.0, National Standard version 1.0, and National Clinical version 1.0.8 Codes for procedures and operations were derived from the Beijing version and National Clinical version. Patients with diabetes and CKD were defined as those diagnosed with both diabetes and CKD but without the presence of nondiabetic kidney diseases evaluated by physicians.12
Kidney biopsy results were unavailable for the majority of patients. Cases with acute kidney diseases and disorders were identified through ICD-10 codes in the HQMS database, despite acknowledging that acute kidney injury may be significantly underestimated by ICD-10 codes; however, we retained this chapter as it could reflect actual diagnoses and we aimed to assess the percentage of acute kidney injury in the overall hospitalized population. All relevant ICD codes can be found in Appendix Table 1, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5, Appendix Table 6, Appendix Table 7.
Identifying patients on dialysis
Patients on dialysis were identified on the basis of the service items in medical billings and ICD-10 codes, specifically categorized as individuals with CKD necessitating dialysis treatment, which includes both hemodialysis (HD) and peritoneal dialysis (PD), while excluding cases of acute renal failure.8 Patients on PD were identified through claim records indicating the use of PD fluid, whereas patients on HD were identified by claim records documenting the utilization of hemodialyzers and associated procedures. HD modalities commonly used in China include HD, hemodiafiltration, high-flux HD, hemoperfusion, and hemofiltration; however, specific details about these modalities were not reflected in this report.
Cardiovascular disease
Patients with cardiovascular disease were identified through the diagnosis of cardiovascular disease using ICD-10 codes as well as claim records of therapeutic drugs for cardiovascular disease based on Anatomical Therapeutic Chemical codes (specifically C01 for cardiac therapy). In addition, related operation procedures such as coronary artery computed tomography and coronary arteriography were taken into consideration. Coronary heart disease, acute myocardial infarction, heart failure, cerebrovascular accident/transient ischemic attack, peripheral arterial disease, atrial fibrillation, and cardiovascular procedures including percutaneous coronary intervention and pacemaker implantation were also identified using ICD-10 codes and relevant claim records.
Diabetes
Patients with diabetes were identified on the basis of the diagnosis of diabetes using ICD-10 codes and claims records of therapeutic drugs (A10, drugs used in diabetes). It should be noted that the subgroup of individuals classified as “patients with diabetes” in the results may not necessarily have kidney disease; thus, patients with both diabetes and CKD could be counted twice in our report.
Hypertension
Patients with hypertension were identified on the basis of the diagnosis of hypertension using ICD-10 codes.
Infectious disease
Infectious disease was identified by the top 3 ICD-10 codes of infection by various pathogens.
Clinical indicators
Laboratory tests and drug use were identified through claim records. Laboratory tests encompassed blood hemoglobin and hemoglobin A1c levels and serum levels of iron, total calcium, phosphorus, parathyroid hormone, albumin, and lipids. A fundoscopic examination was performed for the detection of diabetic retinopathy. However, the outcomes of these tests were not documented in the database. Drug use involved erythropoietin, i.v. and oral iron supplements, calcitriol, phosphate binders, and transfusion therapy.
Vascular access
The definitions of tunneled cuffed catheter, noncuffed catheter, interventions for native arteriovenous fistula (AVF)/arteriovenous graft (AVG), and stable AVF/AVG for patients on HD were established on the basis of the documentation of surgical procedures, medical materials, and nursing interventions. Similarly, the identification of newly inserted peritoneal catheters and stable patients on PD followed the same methodology.
Statistical methods
Statistical methods used encompassed descriptive statistics, including frequency with percentage, median with interquartile range, and mean and SD. The findings were predominantly delineated by sex (defined as biological gender), age groups, geographic distribution, comorbidity status, and dialysis modality. P values were omitted because of the large sample sizes involved.
The comparisons between the 2 groups of patients—one with diabetes and the other with CKD—were performed on the basis of the overall reference population. This approach ensured that we did not exclude individuals who had both diabetes and CKD. The interprovince mobility was defined as the movement of patients leaving their permanent residence to travel to other provinces for hospitalization. The prevalence of dialysis was estimated by multiplying the percentage of patients on dialysis in the sampled data from the CHIRA database with the corresponding Urban Basic Medical Insurance utilization rate (data sourced from the China Statistical Yearbook and Statistical Communiqué of the People’s Republic of China on the National Economic and Social Development).4 The prevalence of dialysis, adjusted for age and sex, was standardized using the direct method with reference to the 2010 national population census data. Dialysis data from the local renal registry systems in 4 provinces—Jiangsu, Ningxia, Zhejiang, and Hunan—were analyzed while ensuring collection of results through a standardized form via email.
In the case where the time between hospital discharge and subsequent readmission was less than 3 days, we considered this as a continuous hospitalization. We excluded 1 hospitalization with a length of stay 180 days or more. In the chapter on vascular access, patients on HD would be categorized into only 1 group on the basis of a specific filter sequence starting from operational AVF/AVG, tunneled cuffed catheter, and noncuffed catheter. If multiple interventions were performed, the preceding filter situation should be selected. Patients without any intervention would be classified as having stable AVF/AVG. Unfortunately, we were unable to differentiate between AVF and AVG in the present database. Patients who underwent new PD catheter placement operations were classified as patients on new-onset PD. Patients who did not undergo new PD catheter placement operations were considered those on maintenance PD. Patients on stable PD were referred to those on maintenance PD without any transient central venous catheter placement procedures. We did not further differentiate between tunneled cuffed catheter and noncuffed catheter in the central venous catheter group because of the infrequent use of the tunneled cuffed catheter.
Chapter 1: Identification and characteristics of hospitalized patients with chronic kidney disease
This article is published as a supplement supported by Peking University.
This chapter describes the prevalence, characteristics, and mobility patterns of hospitalized patients with chronic kidney disease (CKD) in tertiary hospitals in China.
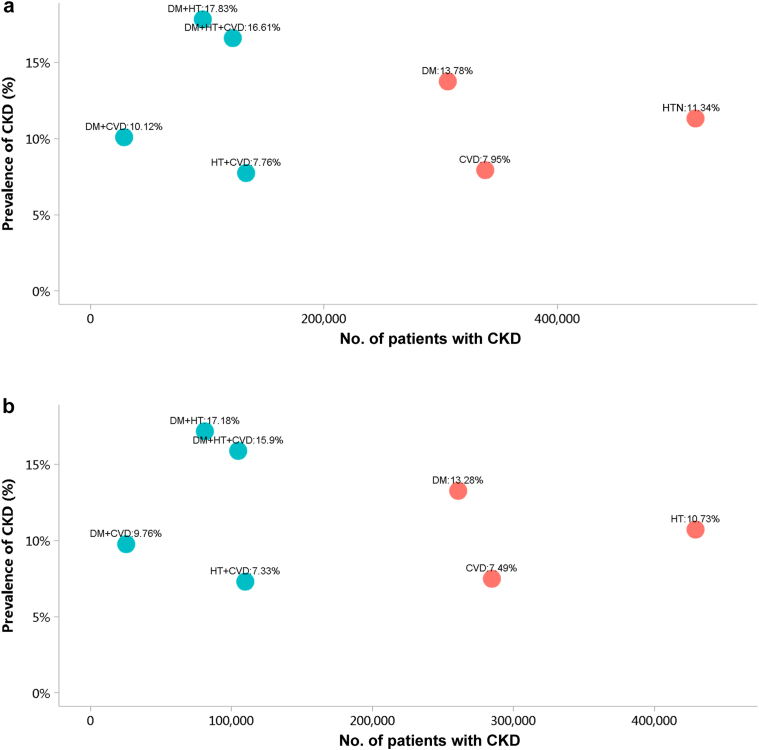
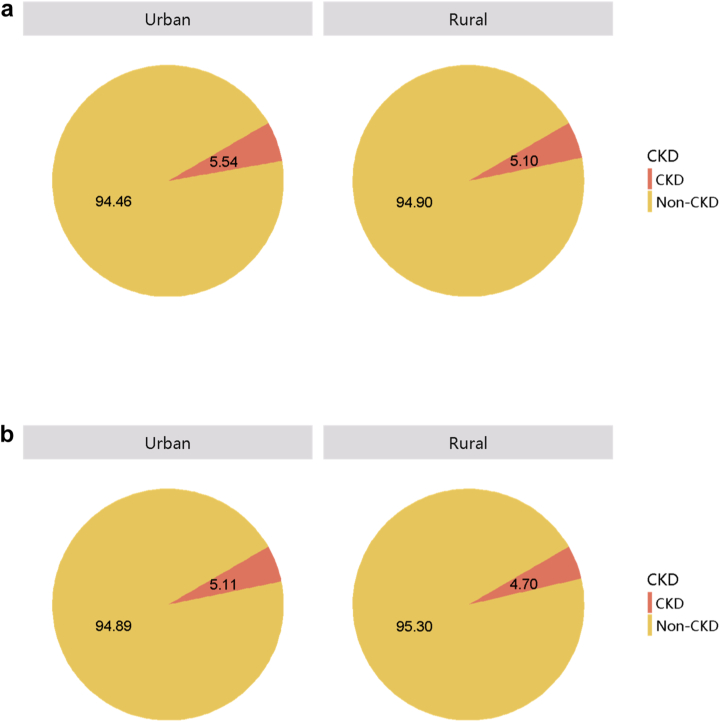
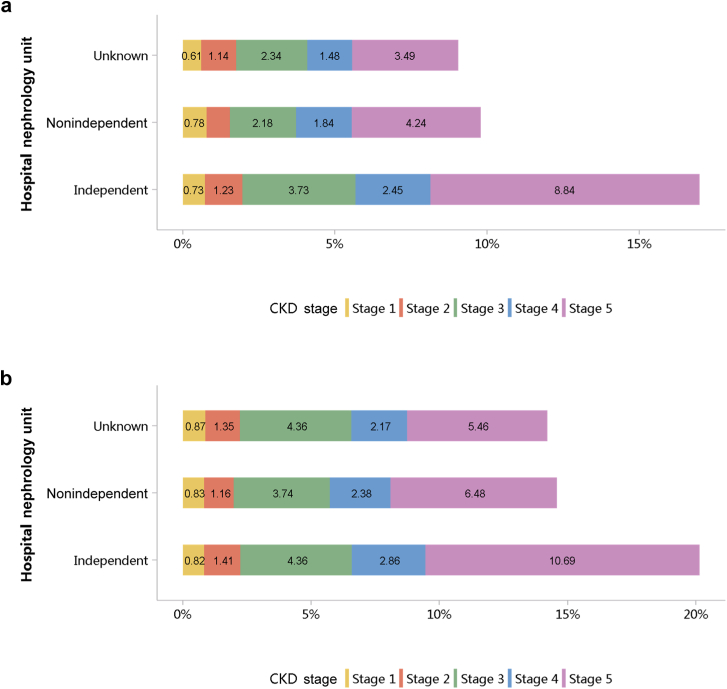
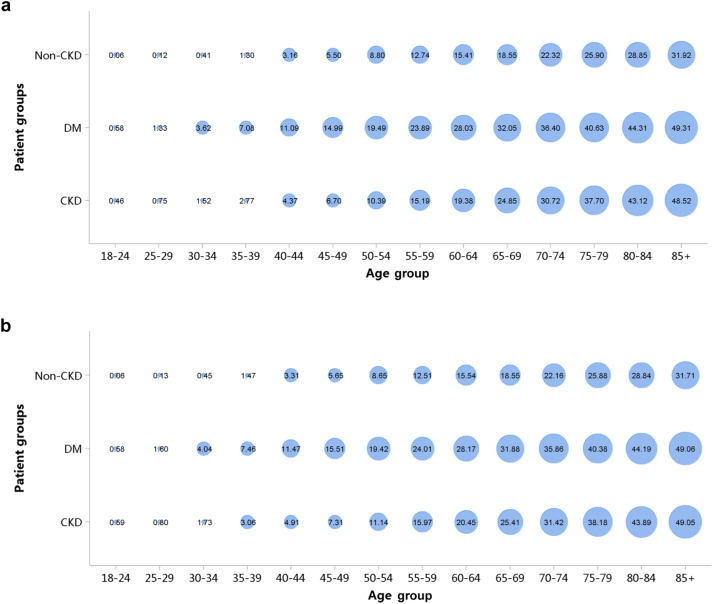
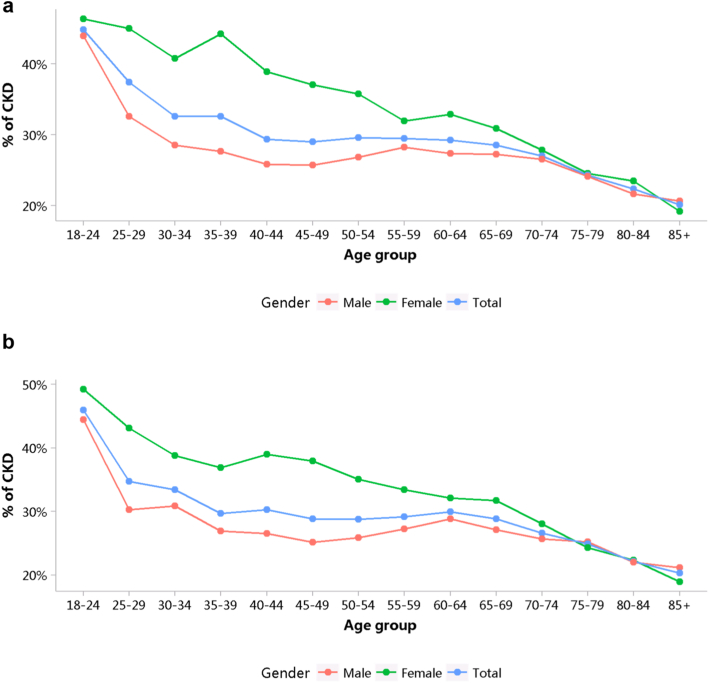
Patients with CKD accounted for 4.95% of all inpatients in 2017 and 4.59% in 2018 (Figure 1; Appendix Table 8), with an overall fluctuating trend over the past 5 years.13 The prevalence of CKD was particularly high among people with diabetes and hypertension. The percentage of CKD increased with age, and the prevalence of CKD was higher among the male population, especially at the age of 45 years or less (Figure 2; Appendix Table 9). Compared with rural areas, urban areas had a higher proportion of CKD (Figure 3; Appendix Table 10). In 2017 and 2018, 15.92% and 19.34% of patients with CKD had a diagnostic code for CKD staging (Figure 4; Appendix Table 11).
Figure 1.
Prevalence of CKD among different types of underlying disease. (a) 2017. (b) 2018. CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; HTN, hypertension.
Figure 2.
Patients with CKD, stratified by sex and age. (a) 2017. (b) 2018. CKD, chronic kidney disease.
Figure 3.
Patients with CKD, stratified by urban versus rural area. (a) 2017. (b) 2018. CKD, chronic kidney disease.
Figure 4.
Staging of CKD, stratified by hospital nephrology unit. (a) 2017. (b) 2018. CKD, chronic kidney disease.
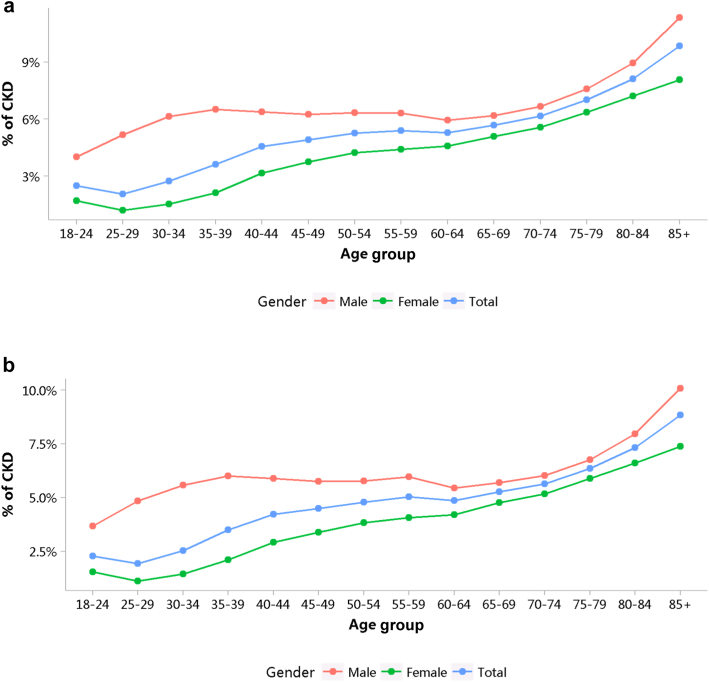
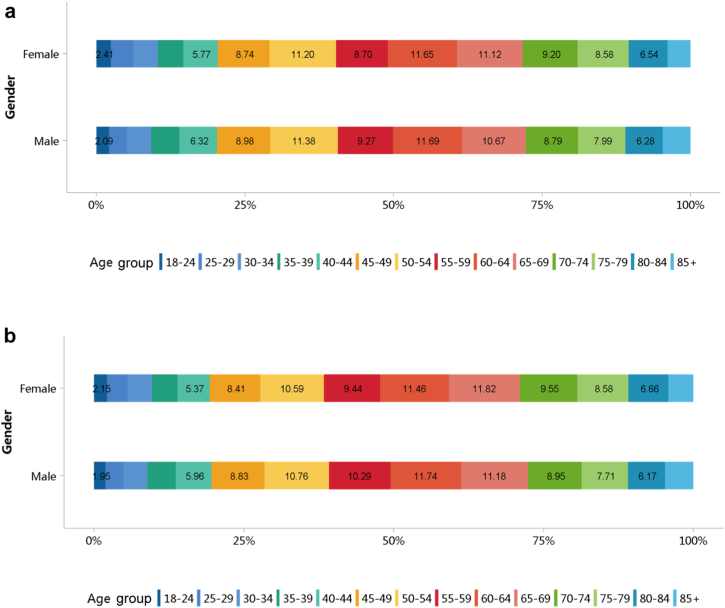
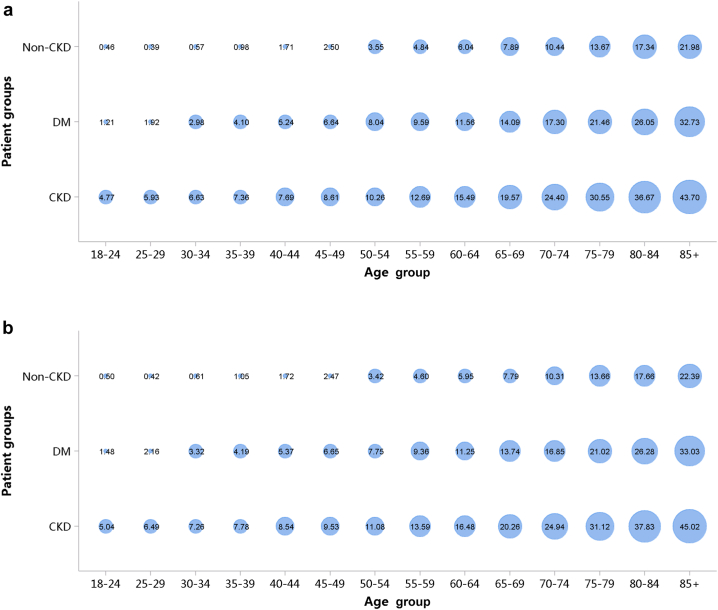
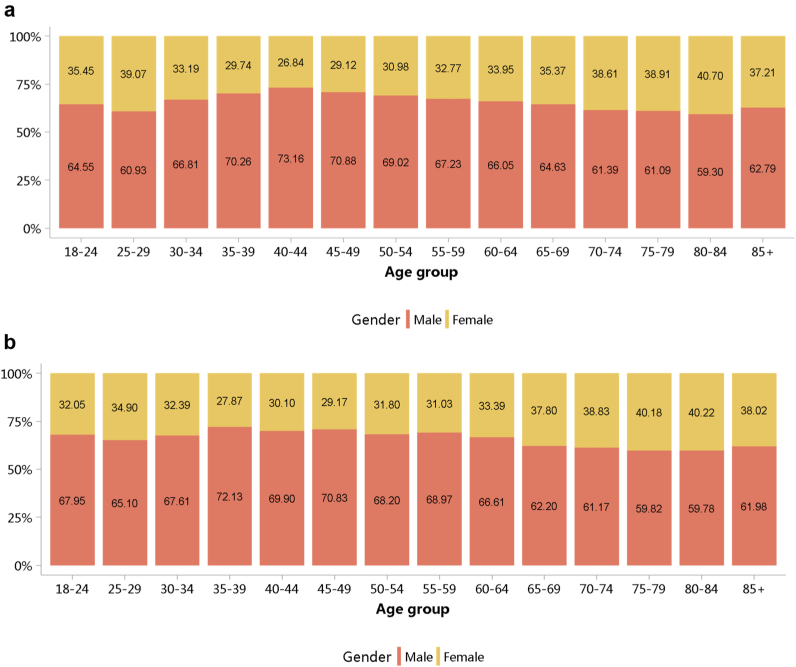
More than half of patients with CKD were 60 years or older (Figure 5; Appendix Table 12), and a male predominance was observed across all age groups (Figure 6; Appendix Table 13). However, it should be noted that these percentages may underestimate the true prevalence of CKD because of potential underdiagnosis; moreover, caution is needed when comparing data from different years, as the coverage of hospitals where the Hospital Quality Monitoring System collects data may vary from year to year.
Figure 5.
Age distribution of patients with CKD, stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease.
Figure 6.
Sex distribution of patients with CKD, stratified by age. (a) 2017. (b) 2018. CKD, chronic kidney disease.
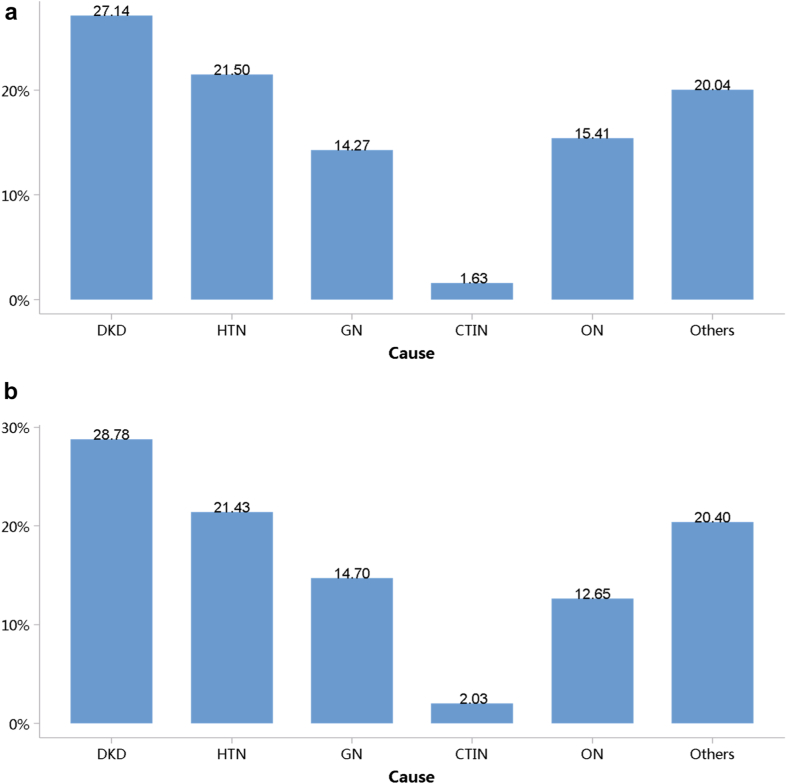
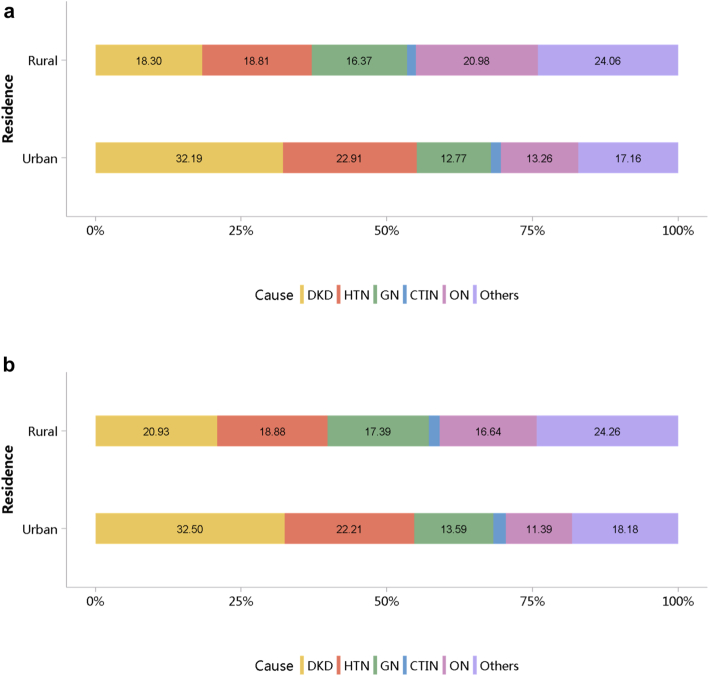
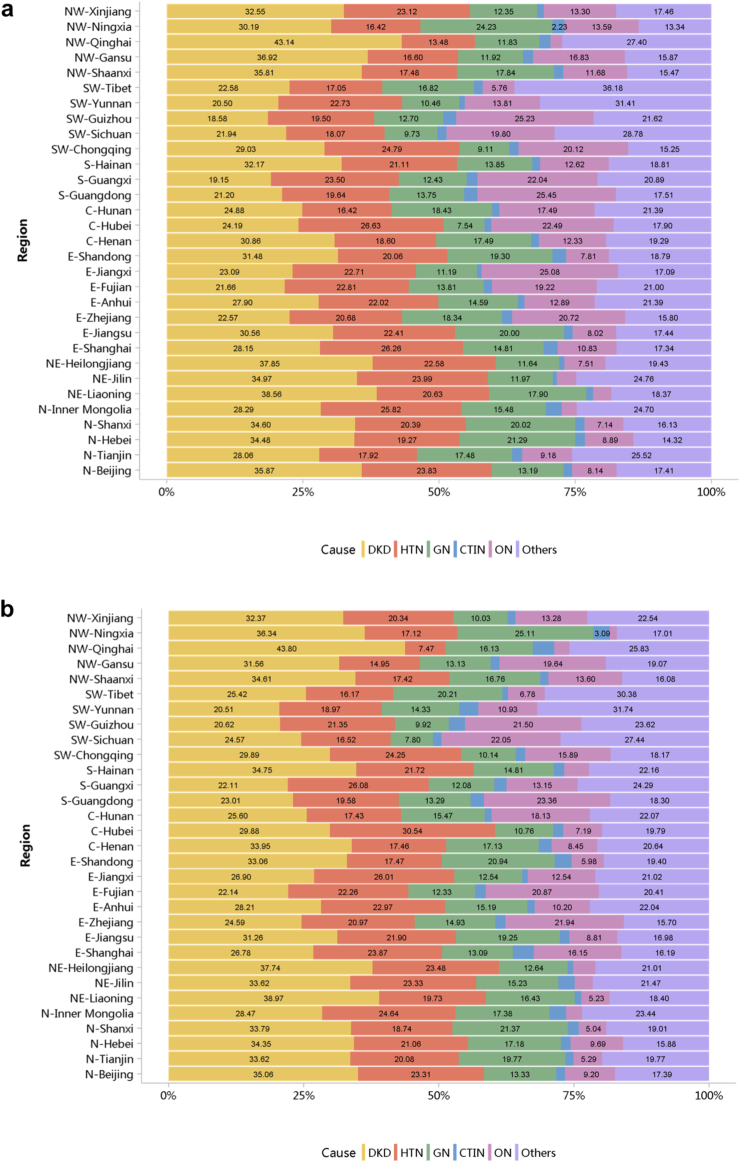
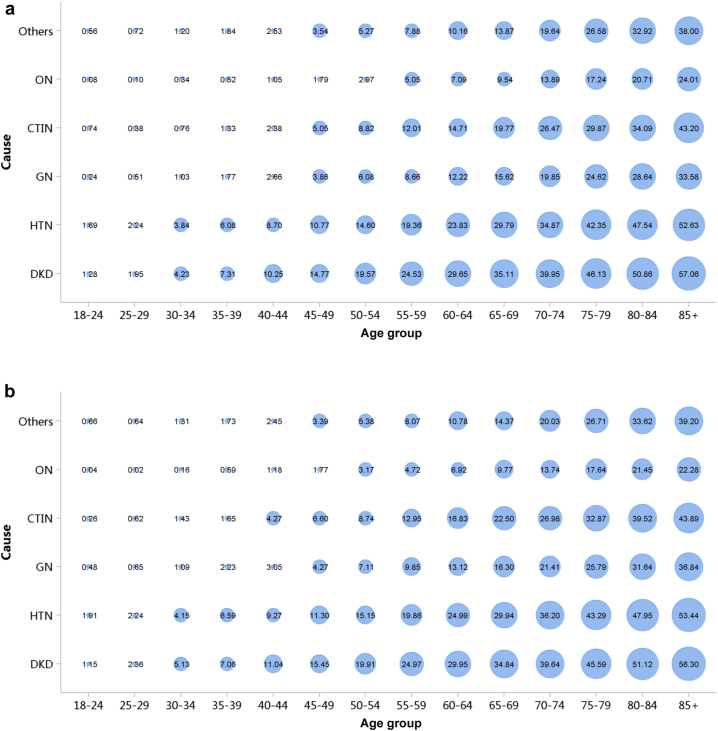
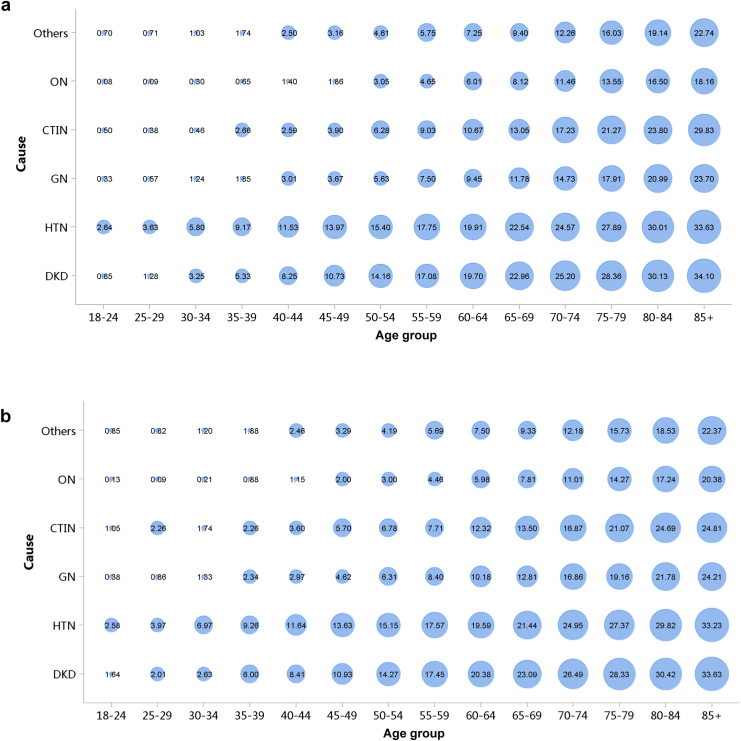
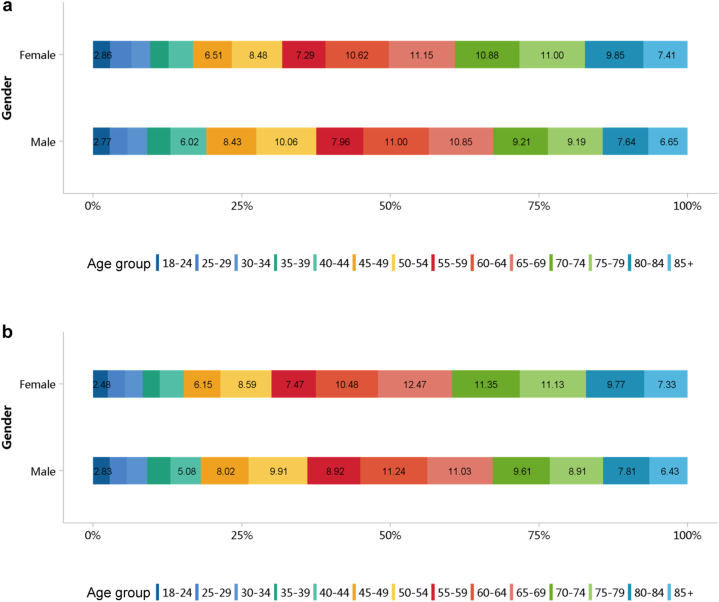
Diabetes was the leading cause of CKD, and its proportion increased slightly in 2018 (28.78%) compared with 2017 (27.14%; Figure 7; Appendix Table 14). The proportion of glomerulonephritis in hospitalized patients with CKD in 2017 and 2018 was 14.27% and 14.70%, respectively. The proportion of other causes of CKD, such as hypertensive nephropathy and obstructive nephropathy, showed slight fluctuations, but the overall trend of change is not significant. It should be noted that we used the term diabetic kidney disease to make the presentation of results more concise, but in fact, these patients should be those with both diabetes and CKD in the absence of a kidney biopsy. The spectrum of CKD varied between urban and rural areas. The causes of CKD in rural areas tended to be similar to those in urban areas, with the proportion of diabetic kidney disease increasing and obstructive nephropathy decreasing (Figure 8; Appendix Table 15). Higher percentages of diabetes and hypertensive nephropathy were observed in the northern China, whereas a higher percentage of obstructive nephropathy was found in the southeast and southwest of the country (Figure 9; Appendix Table 16).
Figure 7.
Cause distribution of patients with CKD. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 8.
Cause distribution of patients with CKD, stratified by urban versus rural area. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 9.
Cause distribution of patients with CKD, stratified by geographic region. (a) 2017. (b) 2018. C, Central China; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; E, East China; GN, glomerulonephritis; HTN, hypertensive nephropathy; N, North China; NE, Northeast China; NW, Northwest China; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons; S, South China; SW, Southwest China.
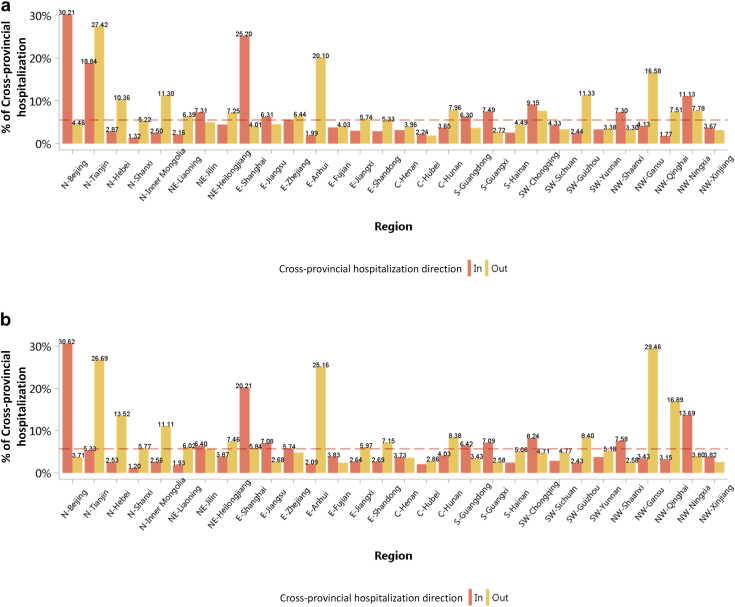
The percentage of interprovince mobility among patients with CKD was 5.53% and 5.66% in 2017 and 2018, respectively (Figure 10; Appendix Table 17). In 2018, the top 3 provinces with the highest proportion of patient outflow were Gansu (29.46%), Tianjin (26.69%), and Anhui (25.16%) while the top 3 provinces with the highest proportion of patient inflow were Beijing (30.62%), Shanghai (20.21%), and Ningxia (13.69%). The mobility patterns revealed that there was a regional imbalance in kidney disease–related medical resources, and optimizing resource allocation should be a policy priority.
Figure 10.
Mobility pattern of patients with CKD. (a) 2017. (b) 2018. C, Central China; CKD, chronic kidney disease; E, East China; N, North China; NE, Northeast China; NW, Northwest China; S, South China; SW, Southwest China. The reference line represents the overall percentage of cross-provincial hospitalization of CKD (2017: 5.53%; 2018: 5.66%).
1.1. Prevalence of CKD among different types of underlying disease
1.2. Staging of CKD
1.3. Demographic characteristics of CKD
1.4. Cause of CKD
1.5. Mobility pattern of hospitalized patients with CKD
Chapter 2: Cardiovascular disease in hospitalized patients with chronic kidney disease
This article is published as a supplement supported by Peking University.
This chapter describes the burden and treatment of cardiovascular disease (CVD) in hospitalized patients with chronic kidney disease (CKD) in China. The clinical pattern of CVD in patients with CKD were compared with that in those with diabetes and those without CKD, and there was overlap between the first 2 groups.
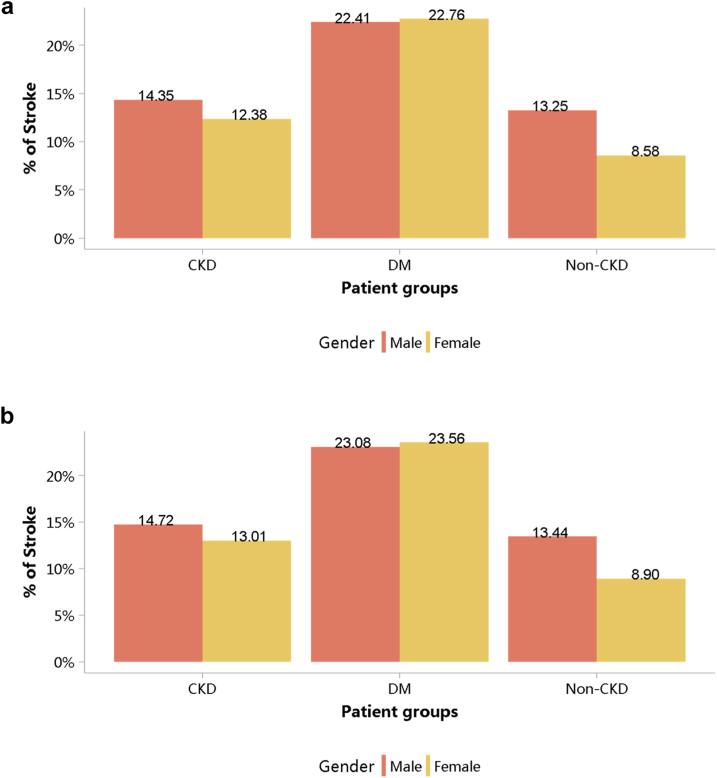
In 2018, coronary heart disease (CHD) was the most common CVD in patients with CKD (20.20%), followed by heart failure (18.28%), stroke (14.01%), and atrial fibrillation (4.38%). These percentages have all increased compared with 2017 (Figure 11; Appendix Table 18). Patients with CKD had lower percentages of CHD and stroke and higher percentages of heart failure and atrial fibrillation than did those with diabetes. These trends were generally consistent across subgroups of sex and age (Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19; Appendix Table 19, Appendix Table 20, Appendix Table 21, Appendix Table 22, Appendix Table 23, Appendix Table 24, Appendix Table 25, Appendix Table 26). In the population with diagnostic codes for CKD staging, in 2017 and 2018, the percentages of CVD for stages 1–2, 3, 4, and 5 of CKD were 31.34% and 32.16%, 40.84% and 43.86%, 41.89% and 44.69%, and 36.27% and 37.71%, respectively.
Figure 11.
Prevalence of CVD, stratified by patient group. (a) 2017. (b) 2018. CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus.
Figure 12.
Prevalence of CHD, stratified by sex. (a) 2017. (b) 2018. CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 13.
Prevalence of CHD, stratified by age. (a) 2017. (b) 2018. CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of CHD.
Figure 14.
Prevalence of stroke, stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 15.
Prevalence of stroke, stratified by age. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of stroke.
Figure 16.
Prevalence of heart failure, stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 17.
Prevalence of heart failure, stratified by age. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of heart failure.
Figure 18.
Prevalence of atrial fibrillation, stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 19.
Prevalence of atrial fibrillation, stratified by age. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of atrial fibrillation.
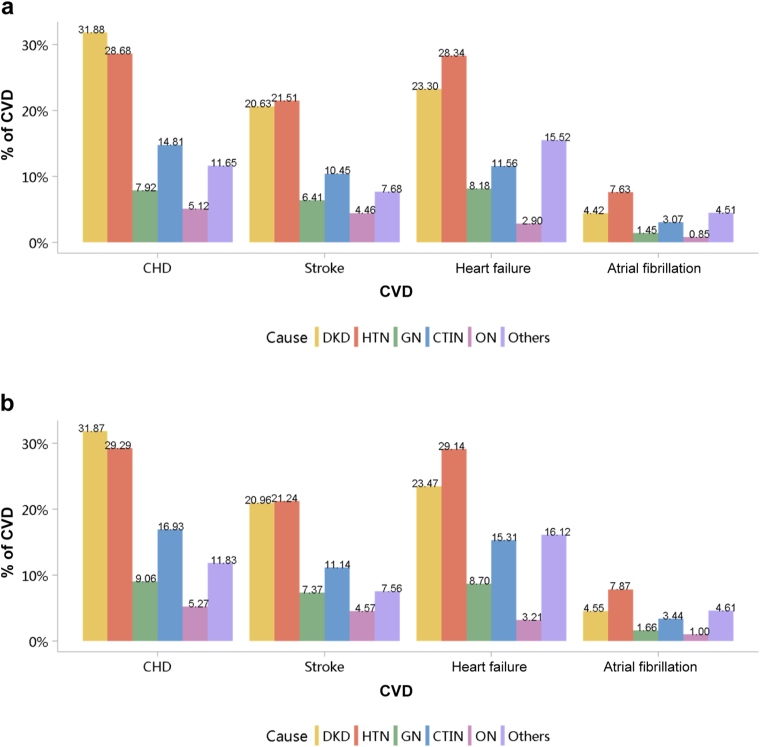
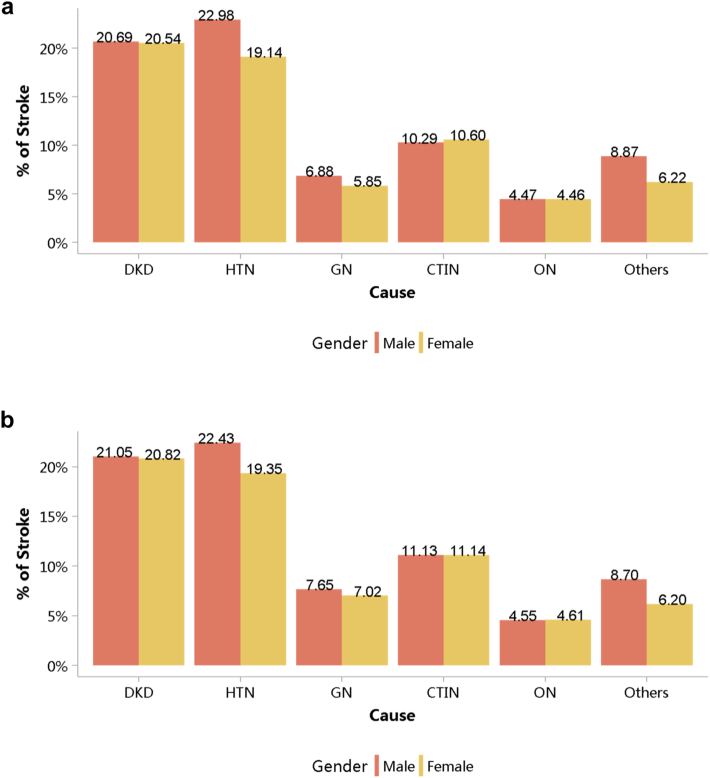
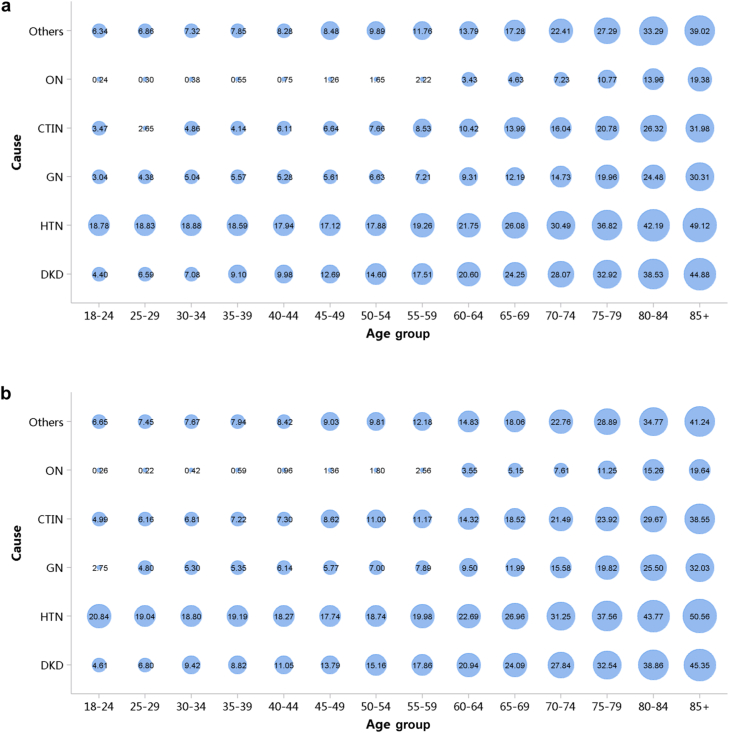
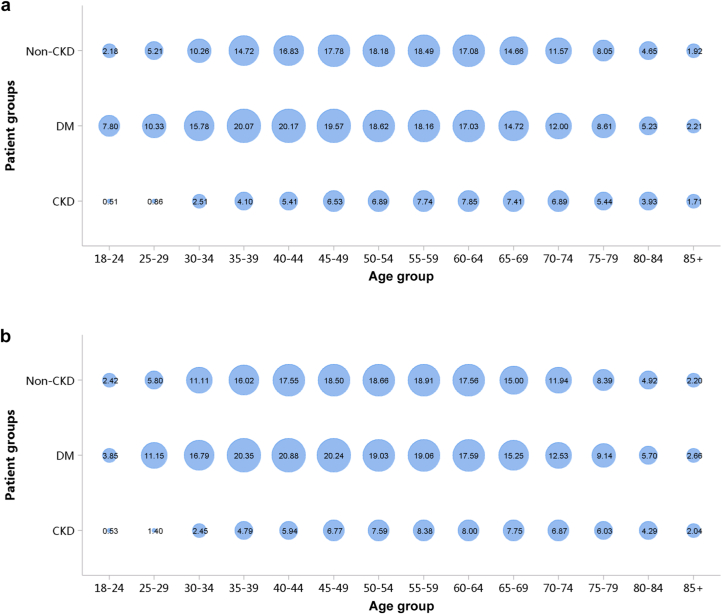
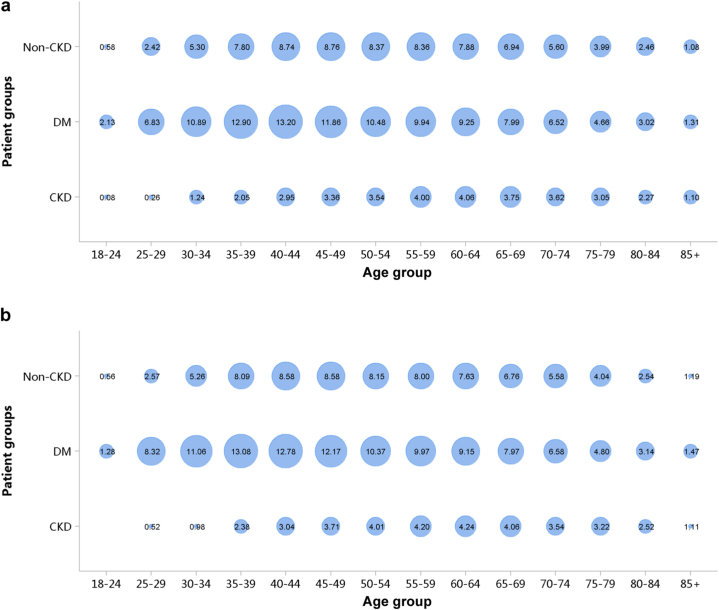
Patients with diabetic kidney disease or hypertensive nephropathy had a higher percentage of CVD, followed by chronic tubulointerstitial nephritis and glomerulonephritis (Figure 20; Appendix Table 27). In 2018, the percentage of CHD among patients with diabetic kidney disease and hypertensive nephropathy was 31.87% and 29.29%, respectively. The trends were generally consistent across subgroups of sex and age (Figure 21, Figure 22, Figure 23, Figure 24, Figure 25, Figure 26, Figure 27, Figure 28; Appendix Table 28, Appendix Table 29, Appendix Table 30, Appendix Table 31, Appendix Table 32, Appendix Table 33, Appendix Table 34, Appendix Table 35). Overall, there was no significant difference in the burden of CVD between male and female patients, and the older the age, the higher the prevalence of various subtypes of CVD.
Figure 20.
Prevalence of CVD among patients with CKD. (a) 2017. (b) 2018. CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; CVD, cardiovascular disease; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 21.
Prevalence of CHD among patients with CKD, stratified by cause and sex. (a) 2017. (b) 2018. CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 22.
Prevalence of CHD among patients with CKD, stratified by cause and age. (a) 2017. (b) 2018. CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons. The point size refers to the percentage of CHD.
Figure 23.
Prevalence of stroke among patients with CKD, stratified by cause and sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 24.
Prevalence of stroke among patients with CKD, stratified by cause and age. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons. The point size refers to the percentage of stroke.
Figure 25.
Prevalence of heart failure among patients with CKD, stratified by cause and sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 26.
Prevalence of heart failure among patients with CKD, stratified by cause and age. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons. The point size refers to the percentage of heart failure.
Figure 27.
Prevalence of atrial fibrillation among patients with CKD, stratified by cause and sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 28.
Prevalence of atrial fibrillation among patients with CKD, stratified by cause and age. (a) 2017. (b) 2018. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons. The point size refers to the percentage of atrial fibrillation.
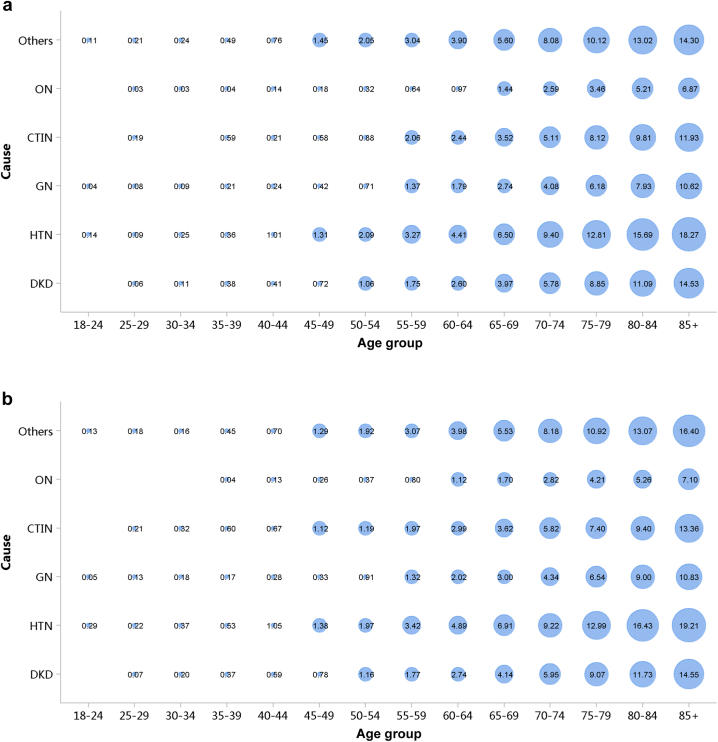
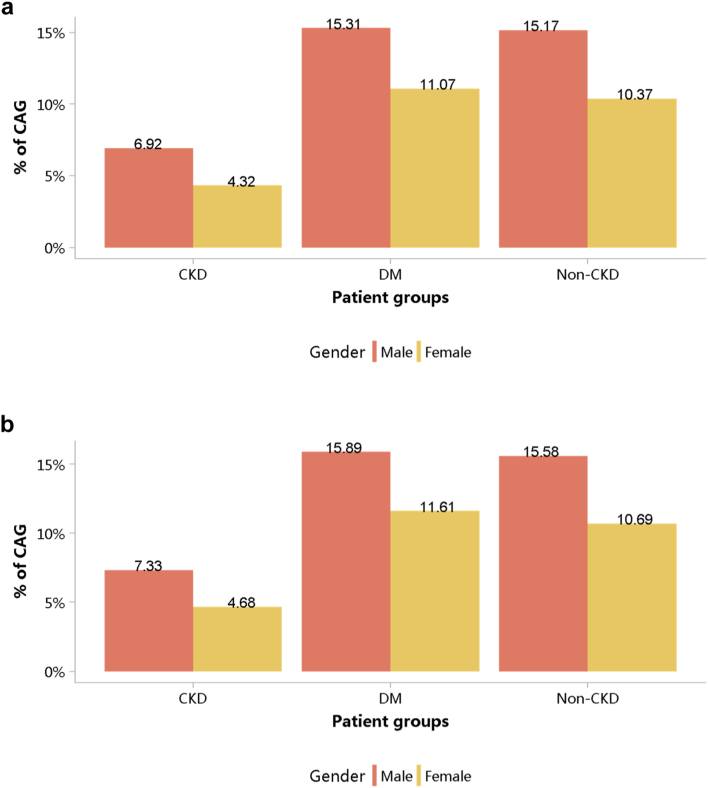
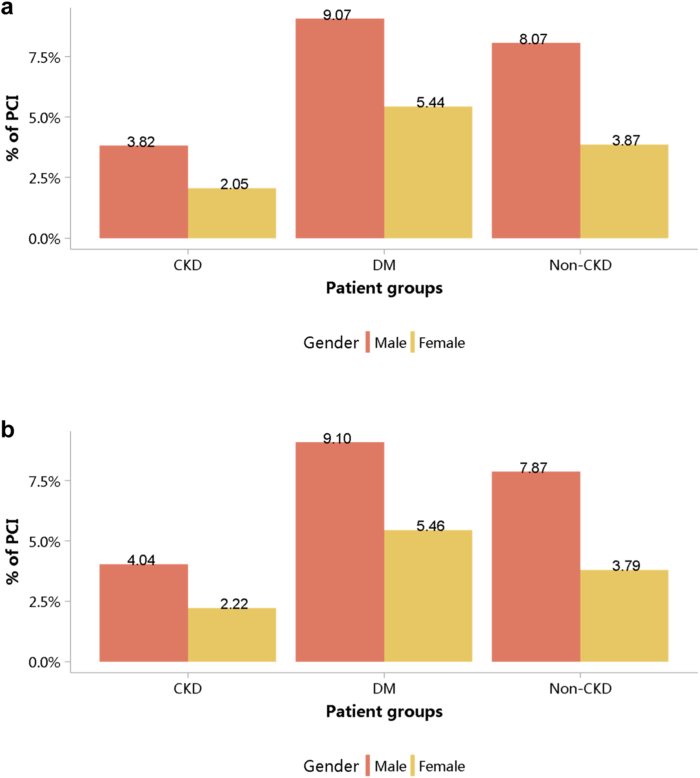
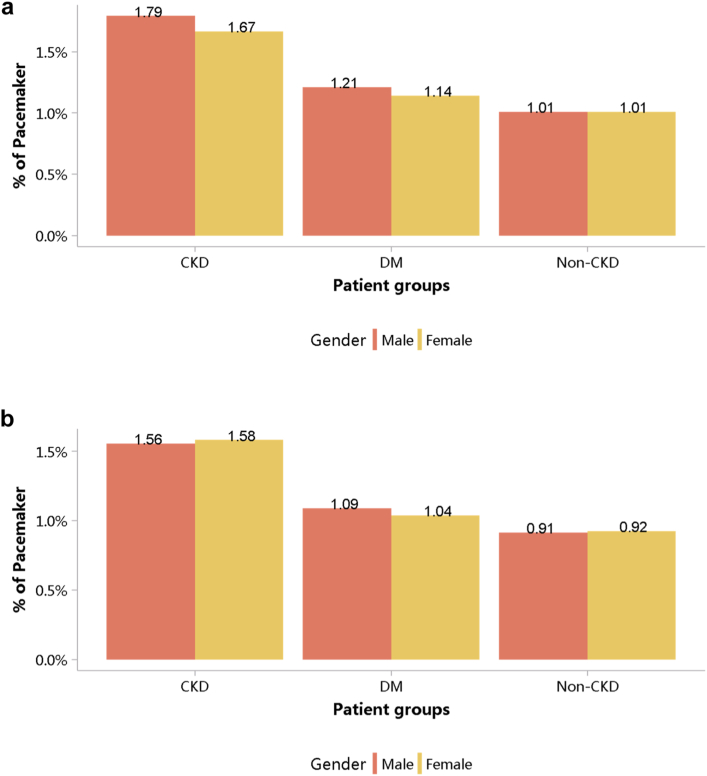
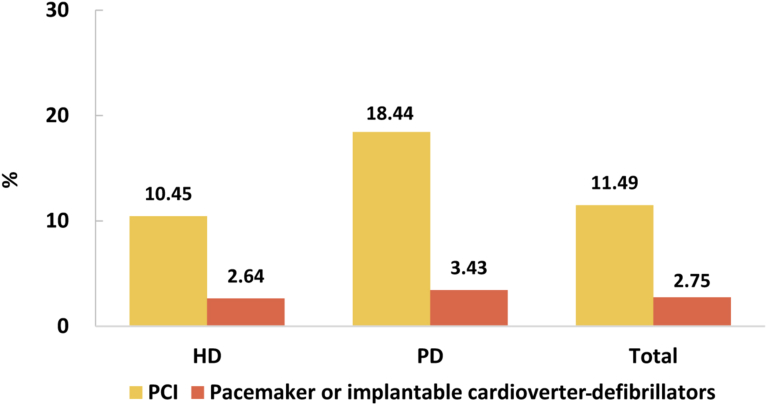
Despite the high burden of CVD in patients with CKD, the percentages of cardiovascular procedures including conventional coronarography, percutaneous coronary intervention, and coronary artery bypass graft were much lower than those among patients without CKD (Figure 29, Figure 30, Figure 31, Figure 32, Figure 33, Figure 34, Figure 35; Appendix Table 36, Appendix Table 37, Appendix Table 38, Appendix Table 39, Appendix Table 40, Appendix Table 41, Appendix Table 42). The percentage of pacemaker implantation among patients with CKD was 1.74% in 2017 and 1.57% in 2018 (Figures 36 and 37; Appendix Tables 43 and 44). The trends did not vary substantially across causes of CKD, except for those with obstructive nephropathy, who had the highest percentage of conventional coronarography (Figure 38; Appendix Table 45).
Figure 29.
Cardiovascular procedures stratified by patient group. (a) 2017. (b) 2018. CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Figure 30.
Cardiovascular procedure: CAG, stratified by sex. (a) 2017. (b) 2018. CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 31.
Cardiovascular procedure: CAG, stratified by age. (a) 2017. (b) 2018. CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of CAG.
Figure 32.
Cardiovascular procedure: PCI, stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Figure 33.
Cardiovascular procedure: PCI, stratified by age. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention. The point size refers to the percentage of PCI.
Figure 34.
Cardiovascular procedure: CABG, stratified by sex. (a) 2017. (b) 2018. CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 35.
Cardiovascular procedure: CABG, stratified by age. (a) 2017. (b) 2018. CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of CABG.
Figure 36.
Cardiovascular procedure: pacemaker, stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 37.
Cardiovascular procedure: pacemaker, stratified by age. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to the percentage of pacemaker.
Figure 38.
Cardiovascular procedures in patients with CKD. (a) 2017. (b) 2018. CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons; PCI, percutaneous coronary intervention.
2.1. Prevalence of CVD, stratified by patient group
2.1.1. Prevalence of CHD
2.1.2. Prevalence of stroke
2.1.3. Prevalence of heart failure
2.1.4. Prevalence of atrial fibrillation
2.2. Prevalence of CVD among patients with CKD
2.2.1. Prevalence of CHD among patients with CKD
2.2.2. Prevalence of stroke among patients with CKD
2.2.3. Prevalence of heart failure among patients with CKD
2.2.4. Prevalence of atrial fibrillation among patients with CKD
2.3. Cardiovascular procedures stratified by patient group
2.3.1. Cardiovascular procedure: coronarography
2.3.2. Cardiovascular procedure: percutaneous coronary intervention
2.3.3. Cardiovascular procedure: coronary artery bypass grafting
2.3.4. Cardiovascular procedure: pacemaker
2.4. Cardiovascular procedures in patients with CKD
Chapter 3: Health care resource utilization in hospitalized patients with chronic kidney disease
This article is published as a supplement supported by Peking University.
This chapter describes the medical expenditure and length of stay (LOS) of patients with chronic kidney disease (CKD), which are both important indicators for health care resource utilization.10 The medical expenditure and LOS of patients with CKD were compared with those of patients with diabetes and those without CKD, and there was overlap between the first 2 groups. The results were displayed as the median and interquartile range, and mean and SD were also provided.
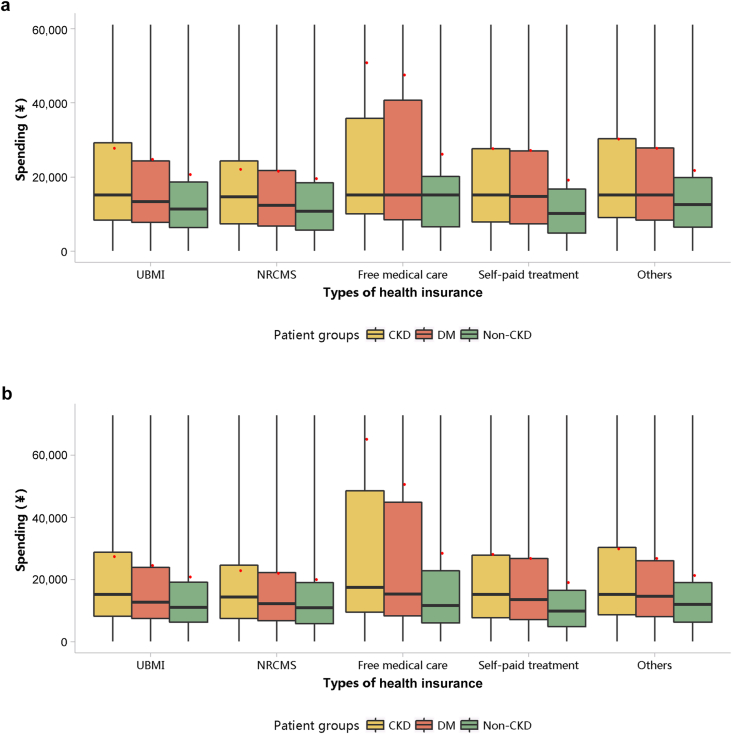
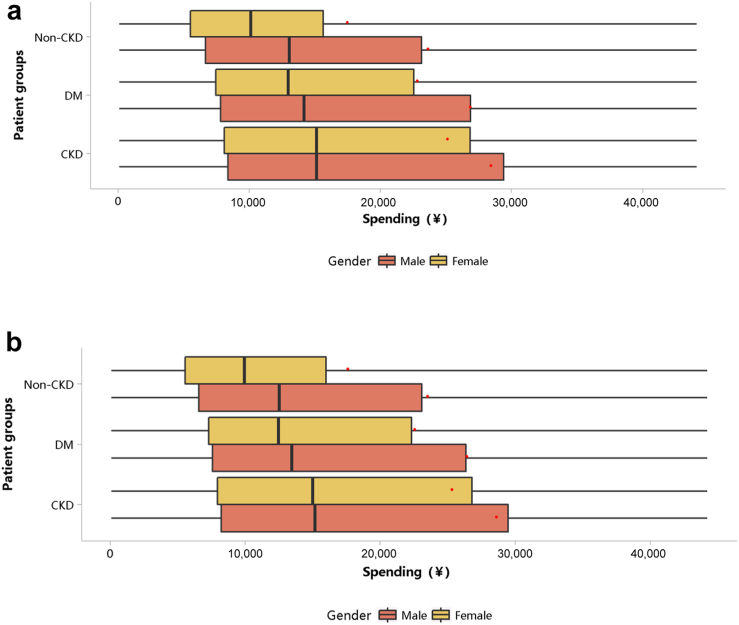
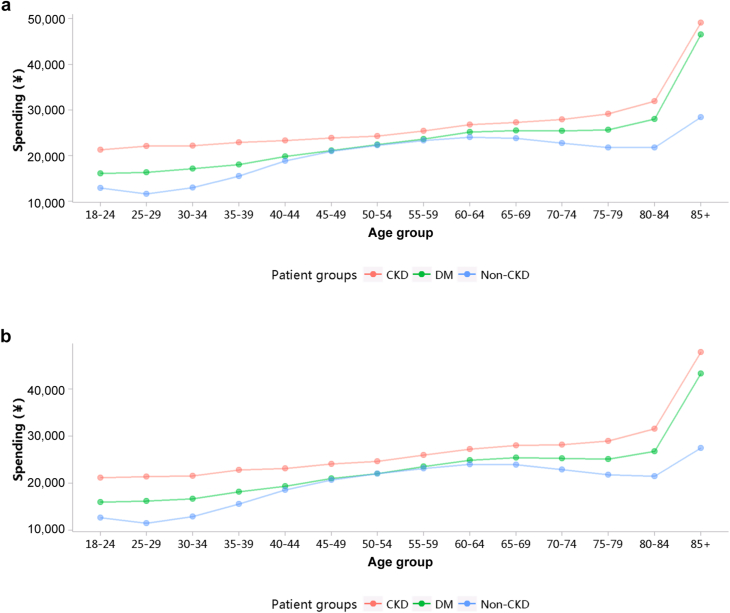
The medical expenditure per person per year was 26,923 renminbi (RMB) (∼3988 USD) in 2017 and 27,115 RMB (∼4099 USD) in 2018 (Table 1). When patients with CKD also had heart failure and diabetes, their medical costs increased significantly. In 2017 and 2018, the median cost per patient with CKD was 15,151 (∼2260 USD; interquartile range 8246–28,305) and 15,175 RMB (∼2293 USD; interquartile range 8100–28,313 RMB), while the mean cost for these 2 years was 26,923 ± 47,110 (∼4142 USD) and 27,115 ± 47,434 RMB (∼4108 USD), respectively (Figure 39; Appendix Tables 46 and 47). The medical expenditure of patients with CKD was higher than that of those with diabetes and those without CKD, and this trend existed across different subgroups of sex and age (Figures 40 and 41; Appendix Table 48, Appendix Table 49, Appendix Table 50, Appendix Table 51). With the increase in age, the hospitalization cost for patients generally showed an upward trend.
Figure 39.
Costs stratified by types of health insurance. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural cooperative medical care; UBMI, Urban Basic Medical Insurance. Limited to 1.5 times the third quartile. The red points refer to cost per person per year.
Figure 40.
Costs stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus. Limited to 1.5 times the third quartile. The red points refer to cost per person per year.
Figure 41.
Costs stratified by age group (mean). (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus.
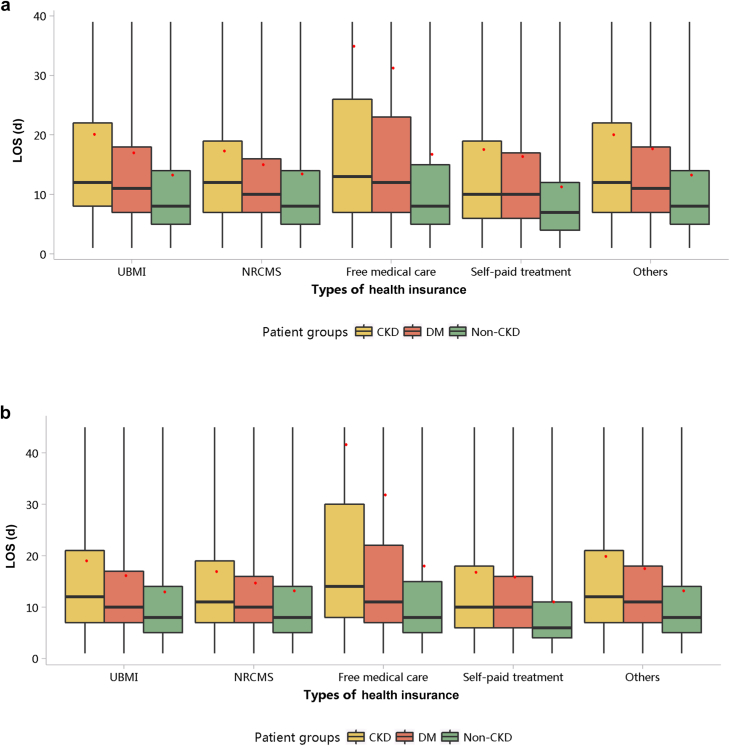
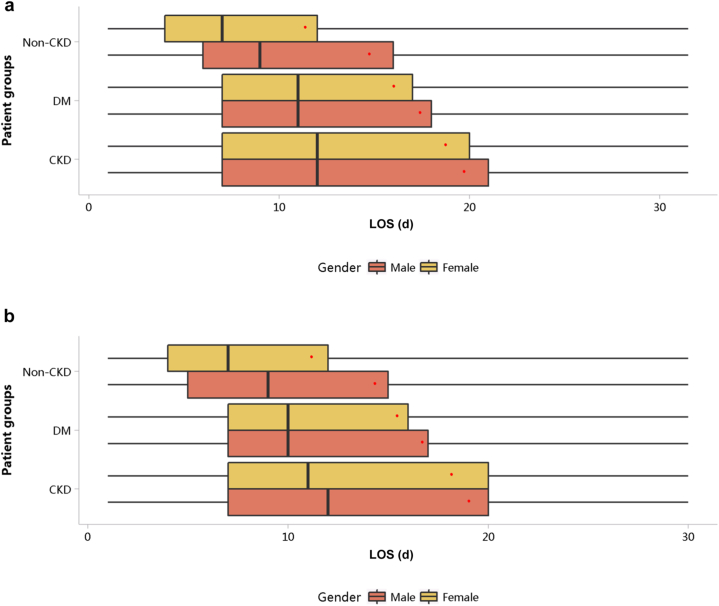
The LOS per person per year was 19.22 days in 2017 and 18.59 days in 2018 (Table 2). Compared with patients with other types of health insurance, those covered by free medical care had the longest LOS (Figure 42; Appendix Tables 52 and 53). The LOS of patients with CKD was longer than that of those with diabetes and those without CKD, and this trend existed across different subgroups of sex and age (Figures 43 and 44; Appendix Table 54, Appendix Table 55, Appendix Table 56, Appendix Table 57). In 2018, patients with CKD who were 85 years or older had the longest hospital stay, with a median of 14 days (interquartile range 8–28 days) and a mean of 32.51 ± 60.43 days.
Figure 42.
LOS stratified by types of health insurance. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; LOS, length of stay; NRCMS, new rural cooperative medical care; UBMI, Urban Basic Medical Insurance. Limited to 1.5 times the third quartile. The red points refer to LOS per person per year.
Figure 43.
LOS stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; LOS, length of stay. Limited to 1.5 times the third quartile. The red points refer to LOS per person per year.
Figure 44.
LOS stratified by age group (mean). (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; LOS, length of stay.
3.1. Costs
3.1.1. Overall medical costs stratified by CKD, diabetes, and heart failure
Table 1.
Overall medical costs stratified by CKD, DM, and HF
| Patient group | 2017 |
2018 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HQMS population | Total costs (millions, ¥) | PPPY (¥) | Population (%) | Costs (%) | HQMS population | Total costs (millions, ¥) | PPPY (¥) | Population (%) | Costs (%) | |
| All | 19,341,078 | 394,164 | 20,380 | 100.00 | 100.00 | 16,805,809 | 343,234 | 20,424 | 100.00 | 100.00 |
| With HF, CKD, or DM | 3,379,917 | 89,755 | 26,555 | 17.48 | 22.77 | 2,937,567 | 78,244 | 26,636 | 17.48 | 22.80 |
| CKD alone | 568,558 | 13,556 | 23,842 | 2.94 | 3.44 | 441,754 | 10,605 | 24,005 | 2.63 | 3.09 |
| DM alone | 1,449,052 | 32,936 | 22,729 | 7.49 | 8.36 | 1,297,749 | 29,023 | 22,364 | 7.72 | 8.46 |
| HF alone | 774,607 | 23,918 | 30,877 | 4.00 | 6.07 | 692,343 | 21,992 | 31,765 | 4.12 | 6.41 |
| CKD and DM alone | 222,605 | 5203 | 23,372 | 1.15 | 1.32 | 188,783 | 4344 | 23,009 | 1.12 | 1.27 |
| CKD and HF alone | 100,439 | 4086 | 40,679 | 0.52 | 1.04 | 84,838 | 3465 | 40,838 | 0.50 | 1.01 |
| DM and HF alone | 199,249 | 7136 | 35,816 | 1.03 | 1.81 | 175,850 | 6307 | 35,864 | 1.05 | 1.84 |
| CKD, HF, and DM | 65,407 | 2921 | 44,655 | 0.34 | 0.74 | 56,250 | 2509 | 44,611 | 0.33 | 0.73 |
| No CKD, HF, or DM | 15,961,161 | 304,408 | 19,072 | 82.52 | 77.23 | 13,868,242 | 264,990 | 19,108 | 82.52 | 77.20 |
| All CKD | 957,009 | 25,765 | 26,923 | 4.95 | 6.54 | 771,625 | 20,922 | 27,115 | 4.59 | 6.10 |
| All DM | 1,936,313 | 48,196 | 24,891 | 10.01 | 12.23 | 1,718,632 | 42,183 | 24,545 | 10.23 | 12.29 |
| All HF | 1,139,702 | 38,061 | 33,395 | 5.89 | 9.66 | 1,009,281 | 34,273 | 33,957 | 6.01 | 9.99 |
| CKD and DM | 288,012 | 8124 | 28,206 | 1.49 | 2.06 | 245,033 | 6853 | 27,968 | 1.46 | 2.00 |
| CKD and HF | 165,846 | 7007 | 42,247 | 0.86 | 1.78 | 141,088 | 5974 | 42,342 | 0.84 | 1.74 |
| DM and HF | 264,656 | 10,057 | 38,000 | 1.37 | 2.55 | 232,100 | 8816 | 37,984 | 1.38 | 2.57 |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HQMS, Hospital Quality Monitoring System; PPPY, per person per year.
3.1.2. Costs stratified by types of health insurance
3.1.3. Costs stratified by sex
3.1.4. Costs stratified by age
3.2. LOS
3.2.1. Overall LOS stratified by CKD, diabetes, and heart failure
Table 2.
Overall LOS stratified by CKD, DM, and HF
| Patient group | 2017 |
2018 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HQMS population | Total LOS (d) | PPPY (d) | Population (%) | LOS (%) | HQMS population | Total LOS (d) | PPPY (d) | Population (%) | LOS (%) | |
| All | 19,341,078 | 252,918 | 13.08 | 100.00 | 100.00 | 16,805,809 | 214,739 | 12.78 | 100.00 | 100.00 |
| With HF, CKD, or DM | 3,379,917 | 57,140 | 16.91 | 17.48 | 22.59 | 2,937,567 | 48,027 | 16.35 | 17.48 | 22.37 |
| CKD alone | 568,558 | 9604 | 16.89 | 2.94 | 3.80 | 441,754 | 7200 | 16.30 | 2.63 | 3.35 |
| DM alone | 1,449,052 | 22,423 | 15.47 | 7.49 | 8.87 | 1,297,749 | 19,343 | 14.90 | 7.72 | 9.01 |
| HF alone | 774,607 | 12,523 | 16.17 | 4.00 | 4.95 | 692,343 | 11,079 | 16.00 | 4.12 | 5.16 |
| CKD and DM alone | 222,605 | 4133 | 18.57 | 1.15 | 1.63 | 188,783 | 3363 | 17.81 | 1.12 | 1.57 |
| CKD and HF alone | 100,439 | 2703 | 26.91 | 0.52 | 1.07 | 84,838 | 2165 | 25.52 | 0.50 | 1.01 |
| DM and HF alone | 199,249 | 3797 | 19.06 | 1.03 | 1.50 | 175,850 | 3264 | 18.56 | 1.05 | 1.52 |
| CKD, HF, and DM | 65,407 | 1957 | 29.92 | 0.34 | 0.77 | 56,250 | 1614 | 28.69 | 0.33 | 0.75 |
| No CKD, HF, or DM | 15,961,161 | 195,778 | 12.27 | 82.52 | 77.41 | 13,868,242 | 166,713 | 12.02 | 82.52 | 77.63 |
| All CKD | 957,009 | 18,397 | 19.22 | 4.95 | 7.27 | 771,625 | 14,341 | 18.59 | 4.59 | 6.68 |
| All DM | 1,936,313 | 32,309 | 16.69 | 10.01 | 12.77 | 1,718,632 | 27,583 | 16.05 | 10.23 | 12.84 |
| All HF | 1,139,702 | 20,980 | 18.41 | 5.89 | 8.30 | 1,009,281 | 18,121 | 17.95 | 6.01 | 8.44 |
| CKD and DM | 288,012 | 6090 | 21.14 | 1.49 | 2.41 | 245,033 | 4976 | 20.31 | 1.46 | 2.32 |
| CKD and HF | 165,846 | 4660 | 28.10 | 0.86 | 1.84 | 141,088 | 3778 | 26.78 | 0.84 | 1.76 |
| DM and HF | 264,656 | 5754 | 21.74 | 1.37 | 2.28 | 232,100 | 4878 | 21.02 | 1.38 | 2.27 |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HQMS, Hospital Quality Monitoring System; LOS, length of stay; PPPY, per person per year.
3.2.2. LOS stratified by types of health insurance
3.2.3. LOS stratified by sex
3.2.4. LOS stratified by age
Chapter 4: In-hospital mortality in patients with chronic kidney disease
This article is published as a supplement supported by Peking University.
This chapter describes the in-hospital mortality in patients with chronic kidney disease (CKD) stratified by types of health insurance, sex, and age group. The in-hospital mortality in patients with CKD was compared with that in those with diabetes and those without CKD, and there was overlap between the first 2 groups.
The in-hospital mortality rate in patients with CKD was decreasing, reaching 2.33% and 2.13% in 2017 and 2018, respectively, which was higher than that in those with diabetes (regardless of whether they had concurrent CKD) but lower than that in those with heart failure (also regardless of CKD status; Table 3). This trend was consistent across different types of health insurance and subgroups of sex and age.
In 2018, patients covered by free medical care had the highest in-hospital mortality rate (6.55%), followed by those with Urban Basic Medical Insurance (2.43%) and others (2.13%; Figure 45; Appendix Table 58). This can be explained by population characteristics with different types of health insurance and the availability and utilization of medical resources. The in-hospital mortality rate in male patients with CKD was higher than that in female patients, but both showed a decreasing trend over time (Figure 46; Appendix Table 59). Moreover, the in-hospital mortality rate increased with age (Figure 47; Appendix Table 60). In 2018, patients with CKD who were 85 years or older had the highest mortality rate (9.59%), 1.7 times that in those with diabetes (5.58%).
Figure 45.
In-hospital mortality stratified by different types of health insurance. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural cooperative medical care; UBMI, Urban Basic medical Insurance.
Figure 46.
In-hospital mortality stratified by sex. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 47.
In-hospital mortality stratified by age group. (a) 2017. (b) 2018. CKD, chronic kidney disease; DM, diabetes mellitus. The point size refers to mortality rate.
4.1. In-hospital mortality stratified by CKD, diabetes alone, and heart failure alone
Table 3.
In-hospital mortality stratified by CKD, DM alone, and HF alone
| Patient group | 2017 |
2018 |
||||||
|---|---|---|---|---|---|---|---|---|
| Hospital mortality | HQMS population | Mortality rate (%) | Proportion (%) | Hospital mortality | HQMS population | Mortality rate (%) | Proportion (%) | |
| All | 146,568 | 19,341,078 | 0.76 | 100.00 | 119,761 | 16,805,809 | 0.71 | 100.00 |
| With HF, CKD, or DM | 69,441 | 3,379,917 | 2.05 | 47.38 | 57,513 | 2,937,567 | 1.96 | 48.02 |
| CKD alone | 7728 | 568,558 | 1.36 | 5.27 | 5325 | 441,754 | 1.21 | 4.45 |
| DM alone | 11,380 | 1,449,052 | 0.79 | 7.76 | 8444 | 1,297,749 | 0.65 | 7.05 |
| HF alone | 29,196 | 774,607 | 3.77 | 19.92 | 26,878 | 692,343 | 3.88 | 22.44 |
| CKD and DM alone | 3036 | 222,605 | 1.36 | 2.07 | 2186 | 188,783 | 1.16 | 1.83 |
| CKD and HF alone | 7539 | 100,439 | 7.51 | 5.14 | 5868 | 84,838 | 6.92 | 4.90 |
| DM and HF alone | 6570 | 199,249 | 3.30 | 4.48 | 5747 | 175,850 | 3.27 | 4.80 |
| CKD, HF, and DM | 3992 | 65,407 | 6.10 | 2.72 | 3065 | 56,250 | 5.45 | 2.56 |
| No CKD, HF, or DM | 77,127 | 15,961,161 | 0.48 | 52.62 | 62,248 | 13,868,242 | 0.45 | 51.98 |
| All CKD | 22,295 | 957,009 | 2.33 | 15.21 | 16,444 | 771,625 | 2.13 | 13.73 |
| All DM | 24,978 | 1,936,313 | 1.29 | 17.04 | 19,442 | 1,718,632 | 1.13 | 16.23 |
| All HF | 47,297 | 1,139,702 | 4.15 | 32.27 | 41,558 | 1,009,281 | 4.12 | 34.70 |
| CKD and DM | 7028 | 288,012 | 2.44 | 4.80 | 5251 | 245,033 | 2.14 | 4.38 |
| CKD and HF | 11,531 | 165,846 | 6.95 | 7.87 | 8933 | 141,088 | 6.33 | 7.46 |
| DM and HF | 10,562 | 264,656 | 3.99 | 7.21 | 8812 | 232,100 | 3.80 | 7.36 |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HQMS, Hospital Quality Monitoring System.
4.2. In-hospital mortality stratified by types of health insurance
4.3. In-hospital mortality stratified by sex
4.4. In-hospital mortality stratified by age
Chapter 5: Acute kidney injury
This article is published as a supplement supported by Peking University.
This chapter focuses on the characteristics of inpatients diagnosed with acute kidney injury (AKI) in tertiary hospitals in China. It should be noted that the results reflect both the reported diagnostic rate and potential burden within the overall hospitalized population because AKI is usually underdiagnosed.
There were significant regional differences in the percentage of AKI among people who stayed in an intensive care unit compared with those without an intensive care unit stay; especially in several southern provinces of China, the percentage of AKI was higher (Figure 48; Appendix Table 61). The percentage of patients with a diagnostic code for AKI was 0.31% in 2017 and 0.30% in 2018, which has remained stable in the past 5 years.6, 7, 8
Figure 48.
Percentage of AKI with and without an ICU stay, stratified by geographic region. (a) 2017. (b) 2018. AKI, acute kidney injury; C, Central China; E, East China; ICU, intensive care unit; N, North China; NE, Northeast China; NW, Northwest China; S, South China; SW, Southwest China.
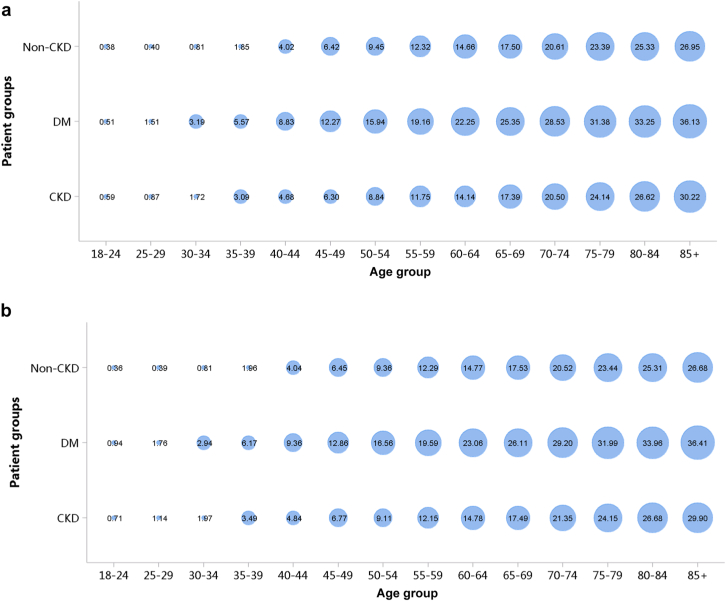
In 2017 and 2018, a total of 1.74% and 1.82% of patients with CKD, respectively, were diagnosed with AKI (Figure 49; Appendix Table 62). Patients with chronic tubulointerstitial nephropathy and glomerulonephritis had higher percentages of AKI, followed by obstructive nephropathy and hypertensive nephropathy. In terms of demographic characteristics of patients with AKI, more than half of them were 60 years or older, in both male and female patients (Figure 50; Appendix Table 63). The proportion of male patients with AKI was almost twice that of female patients (Figure 51; Appendix Table 64).
Figure 49.
Percentage of AKI among patients with CKD. (a) 2017. (b) 2018. AKI, acute kidney injury; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Figure 50.
Age distribution of patients with AKI, stratified by sex. (a) 2017. (b) 2018. AKI, acute kidney injury.
Figure 51.
Sex distribution of patients with AKI, stratified by age. (a) 2017. (b) 2018. AKI, acute kidney injury.
The percentage of CKD among patients with AKI was 28.22% in 2017 and 28.12% in 2018, respectively (Figure 52; Appendix Table 65). The percentage of CKD among female patients was slightly higher than that in male patients, and those percentages decreased with age, which might partly reflect survivorship bias. The percentage of diabetes among patients with AKI was 17.88% in 2017 and 18.33% in 2018, showing a slight upward trend (Figure 53; Appendix Table 66). Patients with AKI who were aged 70 to 74 years had the highest percentage of diabetes.
Figure 52.
Percentage of CKD among patients with AKI, stratified by sex and age. (a) 2017. (b) 2018. AKI, acute kidney injury; CKD, chronic kidney disease.
Figure 53.
Percentage of diabetes mellitus among patients with AKI, stratified by sex and age. (a) 2017. (b) 2018. AKI, acute kidney injury.
5.1. Percentage of AKI
5.2. Characteristics of AKI
5.2.1. Age distribution of AKI, stratified by sex
5.2.2. Sex distribution of AKI, stratified by age
5.3. Percentages of CKD and diabetes among patients with AKI
Chapter 6: Identification and characteristics of patients on dialysis
This article is published as a supplement supported by Peking University.
This chapter describes the prevalence and characteristics of patients receiving hemodialysis (HD) and peritoneal dialysis (PD) in China based on the China Health Insurance Research Association database.
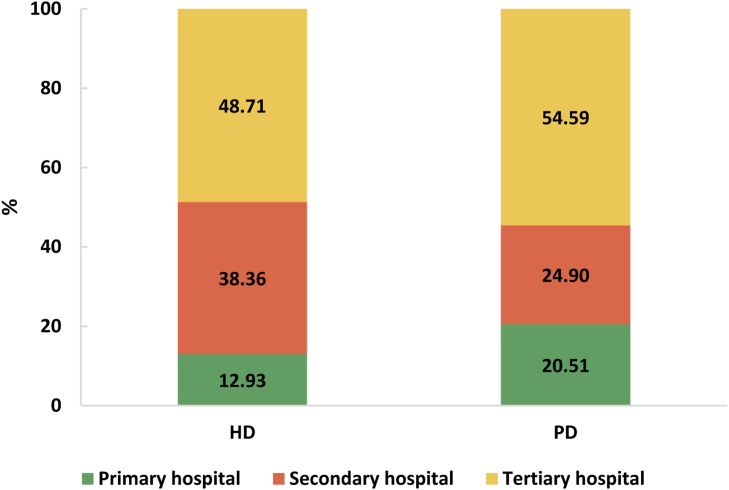
The number of sampled insurance beneficiaries in the China Health Insurance Research Association database in 2017 was 9,765,615, from which we identified 19,923 patients (0.20%) receiving maintenance dialysis. The mean age of patients on dialysis was 55.2 ± 16.2 years, and 58.51% were men (Table 4). Patients 19 years or younger accounted for 1.98%, and patients treated with PD were younger than those treated with HD (Table 5). For all prevalent patients on dialysis, HD was the major treatment modality (92.12%). Patients on HD and PD were mainly treated in tertiary hospitals, whereas among patients on PD, the proportion of patients treated in primary hospitals (20.51%) was slightly lower than that in secondary hospitals (24.90%; Figure 54). Patients on dialysis included in the China Health Insurance Research Association database were mainly the southwestern (32.28%), eastern (30.30%), and central (16.83%) regions of China (Table 6).
Table 4.
Number of patients on dialysis, stratified by sex and modality
| Sex | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Male | 10,779 | 58.73 | 877 | 55.86 | 11,656 | 58.51 |
| Female | 7574 | 41.27 | 693 | 44.14 | 8267 | 41.49 |
| Total | 18,353 | 100 | 1570 | 100 | 19,923 | 100 |
HD, hemodialysis; PD, peritoneal dialysis.
Table 5.
Number of patients on dialysis, stratified by age and modality
| Age (yr) | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Mean ± SD | 55.4 ± 16.2 | 52.8 ± 15.9 | 55.2 ± 16.2 | |||
| 0–19 | 358 | 1.95 | 37 | 2.36 | 395 | 1.98 |
| 20–44 | 4014 | 21.87 | 428 | 27.26 | 4442 | 22.30 |
| 45–64 | 8261 | 45.01 | 718 | 45.73 | 8979 | 45.07 |
| 65–74 | 3511 | 19.13 | 245 | 15.61 | 3756 | 18.85 |
| ≥75 | 2179 | 11.87 | 135 | 8.60 | 2314 | 11.61 |
| Unknown | 30 | 0.16 | 7 | 0.45 | 37 | 0.19 |
| Total | 18,353 | 100 | 1570 | 100 | 19,923 | 100 |
HD, hemodialysis; PD, peritoneal dialysis.
Figure 54.
Distribution of patients on HD and PD among different hospital levels. HD, hemodialysis; PD, peritoneal dialysis.
Table 6.
Number of patients on dialysis, stratified by geographic region and modality
| Geographic region | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| East China | 5544 | 30.21 | 493 | 31.40 | 6037 | 30.30 |
| North China | 604 | 3.29 | 130 | 8.28 | 734 | 3.68 |
| Central China | 3095 | 16.86 | 259 | 16.50 | 3354 | 16.83 |
| South China | 1461 | 7.96 | 228 | 14.52 | 1689 | 8.48 |
| Northwest China | 725 | 3.95 | 135 | 8.60 | 860 | 4.32 |
| Southwest China | 6218 | 33.88 | 214 | 13.63 | 6432 | 32.28 |
| Northeast China | 706 | 3.85 | 111 | 7.07 | 817 | 4.10 |
| Total | 18,353 | 100 | 1570 | 100 | 19,923 | 100 |
HD, hemodialysis; PD, peritoneal dialysis.
The age- and sex-adjusted prevalence of patients receiving dialysis in 2017 was 419.39 per million population (PMP), which has increased rapidly compared with 2015 (311.29 PMP),7 but the increase is not significant compared with 2016 (419.12 PMP; Table 7).8 The age- and sex-adjusted prevalences of HD and PD were 384.41 and 34.98 PMP, respectively. The prevalence of male patients (472.03 PMP) was higher than that of female patients (364.17 PMP), and the trend was increasing more rapidly in male patients. Accordingly, it was estimated that the total number of prevalent patients on dialysis in China in 2017 was 581,273 (HD: 532,791; PD: 48,482).
Table 7.
Age- and sex-adjusted prevalence of patients on dialysis (PMP) in 2015, 2016, and 2017, stratified by sex and modalitya
| Sex | HD |
PD |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | |
| Male | 315.00 | 433.16 | 433.83 | 25.70 | 35.84 | 38.20 | 340.70 | 468.99 | 472.03 |
| Female | 250.23 | 333.21 | 332.56 | 31.73 | 34.05 | 31.60 | 281.97 | 367.26 | 364.17 |
| Total | 282.60 | 384.13 | 384.41 | 28.69 | 34.99 | 34.98 | 311.29 | 419.12 | 419.39 |
HD, hemodialysis; PD, peritoneal dialysis; PMP, per million population.
Age- and sex-adjusted prevalence was standardized using the direct method with reference to the 2010 national population census data.
Given the absence of mortality rates specific to patients on dialysis in the present report, we have drawn on the National Medical Service and Quality Safety Report 2018,14 which revealed an annual mortality rate of 3.4% in patients on HD and a lower rate of 2.3% in patients on PD.
Chapter 7: Examinations and treatments of patients on dialysis
This article is published as a supplement supported by Peking University.
The quality of dialysis services provided to patients differs significantly across nations, underscoring the need for tailored approaches. This chapter delves into the laboratory measurement and management strategies for major complications encountered by patients on dialysis, including anemia, mineral and bone disorders, and malnutrition, aiming to optimize patient outcomes.
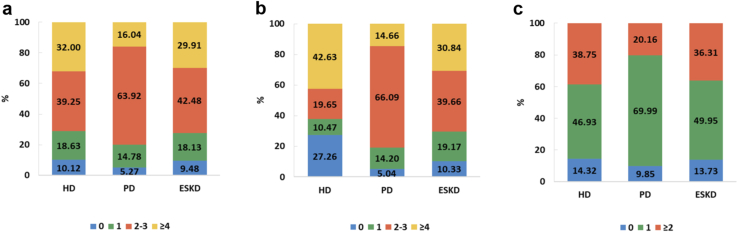
The adherence rates to the Kidney Disease: Improving Global Outcomes guidelines for monitoring hemoglobin, ferritin, phosphorus, and parathyroid hormone were as follows15,16: for patients on hemodialysis, 26.78% achieved the recommended frequency for hemoglobin testing, 60.77% for ferritin testing, 42.63% for phosphorus testing, and 38.75% for parathyroid hormone testing. In contrast, for patients on peritoneal dialysis, the corresponding percentages were 12.14% for hemoglobin, 35.40% for ferritin, 14.66% for phosphorus, and 20.16% for parathyroid hormone (Figures 55 and 56). Overall, these percentages have not increased compared with 2016 or 2015,7,8 but the proportion of patients who have not undergone relevant measurements has significantly decreased in 2017.
Figure 55.
Percentage of patients on dialysis who underwent 1 or more measurements of (a) hemoglobin and (b) serum ferritin in 2017. HD, hemodialysis; PD, peritoneal dialysis.
Figure 56.
Percentage of patients on dialysis who underwent 1 or more measurements of (a) serum calcium, (b) serum phosphorus, and (c) serum parathyroid hormone in 2017. HD, hemodialysis; PD, peritoneal dialysis.
The percentages of patients on hemodialysis using erythropoietin, phosphorus binders, and calcitriol were 90.38%, 46.28%, and 54.24%, respectively, whereas for patients on peritoneal dialysis, these figures were 88.66%, 56.59%, and 61.40%, respectively (Figures 57 and 58). In terms of the frequency of blood albumin monitoring, 39.91% of patients on hemodialysis and 18.44% of patients on peritoneal dialysis reached the suggested threshold (Figure 59). Among patients with diabetes, only 6.45% and 10.65% of patients treated with hemodialysis and peritoneal dialysis, respectively, underwent an ophthalmologic examination, lipid testing, and hemoglobin A1c testing at least once a year (Figure 60). This underscores the urgent need for enhanced adherence to comprehensive diabetes management.
Figure 57.
Percentage of patients on dialysis receiving anemia-related treatment. EPO, erythropoietin; HD, hemodialysis; PD, peritoneal dialysis.
Figure 58.
Percentage of patients on dialysis receiving MBD-related treatment. HD, hemodialysis; MBD, mineral and bone disorder; P, phosphorus; PD, peritoneal dialysis.
Figure 59.
Percentage of patients on dialysis who underwent blood albumin testing. HD, hemodialysis; PD, peritoneal dialysis.
Figure 60.
Diabetes-related examinations in patients with diabetes on dialysis. HbA1c, hemoglobin A1c; HD, hemodialysis; PD, peritoneal dialysis.
Chapter 8: Vascular access
This article is published as a supplement supported by Peking University.
This chapter focuses on vascular access operations in prevalent patients on dialysis. The most common type of vascular access in patients on hemodialysis was arteriovenous fistula or arteriovenous graft, which accounted for 71.57% of cases (Table 8). The age group of 20–44 years had the largest proportion (79.05%), with no difference between male and female patients.
Table 8.
Type of vascular access operations in patients on HD
| Variable | Operations for AVF/AVG |
Tunneled cuffed catheter |
Noncuffed catheter |
Stable AVF /AVG |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 1019 | 9.45 | 181 | 1.68 | 2469 | 22.91 | 7748 | 71.88 |
| Female | 559 | 7.38 | 125 | 1.65 | 1868 | 24.66 | 5388 | 71.14 |
| Age group (yr) | ||||||||
| 0–19 | 2 | 0.56 | 3 | 0.84 | 144 | 40.22 | 211 | 58.94 |
| 20–44 | 305 | 7.60 | 45 | 1.12 | 667 | 16.62 | 3173 | 79.05 |
| 45–64 | 749 | 9.07 | 134 | 1.62 | 1835 | 22.21 | 6026 | 72.95 |
| 65–74 | 323 | 9.20 | 70 | 1.99 | 977 | 27.83 | 2362 | 67.27 |
| ≥75 | 198 | 9.09 | 54 | 2.48 | 711 | 32.63 | 1338 | 61.40 |
| Unknown | 1 | 3.33 | 0.00 | 3 | 10.00 | 26 | 86.67 | |
| Insurance type | ||||||||
| UEBMI | 971 | 8.45 | 164 | 1.43 | 2359 | 20.52 | 8547 | 74.36 |
| URBMI | 607 | 8.85 | 142 | 2.07 | 1978 | 28.84 | 4589 | 66.90 |
| Total | 1578 | 8.60 | 306 | 1.67 | 4337 | 23.63 | 13,136 | 71.57 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; HD, hemodialysis; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Resident Basic Medical Insurance.
Regarding arteriovenous fistula or arteriovenous graft, 8.60% of patients on hemodialysis in the sample underwent new operations. Tunneled cuffed catheters were present in just 1.67% of patients. However, caution is needed in interpreting these findings because of potential biases or limitations in our sample selection and data collection methods, and concurrently, we have made appropriate adjustments to the identification strategy for vascular access.
A total of 16.69% underwent new peritoneal dialysis (PD) catheter placement procedures, signifying that these individuals were diagnosed as patients on new-onset PD (Table 9). There were no discernible differences observed between the 2 distinct types of health insurance. Patients who did not undergo new PD catheter placement procedures were classified as undergoing PD. Patients on stable PD were categorized as those undergoing maintenance PD therapy without the requirement for central venous catheter placement operations, accounting for 98.55% of cases (Table 9). The rate of PD transfer set exchange in patients on maintenance PD was notably low, standing at 34.63%.
Table 9.
Dialysis access operations and PD transfer set exchange rates in patients on new and maintenance PD
| Variable | New PD catheter placement |
Maintenance PD |
Stable PD |
PD transfer set exchange |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 165 | 18.81 | 712 | 81.19 | 701 | 98.46 | 261 | 36.66 |
| Female | 97 | 14.00 | 596 | 86.00 | 588 | 98.66 | 192 | 32.21 |
| Age group (yr) | ||||||||
| 0–19 | 7 | 18.92 | 30 | 81.08 | 30 | 100.00 | 3 | 10.00 |
| 20–44 | 65 | 15.19 | 363 | 84.81 | 358 | 98.62 | 128 | 35.26 |
| 45–64 | 115 | 16.02 | 603 | 83.98 | 596 | 98.84 | 223 | 36.98 |
| 65–74 | 49 | 20.00 | 196 | 80.00 | 194 | 98.98 | 60 | 30.61 |
| ≥75 | 25 | 18.52 | 110 | 81.48 | 105 | 95.45 | 39 | 35.45 |
| Unknown | 1 | 14.29 | 6 | 85.71 | 6 | 100.00 | 0 | 0.00 |
| Insurance type | ||||||||
| UEBMI | 189 | 17.44 | 895 | 82.56 | 881 | 98.44 | 355 | 39.66 |
| URBMI | 73 | 15.02 | 413 | 84.98 | 408 | 98.79 | 98 | 23.73 |
| Total | 262 | 16.69 | 1308 | 83.31 | 1289 | 98.55 | 453 | 34.63 |
PD, peritoneal dialysis; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Resident Basic Medical Insurance.
Chapter 9: Cardiovascular disease and diabetes in patients on dialysis
This article is published as a supplement supported by Peking University.
Patients with chronic kidney disease are most at risk of morbidity and mortality from cardiovascular disease (CVD) and diabetes.1 In this chapter, we provide a description of CVD and diabetes in patients on dialysis, stratified by age, sex, geographic region, and treatment modalities.
Patients on dialysis had a high prevalence of CVD, with a 2017 prevalence rate of 43.01%, which was similar to the 2016 rate of 45.92%.8 Patients receiving hemodialysis had a slightly lower prevalence of CVD (42.08%) than did those receiving peritoneal dialysis (49.14%), and this prevalence generally increased with age (Table 10). Patients on dialysis in North China had the highest prevalence of CVD (72.92%), followed by those in Central China (63.14%).
Table 10.
Prevalence of CVD among patients on dialysis, stratified by modality, age, sex, and geographic region
| Variable | HD | PD | Total |
|---|---|---|---|
| Sex | |||
| Male | 42.47 | 49.60 | 43.37 |
| Female | 41.50 | 48.52 | 42.47 |
| Age group (yr) | |||
| 0–19 | 33.33 | 33.33 | 33.33 |
| 20–44 | 26.65 | 33.05 | 27.70 |
| 45–64 | 40.98 | 51.44 | 42.38 |
| 65–74 | 53.11 | 60.90 | 53.91 |
| ≥75 | 54.56 | 67.50 | 55.92 |
| Unknown | 38.89 | 0.00 | 33.33 |
| Geographic region | |||
| East China | 33.58 | 46.18 | 34.96 |
| North China | 72.13 | 76.29 | 72.92 |
| Central China | 63.55 | 59.26 | 63.14 |
| South China | 40.21 | 33.58 | 38.08 |
| Northwest China | 37.55 | 52.00 | 40.14 |
| Southwest China | 16.32 | 32.26 | 17.59 |
| Northeast China | 44.00 | 48.35 | 44.77 |
| Total | 42.08 | 49.14 | 43.01 |
CVD, cardiovascular disease; HD, hemodialysis; PD, peritoneal dialysis.
Data are expressed as percentage.
The 2 most prevalent CVDs among patients on dialysis were coronary heart disease and heart failure (37.26% and 14.51%, respectively), with cerebrovascular accident/transient ischemic attack (3.39%), peripheral arterial disease (0.74%), acute myocardial infarction (0.72%), and atrial fibrillation (0.12%) being less common (Figure 61). However, it should be noted that both the identification strategy based on claims and missed diagnoses can lead to an underestimation of the percentages of the aforementioned CVD types. It is noteworthy that 11.49% of patients underwent percutaneous coronary intervention while an even smaller percentage of 2.75% received either pacemakers or implantable cardioverter-defibrillators (Figure 62).
Figure 61.
Percentages of different types of CVD among patients on dialysis, stratified by modality. AF, atrial fibrillation; AMI, acute myocardial infarction; CHD, coronary heart disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVD, cardiovascular disease; HD, hemodialysis; HF, heart failure; PAD, peripheral arterial disease; PD, peritoneal dialysis.
Figure 62.
Percentages of patients on dialysis receiving cardiovascular procedures, stratified by modality. HD, hemodialysis; PCI, percutaneous coronary intervention; PD, peritoneal dialysis.
Diabetes affected 26.71% of patients on dialysis in 2017, and it affected patients on peritoneal dialysis (33.33%) more frequently than it did those on hemodialysis (25.71%; Table 11). Similarly, patients on dialysis in North China still had the highest prevalence of diabetes (42.49%). Regardless of the dialysis modality used, the prevalence of CVD was higher in patients with diabetes than in those without the disease (Table 12).
Table 11.
Prevalence of diabetes among patients on dialysis, stratified by modality, age, sex, and geographic region
| Variable | HD | PD | Total |
|---|---|---|---|
| Sex | |||
| Male | 26.52 | 34.46 | 27.52 |
| Female | 24.49 | 31.81 | 25.51 |
| Age group (yr) | |||
| 0–19 | 6.67 | 0.00 | 4.76 |
| 20–44 | 11.00 | 18.03 | 12.16 |
| 45–64 | 26.15 | 33.97 | 27.19 |
| 65–74 | 35.98 | 49.62 | 37.37 |
| ≥75 | 32.21 | 51.25 | 34.21 |
| Unknown | 27.78 | 0.00 | 23.81 |
| Geographic region | |||
| East China | 25.14 | 32.23 | 25.92 |
| North China | 39.85 | 53.61 | 42.49 |
| Central China | 24.14 | 29.63 | 24.67 |
| South China | 29.21 | 26.28 | 28.27 |
| Northwest China | 31.00 | 40.00 | 32.62 |
| Southwest China | 15.89 | 24.19 | 16.56 |
| Northeast China | 31.29 | 34.07 | 31.78 |
| Total | 25.71 | 33.33 | 26.71 |
HD, hemodialysis; PD, peritoneal dialysis.
Data are expressed as percentage.
Table 12.
Prevalence of CVD among patients on dialysis with and without diabetes
| Variable | HD | PD | Total |
|---|---|---|---|
| Diabetes | |||
| Yes | 61.15 | 71.13 | 62.75 |
| No | 35.50 | 38.14 | 35.81 |
| Total | 42.08 | 49.14 | 43.01 |
CVD, cardiovascular disease; HD, hemodialysis; PD, peritoneal dialysis.
Data are expressed as percentage.
Chapter 10: Hospitalization among patients on dialysis
This article is published as a supplement supported by Peking University.
Hospital admissions and readmissions among patients on dialysis are significant markers of the standard of care and the use of medical resources.17 This chapter focuses on the admission rates, length of hospital stay, and 30-day readmission rate of patients on dialysis.
The all-cause hospitalization rate for patients on dialysis in 2017 was 2.42 per person per year, a decrease compared with 2016 data (2.67 per person per year; Table 13).8 For both patients receiving hemodialysis and peritoneal dialysis, the all-cause hospitalization rate in tertiary hospitals was higher than that in secondary and primary hospitals. In 2017, the overall average length of hospital stay of patients on dialysis was 41.88 days; among these patients, women, people 75 years or older, patients with diabetes, and those hospitalized in primary hospitals had a longer length of hospital stay (Table 14).
Table 13.
All-cause hospitalization rate for patients on dialysis, stratified by modality
| Variable | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Sex | ||||||
| Male | 2.32 | 3.36 | 2.79 | 3.38 | 2.40 | 3.37 |
| Female | 2.36 | 3.25 | 2.91 | 3.63 | 2.45 | 3.33 |
| Age group (yr) | ||||||
| 0–19 | 1.67 | 0.58 | 1.33 | 0.58 | 1.50 | 0.55 |
| 20–44 | 2.34 | 3.01 | 2.80 | 3.69 | 2.44 | 3.18 |
| 45–64 | 2.30 | 3.43 | 2.92 | 3.64 | 2.42 | 3.47 |
| 65–74 | 2.39 | 3.65 | 2.89 | 3.04 | 2.45 | 3.58 |
| ≥75 | 2.37 | 2.65 | 2.53 | 2.94 | 2.38 | 2.68 |
| Diabetes | ||||||
| No | 2.32 | 3.48 | 2.91 | 3.68 | 2.42 | 3.52 |
| Yes | 2.36 | 3.02 | 2.74 | 3.16 | 2.43 | 3.04 |
| Hospital level | ||||||
| Primary hospital | 1.80 | 1.56 | 2.19 | 1.61 | 1.88 | 1.57 |
| Secondary hospital | 2.21 | 2.75 | 2.49 | 2.79 | 2.25 | 2.76 |
| Tertiary hospital | 2.53 | 3.90 | 3.12 | 3.97 | 2.65 | 3.91 |
| Admissions PPPY | 2.34 | 3.32 | 2.84 | 3.48 | 2.42 | 3.35 |
HD, hemodialysis; PD, peritoneal dialysis; PPPY, per person per year.
Table 14.
Length of stay of patients on dialysis, stratified by modality
| Variable | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Sex | ||||||
| Male | 41.85 | 67.05 | 34.85 | 53.83 | 40.71 | 65.11 |
| Female | 44.91 | 74.17 | 37.79 | 63.74 | 43.64 | 72.45 |
| Age group (yr) | ||||||
| 0–19 | 26.33 | 15.89 | 6.33 | 3.21 | 16.33 | 15.00 |
| 20–44 | 38.79 | 65.28 | 33.18 | 52.48 | 37.54 | 62.64 |
| 45–64 | 40.09 | 66.06 | 33.54 | 53.26 | 38.88 | 63.92 |
| 65–74 | 47.49 | 74.32 | 39.25 | 61.01 | 46.49 | 72.84 |
| ≥75 | 50.51 | 79.18 | 56.14 | 88.98 | 51.13 | 80.19 |
| Diabetes | ||||||
| No | 41.32 | 70.42 | 28.73 | 46.94 | 39.28 | 67.32 |
| Yes | 46.11 | 69.06 | 47.42 | 70.74 | 46.35 | 69.33 |
| Hospital level | ||||||
| Primary hospital | 79.33 | 87.04 | 43.39 | 71.94 | 72.20 | 85.35 |
| Secondary hospital | 35.56 | 56.42 | 38.11 | 57.77 | 35.87 | 56.56 |
| Tertiary hospital | 41.47 | 73.08 | 33.75 | 55.20 | 39.96 | 69.99 |
| Days PPPY | 43.06 | 69.95 | 36.08 | 58.13 | 41.88 | 68.14 |
HD, hemodialysis; PD, peritoneal dialysis; PPPY, per person per year.
Cardiovascular disease (CVD) was the most frequent cause for hospitalizations among patients on hemodialysis (33.68%; Table 15). For patients undergoing peritoneal dialysis, the proportion of CVD causes in hospitalized patients (28.77%) was slightly higher than that of access events (28.60%; Table 16).
Table 15.
Percentage of cause-specific hospitalizations among patients on HD
| Variable | CVD |
Infectious diseases |
Access events |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sex | ||||||
| Male | 1896 | 32.93 | 532 | 9.24 | 918 | 15.95 |
| Female | 1476 | 34.70 | 373 | 8.77 | 718 | 16.88 |
| Age group (yr) | ||||||
| 0–19 | 91 | 40.63 | 90 | 40.18 | 62 | 27.68 |
| 20–44 | 446 | 24.06 | 155 | 8.36 | 277 | 14.94 |
| 45–64 | 1438 | 32.60 | 313 | 7.10 | 721 | 16.35 |
| 65–74 | 810 | 38.14 | 168 | 7.91 | 346 | 16.29 |
| ≥75 | 583 | 42.03 | 178 | 12.83 | 229 | 16.51 |
| Diabetes | ||||||
| No | 2024 | 28.77 | 664 | 9.44 | 933 | 13.26 |
| Yes | 1348 | 45.30 | 241 | 8.10 | 703 | 23.62 |
| Hospital level | ||||||
| Primary hospital | 599 | 35.83 | 241 | 14.41 | 356 | 21.29 |
| Secondary hospital | 916 | 25.56 | 265 | 7.39 | 397 | 11.08 |
| Tertiary hospital | 1857 | 39.05 | 399 | 8.39 | 883 | 18.57 |
| Total | 3372 | 33.68 | 905 | 9.04 | 1636 | 16.34 |
CVD, cardiovascular disease; HD, hemodialysis.
Table 16.
Percentage of cause-specific hospitalizations among patients on PD
| Variable | CVD |
Infectious diseases |
Access events |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sex | ||||||
| Male | 202 | 30.10 | 56 | 8.35 | 208 | 31.00 |
| Female | 136 | 26.98 | 47 | 9.33 | 128 | 25.40 |
| Age group (yr) | ||||||
| 0–19 | 8 | 22.86 | 6 | 17.14 | 10 | 28.57 |
| 20–44 | 60 | 20.41 | 26 | 8.84 | 81 | 27.55 |
| 45–64 | 143 | 27.03 | 40 | 7.56 | 144 | 27.22 |
| 65–74 | 77 | 38.31 | 24 | 11.94 | 64 | 31.84 |
| ≥75 | 49 | 42.61 | 7 | 6.09 | 36 | 31.30 |
| Diabetes | ||||||
| No | 175 | 21.71 | 70 | 8.68 | 178 | 22.08 |
| Yes | 163 | 44.17 | 33 | 8.94 | 158 | 42.82 |
| Hospital level | ||||||
| Primary hospital | 63 | 24.32 | 31 | 11.97 | 58 | 22.39 |
| Secondary hospital | 91 | 28.00 | 37 | 11.38 | 73 | 22.46 |
| Tertiary hospital | 184 | 31.13 | 35 | 5.92 | 205 | 34.69 |
| Total | 338 | 28.77 | 103 | 8.77 | 336 | 28.60 |
CVD, cardiovascular disease; PD, peritoneal dialysis.
The 30-day readmission rate for patients on dialysis in 2017 was 26.34%, slightly higher than the 24.18% in 2016.8 Patients with diabetes and those 5 years or older had a higher 30-day rehospitalization rate (Table 17). The in-hospital mortality rates for patients on hemodialysis and peritoneal dialysis were 1.23% and 1.45%, respectively (Table 18).
Table 17.
Rehospitalization rate within 30 d for patients on dialysis, stratified by modality
| Variable | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sex | ||||||
| Male | 1510 | 26.23 | 192 | 28.61 | 1702 | 26.48 |
| Female | 1112 | 26.14 | 132 | 26.19 | 1244 | 26.15 |
| Age group (yr) | ||||||
| 0–19 | 34 | 15.18 | 7 | 20.00 | 41 | 15.83 |
| 20–44 | 409 | 22.06 | 74 | 25.17 | 483 | 22.49 |
| 45–64 | 1176 | 26.66 | 148 | 27.98 | 1324 | 26.80 |
| 65–74 | 592 | 27.87 | 56 | 27.86 | 648 | 27.87 |
| ≥75 | 410 | 29.56 | 39 | 33.91 | 449 | 29.89 |
| Diabetes | ||||||
| No | 1591 | 22.62 | 194 | 24.07 | 1785 | 22.76 |
| Yes | 1031 | 34.64 | 130 | 35.23 | 1161 | 34.71 |
| Hospital level | ||||||
| Primary hospital | 432 | 25.84 | 57 | 22.01 | 489 | 25.32 |
| Secondary hospital | 958 | 26.73 | 92 | 28.31 | 1050 | 26.86 |
| Tertiary hospital | 1232 | 25.91 | 175 | 29.61 | 1407 | 26.32 |
| Total | 2622 | 26.19 | 324 | 27.57 | 2946 | 26.34 |
HD, hemodialysis; PD, peritoneal dialysis.
Table 18.
In-hospital mortality of patients on dialysis, stratified by modality
| Variable | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sex | ||||||
| Male | 78 | 1.35 | 8 | 1.19 | 86 | 1.34 |
| Female | 45 | 1.06 | 9 | 1.79 | 54 | 1.13 |
| Age group (yr) | ||||||
| 0–19 | 2 | 0.89 | 1 | 2.86 | 3 | 1.16 |
| 20–44 | 8 | 0.43 | 8 | 0.37 | ||
| 45–64 | 40 | 0.91 | 5 | 0.95 | 45 | 0.91 |
| 65–74 | 23 | 1.08 | 9 | 4.48 | 32 | 1.38 |
| ≥75 | 50 | 3.60 | 2 | 1.74 | 52 | 3.46 |
| Diabetes | ||||||
| No | 59 | 0.84 | 5 | 0.62 | 64 | 0.82 |
| Yes | 64 | 2.15 | 12 | 3.25 | 76 | 2.27 |
| Hospital level | ||||||
| Primary hospital | 15 | 0.90 | 2 | 0.77 | 17 | 0.88 |
| Secondary hospital | 30 | 0.84 | 2 | 0.62 | 32 | 0.82 |
| Tertiary hospital | 15 | 0.90 | 2 | 0.77 | 17 | 0.88 |
| Total | 123 | 1.23 | 17 | 1.45 | 140 | 1.25 |
HD, hemodialysis; PD, peritoneal dialysis.
Chapter 11: Medical expenditures for patients on dialysis
This article is published as a supplement supported by Peking University.
Patients on dialysis typically incur unpredictably high medical costs.18 This chapter focuses on the trends in medical costs associated with dialysis and how they affect the health care system.
In 2017, the total medical costs for 19,923 patients on dialysis amounted to 907.70 million RMB (∼134.47 million USD), of which Urban Basic Medical Insurance paid for 75.6% of the costs. The proportion of medical costs for male patients has reached 60.71% (Table 19). The direct costs related to dialysis were the primary expenses for both patients on hemodialysis and peritoneal dialysis (34.29% vs. 32.78%), followed by medication costs (19.15% vs. 20.70%).
Table 19.
Overall costs for patients on dialysis, stratified by modality
| Variable | HD | PD | Total |
|---|---|---|---|
| Sex | |||
| Male | 60.78 | 60.04 | 60.71 |
| Female | 39.22 | 39.96 | 39.29 |
| Age group (yr) | |||
| 0–19 | 1.20 | 1.74 | 1.25 |
| 20–44 | 18.67 | 22.33 | 19.04 |
| 45–64 | 45.42 | 46.28 | 45.51 |
| 65–74 | 21.01 | 18.93 | 20.80 |
| ≥75 | 13.54 | 10.24 | 13.20 |
| Unknown | 0.16 | 0.48 | 0.20 |
| Breakdown of costs | |||
| Laboratory examinations | 5.40 | 8.15 | 5.68 |
| Other examinations | 1.89 | 2.01 | 1.91 |
| Drugs | 19.15 | 20.70 | 19.31 |
| Direct costs of dialysis | 34.29 | 32.78 | 34.14 |
| Others | 39.26 | 36.36 | 38.97 |
| Pattern of payment | |||
| UBMI paid | 75.60 | 75.83 | 75.62 |
| Out of pocket | 24.40 | 24.17 | 24.38 |
| Hospital level | |||
| Primary hospital | 13.46 | 15.72 | 13.69 |
| Secondary hospital | 30.73 | 22.39 | 29.89 |
| Tertiary hospital | 55.80 | 61.88 | 56.42 |
| Overall costs (RMB) | 815,780,235 | 91,916,489 | 907,696,724 |
HD, hemodialysis; PD, peritoneal dialysis; RMB, renminbi; UBMI, Urban Basic Medical Insurance.
Data are percentage unless otherwise noted.
The median annual cost per patient in 2017 (82,213 RMB [∼12,179 USD]) had decreased compared with 2016 (87,776 RMB [∼12,908 USD]).8 In contrast to patients on peritoneal dialysis, those on hemodialysis had greater outpatient expenditures (51,622 RMB [∼7645 USD] vs. 56,453 RMB [∼8361 USD]); nevertheless, patients on peritoneal dialysis had higher inpatient expenditures (46,446 RMB [∼6880 USD] vs. 31,186 RMB [∼4619 USD]; Table 20).
Table 20.
Costs for patients on dialysis per patient, stratified by modality
| RMB PPPY | HD |
PD |
Total |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Outpatient | 56,453 (32,877–80,924) | 51,622 (18,373–68,212) | 55,789 (31,800–78,372) |
| Inpatient | 31,186 (14,153–59,072) | 46,446 (21,079–82,073) | 32,848 (14,970–62,426) |
| Overall | 82,276 (59,970–114,464) | 81,419 (61,261–113,495) | 82,213 (60,220–114,412) |
HD, hemodialysis; IQR, interquartile range; PD, peritoneal dialysis; PPPY, per patient per year; RMB, renminbi.
There are distinct patterns when comparing the costs for inpatient and outpatient care. Medication was the largest expense for patients receiving hemodialysis and peritoneal dialysis during hospital stays (21.38% vs. 21.44%), whereas direct costs associated with dialysis ranked highest (53.86% vs. 52.69%) among outpatient expenses (Tables 21 and 22). The proportion of expenses incurred in tertiary hospitals exceeded 50%.
Table 21.
Inpatient costs for patients on dialysis, stratified by modality
| Variable | HD | PD | Total |
|---|---|---|---|
| Inpatient costs (RMB) | 439,180,591 | 53,384,389 | 492,564,980 |
| Inpatient/overall | 53.84 | 58.08 | 54.27 |
| Sex | |||
| Male | 60.15 | 59.79 | 60.11 |
| Female | 39.85 | 40.21 | 39.89 |
| Age group (yr) | |||
| 0–19 | 1.80 | 2.76 | 1.91 |
| 20–44 | 16.86 | 20.85 | 17.29 |
| 45–64 | 44.17 | 43.88 | 44.13 |
| 65–74 | 21.63 | 20.62 | 21.52 |
| ≥75 | 15.47 | 11.43 | 15.03 |
| Unknown | 0.07 | 0.45 | 0.11 |
| Breakdown of costs | |||
| Laboratory examinations | 8.50 | 11.68 | 8.85 |
| Other examinations | 3.10 | 3.13 | 3.11 |
| Drugs | 21.38 | 21.44 | 21.39 |
| Direct costs of dialysis | 17.52 | 18.41 | 17.61 |
| Others | 49.50 | 45.35 | 49.05 |
| Pattern of payment | |||
| UBMI paid | 70.86 | 72.53 | 71.04 |
| Out of pocket | 29.14 | 27.47 | 28.96 |
| Hospital level | |||
| Primary hospital | 16.46 | 15.87 | 16.40 |
| Secondary hospital | 29.96 | 21.57 | 29.05 |
| Tertiary hospital | 53.58 | 62.56 | 54.55 |
HD, hemodialysis; PD, peritoneal dialysis; RMB, renminbi; UBMI, Urban Basic Medical Insurance.
Data are percentage unless otherwise noted.
Table 22.
Outpatient costs for patients on dialysis, stratified by treatment modality
| Variable | HD | PD | Total |
|---|---|---|---|
| Outpatient costs (RMB) | 376,599,643 | 38,532,100 | 415,131,744 |
| Outpatient/overall | 46.16 | 41.92 | 45.73 |
| Sex | |||
| Male | 61.52 | 60.37 | 61.41 |
| Female | 38.48 | 39.63 | 38.59 |
| Age group (yr) | |||
| 0–19 | 0.50 | 0.31 | 0.48 |
| 20–44 | 20.79 | 24.37 | 21.12 |
| 45–64 | 46.88 | 49.62 | 47.14 |
| 65–74 | 20.28 | 16.58 | 19.94 |
| ≥75 | 11.27 | 8.59 | 11.02 |
| Unknown | 0.28 | 0.52 | 0.31 |
| Breakdown of costs | |||
| Laboratory examinations | 1.78 | 3.27 | 1.92 |
| Other examinations | 0.48 | 0.47 | 0.48 |
| Drugs | 16.56 | 19.67 | 16.84 |
| Direct costs of dialysis | 53.86 | 52.69 | 53.75 |
| Others | 27.32 | 23.90 | 27.00 |
| Pattern of payment | |||
| UBMI paid | 81.13 | 80.40 | 81.06 |
| Out of pocket | 18.87 | 19.60 | 18.94 |
| Hospital level | |||
| Primary hospital | 9.97 | 15.52 | 10.48 |
| Secondary hospital | 31.64 | 23.53 | 30.89 |
| Tertiary hospital | 58.39 | 60.95 | 58.63 |
HD, hemodialysis; PD, peritoneal dialysis; RMB, renminbi; UBMI, Urban Basic Medical Insurance.
Data are expressed as percentage unless otherwise noted.
Chapter 12: Regional data from the dialysis registry system
This article is published as a supplement supported by Peking University.
This chapter presents regional data from 4 provincial dialysis quality control centers—Jiangsu, Ningxia, Zhejiang, and Hunan—to help better understand the epidemiology and treatment of patients on dialysis in China’s various regions.
Regarding geographic distribution, Zhejiang and Jiangsu are in East China, Ningxia is in North China, and Hunan is in Central China (Figure 63). The general situation, especially population size, gross domestic product, and health expenditure per capita, may vary among different provinces (Table 23). The prevalence and incidence of hemodialysis were observed to be the highest in Hunan (730.8 and 195.4 per million population in 2018), whereas the prevalence of peritoneal dialysis was the highest in Zhejiang (130.3 per million population in 2018; Table 24). On the whole, the prevalence and incidence of dialysis in all the 4 provinces showed an increasing trend. The highest mortality rate in patients treated with hemodialysis was observed in Zhejiang (11.2% in 2017), whereas patients treated with peritoneal dialysis in Ningxia had the highest mortality rate (8.3% in 2017).
Figure 63.
Geographic distribution of the provinces of Hunan, Jiangsu, Ningxia, and Zhejiang in China. Note: Zhejiang and Jiangsu are in East China, Ningxia is in North China, and Hunan is in Central China.
Table 23.
General information on Hunan, Jiangsu, Ningxia, and Zhejiang in 2017 and 2018
| Year | Province | Area (million square kilometers) | Population (million) | Proportion of health expenditure in GDP (%) | GDP per capita (RMB) | Health expenditure per capita (RMB) |
|---|---|---|---|---|---|---|
| 2017 | Hunan | 0.21 | 68.60 | 5.2 | 43,500 | 1788 |
| 2018 | Hunan | 0.21 | 69.10 | 5.3 | 45,200 | 1850 |
| 2017 | Jiangsu | 0.11 | 80.50 | 5.8 | 78,200 | 2954 |
| 2018 | Jiangsu | 0.11 | 81.20 | 5.9 | 81,500 | 3017 |
| 2017 | Ningxia | 0.07 | 6.85 | 5.0 | 45,300 | 2365 |
| 2018 | Ningxia | 0.07 | 6.90 | 5.1 | 46,800 | 2464 |
| 2017 | Zhejiang | 0.11 | 57.37 | 5.5 | 71,000 | 3535 |
| 2018 | Zhejiang | 0.11 | 58.00 | 5.6 | 73,500 | 3621 |
GDP, gross domestic product; RMB, renminbi.
Data from the China Statistical Yearbook and China Health Statistical Yearbook.
Table 24.
Prevalence, incidence, and mortality of patients on dialysis in Hunan, Jiangsu, Ningxia, and Zhejiang in China
| Year | Province | No. of prevalent patients | HD |
No. of prevalent patients | PD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (PMP) | No. of incident patients | Incidence (PMP) | Mortality (%) | Prevalence (PMP) | No. of incident patients | Incidence (PMP) | Mortality (%) | ||||
| 2017 | Hunan | 36,000 | 524.8 | 9500 | 138.5 | 8.1 | 3000 | 43.7 | 2000 | 29.2 | 6.0 |
| 2018 | Hunan | 50,500 | 730.8 | 13,500 | 195.4 | 8.7 | 5800 | 83.9 | 3000 | 43.4 | 2.9 |
| 2017 | Jiangsu | 36,931 | 458.8 | 3321 | 41.3 | 3.2 | 6314 | 78.4 | 159 | 2.0 | 2.6 |
| 2018 | Jiangsu | 39,579 | 487.4 | 3548 | 43.7 | 3.0 | 6480 | 79.8 | 170 | 2.1 | 2.6 |
| 2017 | Ningxia | 1217 | 177.7 | 136 | 19.9 | 3.5 | 495 | 72.3 | 131 | 19.1 | 8.3 |
| 2018 | Ningxia | 1364 | 197.7 | 147 | 21.3 | 4.1 | 533 | 77.2 | 121 | 17.5 | 6.9 |
| 2017 | Zhejiang | 25,065 | 436.9 | 5961 | 103.9 | 11.2 | 7157 | 124.8 | 1528 | 26.6 | 5.4 |
| 2018 | Zhejiang | 28,021 | 483.1 | 6185 | 106.6 | 10.9 | 7560 | 130.3 | 1708 | 29.4 | 4.9 |
HD, hemodialysis; PD, peritoneal dialysis; PMP, per million population.
Note: The numbers provided by Hunan province are accurate to the hundreds place.
The majority of patients on dialysis, whether prevalent or incident, were male. Compared with patients in the other 3 provinces, those receiving dialysis in Zhejiang were older (Tables 25 and 26). Glomerulonephritis was the leading cause in both incident and prevalent patients on dialysis, and diabetic kidney disease or hypertensive nephropathy might follow, but the situation was different in each province (Table 27). Moreover, in most provinces, there was a general trend of a decreasing proportion of glomerulonephritis and an increasing proportion of diabetic kidney disease or hypertensive nephropathy among patients on dialysis, which was similar to the results we observed in the hospitalized population with chronic kidney disease. The leading cause of death was mainly cardiovascular disease, but the first cause of death in Ningxia was infection (Table 28).
Table 25.
Demographic characteristics of prevalent patients on dialysis in Hunan, Jiangsu, Ningxia, and Zhejiang in China
| Year | Province | HD |
PD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Mean age (yr) | 18–44 yr | 45–64 yr | ≥65 yr | Male | Mean age (yr) | 18–44 yr | 45–64 yr | ≥65 yr | ||
| 2017 | Hunan | 59.2 | 56.9 | 15.9 | 47.6 | 36.5 | 52.0 | 40.9 | 51.0 | 42.0 | 7.0 |
| 2018 | Hunan | 59.5 | 57.3 | 16.7 | 46.7 | 32.4 | 50.0 | 51.0 | 50.9 | 41.3 | 7.9 |
| 2017 | Jiangsu | 63.2 | 59.2 | 18.3 | 32.4 | 36.8 | 52.4 | 51.3 | 22.4 | 38.1 | 26.2 |
| 2018 | Jiangsu | 63.1 | 58.1 | 19.2 | 31.8 | 37.1 | 50.1 | 52.8 | 21.7 | 37.9 | 26.8 |
| 2017 | Ningxia | 63.8 | 48.2 | 34.6 | 52.6 | 12.8 | 51.0 | 50.1 | 37.5 | 50.1 | 11.6 |
| 2018 | Ningxia | 62.2 | 47.5 | 38.9 | 49.2 | 11.9 | 55.7 | 49.0 | 32.5 | 49.2 | 18.0 |
| 2017 | Zhejiang | 59.5 | 60.6 | 14.6 | 34.3 | 50.5 | 53.1 | 62.8 | 25.7 | 41.8 | 31.8 |
| 2018 | Zhejiang | 59.9 | 61.2 | 12.7 | 42.3 | 45.0 | 52.8 | 63.8 | 19.0 | 49.4 | 31.6 |
HD, hemodialysis; PD, peritoneal dialysis.
Data are expressed as percentage.
Table 26.
Demographic characteristics of incident patients on dialysis in Hunan, Jiangsu, Ningxia, and Zhejiang in China
| Year | Province | HD |
PD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Mean age (yr) | 18–44 yr | 45–64 yr | ≥65 yr | Male | Mean age (yr) | 18–44 yr | 45–64 yr | ≥65 yr | ||
| 2017 | Hunan | 58.9a | 56.8a | 15.7a | 46.8a | 37.5a | 56.0 | 45.6 | 46.0 | 46.0 | 8.0 |
| 2018 | Hunan | 59.5 | 58.3 | 16.5 | 45.9 | 37.6 | 45.5 | 47.0 | 42.0 | 54.5 | 3.5 |
| 2017 | Jiangsu | 60.5 | 59.4 | 16.2 | 36.7 | 32.9 | 51.8 | 49.6 | 24.8 | 36.9 | 27.7 |
| 2018 | Jiangsu | 61.8 | 57.7 | 16.8 | 35.9 | 30.2 | 49.7 | 52.4 | 26.1 | 34.2 | 26.3 |
| 2017 | Ningxia | 57.3 | 54.8 | 35.6 | 51.3 | 13.0 | 55.7 | 52.2 | 33.6 | 49.6 | 16.8 |
| 2018 | Ningxia | 56.9 | 51.2 | 32.3 | 48.3 | 19.4 | 57.0 | 50.1 | 34.5 | 49.6 | 15.5 |
| 2017 | Zhejiang | 62.3 | 62.0 | 13.4 | 35.6 | 50.4 | 56.9 | 62.1 | 24.6 | 40.7 | 34.1 |
| 2018 | Zhejiang | 61.3 | 62.8 | 12.2 | 34.6 | 52.7 | 59.1 | 63.1 | 23.6 | 44.6 | 31.7 |
HD, hemodialysis; PD, peritoneal dialysis.
Data are expressed as percentage.
Because of the lack of information in Hunan province in 2017, data from the Department of Nephrology, Xiangya Hospital, Central South University, Changsha, China, were used instead.
Table 27.
Top 3 primary causes of incident and prevalent patients on dialysis in Hunan, Jiangsu, Ningxia, and Zhejiang in China
| Year | Province | Incident dialysis |
Prevalent dialysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HD |
PD |
HD |
PD |
||||||||||
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | ||
| 2017 | Hunan | GN (–) | DKD (–) | HTN (–) | GN (–) | PKD (–) | DKD (–) | GN (–) | DKD (–) | HTN (–) | GN (–) | PKD (–) | DKD (–) |
| 2018 | Hunan | GN (–) | DKD (–) | HTN (–) | GN (–) | HTN (–) | DKD (–) | GN (–) | DKD (–) | HTN (–) | GN (–) | HTN (–) | DKD (–) |
| 2017 | Jiangsu | GN (30.2) | DKD (19.8) | HTN (15.8) | GN (29.3) | HTN (16.7) | DKD (12.1) | GN (31.6) | DKD (20.1) | HTN (14.9) | GN (28.1) | HTN (18.9) | DKD (11.9) |
| 2018 | Jiangsu | GN (30.4) | DKD (21.0) | HTN (16.7) | GN (29.5) | HTN (16.1) | DKD (10.8) | GN (31.9) | DKD (22.8) | HTN (16.1) | GN (31.2) | HTN (17.4) | DKD (12.0) |
| 2017 | Ningxia | GN (31.1) | DKD (22.3) | HTN (19.2) | GN (59.5) | DKD (29.8) | HTN (10.4) | GN (33.8) | DKD (20.1) | HTN (16.3) | GN (52.4) | HTN (24.3) | DKD (17.3) |
| 2018 | Ningxia | GN (29.9) | HTN (18.3) | DKD (10.9) | GN (52.1) | HTN (28.1) | DKD (19.8) | GN (27.1) | DKD (19.4) | HTN (18.2) | GN (46.1) | DKD (23.6) | HTN (21.0) |
| 2017 | Zhejiang | GN (42.7) | DKD (33.0) | Others or unknown (12.4) | GN (53.2) | DKD (21.7) | Others or unknown (12.3) | GN (49.4) | DKD (23.0) | Others or unknown (16.0) | GN (50.7) | Others or unknown (21.8) | DKD (17.0) |
| 2018 | Zhejiang | GN (40.7) | DKD (35.3) | Others or unknown (12.6) | GN (52.0) | DKD (26.0) | Others or unknown (10.0) | GN (50.6) | DKD (21.7) | Others or unknown (16.2) | GN (52.4) | Others or unknown (19.6) | DKD (17.0) |
DKD, diabetic kidney disease; GN, glomerulonephritis; HD, hemodialysis; HTN, hypertensive nephropathy; PKD, polycystic kidney disease.
Data within parentheses are expressed as percentage.
Table 28.
Top 3 causes of death of patients on dialysis in Hunan, Jiangsu, Ningxia, and Zhejiang in China
| Year | Province | HD |
PD |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| 2017 | Hunan | Cardiovascular events (45.6)a | Cerebrovascular events (21.5)a | Infection (11.2)a | Others or unknown (50.0) | Cardiovascular events (27.2) | Cerebrovascular events (13.6) |
| 2018 | Hunan | Cardiovascular events (47.2) | Cerebrovascular events (20.6) | Infection (10.5) | Others or unknown (45.4) | Cardiovascular events (28.0) | Infection (18.2) |
| 2017 | Jiangsu | Cardiovascular events (39.3) | Infection (32.1) | Cerebrovascular events (16.4) | Cardiovascular events (40.9) | Infection (33.8) | Cerebrovascular events (10.2) |
| 2018 | Jiangsu | Cardiovascular events (40.2) | Infection (30.8) | Cerebrovascular events (15.9) | Cardiovascular events (41.2) | Infection (36.2) | Cerebrovascular events (10.6) |
| 2017 | Ningxia | Cardiovascular events (50.1) | Infection (19.2) | Cerebrovascular events (14.4) | Infection (24.4) | Cardiovascular events (19.5) | Others or unknown (17.1) |
| 2018 | Ningxia | Cardiovascular events (46.4) | Cerebrovascular events (21.4) | Infection (17.9) | Cardiovascular events (24.3) | Infection (16.2) | Others or unknown (13.5) |
| 2017 | Zhejiang | Cardiovascular events (30.0) | Others or unknown (24.8) | Cerebrovascular events (17.5) | Others or unknown (37.4) | Cardiovascular events (28.0) | Infection (16.5) |
| 2018 | Zhejiang | Cardiovascular events (27.5) | Others or unknown (25.7) | Infection (20.3) | Others or unknown (37.3) | Cardiovascular events (27.0) | Cerebrovascular events (19.6) |
HD, hemodialysis; PD, peritoneal dialysis.
Data within parentheses are expressed as percentage.
Because of the lack of information in Hunan province in 2017, data from the Department of Nephrology, Xiangya Hospital, Central South University, Changsha, China, were used instead.
The hepatitis B/C virus infection rates in patients on hemodialysis in different provinces were comparable (Table 29). In 2018, the prevalence of peritonitis among patients on peritoneal dialysis in Jiangsu and Hunan provinces was reported to be 5.0% and 6.6%, respectively. The percentage of patients on dialysis who achieved the recommended goals for laboratory tests, including hemoglobin, transferrin saturation, serum levels of ferritin, serum calcium, serum phosphorus, intact parathyroid hormone, serum albumin, and single-pool Kt/V, varied greatly among the 4 provinces (Table 30). This indicates that the management of dialysis in China still needs further improvement, and it is necessary to formulate prevention and control strategies tailored to the specific conditions of each province.
Table 29.
Hepatitis B/C virus infection in patients on dialysis in Hunan, Jiangsu, Ningxia, and Zhejiang in China
| Year | Province | HD |
Hepatitis B | Hepatitis C | PD |
|
|---|---|---|---|---|---|---|
| Hepatitis B | Hepatitis C | Peritonitis | ||||
| 2017 | Hunan | 7.9 | 2.5 | 0 | 0 | 10.5 |
| 2018 | Hunan | 7.2 | 2.5 | 0 | 0 | 6.6 |
| 2017 | Jiangsu | 6.2 | 2.0 | 0.9 | 0.2 | 4.9 |
| 2018 | Jiangsu | 6.1 | 2.1 | 0.8 | 0.2 | 5.0 |
| 2017 | Ningxia | – | – | – | – | – |
| 2018 | Ningxia | – | – | – | – | – |
| 2017 | Zhejiang | 6.3 | 1.6 | 6.6 | 0.9 | – |
| 2018 | Zhejiang | 5.3 | 1.2 | 5.8 | 0.6 | – |
HD, hemodialysis; PD, peritoneal dialysis.
Data are expressed as percentage.
Table 30.
Percentage of patients on dialysis who achieved the recommended goals for laboratory tests in Hunan, Jiangsu, Ningxia, and Zhejiang in Chinaa
| Modality | Year | Province | Hemoglobin | Transferrin saturation | Ferritin | Serum calcium (corrected) | Serum phosphorus | iPTH | Serum albumin | spKt/V |
|---|---|---|---|---|---|---|---|---|---|---|
| HD | 2017 | Hunan | 49.0b | 74.6b | 29.9b | 51.0b | 33.0 | 39.5b | 80.9b | 42.4b |
| 2018 | Hunan | 50.0 | 78.9 | 31.0 | 52.0 | 30.0 | 40.0 | 81.9 | 44.4 | |
| PD | 2017 | Hunan | 37.4 | 75.0 | 26.0 | 72.6 | 64.8 | 40.6 | 62.7 | 81.4b |
| 2018 | Hunan | 37.6 | 85.7 | 34.9 | 79.3 | 59.4 | 39.4 | 50.0 | 80.1 | |
| HD | 2017 | Jiangsu | 39.8 | 52.2 | 53.8 | 36.8 | 36.7 | 21.8 | 79.8 | 68.3 |
| 2018 | Jiangsu | 40.1 | 56.4 | 55.3 | 41.1 | 37.3 | 21.7 | 81.5 | 69.1 | |
| PD | 2017 | Jiangsu | 42.4 | 53.8 | 53.2 | 32.1 | 36.9 | 22.9 | 72.3 | 59.2 |
| 2018 | Jiangsu | 38.9 | 56.9 | 55.9 | 33.8 | 39.4 | 22.5 | 73.7 | 60.3 | |
| HD | 2017 | Ningxia | 40.3 | 76.2 | 27.3 | 61.3 | 60.5 | 63.3 | 68.1 | 60.2 |
| 2018 | Ningxia | 46.1 | 81.3 | 36.9 | 59.9 | 64.0 | 60.2 | 62.5 | 59.4 | |
| PD | 2017 | Ningxia | 43.2 | 84.5 | 30.2 | 67.7 | 69.1 | 66.3 | 57.8 | 65.7 |
| 2018 | Ningxia | 37.5 | 84.6 | 33.3 | 52.5 | 66.9 | 54.8 | 51.0 | 68.5 | |
| HD | 2017 | Zhejiang | 25.9 | 74.8 | 31.4 | 61.2 | 28.2 | 25.8 | 86.1 | 82.6 |
| 2018 | Zhejiang | 29.0 | 74.6 | 30.7 | 61.8 | 27.9 | 25.7 | 84.8 | 84.8 | |
| PD | 2017 | Zhejiang | 19.3 | 76.7 | 32.6 | 65.2 | 36.5 | 26.2 | 67.9 | 70.8 |
| 2018 | Zhejiang | 21.6 | 75.6 | 30.5 | 66.2 | 42.2 | 26.1 | 67.3 | 72.2 |
HD, hemodialysis; iPTH, intact parathyroid hormone; PD, peritoneal dialysis; spKt/V, single-pool Kt/V.
Data are expressed as percentage.
The analysis was performed on the basis of the patient’s last values of laboratory tests in that year. The recommended goal for each laboratory test was as follows: (i) hemoglobin 110–130 g/l; (ii) percentage of transferrin saturation >20%; (iii) ferritin 200–500 μg/l; (iv) serum calcium 2.10–2.50 mmol/l; (v) serum phosphorus 0.87–1.45 mmol/l; (vi) iPTH 150–300 pg/ml; (vii) serum albumin (bromocresol green) >35 g/l; and (viii) HD: spKt/V ≥1.2 per week; PD: spKt/V ≥1.7 per week.
Because of the lack of information in Hunan province in 2017, data from the Department of Nephrology, Xiangya Hospital, Central South University, Changsha, China, were used instead.
Chapter 13: Kidney transplantation
This article is published as a supplement supported by Peking University.
Kidney transplantation is an alternative kidney replacement therapy for patients with kidney failure. Over the past 20 years, China has significantly improved the procedures for organ donation and transplantation.19 The China Organ Transplant Response System, a national open and transparent organ allocation computer system, is the foundation of the “China model” of organ transplantation.
The Report on Organ Transplantation Development in China (2015–2018) has provided information on the kidney transplant waiting list.11 Therefore, we presented the relevant data listed in the report. At the end of 2017 and 2018, there were, respectively, 30,502 and 34,567 candidates on the kidney transplant waiting list (excluding Hong Kong, Macao, and Taiwan). In 2018, the top 3 provinces in terms of the number of people waiting for kidney transplantation were Guangdong (4698), Zhejiang (4052), and Hunan (3725).
Since 2015, kidney transplantation from deceased donors has developed rapidly, and the annual number of transplant cases has increased significantly, whereas the number of kidney transplantation procedures from living-related donors has decreased. According to the data from the Chinese Scientific Registry of Kidney Transplantation, there were 9040 kidney transplantation procedures from deceased donors and 1753 from living-related donors in 2017 compared with 11,302 and 1727, respectively, in 2018. The number of pediatric kidney transplantation procedures (age < 18 years) was 217 and 273 in 2017 and 2018, respectively, accounting for 2.0% and 2.1% of all kidney transplantation procedures in mainland China.
Chapter 14: Environmental pollution and kidney disease
This article is published as a supplement supported by Peking University.
Environmental exposures to pollutants are common causes of kidney disease worldwide, especially in developing countries such as China.20 The increasing burden of kidney disease cannot be fully explained by traditional risk factors, such as diabetes and hypertension. The kidney, being a vital organ involved in filtration and excretion, is highly susceptible to environmental toxins. Environmental pollutants, including metals, air pollutants, phthalate, and melamine can potentially increase the risk of chronic kidney disease (CKD) or accelerate the progression of disease.20,21 This chapter focuses on the effects of air pollution and climate change, 2 key environmental factors that are linked to CKD on the basis of evidence from the China Kidney Disease Network.
Air pollution and CKD
Ambient air pollution, especially exposure to particulate matter with an aerodynamic diameter of 2.5 μm or less (PM2.5), has emerged as one of the risk factors for CKD.22,23 A recent systematic review identified 13 epidemiological studies and found a significant relationship between PM2.5 exposure and increased risks of CKD.24 On the basis of a nationwide cross-sectional survey of 47,204 individuals in China, a recent study from the China Kidney Disease Network found that an increase of 10 mg/m3 in PM2.5 was associated with an increased risk of CKD (odds ratio 1.28; 95% confidence interval 1.22–1.35) and urine albumin–creatinine ratio (odds ratio 1.39; 95% confidence interval 1.32–1.47) in the general population.25 Increasing trends in exposure-response curves were also revealed, with the risk increasing at PM2.5 concentrations below the current PM2.5 standards in China. Airborne particulate matter of 1 μm or less might play a leading role in the observed relationship of PM2.5 with the risk of CKD.26
Moreover, the association between air pollution and CKD may be modified by urbanization; specifically, areas with medium urbanization levels were more susceptible to the adverse impact of PM2.5 on CKD,27 which suggests strengthening environmental governance and balancing social resources in similar areas. Currently, the surface ozone air pollution concentration still shows an increasing trend worldwide.28 Another study involving 47,086 participants demonstrated that long-term exposure to ozone pollution was associated with an increased odds of CKD prevalence in the general Chinese population, with a higher effect found in urban areas than in rural areas.29
Climate change and CKD
Climate change, characterized by global warming, poses a serious threat to human health. Heat wave exposures in 2020 in China led to an estimated 92% increase in heat wave–related deaths compared with a historical reference period from 1986 to 2005.30 A recent study evaluated the associations between short-term heat exposure and risks of cause-specific CKD in China by using a sample of 768,129 hospitalizations from the Hospital Quality Monitoring System from 2015 to 2018.31 The temperature increase was consistently positively associated with increased risks of hospitalizations for CKD, especially in cities in the subtropical zone. With a 1 °C increase in daily mean temperature, the cumulative relative risks over 0 to 7 days were 1.008 (95% confidence interval 1.003–1.012) nationwide. Stronger associations were observed in the younger population and patients with obstructive nephropathy (Figure 64).31
Figure 64.

Cumulative association between ambient high temperature and the risk of hospitalizations for CKD over 0–7 days, stratified by age, sex, and cause of CKD. CKD, chronic kidney disease; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy.
A heat wave is an extended period of excessively high temperatures, significantly above normal, that can pose health risks and disrupt daily activities. Another national study, encompassing a total of 47,086 participants from the general Chinese population, has uncovered that long-term exposure to heat waves could increase the odds of CKD prevalence.32 In rural regions, increases in proportions of water bodies and impervious areas could mitigate the associations between heat waves and CKD prevalence, whereas in urban regions, only the effect modification by water body proportion was observed.32
Overall, environmental pollution poses a significant threat to kidney health in China. Findings from the China Kidney Disease Network provide insights for vulnerable population protection and targeted environmental pollution control. Regulatory efforts and public health interventions are essential to control environmental pollution and limit individual exposure. Ongoing research is also needed to better understand the dose-response relationships between pollutants and kidney diseases as well as the interactions between environmental and genetic factors. By addressing these challenges, we can work toward reducing the burden of kidney disease in China and globally.
Chapter 15: Future perspectives
This article is published as a supplement supported by Peking University.
In the era of digital transformation, the field of nephrology is undergoing a paradigm shift as digital intelligence permeates every aspect of diagnosis, treatment, and patient management.33,34 With the advent of advanced information technologies such as artificial intelligence (AI), big data analytics, and particularly large language models (LLMs), nephrology is poised to enter a new era of precision medicine. However, the complexity and heterogeneity of kidney diseases pose significant challenges, often necessitating individualized approaches. The integration of digital intelligence, particularly through the use of AI and big data, offers unprecedented opportunities to address these challenges. This chapter delves into the landscape of digital intelligence in nephrology and explores its implications for clinical practice.
Big data analytics and nephrology
At the core of digital intelligence lies big data, the vast and diverse collection of information generated by health care systems, electronic medical records, and patient-generated data. By leveraging advanced analytics tools, nephrologists can identify patterns, correlations, and predictive models that would otherwise be impossible to discern manually.33 The China Kidney Disease Network aspires to evolve into a comprehensive surveillance system for kidney disease across China by integrating diverse data sources.35 This endeavor aims to furnish evidence for elucidating the epidemiology of chronic kidney disease, thereby fostering targeted management strategies. Moreover, data from regional electronic health records, as well as from external fields such as transportation, environment, socioeconomics, and Internet-based diagnosis and treatment, can contribute to disease surveillance.36, 37, 38
Prediction models are another promising application of big data in nephrology, where high-quality, multisource data hold the key. For instance, predictive analytics can be used to estimate a patient’s risk of kidney failure or identify subgroups of patients who are likely to respond favorably to specific treatments.39 Similarly, real-time monitoring of kidney function using wearable devices and mobile health apps can enable earlier intervention and improved management of chronic kidney disease.40 Moreover, recent findings from the China Kidney Disease Network indicate that the accuracy of a urine quantitative analysis system based on computer vision algorithms has reached 88%, with a sensitivity of 94.0% and a specificity of 81.4%,41 indicating its significant application value in screening large-scale populations.
The rise of LLMs in nephrology
Recent advancements in AI, particularly the emergence of LLMs such as GPT-4,42 have sparked renewed interest in the potential applications of these technologies in nephrology. LLMs, with their ability to understand and generate human language, can facilitate the interpretation of medical literature, support clinical decision making, and even assist in the development of personalized treatment plans.42 In nephrology, LLMs can be trained on vast amounts of medical texts, clinical trials, and patient data to provide clinicians with up-to-date information on disease mechanisms, treatment options, and patient outcomes.
LLMs can be fine-tuned to perform specific tasks such as facilitating the extraction of relevant clinical information for diagnostic and prognostic purposes.43 Furthermore, chatbots can be developed to provide education on kidney health and answer patient queries, allowing for more efficient utilization of health care resources. For example, diabetes-related complications, such as diabetic retinopathy and diabetic kidney disease, can be managed with the assistance of LLMs.44 Patients with diabetes may also benefit from a better understanding of the natural history of their disease.
Ethical and regulatory considerations
As digital intelligence becomes increasingly integrated into nephrology, it is crucial to address the ethical and regulatory challenges. Concerns related to data privacy, informed consent, and algorithmic bias must be addressed to ensure that digital technologies are developed and deployed in a responsible and ethical manner.45 Ethical regulations and data protection mechanisms within the health care sectors without jeopardizing the benefits of patients and the integrity of data are urgently needed.46 Moreover, the validation and regulation of AI-driven tools are essential to ensure their safety, efficacy, and transparency. Regulators must establish clear guidelines for the development, testing, and deployment of AI-based medical products, whereas nephrologists must adhere to rigorous scientific standards to ensure the integrity of their work.
Looking ahead, the integration of digital intelligence into nephrology holds immense promise for advancing the field and improving patient outcomes. As LLMs continue to evolve, we can expect to see more sophisticated applications in clinical decision support and patient education. Moreover, in addressing the traditional management of chronic kidney disease, a more interdisciplinary approach should be contemplated in the future, integrating multifaceted measures to enhance overall care (Figure 65).
Figure 65.
Management of CKD from a multidimensional perspective. CKD, chronic kidney disease.
Chapter 16: Discussion
This article is published as a supplement supported by Peking University.
The China Kidney Disease Network 2017–2018 Annual Data Report presents a comprehensive assessment of the burden of chronic kidney disease (CKD) and kidney failure in China, leveraging national administrative and claims databases from diverse sources. Specifically, in addition to providing epidemiological data on CKD and kidney failure, we have added several hot topics in kidney disease, particularly the impact of environmental pollution and the application of digital technology. This report not only functions as a pivotal surveillance tool for kidney disease but also serves as an insightful resource, offering significant policy implications that can guide future interventions and health care strategies.
CKD is a global health challenge, intertwined with health disparities that significantly amplify inequalities among diverse populations. According to a recent study from 161 countries, the global median prevalence of CKD was 9.5% (interquartile range 5.9%–11.7%), leading to 491.4 per 100,000 population disability-adjusted life years (interquartile range 359.9–636.0 per 100,000 population disability-adjusted life years).47 The results from the Sixth China Chronic Disease and Risk Factor Surveillance, which involved a study population of 176,874 adults, showed that the prevalence of CKD among the adult population was 8.2% during the period of 2018 to 2019.48 The prevalence seems to have decreased by 30% in the past 10 years, but the awareness rate of CKD remains at a relatively low level of 10%. The proportion of patients with CKD among all inpatients has exhibited a fluctuating pattern, accounting for 4.95% in 2017 and slightly declining to 4.59% in 2018. But we should see that the burden of cardiovascular diseases related to CKD and the utilization of health care resources remained high. It should be noted that our analysis comprehensively encapsulates prevalence, hospitalization rates, and diagnostic rates, necessitating caution in international comparisons. Our findings also underscore the elevated burden of CKD in individuals with other major noncommunicable diseases, emphasizing the imperative for targeted management of these high-risk populations.
Rapid urbanization in China has been intimately linked to shifts in the spectrum of CKD, particularly with diabetes as a major underlying cause (28.78% in 2018). This relationship underscores the intricate interplay between societal transformations, environmental factors, and kidney health.49 Given their inherent vulnerability, patients suffering from kidney disease may be particularly prone to the detrimental impacts of environmental pollutants. The plausible biological mechanisms may involve oxidative stress, inflammation, endothelial dysfunction, DNA injuries, and modification of gene expression.50,51 In addition, the proportion of glomerulonephritis remained relatively stable at around 14%, with patients with IgA nephropathy accounting for 2.04% of all inpatients with CKD in 2018. Among them, the young population aged 18 to 34 years was a high-risk group for IgA nephropathy. Furthermore, disparities in medical resource distribution across provinces are evident, with more developed regions enjoying greater access to advanced health care facilities whereas rural and underdeveloped areas lag behind. The percentage of interprovince mobility among inpatients with CKD was 5.66% in 2018. This uneven allocation exacerbates health inequalities, posing challenges for equitable CKD management and prevention. A recent study from the China Kidney Disease Network found significant geographic variations in the nephrology workforce across China, and having 12 to 20 nephrologists per million population could be optimal for addressing local medical needs.38 Together, comprehensive strategies targeting both traditional and new risk factors and equitable resource allocation are imperative to mitigate the burden of CKD in China. We reiterate our earnest appeal for the integration of CKD into the World Health Organization list of priority noncommunicable diseases.
The burden of dialysis in China is substantial, with a rapidly growing patient population and long dialysis durations. The age- and sex-adjusted prevalence of patients receiving dialysis in 2017 was 419.39 per million population, highlighting a substantial treatment gap compared with developed nations. Meanwhile, the epidemiological characteristics of patients on dialysis exhibited significant geographic differences among distinct provinces. The suboptimal management status, characterized by relatively poor long-term outcomes and high mortality rates, underscores the urgency for reform. Digital technologies are emerging as an important tool to address these challenges. Digital tools such as Internet of Things for real-time monitoring and artificial intelligence–driven analytics are enhancing dialysis efficiency and patient outcomes.52 By leveraging these advancements, China can strive toward more efficient and effective dialysis management, mitigating the current burden and improving the quality of life for patients on dialysis. In addition, with the support of the Chinese Preventive Medicine Association for Kidney Disease, the China Kidney Disease Network team recently took the lead in compiling the “Guidelines for the early evaluation and management of chronic kidney disease in China,”53 which also highlights the importance of early screening and intervention for patients with CKD.
When interpreting the results presented in this report, it is important to acknowledge the following limitations: Although our study strives for representativeness, it is based on data from the Hospital Quality Monitoring System and China Health Insurance Research Association databases. The Hospital Quality Monitoring System database, although covering more than 80% of tertiary hospitals in China with more than 100 million hospitalization records, and the China Health Insurance Research Association database, encompassing medical claims for more than 9 million individuals across 31 provinces, may not perfectly represent the entire Chinese population. However, the large sample size and diverse nature of data sets contribute to the statistical power of our study, reducing the potential for sampling bias and increasing the likelihood that our findings are generalizable to a significant portion of the Chinese population. Furthermore, it should be noted that our analysis is based entirely on cross-sectional data, which inherently presents difficulties in establishing causal relationships.
In conclusion, despite the interval since our last report due to the coronavirus disease 2019 outbreak, we remain committed to underscoring the resilience and ongoing relevance of leveraging big data analytics in monitoring kidney diseases in developing nations. Our report serves as a testament to the potential of data-driven approaches in refining diagnostic methods, identifying high-risk populations, and tracking disease progression. We aspire not only to disseminate the China Kidney Disease Network model, a comprehensive framework for CKD surveillance and management, but also to share the invaluable lessons learned and experiences gained through its implementation. We believe that by doing so, we can empower other countries or regions grappling with similar CKD epidemics to tailor their own strategies. Together, we can harness the power of innovation, technology, and collective action to enhance global kidney health, ensuring equitable access to quality care and ultimately reducing the burden of CKD worldwide.
Contributor Information
CK-NET Work Group:
Hong Chu, Lanxia Gan, Bixia Gao, Qi Guo, Jianguo Hao, Daijun He, Shenda Hong, Chenglong Li, Pengfei Li, Jianyan Long, Huijuan Mao, Yingying Qin, Ying Shi, Xiaoyu Sun, Wen Tang, Fang Wang, Fulin Wang, Jinwei Wang, Wanzhou Wang, Shaoqing Wei, Fengyu Wen, Xingchen Yao, Chao Yang, Guang Yang, Ling Yang, Jianhua Ye, Qiongjing Yuan, Dongliang Zhang, Feifei Zhang, Ping Zhang, Zhilong Zhang, Xinju Zhao, and Zhiye Zhou
Appendix I: Definitions of International Classification of Diseases coding
Appendix Table 60.
In-hospital mortality stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 94 (0.44) | 26 (0.39) | 1251 (0.15) | 59 (0.38) | 19 (0.33) | 896 (0.13) |
| 25–29 | 104 (0.32) | 28 (0.22) | 1456 (0.09) | 89 (0.36) | 19 (0.17) | 1112 (0.09) |
| 30–34 | 162 (0.42) | 47 (0.22) | 1900 (0.14) | 147 (0.47) | 45 (0.23) | 1495 (0.12) |
| 35–39 | 277 (0.64) | 146 (0.41) | 2467 (0.21) | 205 (0.59) | 102 (0.32) | 1927 (0.20) |
| 40–44 | 454 (0.78) | 269 (0.41) | 3945 (0.32) | 280 (0.63) | 203 (0.37) | 3055 (0.30) |
| 45–49 | 707 (0.83) | 539 (0.41) | 6334 (0.39) | 551 (0.82) | 484 (0.43) | 5319 (0.37) |
| 50–54 | 1180 (1.09) | 1167 (0.52) | 9082 (0.47) | 803 (0.97) | 852 (0.47) | 7146 (0.43) |
| 55–59 | 1193 (1.38) | 1507 (0.66) | 8898 (0.59) | 923 (1.20) | 1245 (0.58) | 7496 (0.52) |
| 60–64 | 1855 (1.66) | 2494 (0.81) | 13,064 (0.65) | 1440 (1.61) | 1910 (0.70) | 10,748 (0.61) |
| 65–69 | 2261 (2.18) | 3163 (1.07) | 13,426 (0.78) | 1742 (1.97) | 2480 (0.91) | 11,592 (0.73) |
| 70–74 | 2443 (2.85) | 3395 (1.42) | 13,426 (1.03) | 1852 (2.61) | 2604 (1.21) | 11,145 (0.94) |
| 75–79 | 3355 (4.26) | 4102 (2.16) | 16,085 (1.54) | 2286 (3.67) | 3190 (1.93) | 13,243 (1.44) |
| 80–84 | 3920 (6.41) | 4238 (3.53) | 16,498 (2.38) | 2724 (5.54) | 3205 (3.03) | 13,927 (2.24) |
| ≥85 | 4290 (10.30) | 3857 (6.40) | 16,441 (4.32) | 3343 (9.59) | 3084 (5.58) | 14,216 (3.96) |
| Total | 22,295 (2.33) | 24,978 (1.29) | 124,273 (0.68) | 16,444 (2.13) | 19,442 (1.13) | 103,317 (0.64) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 61.
Percentage of AKI with and without an ICU stay, stratified by geographic region
| Geographic region | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| With an ICU stay | Without an ICU stay | Total | With an ICU stay | Without an ICU stay | Total | |
| N-Beijing | 875 (2.70) | 1358 (0.25) | 2233 (0.38) | 841 (2.80) | 1601 (0.29) | 2442 (0.42) |
| N-Tianjin | 24 (3.91) | 96 (0.11) | 120 (0.13) | 29 (4.14) | 49 (0.09) | 78 (0.14) |
| N-Hebei | 389 (7.46) | 2181 (0.32) | 2570 (0.37) | 222 (4.96) | 1884 (0.32) | 2106 (0.36) |
| N-Shanxi | 462 (10.44) | 2002 (0.36) | 2464 (0.43) | 448 (9.24) | 1655 (0.34) | 2103 (0.43) |
| N-Inner Mongolia | 90 (3.54) | 1355 (0.26) | 1445 (0.28) | 90 (4.34) | 1562 (0.30) | 1652 (0.32) |
| NE-Liaoning | 199 (4.55) | 1003 (0.24) | 1202 (0.29) | 161 (3.23) | 747 (0.21) | 908 (0.25) |
| NE-Jilin | 138 (6.67) | 789 (0.21) | 927 (0.25) | 112 (6.19) | 559 (0.18) | 671 (0.21) |
| NE-Heilongjiang | 258 (3.55) | 782 (0.14) | 1040 (0.18) | 206 (3.21) | 781 (0.15) | 987 (0.18) |
| E-Shanghai | 78 (4.43) | 1001 (0.14) | 1079 (0.15) | 63 (2.66) | 437 (0.09) | 500 (0.10) |
| E-Jiangsu | 897 (4.47) | 2803 (0.17) | 3700 (0.23) | 805 (4.06) | 2596 (0.17) | 3401 (0.22) |
| E-Zhejiang | 698 (5.49) | 1941 (0.21) | 2639 (0.29) | 573 (5.60) | 1910 (0.24) | 2483 (0.31) |
| E-Anhui | 115 (2.55) | 893 (0.16) | 1008 (0.18) | 135 (3.34) | 565 (0.13) | 700 (0.16) |
| E-Fujian | 681 (6.40) | 1772 (0.25) | 2453 (0.34) | 875 (5.51) | 2033 (0.22) | 2908 (0.30) |
| E-Jiangxi | 399 (6.17) | 2049 (0.28) | 2448 (0.33) | 356 (6.13) | 1659 (0.25) | 2015 (0.30) |
| E-Shandong | 384 (4.35) | 1359 (0.17) | 1743 (0.22) | 221 (4.57) | 924 (0.17) | 1145 (0.21) |
| C-Henan | 393 (1.90) | 1837 (0.14) | 2230 (0.17) | 342 (1.65) | 1856 (0.15) | 2198 (0.18) |
| C-Hubei | 565 (2.42) | 3050 (0.22) | 3615 (0.25) | 549 (2.72) | 2594 (0.24) | 3143 (0.29) |
| C-Hunan | 216 (2.45) | 1009 (0.22) | 1225 (0.26) | 206 (4.17) | 685 (0.19) | 891 (0.24) |
| S-Guangdong | 1030 (3.60) | 4459 (0.30) | 5489 (0.37) | 977 (4.42) | 3712 (0.31) | 4689 (0.38) |
| S-Guangxi | 647 (5.71) | 2044 (0.41) | 2691 (0.53) | 769 (6.07) | 1767 (0.38) | 2536 (0.53) |
| S-Hainan | 188 (12.25) | 1066 (0.52) | 1254 (0.60) | 179 (7.99) | 779 (0.40) | 958 (0.48) |
| SW-Chongqing | 179 (3.93) | 746 (0.28) | 925 (0.35) | 205 (6.08) | 1039 (0.33) | 1244 (0.40) |
| SW-Sichuan | 745 (3.98) | 3825 (0.30) | 4570 (0.36) | 504 (3.56) | 2457 (0.26) | 2961 (0.31) |
| SW-Guizhou | 78 (4.75) | 1385 (0.40) | 1463 (0.42) | 55 (4.64) | 1150 (0.39) | 1205 (0.41) |
| SW-Yunnan | 763 (8.54) | 3721 (0.43) | 4484 (0.52) | 327 (9.03) | 1765 (0.28) | 2092 (0.33) |
| SW-Tibet | 1 (11.11) | 2 (0.72) | 3 (1.05) | 24 (11.27) | 59 (0.59) | 83 (0.82) |
| NW-Shaanxi | 157 (4.65) | 1286 (0.26) | 1443 (0.29) | 131 (4.76) | 1444 (0.34) | 1575 (0.37) |
| NW-Gansu | 50 (7.18) | 565 (0.34) | 615 (0.36) | 23 (8.68) | 262 (0.26) | 285 (0.29) |
| NW-Qinghai | 78 (4.09) | 157 (0.17) | 235 (0.26) | 7 (1.61) | 38 (0.10) | 45 (0.11) |
| NW-Ningxia | 119 (6.37) | 326 (0.44) | 445 (0.59) | 175 (6.07) | 593 (0.52) | 768 (0.65) |
| NW-Xinjiang | 249 (1.88) | 1039 (0.27) | 1288 (0.32) | 233 (1.69) | 911 (0.23) | 1144 (0.28) |
| Total | 11,145 (4.08) | 47,901 (0.25) | 59,046 (0.31) | 9843 (4.04) | 40,073 (0.24) | 49,916 (0.30) |
AKI, acute kidney injury; C, Central China; E, East China; ICU, intensive care unit; N, North China; NE, Northeast China; NW, Northwest China; S, South China; SW, Southwest China.
Data are expressed as n (%).
Appendix Table 62.
Percentage of AKI among patients with CKD
| Cause | 2017 | 2018 |
|---|---|---|
| DKD | 2645 (1.02) | 2247 (1.01) |
| HTN | 3372 (1.64) | 2720 (1.64) |
| GN | 4139 (3.03) | 3628 (3.20) |
| CTIN | 640 (4.11) | 725 (4.62) |
| ON | 2361 (1.60) | 1656 (1.70) |
| Others | 3507 (1.83) | 3062 (1.95) |
| Total | 16,664 (1.74) | 14,038 (1.82) |
AKI, acute kidney injury; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 63.
Age distribution of patients with AKI, stratified by sex
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 1069 (2.77) | 587 (2.86) | 1656 (2.80) | 918 (2.83) | 433 (2.48) | 1351 (2.71) |
| 25–29 | 1154 (2.99) | 740 (3.61) | 1894 (3.21) | 925 (2.85) | 496 (2.84) | 1421 (2.85) |
| 30–34 | 1274 (3.31) | 633 (3.09) | 1907 (3.23) | 1102 (3.39) | 528 (3.03) | 1630 (3.27) |
| 35–39 | 1512 (3.92) | 640 (3.12) | 2152 (3.64) | 1284 (3.96) | 496 (2.84) | 1780 (3.57) |
| 40–44 | 2320 (6.02) | 851 (4.15) | 3171 (5.37) | 1649 (5.08) | 710 (4.07) | 2359 (4.73) |
| 45–49 | 3249 (8.43) | 1335 (6.51) | 4584 (7.76) | 2605 (8.02) | 1073 (6.15) | 3678 (7.37) |
| 50–54 | 3875 (10.06) | 1739 (8.48) | 5614 (9.51) | 3217 (9.91) | 1500 (8.59) | 4717 (9.45) |
| 55–59 | 3067 (7.96) | 1495 (7.29) | 4562 (7.73) | 2896 (8.92) | 1303 (7.47) | 4199 (8.41) |
| 60–64 | 4237 (11.00) | 2178 (10.62) | 6415 (10.86) | 3648 (11.24) | 1829 (10.48) | 5477 (10.97) |
| 65–69 | 4180 (10.85) | 2288 (11.15) | 6468 (10.95) | 3581 (11.03) | 2176 (12.47) | 5757 (11.53) |
| 70–74 | 3547 (9.21) | 2231 (10.88) | 5778 (9.79) | 3121 (9.61) | 1981 (11.35) | 5102 (10.22) |
| 75–79 | 3543 (9.19) | 2257 (11.00) | 5800 (9.82) | 2893 (8.91) | 1943 (11.13) | 4836 (9.69) |
| 80–84 | 2943 (7.64) | 2020 (9.85) | 4963 (8.41) | 2536 (7.81) | 1706 (9.77) | 4242 (8.50) |
| ≥85 | 2563 (6.65) | 1519 (7.41) | 4082 (6.91) | 2087 (6.43) | 1280 (7.33) | 3367 (6.75) |
| Total | 38,533 (100) | 20,513 (100) | 59,046 (100) | 32,462 (100) | 17,454 (100) | 49,916 (100) |
AKI, acute kidney injury.
Data are expressed as n (%).
Appendix Table 64.
Sex distribution of patients with AKI, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 1069 (64.55) | 587 (35.45) | 1656 | 918 (67.95) | 433 (32.05) | 1351 |
| 25–29 | 1154 (60.93) | 740 (39.07) | 1894 | 925 (65.10) | 496 (34.90) | 1421 |
| 30–34 | 1274 (66.81) | 633 (33.19) | 1907 | 1102 (67.61) | 528 (32.39) | 1630 |
| 35–39 | 1512 (70.26) | 640 (29.74) | 2152 | 1284 (72.13) | 496 (27.87) | 1780 |
| 40–44 | 2320 (73.16) | 851 (26.84) | 3171 | 1649 (69.90) | 710 (30.10) | 2359 |
| 45–49 | 3249 (70.88) | 1335 (29.12) | 4584 | 2605 (70.83) | 1073 (29.17) | 3678 |
| 50–54 | 3875 (69.02) | 1739 (30.98) | 5614 | 3217 (68.20) | 1500 (31.80) | 4717 |
| 55–59 | 3067 (67.23) | 1495 (32.77) | 4562 | 2896 (68.97) | 1303 (31.03) | 4199 |
| 60–64 | 4237 (66.05) | 2178 (33.95) | 6415 | 3648 (66.61) | 1829 (33.39) | 5477 |
| 65–69 | 4180 (64.63) | 2288 (35.37) | 6468 | 3581 (62.20) | 2176 (37.80) | 5757 |
| 70–74 | 3547 (61.39) | 2231 (38.61) | 5778 | 3121 (61.17) | 1981 (38.83) | 5102 |
| 75–79 | 3543 (61.09) | 2257 (38.91) | 5800 | 2893 (59.82) | 1943 (40.18) | 4836 |
| 80–84 | 2943 (59.30) | 2020 (40.70) | 4963 | 2536 (59.78) | 1706 (40.22) | 4242 |
| ≥85 | 2563 (62.79) | 1519 (37.21) | 4082 | 2087 (61.98) | 1280 (38.02) | 3367 |
| Total | 38,533 (65.26) | 20,513 (34.74) | 59,046 | 32,462 (65.03) | 17,454 (34.97) | 49,916 |
AKI, acute kidney injury.
Data are expressed as n (%).
Appendix Table 65.
Percentage of CKD among patients with AKI, stratified by sex and age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 470 (43.97) | 272 (46.34) | 742 (44.81) | 408 (44.44) | 213 (49.19) | 621 (45.97) |
| 25–29 | 376 (32.58) | 333 (45.00) | 709 (37.43) | 280 (30.27) | 214 (43.15) | 494 (34.76) |
| 30–34 | 364 (28.57) | 258 (40.76) | 622 (32.62) | 340 (30.85) | 205 (38.83) | 545 (33.44) |
| 35–39 | 418 (27.65) | 283 (44.22) | 701 (32.57) | 346 (26.95) | 183 (36.90) | 529 (29.72) |
| 40–44 | 600 (25.86) | 331 (38.90) | 931 (29.36) | 438 (26.56) | 277 (39.01) | 715 (30.31) |
| 45–49 | 835 (25.70) | 495 (37.08) | 1330 (29.01) | 655 (25.14) | 407 (37.93) | 1062 (28.87) |
| 50–54 | 1039 (26.81) | 622 (35.77) | 1661 (29.59) | 833 (25.89) | 526 (35.07) | 1359 (28.81) |
| 55–59 | 866 (28.24) | 478 (31.97) | 1344 (29.46) | 789 (27.24) | 436 (33.46) | 1225 (29.17) |
| 60–64 | 1160 (27.38) | 716 (32.87) | 1876 (29.24) | 1052 (28.84) | 588 (32.15) | 1640 (29.94) |
| 65–69 | 1138 (27.22) | 707 (30.90) | 1845 (28.53) | 971 (27.12) | 690 (31.71) | 1661 (28.85) |
| 70–74 | 941 (26.53) | 621 (27.84) | 1562 (27.03) | 803 (25.73) | 556 (28.07) | 1359 (26.64) |
| 75–79 | 855 (24.13) | 554 (24.55) | 1409 (24.29) | 731 (25.27) | 473 (24.34) | 1204 (24.90) |
| 80–84 | 637 (21.64) | 474 (23.47) | 1111 (22.39) | 558 (22.00) | 381 (22.33) | 939 (22.14) |
| ≥85 | 529 (20.64) | 292 (19.22) | 821 (20.11) | 442 (21.18) | 243 (18.98) | 685 (20.34) |
| Total | 10,228 (26.54) | 6436 (31.38) | 16,664 (28.22) | 8646 (26.63) | 5392 (30.89) | 14,038 (28.12) |
AKI, acute kidney injury; CKD, chronic kidney disease.
Data are expressed as n (%).
Appendix Table 66.
Percentage of diabetes mellitus among patients with AKI, stratified by sex and age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 37 (3.46) | 31 (5.28) | 68 (4.11) | 38 (4.14) | 17 (3.93) | 55 (4.07) |
| 25–29 | 74 (6.41) | 39 (5.27) | 113 (5.97) | 78 (8.43) | 14 (2.82) | 92 (6.47) |
| 30–34 | 100 (7.85) | 39 (6.16) | 139 (7.29) | 80 (7.26) | 28 (5.30) | 108 (6.63) |
| 35–39 | 150 (9.92) | 44 (6.88) | 194 (9.01) | 117 (9.11) | 28 (5.65) | 145 (8.15) |
| 40–44 | 263 (11.34) | 58 (6.82) | 321 (10.12) | 167 (10.13) | 62 (8.73) | 229 (9.71) |
| 45–49 | 426 (13.11) | 161 (12.06) | 587 (12.81) | 394 (15.12) | 132 (12.30) | 526 (14.30) |
| 50–54 | 653 (16.85) | 268 (15.41) | 921 (16.41) | 519 (16.13) | 245 (16.33) | 764 (16.20) |
| 55–59 | 638 (20.80) | 336 (22.47) | 974 (21.35) | 603 (20.82) | 313 (24.02) | 916 (21.81) |
| 60–64 | 840 (19.83) | 542 (24.89) | 1382 (21.54) | 737 (20.20) | 430 (23.51) | 1167 (21.31) |
| 65–69 | 827 (19.78) | 592 (25.87) | 1419 (21.94) | 710 (19.83) | 580 (26.65) | 1290 (22.41) |
| 70–74 | 739 (20.83) | 651 (29.18) | 1390 (24.06) | 697 (22.33) | 522 (26.35) | 1219 (23.89) |
| 75–79 | 659 (18.60) | 637 (28.22) | 1296 (22.34) | 590 (20.39) | 547 (28.15) | 1137 (23.51) |
| 80–84 | 517 (17.57) | 495 (24.50) | 1012 (20.39) | 490 (19.32) | 432 (25.32) | 922 (21.74) |
| ≥85 | 425 (16.58) | 318 (20.93) | 743 (18.20) | 347 (16.63) | 233 (18.20) | 580 (17.23) |
| Total | 6348 (16.47) | 4211 (20.53) | 10,559 (17.88) | 5567 (17.15) | 3583 (20.53) | 9150 (18.33) |
AKI, acute kidney injury.
Data are expressed as n (%).
Appendix II: Appendix tables for Chapters 1–5
Appendix Table 1.
ICD coding of various CKD etiologies
| Etiology of CKD | All editions | China edition | Beijing edition | Clinic edition |
|---|---|---|---|---|
| 1. Diabetes mellitus | ||||
| Type 1 diabetes mellitus with renal complications | E10.2 + N08.3 | |||
| Type 2 diabetes mellitus with renal complications | E11.2 + N08.3 | |||
| Unspecified diabetes mellitus with renal complications | E14.2 | |||
| Malnutrition-related diabetes mellitus with renal complications | E12.200 + N08.3 | E12.200 | ||
| Other specified diabetes mellitus with renal complications | E13.2 | E13.200 | ||
| 2. Hypertensive diseases | ||||
| Hypertensive renal disease with renal failure | I12 | |||
| Hypertensive heart and renal disease with (congestive) heart failure | I13 | |||
| Pregnancy with hypertensive heart and renal disease | O10.301 | |||
| Pregnancy with essential hypertension and proteinuria | O11.x01 | |||
| Preexisting hypertensive renal disease during pregnancy, childbirth, and puerperium | O10.200 | O10.200 | ||
| Pregnancy with hypertensive renal disease | O10.201 | O10.201 | ||
| Preexisting hypertensive heart and renal disease during pregnancy, childbirth, and puerperium | O10.300 | O10.300 | ||
| Preexisting hypertension with proteinuria | O11.x00 | O11.x00 | ||
| 3. Glomerular diseases | ||||
| Recurrent and persistent hematuria | N02 | |||
| Chronic nephritic syndrome | N03 | |||
| Nephrotic syndrome | N04 | |||
| Unspecified nephritic syndrome | N05 | |||
| Isolated proteinuria with specified morphological lesion | N06 | |||
| Persistent proteinuria, unspecified | N39.1 | |||
| 4. Renal tubulointerstitial diseases | ||||
| Chronic tubulointerstitial nephritis | N11 | |||
| Tubulointerstitial nephritis, not specified as acute or chronic | N12 | |||
| Drug- and heavy metal–induced tubulointerstitial and tubular conditions | N14 | |||
| Renal tubulointerstitial disorders in diseases classified elsewhere | N16 | |||
| Other specified disorders of carbohydrate metabolism | E74.8 | |||
| Disorders of amino acid transport | E72.0 | |||
| Nephrogenic diabetes insipidus | N25.1 | N25.1 | ||
| Renal tubular acidosis | N25.8 | |||
| Balkan nephropathy | N15.000 | N15.001 | N15.000 | |
| Renal tubulointerstitial disease, specified | N15.800 | N15.800 | ||
| Renal granuloma | N15.801 | N15.801 | ||
| Renal tubulointerstitial disease | N15.900 | N15.900 | ||
| Impaired renal tubular function–related disease | N25.9 | N25.9 | ||
| Liddle syndrome | I15.101 | I15.101 | ||
| Urate nephropathy | M10.001 + N16.8 | N28.905 | M10.001 + N16.8 | |
| Systemic lupus erythematosus + renal tubulointerstitial diseases | M32.102 + N16.4 | M32.113 + N16.4 | M32.102 + N16.4 | |
| Sicca syndrome + renal tubulointerstitial diseases | M35.006 + N16.4 | M35.005 + N16.4 | M35.006 + N16.4 | |
| 5. Obstructive nephropathy | ||||
| Hydronephrosis with ureteropelvic junction obstruction | N13.0 | |||
| Hydronephrosis with ureteral stricture, not elsewhere classified | N13.1 | |||
| Hydronephrosis with renal and ureteral calculous obstruction | N13.2 | N13.2 | N13.200 | |
| Other obstructive nephropathy | N13.8 | N13.8 | N13.801 | |
| 6. Other related diagnosis | ||||
| Hereditary nephropathy, not elsewhere classified | N07 | N07.901 | N07 | |
| Glomerular disorders in diseases classified elsewhere | N08, excluding N08.5 | |||
| Renal agenesis and other reduction defects of the kidney | Q60 | |||
| Polycystic kidney, autosomal recessive | Q61.1 | |||
| Polycystic kidney, autosomal dominant | Q61.2 | |||
| Polycystic kidney, unspecified | Q61.3 | |||
| Medullary cystic kidney, sponge kidney | Q61.5 | |||
| Lobulated, fused, and horseshoe kidney | Q63.1 | |||
| Congenital malformation of the kidney, unspecified | Q63.9 | |||
| Gout due to impairment of renal function | M10.300 | M10.393 | M10.300 | |
| Unspecified contracted kidney | N26 | |||
| Ischemia and infarction of the kidney | N28.0 | |||
| Other specified disorders of the kidney and ureter | N28.8 | |||
| Disorders of the kidney and ureter, unspecified | N28.9 | |||
| Congenital renal failure | P96.0 | P96.0 | P96.000 | |
| Extrarenal uremia | R39.2 | |||
| Aortic arch syndrome + renovascular hypertension | M31.4 + I15.0 | I77.604 + I15.0 | I77.600x004 + I15.0 | |
| Goodpasture syndrome | M31.001 | |||
| Renal osteodystrophy | N25.0 | |||
| Failure and rejection of renal transplantation | T86.1 | |||
| Hemolytic uremic syndrome | D59.3 | |||
| Dialysis | Z49 | |||
| Renal allergic purpura | D69.005 + N08.2 | |||
| Lupus nephritis | M32.101 + N08.5 | M32.105 + N08.5 | M32.101 + N08.5 | |
| Goodpasture syndrome–related glomerulonephritis | M31.003 + N08.5 | M31.003 + N08.5 | ||
| Anti–glomerular basement membrane antibody-related disease | M31.002 + N08.5 | M31.005 + N08.5 | M31.002 + N08.5 | |
| Microscopic polyangiitis | M31.700 | M31.701 | M31.700 | |
| ANCA-related nephritis | M31.701 + N08.5 | M31.802 | M31.701 + N08.5 | |
| Thrombotic thrombocytopenic purpura–related glomerulonephritis | M31.102 + N08.5 | M31.102 + N08.5 | ||
| Wegener granulomatosis–related glomerulonephritis | M31.303 + N08.5 | M31.303 + N08.5 | ||
| Pregnancy with nephrotic syndrome | O26.801 | O26.811 | O26.801 | |
| Pregnancy with glomerulonephritis | O26.804 | O26.812 | O26.804 | |
| Pregnancy with renal failure | O26.802 | O26.813 | O26.802 | |
| HBV-related nephritis | B18.103 + N08.0 | B18.102 | B18.103 + N08.0 | |
| HCV-related nephritis | B18.205 + N08.0 | B18.208 | B18.205 + N08.0 | |
| Cryoglobulinemia-related glomerulonephritis | D89.101 + N08.2 | D89.101 + N08.2 | ||
| Hereditary amyloidosis nephropathy | E85.002 | E85.003 | E85.002 | |
| Amyloidosis-related nephropathy | E85.411 + N29.8 | E85.410 + N08.4 | E85.411 + N29.8 | |
| Psoriatic nephritis | L40.803 | L40.802 + N05.9 | L40.800x002 + N05.9 | |
| Kidney injury–related gout | M10.300 | M10.393 | M10.300 | |
| Syphilitic nephritis | A52.712 + N08.0 | A52.700x012 + N08.0 | ||
| Lupus kidney injury | M32.112 + N08.5 | |||
| Lupus nephritis | M32.101 + N08.5 | M32.105 + N08.5 | M32.101 + N08.5 | |
| Lupus tubulointerstitial kidney | M32.102 + N16.4 | M32.113 + N16.4 | M32.102 + N16.4 | |
| Gouty nephropathy | M10.391 | M10.300x091 | ||
| Gouty nephrolithiasis | M10.005 + N22.8 | M10.392 | M10.005 + N22.8 |
ANCA, antineutrophil cytoplasmic antibody; CKD, chronic kidney disease; HBV, hepatitis B virus; HCV, hepatitis C virus; ICD, International Classification of Diseases.
Blank cells indicate not applicable.
Appendix Table 2.
ICD coding of CKD stages
| CKD stage | China edition | Beijing edition | Clinic edition |
|---|---|---|---|
| CKD stage 1 | N18.801 | N18.914 | N18.801 |
| CKD stage 2 | N18.802 | N18.915 | N18.802 |
| CKD stage 3 | N18.803 | N18.916 | N18.803 |
| CKD stage 4 | N18.804 | N18.917 | N18.804 |
| CKD stage 5 | N18.001 | N18.918 | N18.001 |
CKD, chronic kidney disease; ICD, International Classification of Diseases.
Appendix Table 3.
ICD coding of diabetes mellitus
| Etiology | ICD coding |
|---|---|
| Type 1 diabetes mellitus | E10 |
| Type 2 diabetes mellitus | E11 |
| Malnutrition-related diabetes mellitus | E12 |
| Other specified diabetes mellitus | E13 |
| Unspecified diabetes mellitus | E14 |
ICD, International Classification of Diseases.
Appendix Table 4.
ICD coding of hypertension
| Etiology | ICD coding |
|---|---|
| Essential (primary) hypertension | I10 |
| Hypertensive heart disease | I11 |
| Hypertensive renal disease | I12 |
| Hypertensive heart and renal disease | I13 |
| Secondary hypertension | I15 |
ICD, International Classification of Diseases.
Appendix Table 5.
ICD coding of CVD
| Etiology | All editions | China edition | Beijing edition | Clinic edition |
|---|---|---|---|---|
| 1. Cerebral stroke | ||||
| Subarachnoid hemorrhage | I60 | |||
| Intracerebral hemorrhage | I61 | |||
| Acute ischemic cerebral stroke | I63 | |||
| I64 | ||||
| H34.1 | ||||
| Transient ischemic attack | G45 | |||
| 2. Coronary heart disease | ||||
| Angina pectoris | I20 | |||
| Acute myocardial infarction | I21 | |||
| Subacute myocardial infarction | I22 | |||
| Complications after myocardial infarction | I23 | |||
| Other acute ischemic heart disease | I24 | |||
| Chronic ischemic heart disease | I25 | |||
| 3. Heart failure | ||||
| Whole heart failure | I50.003 | I50.002 | ||
| Right heart failure | I50.001 | I50.004 | I50.001 | |
| Right ventricular failure | I50.005 | I50.000x005 | ||
| Acute right heart failure | I50.000x006 | |||
| Left heart failure | I50.100 | I50.106 | I50.100x006 | |
| Left ventricular failure | I50.100 | |||
| Left atrial failure | I50.102 | |||
| Chronic left heart insufficiency | I50.103 | I50.105 | ||
| Left heart failure with acute pulmonary edema | I50.107 | I50.103 | ||
| Congestive heart failure | I50.000 | I50.001 | I50.000 | |
| Acute heart failure | I50.904 | I50.907 | ||
| Chronic heart failure | I50.905 | I50.908 | ||
| Other heart failure | I50.900 | I50.911 | I50.900 | |
| Postoperative heart failure and pulmonary edema | I97.104 | I97.100x004 | ||
| Heart failure of newborns | P29.000 | P29.001 | P29.000 | |
| Hypertensive heart failure | I11.001 | |||
| Hypertensive heart disease with (congestive) heart failure | I11.000 | I11.000 | ||
| Hypertensive heart disease without (congestive) heart failure | I11.900 | |||
| Hypertensive heart disease and kidney disease with congestive heart failure | I13.000 | I13.000 | ||
| Hypertensive heart disease and kidney disease with congestive heart failure and renal failure | I13.200 | I13.200 | ||
| Intractable heart failure | I50.900x017 | |||
| Heart failure after cardiac surgery | I97.102 | I97.106 | I97.102 | |
| Postoperative heart failure | I97.803 | I97.803 | ||
| Chronic left heart insufficiency | I50.103 | I50.105 | ||
| Cardiac insufficiency | I50.901 | I50.902 | I50.900x002 | |
| Cardiac insufficiency of newborns | P29.001 | |||
| Acute exacerbation of chronic cardiac insufficiency | I50.900x018 | |||
| Acute left heart failure | I50.102 | I50.101 | ||
| Acute pulmonary edema | J81xx02 | J81.x00x002 | ||
| Pregnancy with heart failure | O99.417 | O99.408 | O99.400x008 | |
| Pregnancy with cardiac insufficiency | O99.429 | O99.429 | O99.414 | |
| Childbirth with heart failure | O75.403 | O75.403 | ||
| Pregnancy with left heart failure | O99.423 | O99.424 | ||
| Puerperal cardiac insufficiency | O99.402 | O99.434 | O99.402 | |
| Acute pulmonary edema after postpartum | O99.507 | O99.508 | O99.508 | |
| Heart failure due to anesthesia during pregnancy | O29.102 | O99.500x008 | ||
| Heart failure due to anesthesia during childbirth | O74.202 | O74.200x002 | ||
| Heart failure after obstetric surgery or operation | O75.402 | |||
| Heart failure due to anesthesia during puerperium | O89.102 | O89.100x002 | ||
| Low cardiac output syndrome | I50.901 | I50.901 | ||
| Cardiac function, class I | I50.902 | I50.902 | ||
| Cardiac function, class II | I50.903 | I50.907 | I50.903 | |
| Cardiac function, class III | I50.904 | I50.908 | I50.904 | |
| Cardiac function, class IV | I50.905 | I50.910 | I50.905 | |
| Cardiac function, class II (NYHA) | I50.900x007 | |||
| Cardiac function, class III (NYHA) | I50.900x008 | |||
| Cardiac function, class II–III (NYHA) | I50.900x009 | |||
| Cardiac function, class IV (NYHA) | I50.900x010 | |||
| Circulatory failure | R57.901 | I50.913 | R57.901 | |
| Pulmonary edema | J81.x00 | J81xx03 | J81.x00 | |
| Cardiogenic shock | R57.000 | R57.001 | R57.000 | |
| Respiratory and circulatory failure | J96.102 | J96.900 | ||
| Cardiogenic asthma | I50.104 | I50.104 | ||
| 4. Atrial fibrillation | ||||
| Atrial fibrillation | I48.x01 | I48xx04 | I48.x01 | |
| Idiopathic atrial fibrillation | I48.x02 | I48xx02 | I48.x05 | |
| Persistent atrial fibrillation | I48xx07 | I48.x00x007 | ||
| Chronic atrial fibrillation | I48xx08 | I48.x00x008 | ||
| Pregnancy with atrial fibrillation | O99.427 | O99.427 | O99.400x027 | |
| Atrial fibrillation with flutter | I48.x00 | I48xx01 | I48.x00 | |
| Primary atrial fibrillation | I48.x00x009 | |||
| Long-term persistent atrial fibrillation | I48.x00x011 | |||
| Acute atrial fibrillation | I48.x00x012 | |||
| Permanent atrial fibrillation | I48.x00x013 | |||
| Long-range persistent atrial fibrillation | I48.x00x014 | |||
| New diagnosis of atrial fibrillation | I48.x00x015 | |||
| Paroxysmal atrial fibrillation | I48.x02 | I48xx06 | I48.x02 |
CVD, cardiovascular disease; ICD, International Classification of Diseases; NYHA, New York Heart Association.
Blank cells indicate not applicable.
Appendix Table 6.
ICD coding of CVD operations
| Operation | China edition | Beijing edition | Clinic edition |
|---|---|---|---|
| Coronary angiography | 88.55001 | 88.5500 | |
| 88.5,500x002 | |||
| 88.56001 | 88.5600 | ||
| 88.5,600x002 | |||
| 88.57002 | 88.5701 | ||
| 88.5700 | |||
| 88.5,700x003 | |||
| 88.5900 | |||
| Percutaneous coronary intervention | 36.06003 | 36.0602 | |
| 36.0601 | |||
| 36.06004 | 36.0600 | ||
| 36.07003 | 36.0700 | ||
| 36.0,700x004 | |||
| 36.0701 | |||
| Coronary artery bypass grafting | 36.11001 | ||
| 36.12001 | |||
| 36.13001 | |||
| 36.14001 | |||
| 36.15001 | |||
| 36.16001 | |||
| 36.17001 | |||
| 36.2 001 | |||
| Pacemaker | Z95.000 | Z95.000 | |
| T82.700 | T82.700 | ||
| T82.703 | T82.702 | T82.703 | |
| T82.100 | |||
| T82.101 | |||
| T82.102 | |||
| T82.103 | |||
| T82.800 | T82.800 | ||
| T82.903 | T82.801 | T82.903 | |
| T82.904 | T82.904 | ||
| T85.707 | |||
| Z45.007 | |||
| Z45.001 | Z45.001 | ||
| Z45.002 | |||
| Z95.001 | |||
| Z45.004 | Z45.003 | Z45.004 | |
| T82.100x002 | |||
| T82.100x003 | |||
| T82.702 | T82.700x002 | ||
| Z45.000 | |||
| Z45.003 | Z45.003 | ||
| Z45.005 | Z45.005 | ||
| Z45.006 | Z45.006 | ||
| 37.89001 | 37.8901 | ||
| 89.4500 | |||
| 37.7501 | |||
| 37.7800 | |||
| 37.80001 | 37.8,000x001 | ||
| 37.80002 | 37.8,000x002 | ||
| 37.8001 | |||
| 37.7701 | |||
| 37.7600 | |||
| 37.78001 | |||
| Implantable cardioverter-defibrillator/cardiac resynchronization therapy–defibrillator | Z95.800x007 | ||
| Z45.800x006 | |||
| T82.100x011 | |||
| T82.100x010 | |||
| 00.5100 | |||
| 00.51001 | 00.5,100x001 | ||
| 00.5101 | |||
| 00.5102 | |||
| 00.53001 | 00.5301 | ||
| 00.53002 | 00.5302 | ||
| 00.5400 | |||
| 00.54001 | 00.5401 | ||
| 00.54002 | 00.5402 | ||
| 37.9400 | |||
| 37.94001 | 37.9401 | ||
| 37.9403 | |||
| 37.9404 | |||
| 37.9500 | |||
| 37.9,500x001 | |||
| 37.9600 | |||
| 37.9700 | |||
| 37.9,700x001 | |||
| 37.9,700x002 | |||
| 37.9800 | |||
| 37.9,800x002 | |||
| 99.6202 |
CVD, cardiovascular disease; ICD, International Classification of Diseases.
Blank cells indicate not applicable.
Appendix Table 7.
ICD coding of AKI
| Etiology | All editions | China edition | Beijing edition | Clinic edition |
|---|---|---|---|---|
| Acute renal failure | N17 | |||
| Rapidly progressive nephritic syndrome | N01 | |||
| Traumatic anuria | T79.5 | |||
| Hemolytic uremic syndrome | D59.3 | |||
| Hepatorenal syndrome | K76.7 | |||
| Postpartum acute renal failure | O90.4 | |||
| Renal failure after abortion | O08.4 | |||
| Postprocedural disorders of the genitourinary system, not elsewhere classified | N99.0 | |||
| Acute tubulointerstitial nephritis | N10.x00 | N10.x00 | ||
| Acute interstitial nephritis | N10.x01 | N10.x01 | ||
| Chronic glomerulonephritis with rapidly progressive glomerulonephritis | N00.908 | N00.900x009 | ||
| Acute infectious interstitial nephritis | N10xx03 | N10.x00x003 | ||
| TINU syndrome | N10xx04 + H20.9 | N12.x00x005 |
AKI, acute kidney injury; ICD, International Classification of Diseases; TINU, tubulointerstitial nephritis and uveitis.
Blank cells indicate not applicable.
Appendix Table 8.
Prevalence of CKD among different types of underlying disease
| Patient group | 2017 |
2018 |
||
|---|---|---|---|---|
| No. of patients with CKD | Prevalence of CKD (%) | No. of patients with CKD | Prevalence of CKD (%) | |
| HQMS | 957,009 | 4.95 | 771,625 | 4.59 |
| HTN | 518,103 | 11.34 | 428,999 | 10.73 |
| CVD | 337,814 | 7.95 | 284,706 | 7.49 |
| DM | 305,991 | 13.78 | 260,688 | 13.28 |
| HTN + CVD | 132,971 | 7.76 | 109,526 | 7.33 |
| DM + HTN + CVD | 121,651 | 16.61 | 104,646 | 15.9 |
| DM + HTN | 96,050 | 17.83 | 81,016 | 17.18 |
| DM + CVD | 28,560 | 10.12 | 25,170 | 9.76 |
CKD, chronic kidney disease; CVD cardiovascular disease; DM, diabetes mellitus; HQMS, Hospital Quality Monitoring System; HTN, hypertension.
Appendix Table 9.
Patients with CKD, stratified by sex and age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 11,739 (4.01) | 9512 (1.71) | 21,251 (2.50) | 8837 (3.68) | 6870 (1.53) | 15,707 (2.28) |
| 25–29 | 17,417 (5.17) | 15,006 (1.22) | 32,423 (2.07) | 13,329 (4.85) | 11,122 (1.12) | 24,451 (1.93) |
| 30–34 | 22,661 (6.14) | 16,262 (1.54) | 38,923 (2.73) | 18,192 (5.58) | 13,053 (1.44) | 31,245 (2.54) |
| 35–39 | 26,647 (6.50) | 16,963 (2.14) | 43,610 (3.63) | 21,386 (6.00) | 13,531 (2.10) | 34,917 (3.49) |
| 40–44 | 35,527 (6.39) | 22,776 (3.16) | 58,303 (4.57) | 26,936 (5.89) | 17,172 (2.91) | 44,108 (4.21) |
| 45–49 | 50,486 (6.24) | 34,502 (3.75) | 84,988 (4.92) | 39,923 (5.75) | 26,874 (3.38) | 66,797 (4.49) |
| 50–54 | 63,943 (6.34) | 44,240 (4.23) | 108,183 (5.27) | 48,623 (5.76) | 33,827 (3.83) | 82,450 (4.77) |
| 55–59 | 52,108 (6.31) | 34,342 (4.42) | 86,450 (5.39) | 46,535 (5.97) | 30,153 (4.06) | 76,688 (5.03) |
| 60–64 | 65,688 (5.94) | 45,995 (4.58) | 111,683 (5.29) | 53,069 (5.45) | 36,622 (4.19) | 89,691 (4.85) |
| 65–69 | 59,964 (6.19) | 43,904 (5.09) | 103,868 (5.67) | 50,565 (5.70) | 37,771 (4.77) | 88,336 (5.26) |
| 70–74 | 49,413 (6.66) | 36,328 (5.58) | 85,741 (6.15) | 40,467 (6.03) | 30,514 (5.17) | 70,981 (5.63) |
| 75–79 | 44,915 (7.58) | 33,874 (6.37) | 78,789 (7.01) | 34,833 (6.77) | 27,414 (5.89) | 62,247 (6.35) |
| 80–84 | 35,321 (8.94) | 25,837 (7.20) | 61,158 (8.11) | 27,897 (7.97) | 21,267 (6.60) | 49,164 (7.32) |
| ≥85 | 26,276 (11.33) | 15,363 (8.06) | 41,639 (9.85) | 21,490 (10.07) | 13,353 (7.39) | 34,843 (8.84) |
| Total | 562,105 (6.50) | 394,904 (3.69) | 957,009 (4.95) | 452,082 (5.96) | 319,543 (3.47) | 771,625 (4.59) |
CKD, chronic kidney disease.
Data are expressed as n (%).
Appendix Table 10.
Patients with CKD, stratified by urban vs. rural area
| Residence | 2017 | 2018 |
|---|---|---|
| Urban | 504,862 (5.54) | 470,093 (5.11) |
| Rural | 202,674 (5.10) | 121,373 (4.70) |
| Total | 707,536 (5.41) | 591,466 (5.02) |
CKD, chronic kidney disease.
Patients with missing data for residence were not included in the analysis. 2017: 6,264,702 (32.39%); 2018: 5,026,530 (29.91%).
Data are expressed as n (%).
Appendix Table 11.
Staging of CKD, stratified by hospital nephrology unit
| CKD stage | 2017 |
2018 |
||||||
|---|---|---|---|---|---|---|---|---|
| Independent | Nonindependent | Unknown | Total | Independent | Nonindependent | Unknown | Total | |
| Stage 1 | 6006 (0.73) | 450 (0.78) | 463 (0.61) | 6919 (0.72) | 5471 (0.82) | 364 (0.83) | 547 (0.87) | 6382 (0.83) |
| Stage 2 | 10,125 (1.23) | 439 (0.76) | 861 (1.14) | 11,425 (1.19) | 9386 (1.41) | 509 (1.16) | 844 (1.35) | 10,739 (1.39) |
| Stage 3 | 30,680 (3.73) | 1256 (2.18) | 1772 (2.34) | 33,708 (3.52) | 28,980 (4.36) | 1644 (3.74) | 2731 (4.36) | 33,355 (4.32) |
| Stage 4 | 20,193 (2.45) | 1059 (1.84) | 1122 (1.48) | 22,374 (2.34) | 18,985 (2.86) | 1046 (2.38) | 1363 (2.17) | 21,394 (2.77) |
| Stage 5 | 72,837 (8.84) | 2442 (4.24) | 2644 (3.49) | 77,923 (8.14) | 71,052 (10.69) | 2849 (6.48) | 3421 (5.46) | 77,322 (10.02) |
| Unknown | 683,665 (83.02) | 51,986 (90.20) | 68,905 (90.94) | 804,556 (84.08) | 530,883 (79.86) | 37,559 (85.42) | 53,797 (85.80) | 622,239 (80.66) |
| Total | 823,506 (100) | 57,632 (100) | 75,767 (100) | 956,905 (100) | 664,757 (100) | 43,971 (100) | 62,703 (100) | 771,431 (100) |
CKD, chronic kidney disease.
Patients with missing data and/or controversial data for stage were not included in the analysis. 2017: 104 (0.01%); 2018: 194 (0.03%).
Data are expressed as n (%).
Appendix Table 12.
Age distribution of patients with CKD, stratified by sex
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 11,739 (2.09) | 9512 (2.41) | 21,251 (2.22) | 8837 (1.95) | 6870 (2.15) | 15,707 (2.04) |
| 25–29 | 17,417 (3.10) | 15,006 (3.80) | 32,423 (3.39) | 13,329 (2.95) | 11,122 (3.48) | 24,451 (3.17) |
| 30–34 | 22,661 (4.03) | 16,262 (4.12) | 38,923 (4.07) | 18,192 (4.02) | 13,053 (4.08) | 31,245 (4.05) |
| 35–39 | 26,647 (4.74) | 16,963 (4.30) | 43,610 (4.56) | 21,386 (4.73) | 13,531 (4.23) | 34,917 (4.53) |
| 40–44 | 35,527 (6.32) | 22,776 (5.77) | 58,303 (6.09) | 26,936 (5.96) | 17,172 (5.37) | 44,108 (5.72) |
| 45–49 | 50,486 (8.98) | 34,502 (8.74) | 84,988 (8.88) | 39,923 (8.83) | 26,874 (8.41) | 66,797 (8.66) |
| 50–54 | 63,943 (11.38) | 44,240 (11.20) | 108,183 (11.30) | 48,623 (10.76) | 33,827 (10.59) | 82,450 (10.69) |
| 55–59 | 52,108 (9.27) | 34,342 (8.70) | 86,450 (9.03) | 46,535 (10.29) | 30,153 (9.44) | 76,688 (9.94) |
| 60–64 | 65,688 (11.69) | 45,995 (11.65) | 111,683 (11.67) | 53,069 (11.74) | 36,622 (11.46) | 89,691 (11.62) |
| 65–69 | 59,964 (10.67) | 43,904 (11.12) | 103,868 (10.85) | 50,565 (11.18) | 37,771 (11.82) | 88,336 (11.45) |
| 70–74 | 49,413 (8.79) | 36,328 (9.20) | 85,741 (8.96) | 40,467 (8.95) | 30,514 (9.55) | 70,981 (9.20) |
| 75–79 | 44,915 (7.99) | 33,874 (8.58) | 78,789 (8.23) | 34,833 (7.71) | 27,414 (8.58) | 62,247 (8.07) |
| 80–84 | 35,321 (6.28) | 25,837 (6.54) | 61,158 (6.39) | 27,897 (6.17) | 21,267 (6.66) | 49,164 (6.37) |
| ≥85 | 26,276 (4.67) | 15,363 (3.89) | 41,639 (4.35) | 21,490 (4.75) | 13,353 (4.18) | 34,843 (4.52) |
| Total | 562,105 (100) | 394,904 (100) | 957,009 (100) | 452,082 (100) | 319,543 (100) | 771,625 (100) |
CKD, chronic kidney disease.
Data are expressed as n (%).
Appendix Table 13.
Sex distribution of patients with CKD, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 18–24 | 11,739 (55.24) | 9512 (44.76) | 21,251 | 8837 (56.26) | 6870 (43.74) | 15,707 |
| 25–29 | 17,417 (53.72) | 15,006 (46.28) | 32,423 | 13,329 (54.51) | 11,122 (45.49) | 24,451 |
| 30–34 | 22,661 (58.22) | 16,262 (41.78) | 38,923 | 18,192 (58.22) | 13,053 (41.78) | 31,245 |
| 35–39 | 26,647 (61.10) | 16,963 (38.90) | 43,610 | 21,386 (61.25) | 13,531 (38.75) | 34,917 |
| 40–44 | 35,527 (60.94) | 22,776 (39.06) | 58,303 | 26,936 (61.07) | 17,172 (38.93) | 44,108 |
| 45–49 | 50,486 (59.40) | 34,502 (40.60) | 84,988 | 39,923 (59.77) | 26,874 (40.23) | 66,797 |
| 50–54 | 63,943 (59.11) | 44,240 (40.89) | 108,183 | 48,623 (58.97) | 33,827 (41.03) | 82,450 |
| 55–59 | 52,108 (60.28) | 34,342 (39.72) | 86,450 | 46,535 (60.68) | 30,153 (39.32) | 76,688 |
| 60–64 | 65,688 (58.82) | 45,995 (41.18) | 111,683 | 53,069 (59.17) | 36,622 (40.83) | 89,691 |
| 65–69 | 59,964 (57.73) | 43,904 (42.27) | 103,868 | 50,565 (57.24) | 37,771 (42.76) | 88,336 |
| 70–74 | 49,413 (57.63) | 36,328 (42.37) | 85,741 | 40,467 (57.01) | 30,514 (42.99) | 70,981 |
| 75–79 | 44,915 (57.01) | 33,874 (42.99) | 78,789 | 34,833 (55.96) | 27,414 (44.04) | 62,247 |
| 80–84 | 35,321 (57.75) | 25,837 (42.25) | 61,158 | 27,897 (56.74) | 21,267 (43.26) | 49,164 |
| ≥85 | 26,276 (63.10) | 15,363 (36.90) | 41,639 | 21,490 (61.68) | 13,353 (38.32) | 34,843 |
| Total | 562,105 (58.74) | 394,904 (41.26) | 957,009 | 452,082 (58.59) | 319,543 (41.41) | 771,625 |
CKD, chronic kidney disease.
Data are expressed as n (%).
Appendix Table 14.
Cause distribution of patients with CKD
| Cause | 2017 | 2018 |
|---|---|---|
| DKD | 259,770 (27.14) | 222,079 (28.78) |
| HTN | 205,746 (21.50) | 165,378 (21.43) |
| GN | 136,588 (14.27) | 113,447 (14.70) |
| CTIN | 15,576 (1.63) | 15,679 (2.03) |
| ON | 147,521 (15.41) | 97,618 (12.65) |
| Others | 191,808 (20.04) | 157,424 (20.40) |
| Total | 957,009 (100) | 771,625 (100) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 15.
Cause distribution of patients with CKD, stratified by urban vs. rural area
| Cause | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Urban | Rural | Total | Urban | Rural | Total | |
| DKD | 162,524 (32.19) | 37,090 (18.30) | 199,614 (28.21) | 152,799 (32.50) | 25,404 (20.93) | 178,203 (30.13) |
| HTN | 115,688 (22.91) | 38,128 (18.81) | 153,816 (21.74) | 104,408 (22.21) | 22,920 (18.88) | 127,328 (21.53) |
| GN | 64,476 (12.77) | 33,175 (16.37) | 97,651 (13.80) | 63,875 (13.59) | 21,111 (17.39) | 84,986 (14.37) |
| CTIN | 8600 (1.70) | 3004 (1.48) | 11,604 (1.64) | 9987 (2.12) | 2294 (1.89) | 12,281 (2.08) |
| ON | 66,943 (13.26) | 42,516 (20.98) | 109,459 (15.47) | 53,557 (11.39) | 20,197 (16.64) | 73,754 (12.47) |
| Others | 86,631 (17.16) | 48,761 (24.06) | 135,392 (19.14) | 85,467 (18.18) | 29,447 (24.26) | 114,914 (19.43) |
| Total | 504,862 (100) | 202,674 (100) | 707,536 (100) | 470,093 (100) | 121,373 (100) | 591,466 (100) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Patients with missing data for residence were not included in the analysis. 2017: 249,473 (26.07%); 2018: 180,159 (23.35%).
Appendix Table 16.
Cause distribution of patients with CKD, stratified by geographic region
| Geographic region | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| N-Beijing | 7210 (35.87) | 4790 (23.83) | 2652 (13.19) | 313 (1.56) | 1637 (8.14) | 3499 (17.41) | 20,101 |
| N-Tianjin | 443 (28.06) | 283 (17.92) | 276 (17.48) | 29 (1.84) | 145 (9.18) | 403 (25.52) | 1579 |
| N-Hebei | 11,550 (34.48) | 6456 (19.27) | 7133 (21.29) | 582 (1.74) | 2979 (8.89) | 4797 (14.32) | 33,497 |
| N-Shanxi | 9207 (34.60) | 5426 (20.39) | 5327 (20.02) | 458 (1.72) | 1901 (7.14) | 4293 (16.13) | 26,612 |
| N-Inner Mongolia | 6879 (28.29) | 6280 (25.82) | 3765 (15.48) | 719 (2.96) | 669 (2.75) | 6006 (24.70) | 24,318 |
| NE-Liaoning | 8684 (38.56) | 4645 (20.63) | 4031 (17.90) | 279 (1.24) | 743 (3.30) | 4136 (18.37) | 22,518 |
| NE-Jilin | 5807 (34.97) | 3984 (23.99) | 1987 (11.97) | 119 (0.72) | 597 (3.60) | 4111 (24.76) | 16,605 |
| NE-Heilongjiang | 8976 (37.85) | 5354 (22.58) | 2761 (11.64) | 234 (0.99) | 1782 (7.51) | 4608 (19.43) | 23,715 |
| E-Shanghai | 5201 (28.15) | 4852 (26.26) | 2736 (14.81) | 482 (2.61) | 2001 (10.83) | 3204 (17.34) | 18,476 |
| E-Jiangsu | 18,311 (30.56) | 13,430 (22.41) | 11,986 (20.00) | 941 (1.57) | 4807 (8.02) | 10,451 (17.44) | 59,926 |
| E-Zhejiang | 8791 (22.57) | 8057 (20.68) | 7143 (18.34) | 736 (1.89) | 8073 (20.72) | 6154 (15.80) | 38,954 |
| E-Anhui | 6761 (27.90) | 5336 (22.02) | 3535 (14.59) | 295 (1.22) | 3124 (12.89) | 5183 (21.39) | 24,234 |
| E-Fujian | 6366 (21.66) | 6704 (22.81) | 4059 (13.81) | 442 (1.50) | 5651 (19.22) | 6173 (21.00) | 29,395 |
| E-Jiangxi | 11,573 (23.09) | 11,387 (22.71) | 5608 (11.19) | 423 (0.84) | 12,575 (25.08) | 8565 (17.09) | 50,131 |
| E-Shandong | 9199 (31.48) | 5861 (20.06) | 5638 (19.30) | 750 (2.57) | 2283 (7.81) | 5489 (18.79) | 29,220 |
| C-Henan | 15,933 (30.86) | 9602 (18.60) | 9031 (17.49) | 739 (1.43) | 6366 (12.33) | 9962 (19.29) | 51,633 |
| C-Hubei | 19,778 (24.19) | 21,772 (26.63) | 6168 (7.54) | 1022 (1.25) | 18,385 (22.49) | 14,638 (17.90) | 81,763 |
| C-Hunan | 8890 (24.88) | 5866 (16.42) | 6586 (18.43) | 500 (1.40) | 6249 (17.49) | 7643 (21.39) | 35,734 |
| S-Guangdong | 16,675 (21.20) | 15,451 (19.64) | 10,818 (13.75) | 1923 (2.44) | 20,018 (25.45) | 13,771 (17.51) | 78,656 |
| S-Guangxi | 6394 (19.15) | 7847 (23.50) | 4151 (12.43) | 666 (1.99) | 7359 (22.04) | 6976 (20.89) | 33,393 |
| S-Hainan | 4280 (32.17) | 2809 (21.11) | 1843 (13.85) | 191 (1.44) | 1679 (12.62) | 2502 (18.81) | 13,304 |
| SW-Chongqing | 3817 (29.03) | 3260 (24.79) | 1198 (9.11) | 223 (1.70) | 2646 (20.12) | 2006 (15.25) | 13,150 |
| SW-Sichuan | 13,143 (21.94) | 10,827 (18.07) | 5827 (9.73) | 1008 (1.68) | 11,859 (19.80) | 17,239 (28.78) | 59,903 |
| SW-Guizhou | 3591 (18.58) | 3769 (19.50) | 2454 (12.70) | 457 (2.36) | 4875 (25.23) | 4179 (21.62) | 19,325 |
| SW-Yunnan | 11,865 (20.50) | 13,157 (22.73) | 6054 (10.46) | 629 (1.09) | 7994 (13.81) | 18,182 (31.41) | 57,881 |
| SW-Tibet | 98 (22.58) | 74 (17.05) | 73 (16.82) | 7 (1.61) | 25 (5.76) | 157 (36.18) | 434 |
| NW-Shaanxi | 8692 (35.81) | 4242 (17.48) | 4330 (17.84) | 418 (1.72) | 2834 (11.68) | 3755 (15.47) | 24,271 |
| NW-Gansu | 2873 (36.92) | 1292 (16.60) | 928 (11.92) | 144 (1.85) | 1310 (16.83) | 1235 (15.87) | 7782 |
| NW-Qinghai | 2253 (43.14) | 704 (13.48) | 618 (11.83) | 106 (2.03) | 110 (2.11) | 1431 (27.40) | 5222 |
| NW-Ningxia | 962 (30.19) | 523 (16.42) | 772 (24.23) | 71 (2.23) | 433 (13.59) | 425 (13.34) | 3186 |
| NW-Xinjiang | 6461 (32.55) | 4590 (23.12) | 2452 (12.35) | 241 (1.21) | 2640 (13.30) | 3465 (17.46) | 19,849 |
| Total | 250,663 (27.11) | 198,630 (21.48) | 131,940 (14.27) | 15,147 (1.64) | 143,749 (15.54) | 184,638 (19.97) | 924,767 |
| Year 2018 | |||||||
| N-Beijing | 7216 (35.06) | 4798 (23.31) | 2744 (13.33) | 350 (1.70) | 1893 (9.20) | 3579 (17.39) | 20,580 |
| N-Tianjin | 432 (33.62) | 258 (20.08) | 254 (19.77) | 19 (1.48) | 68 (5.29) | 254 (19.77) | 1285 |
| N-Hebei | 8562 (34.35) | 5248 (21.06) | 4283 (17.18) | 457 (1.83) | 2415 (9.69) | 3959 (15.88) | 24,924 |
| N-Shanxi | 7919 (33.79) | 4391 (18.74) | 5009 (21.37) | 479 (2.04) | 1181 (5.04) | 4456 (19.01) | 23,435 |
| N-Inner Mongolia | 6993 (28.47) | 6052 (24.64) | 4269 (17.38) | 762 (3.10) | 730 (2.97) | 5757 (23.44) | 24,563 |
| NE-Liaoning | 7852 (38.97) | 3976 (19.73) | 3310 (16.43) | 252 (1.25) | 1053 (5.23) | 3707 (18.40) | 20,150 |
| NE-Jilin | 4089 (33.62) | 2838 (23.33) | 1852 (15.23) | 360 (2.96) | 413 (3.40) | 2611 (21.47) | 12,163 |
| NE-Heilongjiang | 7830 (37.74) | 4870 (23.48) | 2623 (12.64) | 218 (1.05) | 845 (4.07) | 4359 (21.01) | 20,745 |
| E-Shanghai | 3036 (26.78) | 2706 (23.87) | 1484 (13.09) | 445 (3.92) | 1831 (16.15) | 1836 (16.19) | 11,338 |
| E-Jiangsu | 18,226 (31.26) | 12,770 (21.90) | 11,222 (19.25) | 1042 (1.79) | 5139 (8.81) | 9900 (16.98) | 58,299 |
| E-Zhejiang | 7957 (24.59) | 6785 (20.97) | 4831 (14.93) | 604 (1.87) | 7101 (21.94) | 5082 (15.70) | 32,360 |
| E-Anhui | 4865 (28.21) | 3961 (22.97) | 2620 (15.19) | 239 (1.39) | 1760 (10.20) | 3802 (22.04) | 17,247 |
| E-Fujian | 8635 (22.14) | 8683 (22.26) | 4809 (12.33) | 777 (1.99) | 8142 (20.87) | 7961 (20.41) | 39,007 |
| E-Jiangxi | 10,642 (26.90) | 10,290 (26.01) | 4961 (12.54) | 386 (0.98) | 4962 (12.54) | 8314 (21.02) | 39,555 |
| E-Shandong | 6734 (33.06) | 3558 (17.47) | 4265 (20.94) | 641 (3.15) | 1217 (5.98) | 3951 (19.40) | 20,366 |
| C-Henan | 15,162 (33.95) | 7798 (17.46) | 7651 (17.13) | 1056 (2.36) | 3773 (8.45) | 9217 (20.64) | 44,657 |
| C-Hubei | 14,452 (29.88) | 14,774 (30.54) | 5206 (10.76) | 885 (1.83) | 3480 (7.19) | 9573 (19.79) | 48,370 |
| C-Hunan | 6610 (25.60) | 4501 (17.43) | 3994 (15.47) | 338 (1.31) | 4681 (18.13) | 5698 (22.07) | 25,822 |
| S-Guangdong | 15,451 (23.01) | 13,148 (19.58) | 8921 (13.29) | 1654 (2.46) | 15,681 (23.36) | 12,283 (18.30) | 67,138 |
| S-Guangxi | 6509 (22.11) | 7678 (26.08) | 3558 (12.08) | 674 (2.29) | 3873 (13.15) | 7153 (24.29) | 29,445 |
| S-Hainan | 4184 (34.75) | 2615 (21.72) | 1784 (14.81) | 239 (1.98) | 551 (4.58) | 2669 (22.16) | 12,042 |
| SW-Chongqing | 4222 (29.89) | 3426 (24.25) | 1433 (10.14) | 235 (1.66) | 2244 (15.89) | 2566 (18.17) | 14,126 |
| SW-Sichuan | 9280 (24.57) | 6239 (16.52) | 2945 (7.80) | 609 (1.61) | 8327 (22.05) | 10,364 (27.44) | 37,764 |
| SW-Guizhou | 3444 (20.62) | 3565 (21.35) | 1657 (9.92) | 499 (2.99) | 3590 (21.50) | 3945 (23.62) | 16,700 |
| SW-Yunnan | 5542 (20.51) | 5125 (18.97) | 3871 (14.33) | 954 (3.53) | 2953 (10.93) | 8577 (31.74) | 27,022 |
| SW-Tibet | 195 (25.42) | 124 (16.17) | 155 (20.21) | 8 (1.04) | 52 (6.78) | 233 (30.38) | 767 |
| NW-Shaanxi | 8304 (34.61) | 4180 (17.42) | 4021 (16.76) | 365 (1.52) | 3263 (13.60) | 3859 (16.08) | 23,992 |
| NW-Gansu | 1438 (31.56) | 681 (14.95) | 598 (13.13) | 75 (1.65) | 895 (19.64) | 869 (19.07) | 4556 |
| NW-Qinghai | 809 (43.80) | 138 (7.47) | 298 (16.13) | 73 (3.95) | 52 (2.82) | 477 (25.83) | 1847 |
| NW-Ningxia | 1634 (36.34) | 770 (17.12) | 1129 (25.11) | 139 (3.09) | 60 (1.33) | 765 (17.01) | 4497 |
| NW-Xinjiang | 5904 (32.37) | 3711 (20.34) | 1830 (10.03) | 263 (1.44) | 2422 (13.28) | 4111 (22.54) | 18,241 |
| Total | 214,128 (28.82) | 159,657 (21.49) | 107,587 (14.48) | 15,097 (2.03) | 94,647 (12.74) | 151,887 (20.44) | 743,003 |
C, Central China; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; E, East China; GN, glomerulonephritis; HTN, hypertensive nephropathy; N, North China; NE, Northeast China; NW, Northwest China; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons; S, South China; SW, Southwest China.
Data are expressed as n (%).
Patients with missing data for geographic region were not included in the analysis. 2017: 32,215 (3.37%); 2018: 28,612 (3.71%).
Appendix Table 17.
Mobility pattern of patients with CKD
| Province | Hospital location |
Patient residence |
||
|---|---|---|---|---|
| Local | In | Local | Out | |
| Year 2017 | ||||
| Overall | 873,633 (94.47) | 51,161 (5.53) | 873,633 (94.47) | 51,161 (5.53) |
| N-Beijing | 19,205 (69.79) | 8314 (30.21) | 19,205 (95.54) | 896 (4.46) |
| N-Tianjin | 1146 (81.16) | 266 (18.84) | 1146 (72.58) | 433 (27.42) |
| N-Hebei | 30,027 (97.13) | 886 (2.87) | 30,027 (89.64) | 3470 (10.36) |
| N-Shanxi | 25,223 (98.68) | 338 (1.32) | 25,223 (94.78) | 1389 (5.22) |
| N-Inner Mongolia | 21,570 (97.50) | 554 (2.50) | 21,570 (88.70) | 2748 (11.30) |
| NE-Liaoning | 21,080 (97.84) | 466 (2.16) | 21,080 (93.61) | 1438 (6.39) |
| NE-Jilin | 15,777 (92.69) | 1244 (7.31) | 15,777 (95.01) | 828 (4.99) |
| NE-Heilongjiang | 21,995 (95.51) | 1033 (4.49) | 21,995 (92.75) | 1720 (7.25) |
| E-Shanghai | 17,736 (74.80) | 5974 (25.20) | 17,736 (95.99) | 740 (4.01) |
| E-Jiangsu | 57,181 (93.69) | 3849 (6.31) | 57,181 (95.42) | 2745 (4.58) |
| E-Zhejiang | 36,447 (94.43) | 2149 (5.57) | 36,447 (93.56) | 2507 (6.44) |
| E-Anhui | 19,362 (98.01) | 393 (1.99) | 19,362 (79.90) | 4872 (20.10) |
| E-Fujian | 28,210 (96.26) | 1095 (3.74) | 28,210 (95.97) | 1185 (4.03) |
| E-Jiangxi | 47,254 (96.99) | 1468 (3.01) | 47,254 (94.26) | 2877 (5.74) |
| E-Shandong | 27,663 (97.06) | 837 (2.94) | 27,663 (94.67) | 1557 (5.33) |
| C-Henan | 49,587 (96.80) | 1637 (3.20) | 49,587 (96.04) | 2046 (3.96) |
| C-Hubei | 80,258 (97.76) | 1837 (2.24) | 80,258 (98.16) | 1505 (1.84) |
| C-Hunan | 32,888 (96.35) | 1245 (3.65) | 32,888 (92.04) | 2846 (7.96) |
| S-Guangdong | 75,744 (93.70) | 5091 (6.30) | 75,744 (96.30) | 2912 (3.70) |
| S-Guangxi | 32,484 (92.51) | 2629 (7.49) | 32,484 (97.28) | 909 (2.72) |
| S-Hainan | 12,707 (97.41) | 338 (2.59) | 12,707 (95.51) | 597 (4.49) |
| SW-Chongqing | 12,146 (90.85) | 1224 (9.15) | 12,146 (92.37) | 1004 (7.63) |
| SW-Sichuan | 57,912 (95.67) | 2623 (4.33) | 57,912 (96.68) | 1991 (3.32) |
| SW-Guizhou | 17,136 (97.56) | 429 (2.44) | 17,136 (88.67) | 2189 (11.33) |
| SW-Yunnan | 55,923 (96.67) | 1925 (3.33) | 55,923 (96.62) | 1958 (3.38) |
| SW-Tibet | 21 (95.45) | 1 (4.55) | 21 (4.84) | 413 (95.16) |
| NW-Shaanxi | 23,470 (92.70) | 1849 (7.30) | 23,470 (96.70) | 801 (3.30) |
| NW-Gansu | 6492 (95.87) | 280 (4.13) | 6,492 (83.42) | 1290 (16.58) |
| NW-Qinghai | 4830 (98.23) | 87 (1.77) | 4830 (92.49) | 392 (7.51) |
| NW-Ningxia | 2938 (88.87) | 368 (11.13) | 2938 (92.22) | 248 (7.78) |
| NW-Xinjiang | 19,221 (96.33) | 732 (3.67) | 19,221 (96.84) | 628 (3.16) |
| Year 2018 | ||||
| Overall | 700,980 (94.34) | 42,033 (5.66) | 700,980 (94.34) | 42,033 (5.66) |
| N-Beijing | 19,816 (69.38) | 8744 (30.62) | 19,816 (96.29) | 764 (3.71) |
| N-Tianjin | 942 (94.67) | 53 (5.33) | 942 (73.31) | 343 (26.69) |
| N-Hebei | 21,554 (97.47) | 559 (2.53) | 21,554 (86.48) | 3370 (13.52) |
| N-Shanxi | 22,082 (98.80) | 268 (1.20) | 22,082 (94.23) | 1353 (5.77) |
| N-Inner Mongolia | 21,834 (97.44) | 574 (2.56) | 21,834 (88.89) | 2729 (11.11) |
| NE-Liaoning | 18,937 (98.07) | 373 (1.93) | 18,937 (93.98) | 1213 (6.02) |
| NE-Jilin | 11,473 (93.60) | 785 (6.40) | 11,473 (94.33) | 690 (5.67) |
| NE-Heilongjiang | 19,198 (96.13) | 772 (3.87) | 19,198 (92.54) | 1547 (7.46) |
| E-Shanghai | 10,676 (79.79) | 2704 (20.21) | 10,676 (94.16) | 662 (5.84) |
| E-Jiangsu | 56,737 (92.92) | 4321 (7.08) | 56,737 (97.32) | 1562 (2.68) |
| E-Zhejiang | 30,806 (94.26) | 1877 (5.74) | 30,806 (95.20) | 1554 (4.80) |
| E-Anhui | 12,907 (97.91) | 276 (2.09) | 12,907 (74.84) | 4340 (25.16) |
| E-Fujian | 38,066 (96.17) | 1515 (3.83) | 38,066 (97.59) | 941 (2.41) |
| E-Jiangxi | 37,192 (97.36) | 1010 (2.64) | 37,192 (94.03) | 2363 (5.97) |
| E-Shandong | 18,909 (97.31) | 523 (2.69) | 18,909 (92.85) | 1457 (7.15) |
| C-Henan | 43,052 (96.27) | 1666 (3.73) | 43,052 (96.41) | 1605 (3.59) |
| C-Hubei | 46,985 (97.94) | 986 (2.06) | 46,985 (97.14) | 1385 (2.86) |
| C-Hunan | 23,658 (95.97) | 993 (4.03) | 23,658 (91.62) | 2164 (8.38) |
| S-Guangdong | 64,837 (93.58) | 4445 (6.42) | 64,837 (96.57) | 2301 (3.43) |
| S-Guangxi | 28,684 (92.91) | 2190 (7.09) | 28,684 (97.42) | 761 (2.58) |
| S-Hainan | 11,433 (97.63) | 278 (2.37) | 11,433 (94.94) | 609 (5.06) |
| SW-Chongqing | 13,460 (91.76) | 1209 (8.24) | 13,460 (95.29) | 666 (4.71) |
| SW-Sichuan | 35,963 (97.13) | 1064 (2.87) | 35,963 (95.23) | 1801 (4.77) |
| SW-Guizhou | 15,297 (97.57) | 381 (2.43) | 15,297 (91.60) | 1403 (8.40) |
| SW-Yunnan | 25,623 (96.31) | 982 (3.69) | 25,623 (94.82) | 1399 (5.18) |
| SW-Tibet | 649 (97.89) | 14 (2.11) | 649 (84.62) | 118 (15.38) |
| NW-Shaanxi | 23,372 (92.42) | 1916 (7.58) | 23,372 (97.42) | 620 (2.58) |
| NW-Gansu | 3214 (96.57) | 114 (3.43) | 3214 (70.54) | 1342 (29.46) |
| NW-Qinghai | 1535 (96.85) | 50 (3.15) | 1535 (83.11) | 312 (16.89) |
| NW-Ningxia | 4326 (86.31) | 686 (13.69) | 4326 (96.20) | 171 (3.80) |
| NW-Xinjiang | 17,763 (96.18) | 705 (3.82) | 17,763 (97.38) | 478 (2.62) |
C, Central China; CKD, chronic kidney disease; E, East China; N, North China; NE, Northeast China; NW, Northwest China; S, South China; SW, Southwest China.
Patients with missing data for residence were not included in the analysis. 2017: 32,215 (3.37%); 2018: 28,612 (3.71%).
Data are expressed as n (%).
Appendix Table 18.
Prevalence of CVD, stratified by patient group
| Patient group | 2017 |
2018 |
||||||
|---|---|---|---|---|---|---|---|---|
| CHD | Stroke | Heart failure | Atrial fibrillation | CHD | Stroke | Heart failure | Atrial fibrillation | |
| CKD | 184,832 (19.31) | 129,536 (13.54) | 165,846 (17.33) | 39,550 (4.13) | 155,902 (20.20) | 108,134 (14.01) | 141,088 (18.28) | 33,771 (4.38) |
| DM | 556,473 (28.74) | 436,910 (22.56) | 264,656 (13.67) | 73,276 (3.78) | 495,409 (28.83) | 400,311 (23.29) | 232,100 (13.50) | 67,668 (3.94) |
| Non-CKD | 2,028,651 (11.03) | 1,954,683 (10.63) | 973,856 (5.30) | 375,766 (2.04) | 1821,659 (11.36) | 1751,261 (10.92) | 868,193 (5.41) | 342,876 (2.14) |
CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 19.
Prevalence of CHD, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 112,073 (19.94) | 304,295 (28.66) | 1131,029 (14.00) | 93,994 (20.79) | 272,698 (28.88) | 1023,173 (14.35) |
| Female | 72,759 (18.42) | 252,178 (28.84) | 897,622 (8.71) | 61,908 (19.37) | 222,711 (28.76) | 798,486 (8.97) |
| Total | 184,832 (19.31) | 556,473 (28.74) | 2,028,651 (11.03) | 155,902 (20.20) | 495,409 (28.83) | 1821,659 (11.36) |
CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 20.
Prevalence of CHD, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 98 (0.46) | 38 (0.58) | 457 (0.06) | 93 (0.59) | 33 (0.58) | 433 (0.06) |
| 25–29 | 242 (0.75) | 167 (1.33) | 1826 (0.12) | 196 (0.80) | 174 (1.60) | 1624 (0.13) |
| 30–34 | 592 (1.52) | 771 (3.62) | 5717 (0.41) | 539 (1.73) | 777 (4.04) | 5437 (0.45) |
| 35–39 | 1206 (2.77) | 2506 (7.08) | 15,044 (1.30) | 1070 (3.06) | 2366 (7.46) | 14,227 (1.47) |
| 40–44 | 2548 (4.37) | 7309 (11.09) | 38,517 (3.16) | 2165 (4.91) | 6373 (11.47) | 33,184 (3.31) |
| 45–49 | 5697 (6.70) | 19,546 (14.99) | 90,331 (5.50) | 4882 (7.31) | 17,527 (15.51) | 80,352 (5.65) |
| 50–54 | 11,237 (10.39) | 43,485 (19.49) | 171,076 (8.80) | 9185 (11.14) | 35,237 (19.42) | 142,265 (8.65) |
| 55–59 | 13,133 (15.19) | 54,447 (23.89) | 193,162 (12.74) | 12,249 (15.97) | 51,813 (24.01) | 180,963 (12.51) |
| 60–64 | 21,649 (19.38) | 86,333 (28.03) | 308,019 (15.41) | 18,338 (20.45) | 76,445 (28.17) | 273,236 (15.54) |
| 65–69 | 25,813 (24.85) | 94,879 (32.05) | 320,406 (18.55) | 22,449 (25.41) | 86,690 (31.88) | 295,246 (18.55) |
| 70–74 | 26,343 (30.72) | 86,991 (36.40) | 291,849 (22.32) | 22,299 (31.42) | 77,208 (35.86) | 263,674 (22.16) |
| 75–79 | 29,702 (37.70) | 77,157 (40.63) | 270,844 (25.90) | 23,768 (38.18) | 66,816 (40.38) | 237,524 (25.88) |
| 80–84 | 26,369 (43.12) | 53,124 (44.31) | 199,787 (28.85) | 21,578 (43.89) | 46,813 (44.19) | 179,618 (28.84) |
| ≥85 | 20,203 (48.52) | 29,720 (49.31) | 121,616 (31.92) | 17,091 (49.05) | 27,137 (49.06) | 113,876 (31.71) |
| Total | 184,832 (19.31) | 556,473 (28.74) | 2,028,651 (11.03) | 155,902 (20.20) | 495,409 (28.83) | 1821,659 (11.36) |
CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 21.
Prevalence of stroke, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 80,652 (14.35) | 237,922 (22.41) | 1,071,061 (13.25) | 66,562 (14.72) | 217,894 (23.08) | 958,536 (13.44) |
| Female | 48,884 (12.38) | 198,988 (22.76) | 883,622 (8.58) | 41,572 (13.01) | 182,417 (23.56) | 792,725 (8.90) |
| Total | 129,536 (13.54) | 436,910 (22.56) | 1,954,683 (10.63) | 108,134 (14.01) | 400,311 (23.29) | 1751,261 (10.92) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 22.
Prevalence of stroke, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 125 (0.59) | 34 (0.51) | 3181 (0.38) | 112 (0.71) | 54 (0.94) | 2456 (0.36) |
| 25–29 | 283 (0.87) | 190 (1.51) | 6080 (0.40) | 278 (1.14) | 191 (1.76) | 4820 (0.39) |
| 30–34 | 668 (1.72) | 679 (3.19) | 11,182 (0.81) | 614 (1.97) | 565 (2.94) | 9732 (0.81) |
| 35–39 | 1346 (3.09) | 1970 (5.57) | 21,495 (1.85) | 1220 (3.49) | 1955 (6.17) | 18,903 (1.96) |
| 40–44 | 2730 (4.68) | 5815 (8.83) | 48,942 (4.02) | 2133 (4.84) | 5198 (9.36) | 40,568 (4.04) |
| 45–49 | 5350 (6.30) | 15,992 (12.27) | 105,554 (6.42) | 4522 (6.77) | 14,535 (12.86) | 91,679 (6.45) |
| 50–54 | 9563 (8.84) | 35,575 (15.94) | 183,780 (9.45) | 7511 (9.11) | 30,050 (16.56) | 153,986 (9.36) |
| 55–59 | 10,155 (11.75) | 43,656 (19.16) | 186,850 (12.32) | 9315 (12.15) | 42,265 (19.59) | 177,759 (12.29) |
| 60–64 | 15,790 (14.14) | 68,524 (22.25) | 293,167 (14.66) | 13,259 (14.78) | 62,590 (23.06) | 259,673 (14.77) |
| 65–69 | 18,062 (17.39) | 75,062 (25.35) | 302,349 (17.50) | 15,449 (17.49) | 70,998 (26.11) | 279,014 (17.53) |
| 70–74 | 17,577 (20.50) | 68,180 (28.53) | 269,412 (20.61) | 15,157 (21.35) | 62,865 (29.20) | 244,124 (20.52) |
| 75–79 | 19,022 (24.14) | 59,597 (31.38) | 244,580 (23.39) | 15,032 (24.15) | 52,935 (31.99) | 215,107 (23.44) |
| 80–84 | 16,283 (26.62) | 39,863 (33.25) | 175,431 (25.33) | 13,115 (26.68) | 35,972 (33.96) | 157,630 (25.31) |
| ≥85 | 12,582 (30.22) | 21,773 (36.13) | 102,680 (26.95) | 10,417 (29.90) | 20,138 (36.41) | 95,810 (26.68) |
| Total | 129,536 (13.54) | 436,910 (22.56) | 1,954,683 (10.63) | 108,134 (14.01) | 400,311 (23.29) | 1751,261 (10.92) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 23.
Prevalence of heart failure, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 99,208 (17.65) | 145,819 (13.73) | 546,756 (6.77) | 83,882 (18.55) | 127,753 (13.53) | 490,659 (6.88) |
| Female | 66,638 (16.87) | 118,837 (13.59) | 427,100 (4.15) | 57,206 (17.90) | 104,347 (13.48) | 377,534 (4.24) |
| Total | 165,846 (17.33) | 264,656 (13.67) | 973,856 (5.30) | 141,088 (18.28) | 232,100 (13.50) | 868,193 (5.41) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 24.
Prevalence of heart failure, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 1014 (4.77) | 80 (1.21) | 3780 (0.46) | 791 (5.04) | 85 (1.48) | 3332 (0.50) |
| 25–29 | 1922 (5.93) | 242 (1.92) | 5995 (0.39) | 1587 (6.49) | 235 (2.16) | 5222 (0.42) |
| 30–34 | 2581 (6.63) | 634 (2.98) | 7954 (0.57) | 2268 (7.26) | 638 (3.32) | 7369 (0.61) |
| 35–39 | 3210 (7.36) | 1449 (4.10) | 11,354 (0.98) | 2715 (7.78) | 1328 (4.19) | 10,125 (1.05) |
| 40–44 | 4485 (7.69) | 3450 (5.24) | 20,797 (1.71) | 3768 (8.54) | 2981 (5.37) | 17,218 (1.72) |
| 45–49 | 7315 (8.61) | 8651 (6.64) | 41,151 (2.50) | 6364 (9.53) | 7520 (6.65) | 35,146 (2.47) |
| 50–54 | 11,103 (10.26) | 17,936 (8.04) | 69,100 (3.55) | 9137 (11.08) | 14,071 (7.75) | 56,218 (3.42) |
| 55–59 | 10,968 (12.69) | 21,862 (9.59) | 73,414 (4.84) | 10,420 (13.59) | 20,197 (9.36) | 66,593 (4.60) |
| 60–64 | 17,301 (15.49) | 35,595 (11.56) | 120,721 (6.04) | 14,785 (16.48) | 30,534 (11.25) | 104,656 (5.95) |
| 65–69 | 20,327 (19.57) | 41,715 (14.09) | 136,311 (7.89) | 17,900 (20.26) | 37,355 (13.74) | 123,901 (7.79) |
| 70–74 | 20,922 (24.40) | 41,333 (17.30) | 136,513 (10.44) | 17,702 (24.94) | 36,277 (16.85) | 122,680 (10.31) |
| 75–79 | 24,071 (30.55) | 40,745 (21.46) | 142,962 (13.67) | 19,369 (31.12) | 34,775 (21.02) | 125,344 (13.66) |
| 80–84 | 22,429 (36.67) | 31,235 (26.05) | 120,060 (17.34) | 18,597 (37.83) | 27,836 (26.28) | 109,973 (17.66) |
| ≥85 | 18,198 (43.70) | 19,729 (32.73) | 83,744 (21.98) | 15,685 (45.02) | 18,268 (33.03) | 80,416 (22.39) |
| Total | 165,846 (17.33) | 264,656 (13.67) | 973,856 (5.30) | 141,088 (18.28) | 232,100 (13.50) | 868,193 (5.41) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 25.
Prevalence of atrial fibrillation, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 23,538 (4.19) | 38,335 (3.61) | 203,512 (2.52) | 19,957 (4.41) | 35,794 (3.79) | 187,408 (2.63) |
| Female | 16,012 (4.05) | 34,941 (4.00) | 172,254 (1.67) | 13,814 (4.32) | 31,874 (4.12) | 155,468 (1.75) |
| Total | 39,550 (4.13) | 73,276 (3.78) | 375,766 (2.04) | 33,771 (4.38) | 67,668 (3.94) | 342,876 (2.14) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 26.
Prevalence of atrial fibrillation, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 13 (0.06) | 1 (0.02) | 313 (0.04) | 13 (0.08) | 3 (0.05) | 300 (0.04) |
| 25–29 | 36 (0.11) | 13 (0.10) | 763 (0.05) | 32 (0.13) | 5 (0.05) | 601 (0.05) |
| 30–34 | 54 (0.14) | 44 (0.21) | 1268 (0.09) | 53 (0.17) | 54 (0.28) | 1148 (0.10) |
| 35–39 | 125 (0.29) | 115 (0.33) | 2267 (0.20) | 108 (0.31) | 103 (0.32) | 1914 (0.20) |
| 40–44 | 284 (0.49) | 299 (0.45) | 4708 (0.39) | 238 (0.54) | 320 (0.58) | 3970 (0.40) |
| 45–49 | 675 (0.79) | 1014 (0.78) | 10,668 (0.65) | 548 (0.82) | 904 (0.80) | 9014 (0.63) |
| 50–54 | 1310 (1.21) | 2587 (1.16) | 19,578 (1.01) | 1053 (1.28) | 2142 (1.18) | 16,195 (0.98) |
| 55–59 | 1717 (1.99) | 4060 (1.78) | 22,924 (1.51) | 1604 (2.09) | 3944 (1.83) | 21,521 (1.49) |
| 60–64 | 3151 (2.82) | 7682 (2.49) | 42,128 (2.11) | 2764 (3.08) | 7086 (2.61) | 37,726 (2.15) |
| 65–69 | 4512 (4.34) | 11,003 (3.72) | 52,927 (3.06) | 4047 (4.58) | 10,292 (3.79) | 49,531 (3.11) |
| 70–74 | 5713 (6.66) | 12,715 (5.32) | 57,338 (4.39) | 4793 (6.75) | 11,695 (5.43) | 52,764 (4.43) |
| 75–79 | 7703 (9.78) | 14,546 (7.66) | 65,843 (6.30) | 6299 (10.12) | 13,230 (8.00) | 58,535 (6.38) |
| 80–84 | 7761 (12.69) | 11,755 (9.80) | 56,291 (8.13) | 6484 (13.19) | 10,847 (10.24) | 52,293 (8.40) |
| ≥85 | 6496 (15.60) | 7442 (12.35) | 38,750 (10.17) | 5735 (16.46) | 7043 (12.73) | 37,364 (10.40) |
| Total | 39,550 (4.13) | 73,276 (3.78) | 375,766 (2.04) | 33,771 (4.38) | 67,668 (3.94) | 342,876 (2.14) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 27.
Prevalence of CVD among patients with CKD
| Cause | 2017 |
2018 |
||||||
|---|---|---|---|---|---|---|---|---|
| CHD | Stroke | Heart failure | Atrial fibrillation | CHD | Stroke | Heart failure | Atrial fibrillation | |
| DKD | 82,812 (31.88) | 53,596 (20.63) | 60,521 (23.30) | 11,482 (4.42) | 70,777 (31.87) | 46,541 (20.96) | 52,129 (23.47) | 10,097 (4.55) |
| HTN | 58,999 (28.68) | 44,246 (21.51) | 58,311 (28.34) | 15,705 (7.63) | 48,441 (29.29) | 35,120 (21.24) | 48,187 (29.14) | 13,012 (7.87) |
| GN | 10,811 (7.92) | 8751 (6.41) | 11,176 (8.18) | 1981 (1.45) | 10,273 (9.06) | 8363 (7.37) | 9867 (8.70) | 1879 (1.66) |
| CTIN | 2307 (14.81) | 1628 (10.45) | 1800 (11.56) | 478 (3.07) | 2654 (16.93) | 1746 (11.14) | 2400 (15.31) | 539 (3.44) |
| ON | 7550 (5.12) | 6583 (4.46) | 4271 (2.90) | 1256 (0.85) | 5141 (5.27) | 4463 (4.57) | 3135 (3.21) | 980 (1.00) |
| Others | 22,353 (11.65) | 14,732 (7.68) | 29,767 (15.52) | 8648 (4.51) | 18,616 (11.83) | 11,901 (7.56) | 25,370 (16.12) | 7264 (4.61) |
| Total | 184,832 (19.31) | 129,536 (13.54) | 165,846 (17.33) | 39,550 (4.13) | 155,902 (20.20) | 108,134 (14.01) | 141,088 (18.28) | 33,771 (4.38) |
CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; CVD, cardiovascular disease; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 28.
Prevalence of CHD among patients with CKD, stratified by cause and sex
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| Male | 47,840 (30.83) | 37,922 (29.87) | 6116 (8.24) | 1033 (14.02) | 4696 (5.06) | 14,466 (13.70) | 112,073 (19.94) |
| Female | 34,972 (33.43) | 21,077 (26.75) | 4695 (7.53) | 1274 (15.52) | 2854 (5.22) | 7887 (9.15) | 72,759 (18.42) |
| Total | 82,812 (31.88) | 58,999 (28.68) | 10,811 (7.92) | 2307 (14.81) | 7550 (5.12) | 22,353 (11.65) | 184,832 (19.31) |
| Year 2018 | |||||||
| Male | 40,917 (30.84) | 30,667 (30.31) | 5944 (9.49) | 1287 (16.66) | 3242 (5.22) | 11,937 (13.92) | 93,994 (20.79) |
| Female | 29,860 (33.40) | 17,774 (27.69) | 4329 (8.52) | 1367 (17.19) | 1899 (5.35) | 6679 (9.32) | 61,908 (19.37) |
| Total | 70,777 (31.87) | 48,441 (29.29) | 10,273 (9.06) | 2654 (16.93) | 5141 (5.27) | 18,616 (11.83) | 155,902 (20.20) |
CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 29.
Prevalence of CHD among patients with CKD, stratified by cause and age
| Age group (yr) | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| 18–24 | 9 (1.28) | 25 (1.69) | 19 (0.24) | 3 (0.74) | 3 (0.08) | 39 (0.56) | 98 (0.46) |
| 25–29 | 32 (1.95) | 77 (2.24) | 51 (0.51) | 2 (0.38) | 7 (0.10) | 73 (0.72) | 242 (0.75) |
| 30–34 | 117 (4.23) | 203 (3.84) | 107 (1.03) | 5 (0.76) | 30 (0.34) | 130 (1.20) | 592 (1.52) |
| 35–39 | 347 (7.31) | 417 (6.08) | 183 (1.77) | 9 (1.33) | 53 (0.52) | 197 (1.84) | 1206 (2.77) |
| 40–44 | 894 (10.25) | 824 (8.70) | 327 (2.66) | 23 (2.38) | 142 (1.05) | 338 (2.53) | 2548 (4.37) |
| 45–49 | 2539 (14.77) | 1509 (10.77) | 610 (3.86) | 70 (5.05) | 334 (1.79) | 635 (3.54) | 5697 (6.70) |
| 50–54 | 5716 (19.57) | 2616 (14.60) | 1007 (6.08) | 160 (8.82) | 657 (2.97) | 1081 (5.27) | 11,237 (10.39) |
| 55–59 | 7237 (24.53) | 2849 (19.36) | 975 (8.66) | 169 (12.01) | 761 (5.05) | 1142 (7.88) | 13,133 (15.19) |
| 60–64 | 11,367 (29.65) | 5197 (23.83) | 1671 (12.22) | 295 (14.71) | 1209 (7.09) | 1910 (10.16) | 21,649 (19.38) |
| 65–69 | 13,248 (35.11) | 6839 (29.79) | 1733 (15.62) | 359 (19.77) | 1240 (9.54) | 2394 (13.87) | 25,813 (24.85) |
| 70–74 | 12,602 (39.95) | 7916 (34.87) | 1477 (19.85) | 378 (26.47) | 1142 (13.89) | 2828 (19.64) | 26,343 (30.72) |
| 75–79 | 12,683 (46.13) | 10,624 (42.35) | 1311 (24.62) | 368 (29.87) | 938 (17.24) | 3778 (26.58) | 29,702 (37.70) |
| 80–84 | 9777 (50.86) | 10,727 (47.54) | 888 (28.64) | 285 (34.09) | 660 (20.71) | 4032 (32.92) | 26,369 (43.12) |
| ≥85 | 6244 (57.06) | 9176 (52.63) | 452 (33.58) | 181 (43.20) | 374 (24.01) | 3776 (38.00) | 20,203 (48.52) |
| Total | 82,812 (31.88) | 58,999 (28.68) | 10,811 (7.92) | 2307 (14.81) | 7550 (5.12) | 22,353 (11.65) | 184,832 (19.31) |
| Year 2018 | |||||||
| 18–24 | 7 (1.15) | 20 (1.91) | 29 (0.48) | 1 (0.26) | 1 (0.04) | 35 (0.66) | 93 (0.59) |
| 25–29 | 34 (2.36) | 60 (2.24) | 48 (0.65) | 3 (0.62) | 1 (0.02) | 50 (0.64) | 196 (0.80) |
| 30–34 | 127 (5.13) | 180 (4.15) | 93 (1.09) | 9 (1.43) | 10 (0.16) | 120 (1.31) | 539 (1.73) |
| 35–39 | 306 (7.06) | 370 (6.59) | 184 (2.23) | 11 (1.65) | 41 (0.59) | 158 (1.73) | 1070 (3.06) |
| 40–44 | 799 (11.04) | 673 (9.27) | 293 (3.05) | 38 (4.27) | 98 (1.18) | 264 (2.45) | 2165 (4.91) |
| 45–49 | 2269 (15.45) | 1258 (11.30) | 554 (4.27) | 88 (6.60) | 210 (1.77) | 503 (3.39) | 4882 (7.31) |
| 50–54 | 4655 (19.91) | 2120 (15.15) | 949 (7.11) | 147 (8.74) | 434 (3.17) | 880 (5.38) | 9185 (11.14) |
| 55–59 | 6793 (24.97) | 2628 (19.86) | 1021 (9.85) | 210 (12.95) | 509 (4.72) | 1088 (8.07) | 12,249 (15.97) |
| 60–64 | 9710 (29.95) | 4375 (24.99) | 1536 (13.12) | 321 (16.83) | 760 (6.92) | 1636 (10.78) | 18,338 (20.45) |
| 65–69 | 11,635 (34.84) | 5742 (29.94) | 1633 (16.30) | 435 (22.50) | 889 (9.77) | 2115 (14.37) | 22,449 (25.41) |
| 70–74 | 10,700 (39.64) | 6623 (36.20) | 1417 (21.41) | 408 (26.98) | 800 (13.74) | 2351 (20.03) | 22,299 (31.42) |
| 75–79 | 10,234 (45.59) | 8335 (43.29) | 1175 (25.79) | 404 (32.87) | 629 (17.64) | 2991 (26.71) | 23,768 (38.18) |
| 80–84 | 8175 (51.12) | 8461 (47.95) | 851 (31.64) | 349 (39.52) | 489 (21.45) | 3253 (33.62) | 21,578 (43.89) |
| ≥85 | 5333 (56.30) | 7596 (53.44) | 490 (36.84) | 230 (43.89) | 270 (22.28) | 3172 (39.20) | 17,091 (49.05) |
| Total | 70,777 (31.87) | 48,441 (29.29) | 10,273 (9.06) | 2654 (16.93) | 5141 (5.27) | 18,616 (11.83) | 155,902 (20.20) |
CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 30.
Prevalence of stroke among patients with CKD, stratified by cause and sex
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| Male | 32,106 (20.69) | 29,171 (22.98) | 5103 (6.88) | 758 (10.29) | 4144 (4.47) | 9370 (8.87) | 80,652 (14.35) |
| Female | 21,490 (20.54) | 15,075 (19.14) | 3648 (5.85) | 870 (10.60) | 2439 (4.46) | 5362 (6.22) | 48,884 (12.38) |
| Total | 53,596 (20.63) | 44,246 (21.51) | 8751 (6.41) | 1628 (10.45) | 6583 (4.46) | 14,732 (7.68) | 129,536 (13.54) |
| Year 2018 | |||||||
| Male | 27,928 (21.05) | 22,699 (22.43) | 4794 (7.65) | 860 (11.13) | 2824 (4.55) | 7457 (8.70) | 66,562 (14.72) |
| Female | 18,613 (20.82) | 12,421 (19.35) | 3569 (7.02) | 886 (11.14) | 1639 (4.61) | 4444 (6.20) | 41,572 (13.01) |
| Total | 46,541 (20.96) | 35,120 (21.24) | 8363 (7.37) | 1746 (11.14) | 4463 (4.57) | 11,901 (7.56) | 108,134 (14.01) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 31.
Prevalence of stroke among patients with CKD, stratified by cause and age
| Age group (yr) | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| 18–24 | 6 (0.85) | 39 (2.64) | 26 (0.33) | 2 (0.50) | 3 (0.08) | 49 (0.70) | 125 (0.59) |
| 25–29 | 21 (1.28) | 125 (3.63) | 57 (0.57) | 2 (0.38) | 6 (0.09) | 72 (0.71) | 283 (0.87) |
| 30–34 | 90 (3.25) | 307 (5.80) | 129 (1.24) | 3 (0.46) | 27 (0.30) | 112 (1.03) | 668 (1.72) |
| 35–39 | 253 (5.33) | 629 (9.17) | 192 (1.85) | 18 (2.66) | 67 (0.65) | 187 (1.74) | 1346 (3.09) |
| 40–44 | 720 (8.25) | 1092 (11.53) | 371 (3.01) | 25 (2.59) | 189 (1.40) | 333 (2.50) | 2730 (4.68) |
| 45–49 | 1844 (10.73) | 1958 (13.97) | 580 (3.67) | 54 (3.90) | 347 (1.86) | 567 (3.16) | 5350 (6.30) |
| 50–54 | 4136 (14.16) | 2760 (15.40) | 932 (5.63) | 114 (6.28) | 675 (3.05) | 946 (4.61) | 9563 (8.84) |
| 55–59 | 5037 (17.08) | 2612 (17.75) | 845 (7.50) | 127 (9.03) | 701 (4.65) | 833 (5.75) | 10,155 (11.75) |
| 60–64 | 7553 (19.70) | 4343 (19.91) | 1292 (9.45) | 214 (10.67) | 1024 (6.01) | 1364 (7.25) | 15,790 (14.14) |
| 65–69 | 8666 (22.96) | 5174 (22.54) | 1307 (11.78) | 237 (13.05) | 1056 (8.12) | 1622 (9.40) | 18,062 (17.39) |
| 70–74 | 7951 (25.20) | 5577 (24.57) | 1096 (14.73) | 246 (17.23) | 942 (11.46) | 1765 (12.26) | 17,577 (20.50) |
| 75–79 | 7796 (28.36) | 6995 (27.89) | 954 (17.91) | 262 (21.27) | 737 (13.55) | 2278 (16.03) | 19,022 (24.14) |
| 80–84 | 5792 (30.13) | 6771 (30.01) | 651 (20.99) | 199 (23.80) | 526 (16.50) | 2344 (19.14) | 16,283 (26.62) |
| ≥85 | 3731 (34.10) | 5864 (33.63) | 319 (23.70) | 125 (29.83) | 283 (18.16) | 2260 (22.74) | 12,582 (30.22) |
| Total | 53,596 (20.63) | 44,246 (21.51) | 8751 (6.41) | 1628 (10.45) | 6583 (4.46) | 14,732 (7.68) | 129,536 (13.54) |
| Year 2018 | |||||||
| 18–24 | 10 (1.64) | 27 (2.58) | 23 (0.38) | 4 (1.05) | 3 (0.13) | 45 (0.85) | 112 (0.71) |
| 25–29 | 29 (2.01) | 106 (3.97) | 64 (0.86) | 11 (2.26) | 4 (0.09) | 64 (0.82) | 278 (1.14) |
| 30–34 | 65 (2.63) | 302 (6.97) | 113 (1.33) | 11 (1.74) | 13 (0.21) | 110 (1.20) | 614 (1.97) |
| 35–39 | 260 (6.00) | 520 (9.26) | 193 (2.34) | 15 (2.26) | 61 (0.88) | 171 (1.88) | 1220 (3.49) |
| 40–44 | 609 (8.41) | 845 (11.64) | 286 (2.97) | 32 (3.60) | 96 (1.15) | 265 (2.46) | 2133 (4.84) |
| 45–49 | 1605 (10.93) | 1517 (13.63) | 599 (4.62) | 76 (5.70) | 237 (2.00) | 488 (3.29) | 4522 (6.77) |
| 50–54 | 3337 (14.27) | 2120 (15.15) | 843 (6.31) | 114 (6.78) | 411 (3.00) | 686 (4.19) | 7511 (9.11) |
| 55–59 | 4747 (17.45) | 2325 (17.57) | 870 (8.40) | 125 (7.71) | 481 (4.46) | 767 (5.69) | 9315 (12.15) |
| 60–64 | 6608 (20.38) | 3429 (19.59) | 1192 (10.18) | 235 (12.32) | 657 (5.98) | 1138 (7.50) | 13,259 (14.78) |
| 65–69 | 7711 (23.09) | 4111 (21.44) | 1283 (12.81) | 261 (13.50) | 710 (7.81) | 1373 (9.33) | 15,449 (17.49) |
| 70–74 | 7151 (26.49) | 4564 (24.95) | 1116 (16.86) | 255 (16.87) | 641 (11.01) | 1430 (12.18) | 15,157 (21.35) |
| 75–79 | 6360 (28.33) | 5270 (27.37) | 873 (19.16) | 259 (21.07) | 509 (14.27) | 1761 (15.73) | 15,032 (24.15) |
| 80–84 | 4864 (30.42) | 5261 (29.82) | 586 (21.78) | 218 (24.69) | 393 (17.24) | 1793 (18.53) | 13,115 (26.68) |
| ≥85 | 3185 (33.63) | 4723 (33.23) | 322 (24.21) | 130 (24.81) | 247 (20.38) | 1810 (22.37) | 10,417 (29.90) |
| Total | 46,541 (20.96) | 35,120 (21.24) | 8363 (7.37) | 1746 (11.14) | 4463 (4.57) | 11,901 (7.56) | 108,134 (14.01) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 32.
Prevalence of heart failure among patients with CKD, stratified by cause and sex
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| Male | 34,554 (22.27) | 36,339 (28.62) | 6601 (8.89) | 940 (12.76) | 2684 (2.89) | 18,090 (17.13) | 99,208 (17.65) |
| Female | 25,967 (24.82) | 21,972 (27.89) | 4575 (7.33) | 860 (10.48) | 1587 (2.90) | 11,677 (13.55) | 66,638 (16.87) |
| Total | 60,521 (23.30) | 58,311 (28.34) | 11,176 (8.18) | 1800 (11.56) | 4271 (2.90) | 29,767 (15.52) | 165,846 (17.33) |
| Year 2018 | |||||||
| Male | 29,677 (22.37) | 29,790 (29.44) | 5835 (9.32) | 1305 (16.89) | 1994 (3.21) | 15,281 (17.82) | 83,882 (18.55) |
| Female | 22,452 (25.11) | 18,397 (28.66) | 4032 (7.94) | 1095 (13.77) | 1141 (3.21) | 10,089 (14.08) | 57,206 (17.90) |
| Total | 52,129 (23.47) | 48,187 (29.14) | 9867 (8.70) | 2400 (15.31) | 3135 (3.21) | 25,370 (16.12) | 141,088 (18.28) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 33.
Prevalence of heart failure among patients with CKD, stratified by cause and age
| Age group (yr) | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| 18–24 | 31 (4.40) | 278 (18.78) | 240 (3.04) | 14 (3.47) | 9 (0.24) | 442 (6.34) | 1014 (4.77) |
| 25–29 | 108 (6.59) | 648 (18.83) | 439 (4.38) | 14 (2.65) | 20 (0.30) | 693 (6.86) | 1922 (5.93) |
| 30–34 | 196 (7.08) | 999 (18.88) | 524 (5.04) | 32 (4.86) | 34 (0.38) | 796 (7.32) | 2581 (6.63) |
| 35–39 | 432 (9.10) | 1275 (18.59) | 577 (5.57) | 28 (4.14) | 56 (0.55) | 842 (7.85) | 3210 (7.36) |
| 40–44 | 871 (9.98) | 1699 (17.94) | 650 (5.28) | 59 (6.11) | 101 (0.75) | 1105 (8.28) | 4485 (7.69) |
| 45–49 | 2182 (12.69) | 2400 (17.12) | 886 (5.61) | 92 (6.64) | 236 (1.26) | 1519 (8.48) | 7315 (8.61) |
| 50–54 | 4266 (14.60) | 3205 (17.88) | 1098 (6.63) | 139 (7.66) | 366 (1.65) | 2029 (9.89) | 11,103 (10.26) |
| 55–59 | 5164 (17.51) | 2834 (19.26) | 812 (7.21) | 120 (8.53) | 335 (2.22) | 1703 (11.76) | 10,968 (12.69) |
| 60–64 | 7898 (20.60) | 4743 (21.75) | 1272 (9.31) | 209 (10.42) | 585 (3.43) | 2594 (13.79) | 17,301 (15.49) |
| 65–69 | 9150 (24.25) | 5987 (26.08) | 1352 (12.19) | 254 (13.99) | 602 (4.63) | 2982 (17.28) | 20,327 (19.57) |
| 70–74 | 8854 (28.07) | 6922 (30.49) | 1096 (14.73) | 229 (16.04) | 594 (7.23) | 3227 (22.41) | 20,922 (24.40) |
| 75–79 | 9051 (32.92) | 9236 (36.82) | 1063 (19.96) | 256 (20.78) | 586 (10.77) | 3879 (27.29) | 24,071 (30.55) |
| 80–84 | 7407 (38.53) | 9520 (42.19) | 759 (24.48) | 220 (26.32) | 445 (13.96) | 4078 (33.29) | 22,429 (36.67) |
| ≥85 | 4911 (44.88) | 8565 (49.12) | 408 (30.31) | 134 (31.98) | 302 (19.38) | 3878 (39.02) | 18,198 (43.70) |
| Total | 60,521 (23.30) | 58,311 (28.34) | 11,176 (8.18) | 1800 (11.56) | 4271 (2.90) | 29,767 (15.52) | 165,846 (17.33) |
| Year 2018 | |||||||
| 18–24 | 28 (4.61) | 218 (20.84) | 166 (2.75) | 19 (4.99) | 6 (0.26) | 354 (6.65) | 791 (5.04) |
| 25–29 | 98 (6.80) | 509 (19.04) | 357 (4.80) | 30 (6.16) | 10 (0.22) | 583 (7.45) | 1587 (6.49) |
| 30–34 | 233 (9.42) | 815 (18.80) | 450 (5.30) | 43 (6.81) | 26 (0.42) | 701 (7.67) | 2268 (7.26) |
| 35–39 | 382 (8.82) | 1078 (19.19) | 442 (5.35) | 48 (7.22) | 41 (0.59) | 724 (7.94) | 2715 (7.78) |
| 40–44 | 800 (11.05) | 1326 (18.27) | 591 (6.14) | 65 (7.30) | 80 (0.96) | 906 (8.42) | 3768 (8.54) |
| 45–49 | 2025 (13.79) | 1975 (17.74) | 749 (5.77) | 115 (8.62) | 161 (1.36) | 1339 (9.03) | 6364 (9.53) |
| 50–54 | 3544 (15.16) | 2622 (18.74) | 935 (7.00) | 185 (11.00) | 247 (1.80) | 1604 (9.81) | 9137 (11.08) |
| 55–59 | 4858 (17.86) | 2644 (19.98) | 818 (7.89) | 181 (11.17) | 276 (2.56) | 1643 (12.18) | 10,420 (13.59) |
| 60–64 | 6788 (20.94) | 3972 (22.69) | 1112 (9.50) | 273 (14.32) | 390 (3.55) | 2250 (14.83) | 14,785 (16.48) |
| 65–69 | 8044 (24.09) | 5170 (26.96) | 1201 (11.99) | 358 (18.52) | 468 (5.15) | 2659 (18.06) | 17,900 (20.26) |
| 70–74 | 7515 (27.84) | 5717 (31.25) | 1031 (15.58) | 325 (21.49) | 443 (7.61) | 2671 (22.76) | 17,702 (24.94) |
| 75–79 | 7304 (32.54) | 7232 (37.56) | 903 (19.82) | 294 (23.92) | 401 (11.25) | 3235 (28.89) | 19,369 (31.12) |
| 80–84 | 6214 (38.86) | 7723 (43.77) | 686 (25.50) | 262 (29.67) | 348 (15.26) | 3364 (34.77) | 18,597 (37.83) |
| ≥85 | 4296 (45.35) | 7186 (50.56) | 426 (32.03) | 202 (38.55) | 238 (19.64) | 3337 (41.24) | 15,685 (45.02) |
| Total | 52,129 (23.47) | 48,187 (29.14) | 9867 (8.70) | 2400 (15.31) | 3135 (3.21) | 25,370 (16.12) | 141,088 (18.28) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 34.
Prevalence of atrial fibrillation among patients with CKD, stratified by cause and sex
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| Male | 6496 (4.19) | 9480 (7.47) | 1200 (1.62) | 243 (3.30) | 806 (0.87) | 5313 (5.03) | 23,538 (4.19) |
| Female | 4986 (4.77) | 6225 (7.90) | 781 (1.25) | 235 (2.86) | 450 (0.82) | 3335 (3.87) | 16,012 (4.05) |
| Total | 11,482 (4.42) | 15,705 (7.63) | 1981 (1.45) | 478 (3.07) | 1256 (0.85) | 8648 (4.51) | 39,550 (4.13) |
| Year 2018 | |||||||
| Male | 5657 (4.26) | 7841 (7.75) | 1132 (1.81) | 303 (3.92) | 660 (1.06) | 4364 (5.09) | 19,957 (4.41) |
| Female | 4440 (4.97) | 5171 (8.06) | 747 (1.47) | 236 (2.97) | 320 (0.90) | 2900 (4.05) | 13,814 (4.32) |
| Total | 10,097 (4.55) | 13,012 (7.87) | 1879 (1.66) | 539 (3.44) | 980 (1.00) | 7264 (4.61) | 33,771 (4.38) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 35.
Prevalence of atrial fibrillation among patients with CKD, stratified by cause and age
| Age group (yr) | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Year 2017 | |||||||
| 18–24 | 0 (0.00) | 2 (0.14) | 3 (0.04) | 0 (0.00) | 0 (0.00) | 8 (0.11) | 13 (0.06) |
| 25–29 | 1 (0.06) | 3 (0.09) | 8 (0.08) | 1 (0.19) | 2 (0.03) | 21 (0.21) | 36 (0.11) |
| 30–34 | 3 (0.11) | 13 (0.25) | 9 (0.09) | 0 (0.00) | 3 (0.03) | 26 (0.24) | 54 (0.14) |
| 35–39 | 18 (0.38) | 25 (0.36) | 22 (0.21) | 4 (0.59) | 4 (0.04) | 52 (0.49) | 125 (0.29) |
| 40–44 | 36 (0.41) | 96 (1.01) | 29 (0.24) | 2 (0.21) | 19 (0.14) | 102 (0.76) | 284 (0.49) |
| 45–49 | 123 (0.72) | 184 (1.31) | 67 (0.42) | 8 (0.58) | 34 (0.18) | 259 (1.45) | 675 (0.79) |
| 50–54 | 311 (1.06) | 374 (2.09) | 118 (0.71) | 16 (0.88) | 71 (0.32) | 420 (2.05) | 1310 (1.21) |
| 55–59 | 516 (1.75) | 481 (3.27) | 154 (1.37) | 29 (2.06) | 96 (0.64) | 441 (3.04) | 1717 (1.99) |
| 60–64 | 995 (2.60) | 962 (4.41) | 245 (1.79) | 49 (2.44) | 166 (0.97) | 734 (3.90) | 3151 (2.82) |
| 65–69 | 1500 (3.97) | 1491 (6.50) | 304 (2.74) | 64 (3.52) | 187 (1.44) | 966 (5.60) | 4512 (4.34) |
| 70–74 | 1824 (5.78) | 2135 (9.40) | 304 (4.08) | 73 (5.11) | 213 (2.59) | 1164 (8.08) | 5713 (6.66) |
| 75–79 | 2433 (8.85) | 3214 (12.81) | 329 (6.18) | 100 (8.12) | 188 (3.46) | 1439 (10.12) | 7703 (9.78) |
| 80–84 | 2132 (11.09) | 3540 (15.69) | 246 (7.93) | 82 (9.81) | 166 (5.21) | 1595 (13.02) | 7761 (12.69) |
| ≥85 | 1590 (14.53) | 3185 (18.27) | 143 (10.62) | 50 (11.93) | 107 (6.87) | 1421 (14.30) | 6496 (15.60) |
| Total | 11,482 (4.42) | 15,705 (7.63) | 1981 (1.45) | 478 (3.07) | 1256 (0.85) | 8648 (4.51) | 39,550 (4.13) |
| Year 2018 | |||||||
| 18–24 | 0 (0.00) | 3 (0.29) | 3 (0.05) | 0 (0.00) | 0 (0.00) | 7 (0.13) | 13 (0.08) |
| 25–29 | 1 (0.07) | 6 (0.22) | 10 (0.13) | 1 (0.21) | 0 (0.00) | 14 (0.18) | 32 (0.13) |
| 30–34 | 5 (0.20) | 16 (0.37) | 15 (0.18) | 2 (0.32) | 0 (0.00) | 15 (0.16) | 53 (0.17) |
| 35–39 | 16 (0.37) | 30 (0.53) | 14 (0.17) | 4 (0.60) | 3 (0.04) | 41 (0.45) | 108 (0.31) |
| 40–44 | 43 (0.59) | 76 (1.05) | 27 (0.28) | 6 (0.67) | 11 (0.13) | 75 (0.70) | 238 (0.54) |
| 45–49 | 114 (0.78) | 154 (1.38) | 43 (0.33) | 15 (1.12) | 31 (0.26) | 191 (1.29) | 548 (0.82) |
| 50–54 | 272 (1.16) | 275 (1.97) | 121 (0.91) | 20 (1.19) | 51 (0.37) | 314 (1.92) | 1053 (1.28) |
| 55–59 | 482 (1.77) | 453 (3.42) | 137 (1.32) | 32 (1.97) | 86 (0.80) | 414 (3.07) | 1604 (2.09) |
| 60–64 | 887 (2.74) | 856 (4.89) | 237 (2.02) | 57 (2.99) | 123 (1.12) | 604 (3.98) | 2764 (3.08) |
| 65–69 | 1381 (4.14) | 1326 (6.91) | 301 (3.00) | 70 (3.62) | 155 (1.70) | 814 (5.53) | 4047 (4.58) |
| 70–74 | 1607 (5.95) | 1687 (9.22) | 287 (4.34) | 88 (5.82) | 164 (2.82) | 960 (8.18) | 4793 (6.75) |
| 75–79 | 2036 (9.07) | 2501 (12.99) | 298 (6.54) | 91 (7.40) | 150 (4.21) | 1223 (10.92) | 6299 (10.12) |
| 80–84 | 1875 (11.73) | 2899 (16.43) | 242 (9.00) | 83 (9.40) | 120 (5.26) | 1265 (13.07) | 6484 (13.19) |
| ≥85 | 1378 (14.55) | 2730 (19.21) | 144 (10.83) | 70 (13.36) | 86 (7.10) | 1327 (16.40) | 5735 (16.46) |
| Total | 10,097 (4.55) | 13,012 (7.87) | 1879 (1.66) | 539 (3.44) | 980 (1.00) | 7264 (4.61) | 33,771 (4.38) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons.
Data are expressed as n (%).
Appendix Table 36.
Cardiovascular procedures stratified by patient group
| Patient group | 2017 |
2018 |
||||||
|---|---|---|---|---|---|---|---|---|
| CAG | PCI | CABG | Pacemaker | CAG | PCI | CABG | Pacemaker | |
| CKD | 19,928 (5.90) | 10,557 (3.13) | 915 (0.27) | 5889 (1.74) | 17,897 (6.29) | 9448 (3.32) | 662 (0.23) | 4458 (1.57) |
| DM | 122,815 (13.39) | 68,139 (7.43) | 6229 (0.68) | 10,811 (1.18) | 115,527 (13.97) | 61,709 (7.46) | 4575 (0.55) | 8809 (1.07) |
| Non-CKD | 509,699 (13.03) | 242,434 (6.20) | 18,938 (0.48) | 39,504 (1.01) | 471,652 (13.42) | 213,313 (6.07) | 14,182 (0.40) | 32,274 (0.92) |
CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Data are expressed as n (%).
Appendix Table 37.
Cardiovascular procedure: CAG, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 14,214 (6.92) | 77,064 (15.31) | 329,606 (15.17) | 12,629 (7.33) | 72,427 (15.89) | 305,759 (15.58) |
| Female | 5714 (4.32) | 45,751 (11.07) | 180,093 (10.37) | 5268 (4.68) | 43,100 (11.61) | 165,893 (10.69) |
| Total | 19,928 (5.90) | 122,815 (13.39) | 509,699 (13.03) | 17,897 (6.29) | 115,527 (13.97) | 471,652 (13.42) |
CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 38.
Cardiovascular procedure: CAG, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 6 (0.51) | 11 (7.80) | 162 (2.18) | 5 (0.53) | 6 (3.85) | 151 (2.42) |
| 25–29 | 20 (0.86) | 56 (10.33) | 716 (5.21) | 27 (1.40) | 59 (11.15) | 666 (5.80) |
| 30–34 | 87 (2.51) | 281 (15.78) | 2430 (10.26) | 75 (2.45) | 287 (16.79) | 2385 (11.11) |
| 35–39 | 206 (4.10) | 996 (20.07) | 6484 (14.72) | 207 (4.79) | 969 (20.35) | 6367 (16.02) |
| 40–44 | 449 (5.41) | 2760 (20.17) | 16,275 (16.83) | 400 (5.94) | 2524 (20.88) | 14,313 (17.55) |
| 45–49 | 983 (6.53) | 7029 (19.57) | 36,949 (17.78) | 871 (6.77) | 6556 (20.24) | 33,759 (18.50) |
| 50–54 | 1741 (6.89) | 14,415 (18.62) | 66,060 (18.18) | 1542 (7.59) | 12,154 (19.03) | 56,839 (18.66) |
| 55–59 | 2022 (7.74) | 17,025 (18.16) | 69,991 (18.49) | 2036 (8.38) | 17,141 (19.06) | 67,812 (18.91) |
| 60–64 | 3177 (7.85) | 24,708 (17.03) | 100,852 (17.08) | 2739 (8.00) | 22,892 (17.59) | 91,998 (17.56) |
| 65–69 | 3388 (7.41) | 23,024 (14.72) | 88,429 (14.66) | 3092 (7.75) | 22,145 (15.25) | 83,670 (15.00) |
| 70–74 | 3086 (6.89) | 16,819 (12.00) | 61,824 (11.57) | 2619 (6.87) | 15,860 (12.53) | 57,937 (11.94) |
| 75–79 | 2614 (5.44) | 10,453 (8.61) | 39,150 (8.05) | 2306 (6.03) | 9706 (9.14) | 35,798 (8.39) |
| 80–84 | 1624 (3.93) | 4262 (5.23) | 16,346 (4.65) | 1447 (4.29) | 4151 (5.70) | 15,632 (4.92) |
| ≥85 | 525 (1.71) | 976 (2.21) | 4031 (1.92) | 531 (2.04) | 1077 (2.66) | 4325 (2.20) |
| Total | 19,928 (5.90) | 122,815 (13.39) | 509,699 (13.03) | 17,897 (6.29) | 115,527 (13.97) | 471,652 (13.42) |
CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 39.
Cardiovascular procedure: PCI, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 7843 (3.82) | 45,665 (9.07) | 175,273 (8.07) | 6956 (4.04) | 41,463 (9.10) | 154,474 (7.87) |
| Female | 2714 (2.05) | 22,474 (5.44) | 67,161 (3.87) | 2492 (2.22) | 20,246 (5.46) | 58,839 (3.79) |
| Total | 10,557 (3.13) | 68,139 (7.43) | 242,434 (6.20) | 9448 (3.32) | 61,709 (7.46) | 213,313 (6.07) |
CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Data are expressed as n (%).
Appendix Table 40.
Cardiovascular procedure: PCI, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 1 (0.08) | 3 (2.13) | 43 (0.58) | 0 (0.00) | 2 (1.28) | 35 (0.56) |
| 25–29 | 6 (0.26) | 37 (6.83) | 332 (2.42) | 10 (0.52) | 44 (8.32) | 295 (2.57) |
| 30–34 | 43 (1.24) | 194 (10.89) | 1254 (5.30) | 30 (0.98) | 189 (11.06) | 1129 (5.26) |
| 35–39 | 103 (2.05) | 640 (12.90) | 3436 (7.80) | 103 (2.38) | 623 (13.08) | 3217 (8.09) |
| 40–44 | 245 (2.95) | 1806 (13.20) | 8449 (8.74) | 205 (3.04) | 1545 (12.78) | 6999 (8.58) |
| 45–49 | 506 (3.36) | 4260 (11.86) | 18,206 (8.76) | 477 (3.71) | 3942 (12.17) | 15,652 (8.58) |
| 50–54 | 894 (3.54) | 8116 (10.48) | 30,416 (8.37) | 814 (4.01) | 6626 (10.37) | 24,834 (8.15) |
| 55–59 | 1046 (4.00) | 9318 (9.94) | 31,648 (8.36) | 1022 (4.20) | 8960 (9.97) | 28,683 (8.00) |
| 60–64 | 1643 (4.06) | 13,429 (9.25) | 46,536 (7.88) | 1452 (4.24) | 11,904 (9.15) | 39,998 (7.63) |
| 65–69 | 1712 (3.75) | 12,497 (7.99) | 41,871 (6.94) | 1618 (4.06) | 11,572 (7.97) | 37,727 (6.76) |
| 70–74 | 1620 (3.62) | 9145 (6.52) | 29,935 (5.60) | 1348 (3.54) | 8324 (6.58) | 27,072 (5.58) |
| 75–79 | 1463 (3.05) | 5651 (4.66) | 19,393 (3.99) | 1232 (3.22) | 5098 (4.80) | 17,258 (4.04) |
| 80–84 | 938 (2.27) | 2464 (3.02) | 8649 (2.46) | 848 (2.52) | 2286 (3.14) | 8078 (2.54) |
| ≥85 | 337 (1.10) | 579 (1.31) | 2266 (1.08) | 289 (1.11) | 594 (1.47) | 2336 (1.19) |
| Total | 10,557 (3.13) | 68,139 (7.43) | 242,434 (6.20) | 9448 (3.32) | 61,709 (7.46) | 213,313 (6.07) |
CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Data are expressed as n (%).
Appendix Table 41.
Cardiovascular procedure: CABG, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 728 (0.35) | 4426 (0.88) | 14,008 (0.64) | 501 (0.29) | 3331 (0.73) | 10,643 (0.54) |
| Female | 187 (0.14) | 1803 (0.44) | 4930 (0.28) | 161 (0.14) | 1244 (0.34) | 3539 (0.23) |
| Total | 915 (0.27) | 6229 (0.68) | 18,938 (0.48) | 662 (0.23) | 4575 (0.55) | 14,182 (0.40) |
CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 42.
Cardiovascular procedure: CABG, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 0 (0.00) | 0 (0.00) | 7 (0.09) | 0 (0.00) | 0 (0.00) | 8 (0.13) |
| 25–29 | 0 (0.00) | 1 (0.18) | 21 (0.15) | 2 (0.10) | 0 (0.00) | 8 (0.07) |
| 30–34 | 2 (0.06) | 5 (0.28) | 48 (0.20) | 3 (0.10) | 10 (0.59) | 53 (0.25) |
| 35–39 | 13 (0.26) | 29 (0.58) | 116 (0.26) | 7 (0.16) | 22 (0.46) | 104 (0.26) |
| 40–44 | 18 (0.22) | 109 (0.80) | 399 (0.41) | 10 (0.15) | 74 (0.61) | 304 (0.37) |
| 45–49 | 51 (0.34) | 355 (0.99) | 1137 (0.55) | 33 (0.26) | 269 (0.83) | 822 (0.45) |
| 50–54 | 89 (0.35) | 773 (1.00) | 2318 (0.64) | 62 (0.31) | 485 (0.76) | 1624 (0.53) |
| 55–59 | 106 (0.41) | 956 (1.02) | 2813 (0.74) | 99 (0.41) | 744 (0.83) | 2052 (0.57) |
| 60–64 | 185 (0.46) | 1548 (1.07) | 4577 (0.78) | 112 (0.33) | 1049 (0.81) | 3231 (0.62) |
| 65–69 | 199 (0.44) | 1359 (0.87) | 4013 (0.67) | 113 (0.28) | 1021 (0.70) | 3138 (0.56) |
| 70–74 | 143 (0.32) | 720 (0.51) | 2276 (0.43) | 110 (0.29) | 593 (0.47) | 1794 (0.37) |
| 75–79 | 75 (0.16) | 298 (0.25) | 930 (0.19) | 74 (0.19) | 243 (0.23) | 764 (0.18) |
| 80–84 | 24 (0.06) | 61 (0.07) | 229 (0.07) | 21 (0.06) | 44 (0.06) | 223 (0.07) |
| ≥85 | 10 (0.03) | 15 (0.03) | 54 (0.03) | 16 (0.06) | 21 (0.05) | 57 (0.03) |
| Total | 915 (0.27) | 6229 (0.68) | 18,938 (0.48) | 662 (0.23) | 4575 (0.55) | 14,182 (0.40) |
CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 43.
Cardiovascular procedure: pacemaker, stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 3683 (1.79) | 6098 (1.21) | 21,949 (1.01) | 2679 (1.56) | 4963 (1.09) | 17,945 (0.91) |
| Female | 2206 (1.67) | 4713 (1.14) | 17,555 (1.01) | 1779 (1.58) | 3846 (1.04) | 14,329 (0.92) |
| Total | 5889 (1.74) | 10,811 (1.18) | 39,504 (1.01) | 4458 (1.57) | 8809 (1.07) | 32,274 (0.92) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 44.
Cardiovascular procedure: pacemaker, stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 3 (0.25) | 0 (0.00) | 57 (0.77) | 2 (0.21) | 0 (0.00) | 36 (0.58) |
| 25–29 | 4 (0.17) | 0 (0.00) | 79 (0.57) | 8 (0.42) | 3 (0.57) | 56 (0.49) |
| 30–34 | 6 (0.17) | 5 (0.28) | 100 (0.42) | 4 (0.13) | 3 (0.18) | 103 (0.48) |
| 35–39 | 12 (0.24) | 17 (0.34) | 174 (0.40) | 13 (0.30) | 12 (0.25) | 135 (0.34) |
| 40–44 | 19 (0.23) | 28 (0.20) | 340 (0.35) | 10 (0.15) | 20 (0.17) | 219 (0.27) |
| 45–49 | 67 (0.45) | 106 (0.30) | 777 (0.37) | 45 (0.35) | 72 (0.22) | 516 (0.28) |
| 50–54 | 145 (0.57) | 268 (0.35) | 1426 (0.39) | 90 (0.44) | 179 (0.28) | 985 (0.32) |
| 55–59 | 165 (0.63) | 373 (0.40) | 1744 (0.46) | 127 (0.52) | 312 (0.35) | 1346 (0.38) |
| 60–64 | 295 (0.73) | 763 (0.53) | 3423 (0.58) | 245 (0.72) | 637 (0.49) | 2677 (0.51) |
| 65–69 | 505 (1.10) | 1200 (0.77) | 4621 (0.77) | 392 (0.98) | 939 (0.65) | 3826 (0.69) |
| 70–74 | 760 (1.70) | 1780 (1.27) | 5937 (1.11) | 567 (1.49) | 1395 (1.10) | 4882 (1.01) |
| 75–79 | 1119 (2.33) | 2238 (1.84) | 7651 (1.57) | 846 (2.21) | 1869 (1.76) | 6150 (1.44) |
| 80–84 | 1395 (3.38) | 2225 (2.73) | 7374 (2.10) | 992 (2.94) | 1815 (2.49) | 6217 (1.96) |
| ≥85 | 1394 (4.54) | 1808 (4.10) | 5801 (2.77) | 1117 (4.30) | 1553 (3.83) | 5126 (2.60) |
| Total | 5889 (1.74) | 10,811 (1.18) | 39,504 (1.01) | 4458 (1.57) | 8809 (1.07) | 32,274 (0.92) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
Appendix Table 45.
Cardiovascular procedures in patients with CKD, stratified by cause
| Cause | 2017 |
2018 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAG | PCI | CABG | Pacemaker | Total | CAG | PCI | CABG | Pacemaker | Total | |
| DKD | 7915 (5.96) | 4547 (3.43) | 401 (0.30) | 2110 (1.59) | 14,973 (2.82) | 7324 (6.39) | 4160 (3.63) | 297 (0.26) | 1692 (1.48) | 13,473 (2.94) |
| HTN | 7193 (6.51) | 3755 (3.40) | 308 (0.28) | 2349 (2.13) | 13,605 (3.08) | 6420 (7.15) | 3248 (3.61) | 199 (0.22) | 1661 (1.85) | 11,528 (3.21) |
| GN | 1078 (4.35) | 509 (2.06) | 39 (0.16) | 199 (0.80) | 1825 (1.84) | 1084 (4.78) | 531 (2.34) | 44 (0.19) | 155 (0.68) | 1814 (2.00) |
| CTIN | 232 (5.45) | 115 (2.70) | 2 (0.05) | 46 (1.08) | 395 (2.32) | 249 (5.00) | 172 (3.46) | 6 (0.12) | 50 (1.00) | 477 (2.40) |
| ON | 1135 (7.60) | 504 (3.37) | 42 (0.28) | 104 (0.70) | 1785 (2.99) | 784 (7.49) | 381 (3.64) | 20 (0.19) | 72 (0.69) | 1257 (3.00) |
| Others | 2375 (4.69) | 1127 (2.23) | 123 (0.24) | 1081 (2.14) | 4706 (2.32) | 2036 (4.83) | 956 (2.27) | 96 (0.23) | 828 (1.96) | 3916 (2.32) |
| Total | 19,928 (5.90) | 10,557 (3.13) | 915 (0.27) | 5889 (1.74) | 37,289 (2.76) | 17,897 (6.29) | 9448 (3.32) | 662 (0.23) | 4458 (1.57) | 32,465 (2.85) |
CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, chronic kidney disease due to other reasons; PCI, percutaneous coronary intervention.
Data are expressed as n (%).
Appendix Table 46.
Costs stratified by types of health insurance
| Types of health insurance | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| UBMI | 15,151 (8434–29,302) | 13,336 (7783–24,333) | 11,411 (6374–18,720) | 15,175 (8216–28,769) | 12,771 (7531–23,925) | 11,074 (6277–19,169) |
| NRCMS | 14,673 (7432–24,370) | 12,392 (6754–21,792) | 10,789 (5685–18,433) | 14,387 (7442–24,679) | 12,296 (6755–22,263) | 10,879 (5813–18,988) |
| Free medical care | 15,151 (10,075–35,801) | 15,151 (8522–40,742) | 15,151 (6630–20,183) | 17,469 (9505–48,567) | 15,326 (8272–44,843) | 11,601 (6112–22,801) |
| Self-paid treatment | 15,151 (7895–27,688) | 14,755 (7380–27,057) | 10,221 (4930–16,728) | 15,175 (7684–27,867) | 13,544 (7136–26,731) | 9889 (4839–16,568) |
| Others | 15,151 (9063–30,302) | 15,151 (8379–27,847) | 12,546 (6523–19,874) | 15,175 (8691–30,350) | 14,652 (8029–26,092) | 11,989 (6350–18,997) |
| Total | 15,151 (8246–28,305) | 13,606 (7641–24,743) | 11,237 (5982–18,442) | 15,175 (8100–28,313) | 12,985 (7455–24,370) | 10,952 (5972–18,700) |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural cooperative medical care; UBMI, Urban Basic Medical Insurance.
Data are expressed as median (interquartile range).
Appendix Table 47.
Costs stratified by types of health insurance
| Types of health insurance | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| UBMI | 27,451 ± 45,487 | 24,479 ± 38,654 | 20,350 ± 32,166 | 26,959 ± 43,494 | 24,072 ± 36,875 | 20,385 ± 31,622 |
| NRCMS | 21,699 ± 29,741 | 21,272 ± 29,518 | 19,219 ± 27,586 | 22,462 ± 32,281 | 21,553 ± 30,434 | 19,628 ± 28,384 |
| Free medical care | 50,524 ±125,431 | 47,217 ± 114,761 | 25,795 ± 61,824 | 64,693 ± 150,800 | 50,137 ± 114,081 | 27,987 ± 67,512 |
| Self-paid treatment | 27,349 ± 51,975 | 26,797 ± 47,749 | 18,868 ± 32,714 | 27,699 ± 52,872 | 26,371 ± 45,906 | 18,654 ± 32,403 |
| Others | 29,901 ± 50,990 | 27,393 ± 45,019 | 21,406 ± 34,351 | 29,520 ± 52,608 | 26,418 ± 45,380 | 20,877 ± 34,070 |
| Total | 26,923 ± 47,110 | 24,891 ± 41,607 | 20,039 ± 32,309 | 27,115 ± 47,434 | 24,545 ± 40,090 | 20,102 ± 32,178 |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural co-operative medical care; UBMI, Urban Basic Medical Insurance.
Data are expressed as mean ± SD.
Appendix Table 48.
Costs stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 15,151 (8369–29,413) | 14,186 (7805–26,887) | 13,078 (6669–23,135) | 15,175 (8226–29,487) | 13,451 (7588–26,351) | 12,536 (6578–23,092) |
| Female | 15,151 (8085–26,860) | 12,989 (749–22,556) | 10,110 (5520–15,654) | 15,005 (7935–26,799) | 12,476 (7301–22,315) | 9958 (5556–16,000) |
| Total | 15,151 (8246–28,305) | 13,606 (7641–24,743) | 11,237 (5982–18,442) | 15,175 (8100–28,313) | 12,985 (7455–24,370) | 10,952 (5972–18,700) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as median (interquartile range).
Appendix Table 49.
Costs stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 28,291 ± 51,241 | 26,714 ± 45,635 | 23,488 ± 37,739 | 28,469 ± 51,388 | 26,280 ± 44,107 | 23,394 ± 37,385 |
| Female | 24,974 ± 40,431 | 22,677 ± 35,993 | 17,334 ± 26,998 | 25,198 ± 41,122 | 22,429 ± 34,448 | 17,464 ± 27,012 |
| Total | 26,923 ± 47,110 | 24,891 ± 41,607 | 20,039 ± 32,309 | 27,115 ± 47,434 | 24,545 ± 40,090 | 20,102 ± 32,178 |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as mean ± SD.
Appendix Table 50.
Costs stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 12,805 (6377–21,362) | 8552 (5369–15,151) | 7496 (3877–15,151) | 12,072 (6369–20,205) | 8465 (5372–15,175) | 7421 (3886–15,175) |
| 25–29 | 12,969 (6579–21,533) | 9209 (5755–15,151) | 7567 (4173–15,151) | 12,454 (6423–21,038) | 8842 (5649–15,175) | 7554 (4248–14,593) |
| 30–34 | 13,377 (6755–22,419) | 9514 (5900–15,151) | 8349 (4537–15,151) | 12,757 (6678–21,286) | 9188 (5800–15,175) | 8266 (4563–15,175) |
| 35–39 | 14,099 (7107–23,176) | 10,013 (6170–15,719) | 9432 (5090–15,151) | 13,380 (6992–22,907) | 9754 (6101–15,811) | 9291 (5067–15,175) |
| 40–44 | 14,576 (7319–23,811) | 10,772 (6459–17,723) | 10,984 (5706–17,259) | 13,726 (7094–23,852) | 10,356 (6316–17,597) | 10,546 (5603–17,330) |
| 45–49 | 15,068 (7598–24,944) | 11,480 (6765–19,570) | 12,066 (6116–19,930) | 14,423 (7413–25,052) | 11,115 (6633–19,592) | 11,550 (6013–19,864) |
| 50–54 | 15,151 (7886–26,042) | 12,204 (7080–21,406) | 12,452 (6385–21,606) | 14,886 (7706–26,129) | 11,686 (6917–21,130) | 11,892 (6261–21,488) |
| 55–59 | 15,151 (8276–27,760) | 12,852 (7416–23,230) | 12,887 (6743–23,325) | 15,175 (8155–28,001) | 12,317 (7231–23,043) | 12,293 (6592–23,064) |
| 60–64 | 15,151 (8586–29,154) | 13,754 (7765–25,582) | 13,420 (6975–24,662) | 15,175 (8450–29,691) | 13,121 (7571–25,242) | 12,916 (6873–24,767) |
| 65–69 | 15,151 (8820–30,302) | 14,269 (8009–26,496) | 13,638 (7135–24,677) | 15,175 (8634–30,350) | 13,720 (7808–26,435) | 13,156 (7048–24,854) |
| 70–74 | 15,151 (8970–30,302) | 14,681 (8167–26,955) | 13,432 (7161–23,295) | 15,175 (8803–30,350) | 13,966 (7939–26,345) | 12,966 (7075–23,529) |
| 75–79 | 15,151 (9182–31,105) | 15,151 (8444–27,537) | 13,357 (7213–22,300) | 15,175 (8968–30,646) | 14,431 (8149–26,616) | 12,805 (7109–22,318) |
| 80–84 | 15,571 (9460–33,112) | 15,151 (8737–29,488) | 13,370 (7222–22,155) | 15,319 (9275–32,377) | 15,072 (8367–27,815) | 12,828 (7112–21,913) |
| ≥85 | 18,655 (10,422–43,254) | 16,967 (10,014–39,824) | 14,877 (7478–26,233) | 17,935 (10,031–41,362) | 15,732 (9416–37,072) | 13,989 (7326–25,130) |
| Total | 15,151 (8246–28,305) | 13,606 (7641–24,743) | 11,237 (5982–18,442) | 15,175 (8100–28,313) | 12,985 (7455–24,370) | 10,952 (5972–18,700) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as median (interquartile range).
Appendix Table 51.
Costs stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 21,260 ± 37,121 | 16,075 ± 40,263 | 12,894 ± 25,340 | 21,202 ± 41,664 | 16,013 ± 40,248 | 12,693 ± 23,999 |
| 25–29 | 22,070 ± 41,788 | 16,308 ± 32,605 | 11,670 ± 20,127 | 21,435 ± 41,720 | 16,258 ± 32,641 | 11,543 ± 19,598 |
| 30–34 | 22,199 ± 38,635 | 17,115 ± 32,340 | 13,017 ± 22,422 | 21,560 ± 39,534 | 16,704 ± 32,926 | 12,960 ± 22,254 |
| 35–39 | 22,881 ± 39,987 | 18,072 ± 33,759 | 15,529 ± 26,244 | 22,862 ± 40,857 | 18,199 ± 33,395 | 15,635 ± 26,422 |
| 40–44 | 23,305 ± 41,385 | 19,813 ± 34,896 | 18,862 ± 30,367 | 23,145 ± 39,071 | 19,363 ± 32,605 | 18,616 ± 30,217 |
| 45–49 | 23,915 ± 39,276 | 21,095 ± 34,307 | 20,949 ± 32,431 | 24,068 ± 40,550 | 21,002 ± 33,532 | 20,723 ± 31,981 |
| 50–54 | 24,299 ± 37,534 | 22,429 ± 34,992 | 22,293 ± 33,649 | 24,630 ± 39,684 | 22,059 ± 34,461 | 22,039 ± 33,291 |
| 55–59 | 25,401 ± 37,571 | 23,632 ± 35,317 | 23,346 ± 34,219 | 26,012 ± 39,844 | 23,530 ± 35,470 | 23,160 ± 34,181 |
| 60–64 | 26,826 ± 41,131 | 25,226 ± 37,512 | 24,020 ± 34,406 | 27,245 ± 41,467 | 24,864 ± 36,657 | 24,032 ± 34,527 |
| 65–69 | 27,274 ± 40,011 | 25,504 ± 36,810 | 23,835 ± 33,652 | 28,007 ± 42,225 | 25,417 ± 36,957 | 23,966 ± 34,234 |
| 70–74 | 27,908 ± 40,251 | 25,408 ± 36,417 | 22,737 ± 32,140 | 28,164 ± 41,422 | 25,261 ± 36,841 | 22,912 ± 32,759 |
| 75–79 | 29,177 ± 44,385 | 25,664 ± 37,663 | 21,781 ± 31,296 | 28,951 ± 45,359 | 25,159 ± 37,034 | 21,809 ± 31,700 |
| 80–84 | 31,963 ± 56,959 | 28,056 ± 50,201 | 21,753 ± 36,110 | 31,595 ± 55,206 | 26,742 ± 45,113 | 21,484 ± 33,793 |
| ≥85 | 49,104 ± 112,722 | 46,516 ± 108,669 | 28,446 ± 67,617 | 47,841 ± 106,864 | 43,286 ± 96,310 | 27,486 ± 62,472 |
| Total | 26,923 ± 47,110 | 24,891 ± 41,607 | 20,039 ± 32,309 | 27,115 ± 47,434 | 24,545 ± 40,090 | 20,102 ± 32,178 |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as mean ± SD.
Appendix Table 52.
Length of hospital stay stratified by types of health insurance
| Types of health insurance | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| UBMI | 12 (8–22) | 11 (7–18) | 8 (5–14) | 12 (7–21) | 10 (7–17) | 8 (5–14) |
| NRCMS | 12 (7–19) | 10 (7–16) | 8 (5–14) | 11 (7–19) | 10 (7–16) | 8 (5–14) |
| Free medical care | 13 (7–26) | 12 (7–23) | 8 (5–15) | 14 (8–30) | 11 (7–22) | 8 (5–15) |
| Self-paid treatment | 10 (6–19) | 10 (6–17) | 7 (4–12) | 10 (6–18) | 10 (6–16) | 6 (4–11) |
| Others | 12 (7–22) | 11 (7–18) | 8 (5–14) | 12 (7–21) | 11 (7–18) | 8 (5–14) |
| Total | 12 (7–21) | 11 (7–17) | 8 (5–14) | 12 (7–20) | 10 (7–17) | 8 (5–13) |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural cooperative medical care; UBMI, Urban Basic Medical Insurance.
Data are expressed as median (interquartile range).
Appendix Table 53.
Length of hospital stay stratified by types of health insurance
| Types of health insurance | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| UBMI | 19.89 ± 29.13 | 16.76 ± 24.46 | 13.07 ± 20.16 | 18.76 ± 27.40 | 15.92 ± 23.27 | 12.74 ± 19.77 |
| NRCMS | 17.06 ± 22.38 | 14.79 ± 17.51 | 13.21 ± 17.52 | 16.70 ± 22.84 | 14.46 ± 17.73 | 12.95 ± 17.56 |
| Free medical care | 34.70 ± 74.85 | 31.02 ± 66.08 | 16.51 ± 37.26 | 41.37 ± 83.36 | 31.57 ± 65.89 | 17.72 ± 40.50 |
| Self-paid treatment | 17.32 ± 27.69 | 16.16 ± 25.08 | 11.05 ± 17.94 | 16.49 ± 26.41 | 15.62 ± 24.70 | 10.76 ± 17.97 |
| Others | 19.81 ± 28.54 | 17.46 ± 26.41 | 13.05 ± 20.55 | 19.63 ± 30.21 | 17.27 ± 27.22 | 12.92 ± 20.50 |
| Total | 19.22 ± 29.17 | 16.69 ± 25.09 | 12.76 ± 19.67 | 18.59 ± 28.57 | 16.05 ± 24.34 | 12.50 ± 19.58 |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural cooperative medical care; UBMI, Urban Basic Medical Insurance.
Data are expressed as mean ± SD.
Appendix Table 54.
Length of hospital stay stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 12 (7–21) | 11 (7–18) | 9 (6–16) | 12 (7–20) | 10 (7–17) | 9 (5–15) |
| Female | 12 (7–20) | 11 (7–17) | 7 (4–12) | 11 (7–20) | 10 (7–16) | 7 (4–12) |
| Total | 12 (7–21) | 11 (7–17) | 8 (5–14) | 12 (7–20) | 10 (7–17) | 8 (5–13) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as median (interquartile range).
Appendix Table 55.
Length of hospital stay stratified by sex
| Sex | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| Male | 19.63 ± 30.52 | 17.31 ± 26.73 | 14.64 ± 22.54 | 18.95 ± 29.74 | 16.61 ± 25.94 | 14.25 ± 22.39 |
| Female | 18.65 ± 27.13 | 15.93 ± 22.91 | 11.28 ± 16.93 | 18.07 ± 26.83 | 15.36 ± 22.22 | 11.10 ± 16.87 |
| Total | 19.22 ± 29.17 | 16.69 ± 25.09 | 12.76 ± 19.67 | 18.59 ± 28.57 | 16.05 ± 24.34 | 12.50 ± 19.58 |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as mean ± SD.
Appendix Table 56.
Length of hospital stay stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 11 (6–19) | 10 (7–14) | 6 (4–9) | 10 (6–17) | 10 (7–14) | 6 (3–9) |
| 25–29 | 10 (6–17) | 10 (7–15) | 5 (4–8) | 9 (6–17) | 10 (7–14) | 5 (4–8) |
| 30–34 | 10 (6–17) | 10 (7–14) | 6 (4–9) | 9 (6–16) | 10 (7–14) | 6 (4–9) |
| 35–39 | 10 (6–17) | 10 (7–15) | 6 (4–10) | 10 (6–17) | 10 (7–14) | 6 (4–10) |
| 40–44 | 11 (7–18) | 10 (7–15) | 8 (5–13) | 10 (6–17) | 10 (7–15) | 7 (5–12) |
| 45–49 | 11 (7–19) | 10 (7–16) | 8 (5–14) | 11 (7–18) | 10 (7–15) | 8 (5–14) |
| 50–54 | 12 (7–19) | 10 (7–16) | 9 (5–15) | 11 (7–19) | 10 (7–15) | 8 (5–14) |
| 55–59 | 12 (7–20) | 10 (7–16) | 9 (6–15) | 11 (7–20) | 10 (7–16) | 9 (5–14) |
| 60–64 | 12 (8–21) | 11 (7–17) | 9 (6–15) | 12 (7–20) | 10 (7–16) | 9 (6–15) |
| 65–69 | 13 (8–22) | 11 (7–18) | 10 (6–16) | 12 (8–21) | 10 (7–17) | 9 (6–15) |
| 70–74 | 13 (8–23) | 11 (7–18) | 10 (6–16) | 12 (8–22) | 11 (7–17) | 9 (6–15) |
| 75–79 | 13 (8–24) | 12 (8–19) | 10 (6–16) | 13 (8–22) | 11 (7–18) | 10 (6–16) |
| 80–84 | 14 (8–25) | 12 (8–21) | 10 (6–16) | 13 (8–23) | 11 (7–19) | 10 (6–16) |
| ≥85 | 15 (8–30) | 14 (8–27) | 10 (6–18) | 14 (8–28) | 13 (8–25) | 10 (6–17) |
| Total | 12 (7–21) | 11 (7–17) | 8 (5–14) | 12 (7–20) | 10 (7–17) | 8 (5–13) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as median (interquartile range).
Appendix Table 57.
Length of hospital stay stratified by age
| Age group (yr) | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| 18–24 | 16.27 ± 22.51 | 13.68 ± 19.17 | 9.16 ± 16.38 | 15.28 ± 21.30 | 13.24 ± 16.48 | 8.96 ± 15.60 |
| 25–29 | 15.74 ± 22.85 | 13.72 ± 17.70 | 8.09 ± 13.74 | 15.06 ± 26.04 | 13.53 ± 18.16 | 7.92 ± 13.57 |
| 30–34 | 15.68 ± 23.83 | 14.02 ± 19.43 | 8.73 ± 14.41 | 14.81 ± 22.51 | 13.51 ± 18.98 | 8.60 ± 14.57 |
| 35–39 | 16.30 ± 24.80 | 14.01 ± 19.49 | 10.17 ± 17.38 | 15.52 ± 24.27 | 13.77 ± 21.31 | 10.03 ± 17.66 |
| 40–44 | 16.79 ± 24.96 | 14.74 ± 22.30 | 12.34 ± 20.08 | 16.39 ± 26.35 | 14.24 ± 20.50 | 11.91 ± 19.75 |
| 45–49 | 17.44 ± 25.30 | 14.97 ± 21.96 | 13.39 ± 20.14 | 16.87 ± 24.78 | 14.54 ± 20.05 | 12.97 ± 20.47 |
| 50–54 | 17.84 ± 25.32 | 15.27 ± 21.16 | 13.89 ± 20.33 | 17.32 ± 24.67 | 14.78 ± 20.53 | 13.48 ± 19.92 |
| 55–59 | 18.23 ± 24.77 | 15.42 ± 20.61 | 14.05 ± 19.74 | 17.74 ± 26.25 | 14.87 ± 20.37 | 13.57 ± 19.82 |
| 60–64 | 18.82 ± 25.13 | 15.91 ± 21.47 | 14.27 ± 19.38 | 18.23 ± 24.41 | 15.23 ± 20.73 | 13.88 ± 19.19 |
| 65–69 | 19.33 ± 25.76 | 16.20 ± 20.90 | 14.32 ± 18.61 | 18.94 ± 25.45 | 15.63 ± 20.48 | 14.01 ± 18.54 |
| 70–74 | 19.88 ± 26.74 | 16.60 ± 21.39 | 14.24 ± 17.92 | 19.11 ± 25.09 | 15.97 ± 21.27 | 13.93 ± 18.26 |
| 75–79 | 20.85 ± 30.82 | 17.40 ± 23.32 | 14.39 ± 19.28 | 19.91 ± 27.50 | 16.83 ± 23.99 | 14.07 ± 19.31 |
| 80–84 | 22.76 ± 36.83 | 20.07 ± 34.54 | 15.22 ± 24.74 | 21.41 ± 33.89 | 18.71 ± 31.42 | 14.63 ± 23.64 |
| ≥85 | 33.70 ± 60.86 | 33.73 ± 64.42 | 20.50 ± 43.01 | 32.51 ± 60.43 | 31.68 ± 62.12 | 19.64 ± 41.45 |
| Total | 19.22 ± 29.17 | 16.69 ± 25.09 | 12.76 ± 19.67 | 18.59 ± 28.57 | 16.05 ± 24.34 | 12.50 ± 19.58 |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as mean ± SD.
Appendix Table 58.
In-hospital mortality stratified by different types of insurance
| Type of health insurance | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| CKD | DM | Non-CKD | CKD | DM | Non-CKD | |
| UBMI | 15,042 (2.98) | 17,210 (1.47) | 74,760 (0.87) | 11,416 (2.43) | 13,880 (1.21) | 66,067 (0.76) |
| NRCMS | 1505 (0.74) | 1499 (0.48) | 11,687 (0.31) | 944 (0.78) | 987 (0.45) | 8082 (0.33) |
| Free medical care | 922 (5.42) | 883 (2.87) | 3574 (1.36) | 663 (6.55) | 619 (2.78) | 2556 (1.55) |
| Self-paid treatment | 2282 (1.93) | 2383 (1.20) | 18,741 (0.53) | 1571 (1.89) | 1785 (1.15) | 14,593 (0.52) |
| Others | 2544 (2.23) | 3003 (1.37) | 15,511 (0.70) | 1850 (2.13) | 2171 (1.21) | 12,019 (0.64) |
| Total | 22,295 (2.33) | 24,978 (1.29) | 124,273 (0.68) | 16,444 (2.13) | 19,442 (1.13) | 103,317 (0.64) |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural cooperative medical care; UBMI, Urban Basic Medical Insurance.
Data are expressed as n (%).
Appendix Table 59.
In-hospital mortality stratified by sex
| Patient group | 2017 |
2018 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| CKD | 13,985 (2.49) | 8310 (2.10) | 22,295 (2.33) | 10,318 (2.28) | 6126 (1.92) | 16,444 (2.13) |
| DM | 15,336 (1.44) | 9642 (1.10) | 24,978 (1.29) | 12,006 (1.27) | 7436 (0.96) | 19,442 (1.13) |
| Non-CKD | 80,209 (0.99) | 44,064 (0.43) | 124,273 (0.68) | 66,615 (0.93) | 36,702 (0.41) | 103,317 (0.64) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Data are expressed as n (%).
References
- 1.Kalantar-Zadeh K., Jafar T.H., Nitsch D., et al. Chronic kidney disease. Lancet. 2021;398:786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 2.Francis A., Harhay M.N., Ong A.C.M., et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol. 2024;20:473–485. doi: 10.1038/s41581-024-00820-6. [DOI] [PubMed] [Google Scholar]
- 3.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang C., Yang Z., Wang J., et al. Estimation of prevalence of kidney disease treated with dialysis in China: a study of insurance claims data. Am J Kidney Dis. 2021;77:889–897.e1. doi: 10.1053/j.ajkd.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Jdiaa S.S., Mansour R., El Alayli A., et al. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022;35:69–85. doi: 10.1007/s40620-021-01206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Wang H., Long J., et al. China Kidney Disease Network (CK-NET) 2014 Annual Data Report. Am J Kidney Dis. 2017;69(6, suppl 2):A4. [Google Scholar]
- 7.Zhang L., Zhao M.-H., Zuo L., et al. China Kidney Disease Network (CK-NET) 2015 Annual Data Report. Kidney Int Suppl. 2019;9:e1–e81. [Google Scholar]
- 8.Zhang L., Zhao M.H., Zuo L., et al. China Kidney Disease Network (CK-NET) 2016 Annual Data Report. Kidney Int Suppl. 2020;10:e97–e185. [Google Scholar]
- 9.Yang C., Gao B., Zhao X., et al. Executive summary for China Kidney Disease Network (CK-NET) 2016 Annual Data Report. Kidney Int. 2020;98:1419–1423. doi: 10.1016/j.kint.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Yang C., Long J., Shi Y., et al. Healthcare resource utilisation for chronic kidney disease and other major non-communicable chronic diseases in China: a cross-sectional study. BMJ Open. 2022;12 [Google Scholar]
- 11.China Organ Transplantation Development Foundation Report on Organ Transplantation Development in China (2015–2018). Accessed May 15, 2024. http://www.cotdf.org.cn/
- 12.Afkarian M., Zelnick L.R., Hall Y.N., et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C., Wang H., Zhao X., et al. CKD in China: evolving spectrum and public health implications. Am J Kidney Dis. 2020;76:258–264. doi: 10.1053/j.ajkd.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 14.National Health Commission . Scientific and Technical Documentation Press; August 2019. National Medical Service and Quality Safety Report, 2018. [Google Scholar]
- 15.Sola L., Levin N.W., Johnson D.W., et al. Development of a framework for minimum and optimal safety and quality standards for hemodialysis and peritoneal dialysis. Kidney Int Suppl. 2020;10:e55–e62. [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. [Google Scholar]
- 17.Mathew A.T., Strippoli G.F., Ruospo M., Fishbane S. Reducing hospital readmissions in patients with end-stage kidney disease. Kidney Int. 2015;88:1250–1260. doi: 10.1038/ki.2015.307. [DOI] [PubMed] [Google Scholar]
- 18.Jha V., Al-Ghamdi S.M.G., Li G., et al. Global economic burden associated with chronic kidney disease: a pragmatic review of medical costs for the Inside CKD research programme. Adv Ther. 2023;40:4405–4420. doi: 10.1007/s12325-023-02608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J.F., Wang H.B., Zheng S.S., et al. The new era of organ transplantation in China. Chin Med J. 2016;129:1891–1893. doi: 10.4103/0366-6999.187865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X., Nie S., Ding H., Hou F.F. Environmental pollution and kidney diseases. Nat Rev Nephrol. 2018;14:313–324. doi: 10.1038/nrneph.2018.11. [DOI] [PubMed] [Google Scholar]
- 21.Tsai H.J., Wu P.Y., Huang J.C., Chen S.C. Environmental pollution and chronic kidney disease. Int J Med Sci. 2021;18:1121–1129. doi: 10.7150/ijms.51594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowe B., Artimovich E., Xie Y., et al. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: a modelling study. BMJ Glob Health. 2020;5 [Google Scholar]
- 23.Bowe B., Xie Y., Li T., et al. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2018;29:218–230. doi: 10.1681/ASN.2017030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye J.J., Wang S.S., Fang Y., et al. Ambient air pollution exposure and risk of chronic kidney disease: a systematic review of the literature and meta-analysis. Environ Res. 2021;195 [Google Scholar]
- 25.Li G., Huang J., Wang J., et al. Long-term exposure to ambient PM2.5 and increased risk of CKD prevalence in China. J Am Soc Nephrol. 2021;32:448–458. doi: 10.1681/ASN.2020040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R., Yang C., Guo Y., et al. Association between ambient PM1 and the prevalence of chronic kidney disease in China: a nationwide study. J Hazard Mater. 2024;468 [Google Scholar]
- 27.Liang Z., Wang W., Wang Y., et al. Urbanization, ambient air pollution, and prevalence of chronic kidney disease: a nationwide cross-sectional study. Environ Int. 2021;156 [Google Scholar]
- 28.Zhang J.J., Wei Y., Fang Z. Ozone pollution: a major health hazard worldwide. Front Immunol. 2019;10:2518. doi: 10.3389/fimmu.2019.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C., Wang W., Wang Y., et al. Ambient ozone pollution and prevalence of chronic kidney disease: a nationwide study based on the China National Survey of Chronic Kidney Disease. Chemosphere. 2022;306 [Google Scholar]
- 30.Cai W., Zhang C., Zhang S., et al. The 2021 China report of the Lancet Countdown on health and climate change: seizing the window of opportunity. Lancet Public Health. 2021;6:e932–e947. doi: 10.1016/S2468-2667(21)00209-7. [DOI] [PubMed] [Google Scholar]
- 31.Wang F.L., Wang W.Z., Zhang F.F., et al. Heat exposure and hospitalizations for chronic kidney disease in China: a nationwide time series study in 261 major Chinese cities. Mil Med Res. 2023;10:41. doi: 10.1186/s40779-023-00478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W., Wang F., Yang C., et al. Associations between heat waves and chronic kidney disease in China: the modifying role of land cover. Environ Int. 2024;186 [Google Scholar]
- 33.Yang C., Kong G., Wang L., et al. Big data in nephrology: are we ready for the change? Nephrology. 2019;24:1097–1102. doi: 10.1111/nep.13636. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L., Wang H., Li Q., et al. Big data and medical research in China. BMJ. 2018;360 [Google Scholar]
- 35.Saran R., Steffick D., Bragg-Gresham J. The China Kidney Disease Network (CK-NET): “Big Data-Big Dreams.”. Am J Kidney Dis. 2017;69:713–716. doi: 10.1053/j.ajkd.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Bao B., Shen P., et al. Using electronic health record data to establish a chronic kidney disease surveillance system in China: protocol for the China Kidney Disease Network (CK-NET)-Yinzhou Study. BMJ Open. 2019;9 [Google Scholar]
- 37.Ding J., Yang C., Wang Y., et al. Influential factors of intercity patient mobility and its network structure in China. Cities. 2023;132 [Google Scholar]
- 38.Wu J., Li Q., Bao C., et al. Nephrology workforce in China: describing current status and evaluating the optimal capacity based on real-world data. Hum Resour Health. 2023;21:62. doi: 10.1186/s12960-023-00851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grams M.E., Brunskill N.J., Ballew S.H., et al. The kidney failure risk equation: evaluation of novel input variables including eGFR estimated using the CKD-EPI 2021 equation in 59 cohorts. J Am Soc Nephrol. 2023;34:482–494. doi: 10.1681/ASN.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S.H., Zhang Y., Rogers J.A., Gallon L. Recent advances of biosensors for hypertension and nephrology. Curr Opin Nephrol Hypertens. 2019;28:390–396. doi: 10.1097/MNH.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 41.Wu J., Wang F., Li Q., et al. The application performance of urine quantitative analysis system based on computer vision in the screening of albuminuria. Chin J Blood Purif. 2022;21:98–102. [Google Scholar]
- 42.Thirunavukarasu A.J., Ting D.S.J., Elangovan K., et al. Large language models in medicine. Nat Med. 2023;29:1930–1940. doi: 10.1038/s41591-023-02448-8. [DOI] [PubMed] [Google Scholar]
- 43.Shah N.H., Entwistle D., Pfeffer M.A. Creation and adoption of large language models in medicine. JAMA. 2023;330:866–869. doi: 10.1001/jama.2023.14217. [DOI] [PubMed] [Google Scholar]
- 44.Sheng B., Guan Z., Lim L.L., et al. Large language models for diabetes care: potentials and prospects. Sci Bull. 2024;69:583–588. [Google Scholar]
- 45.Brall C., Schröder-Bäck P., Maeckelberghe E. Ethical aspects of digital health from a justice point of view. Eur J Public Health. 2019;29(suppl 3):18–22. doi: 10.1093/eurpub/ckz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Char D.S., Shah N.H., Magnus D. Implementing machine learning in health care—addressing ethical challenges. N Engl J Med. 2018;378:981–983. doi: 10.1056/NEJMp1714229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bello A.K., Okpechi I.G., Levin A., et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health. 2024;12:e382–e395. doi: 10.1016/S2214-109X(23)00570-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Xu X., Zhang M., et al. Prevalence of chronic kidney disease in China: results from the Sixth China Chronic Disease and Risk Factor Surveillance. JAMA Intern Med. 2023;183:298–310. doi: 10.1001/jamainternmed.2022.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L., Long J., Jiang W., et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375:905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 50.Kataria A., Trasande L., Trachtman H. The effects of environmental chemicals on renal function. Nat Rev Nephrol. 2015;11:610–625. doi: 10.1038/nrneph.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lippmann M., Chen L.C., Gordon T., et al. National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res Rep Health Eff Inst. 2013;177:5–13. [Google Scholar]
- 52.Thompson S., Schick-Makaroff K., Bello A., et al. Voicing Individual Concerns for Engagement in Hemodialysis (VOICE-HD): a mixed method, randomized pilot trial of digital health in dialysis care delivery. Can J Kidney Health Dis. 2021;8 [Google Scholar]
- 53.Chinese Preventive Medicine Association for Kidney Disease Guidelines for the early evaluation and management of chronic kidney disease in China. Chin J Intern Med. 2023;62:902–930. [Google Scholar]