Key Teaching Points.
-
•
Cardiovascular screening serves a vital role in tactical athletes and military populations with high-risk occupations.
-
•
Mitral annular disjunction is associated with ventricular arrhythmias and may warrant more detailed screening in particular subsets of tactical and competitive athletes alike.
-
•
Clinicians caring for athletes with mitral valve prolapse should be aware of risk markers of arrhythmic mitral valve prolapse that may preclude individuals from performing high-risk activities and occupations.
Introduction
Mitral annular disjunction (MAD) is characterized by an anatomic separation between the left ventricular myocardium and mitral valve annulus and is an increasingly recognized contributor to ventricular arrhythmias and sudden cardiac arrest.1 Despite significant advances in identifying high-risk markers in patients with MAD, less is understood regarding appropriate screening and management of competitive and tactical athletes with MAD. We present a case of a military helicopter pilot who experienced sudden cardiac arrest during a nighttime flying exercise whose evaluation revealed bileaflet mitral valve prolapse (MVP) with MAD. This case highlights the complexities and importance of continued debates regarding appropriate screening for tactical athletes with high-risk occupations.
Case report
A 29-year-old US Army helicopter pilot suffered sudden cardiac arrest while performing a nighttime flying exercise. After the helicopter was safely landed by a co-pilot, resuscitation was initiated, and the automated external defibrillator indicated ventricular fibrillation (Figure 1). After prolonged cardiopulmonary resuscitation with 6 total defibrillations, spontaneous circulation was restored, and he was taken to a local hospital for supportive care. He was later transferred to a tertiary care facility for further evaluation and management of sudden cardiac arrest. Medical history obtained at admission disclosed an episode of exertional heat illness while completing prior Army Ranger School and a prior screening electrocardiogram (ECG) with premature ventricular contractions for which a treadmill ECG stress test was performed 1 year before the event. Treadmill exercise testing showed no evidence of ischemia, an excellent functional capacity, and premature ventricular contractions (PVCs) only during recovery. No echocardiogram was performed based on the results of the screening ECG and stress test because there was no standing military policy recommending such an evaluation at that time.
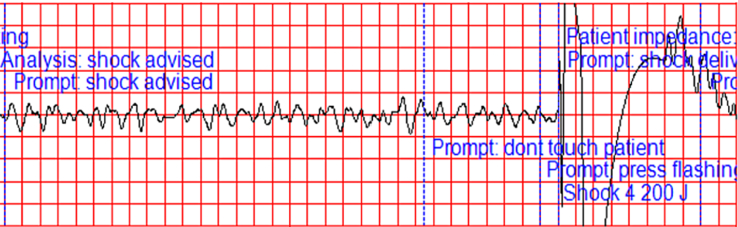
Figure 1.
Automated external defibrillator tracing demonstrating ventricular fibrillation and appropriate delivery of 200 J defibrillation during cardiopulmonary resuscitation efforts.
Review of the ECG obtained immediately post arrest showed a normal sinus rhythm with PVCs in a bigeminal pattern with left ventricle origin (Figure 2). Repeat baseline electrocardiogram performed at arrival to the tertiary care facility showed a normal sinus rhythm with first-degree AV delay, intraventricular conduction delay, QRS fractionation, and T-wave inversions in the inferior leads (Figure 3). Transthoracic echocardiogram revealed bileaflet MVP with trivial mitral valve regurgitation and MAD with a 15-mm distance between the mitral valve annulus and left ventricular myocardium. Coronary computed tomography angiography showed normal coronary origins with no evidence of coronary disease. Cardiac magnetic resonance imaging confirmed MAD findings and additionally identified systolic curling and focal late gadolinium enhancement in the basal inferior posterior wall (Figure 4). The service member was diagnosed with MVP with MAD, and beta-blocker medical therapy was initiated. Catheter ablation was discussed at length with the patient and his family, although it was ultimately deferred because of a low residual PVC burden. Furthermore, electrophysiology study with programmed ventricular stimulation was not performed given the demonstrated substrate for sustained ventricular arrhythmias and presenting cardiac arrest. It was determined that no additional sudden cardiac death risk stratification would be safely warranted in light of the known presenting information. He received a secondary prevention subcutaneous implantable cardioverter-defibrillator and was discharged from the hospital with flying and military physical activity restrictions before being medically discharged from the military. Although no formal exercise ECG stress test was performed after the event, he completed an intensive cardiac rehabilitation program, achieving up to 9.3 Metabolic Equivalents of Task without cardiopulmonary symptoms or ventricular arrhythmias. A 2-week outpatient ambulatory rhythm monitoring performed 4 months after hospital discharge showed occasional ventricular ectopy with a 1.9% PVC burden. Given the persistently low PVC burden and lack of recurrent sustained ventricular arrhythmias on beta-blocker medical therapy, long-term monitoring with an implantable loop recorder was not pursued.
Figure 2.
Electrocardiogram obtained immediately post arrest demonstrating a normal sinus rhythm with PVCs in a bigeminal pattern with a left ventricle origin.
Figure 3.
Baseline electrocardiogram performed at admission to a tertiary care facility showing a normal sinus rhythm with first-degree AV delay, intraventricular conduction delay, QRS fractionation, and T-wave inversions in the inferior leads.
Figure 4.
Cardiac magnetic resonance imaging demonstrating the bileaflet mitral valve prolapse and mitral annular disjunction (A) and the basal inferior posterior wall late gadolinium enhancement (B).
Discussion
Sudden unexplained death has been reported as the leading cause of nontraumatic sudden death in US military populations.2,3 Of US military service members with nontraumatic sudden death, sudden cardiac death (SCD) prevails as the leading cause. There is an estimated incidence ranging from 1.63 per 100,000 person-years in active-duty service members4 to 6.6 per 100,000 person-years in US military recruits under the age of 19.5 In recruits 25 years of age and older, there is an estimated incidence of 14.4 per 100,000 person-years.5 Accordingly, screening for cardiovascular disease has garnered much attention and debate. Since the development of US military medical accession standards during World War I to identify prevalent diseases with high rates of morbidity and mortality,5 various modalities have been used to screen for cardiovascular disease. Universal ECG screening has not been used in the medical evaluation of military applicants since 2002, although it is expected to be reinstituted based on the results of a pilot study performed at the US Naval Academy in which the incorporation of ECG screening in addition to the standard American Heart Association–recommended questionnaire and physical examination identified 16 of 19 cardiac abnormalities with potential vulnerability to SCD.6 We propose that the addition of screening ECG alone may have been insufficient in this case, and tactical athletes with high-risk occupations may require more detailed screening with additional modalities such as echocardiography. However, the appropriateness of screening echocardiography is debated because of the infrequency of positive findings, with only 9 of 20,208 (0.0445%) United States Air Force pilot applicants with findings that would lead to disqualification without the potential for a waiver.7
MVP accounts for approximately 4% of SCD in young competitive athletes in the United States,8 and MAD is frequently seen in patients with arrhythmic mitral valve prolapse.9 MAD is thought to play a pivotal role in arrhythmogenesis through excess mitral valve apparatus mobility, leading to traction on the papillary muscles and posterobasal left ventricular myocardium, which serves as the trigger for ventricular arrhythmias.9 The repeated mechanical injury caused by this increased traction leads to activation of apoptosis pathways, resulting in papillary muscle and left ventricular fibrosis, which provides the arrhythmic substrate.10 It is postulated that the combination of substrate and trigger produced by MAD may provoke early after-depolarizations leading to PVCs, ventricular arrhythmias, and SCD.10,11
Despite a low but statistically increased prevalence of incapacitating aeromedical events in military aviators with echocardiographic MVP,12 waivers may be granted to US Air Force (USAF) pilots depending on the severity of the associated mitral valve regurgitation.13 Federal Aviation Administration standards allow pilots with asymptomatic mitral valve prolapse to fly with regular monitoring.14 United States Army (USA) regulations stipulate that individuals with MVP do not meet the standards of medical fitness for flying duty.15 Similarly, the US Navy only considers waivers for MVP in pilots that have no history of significant arrhythmias, minimal mitral regurgitation, no symptoms, and no requirement for medication for the condition.16
Medical standards and regulations also differ between military branches regarding the allowable burden of ventricular ectopy. For example, USAF medical standards allow for up to 10% burden of ventricular ectopy without requiring further testing or a waiver.13 In contrast, USA pilots with frequent monomorphic or polymorphic PVCs are disqualified, although no specific PVC burden threshold for frequent ventricular ectopy is defined.15 We recommend revision of current military and civilian aeromedical regulations to screen for and identify markers of arrhythmic MVP as described by Lancellotti et al17 and presented in Figure 5. We collectively recommend using a lower 5% PVC burden cutoff to increase the likelihood of identifying patients with arrhythmic mitral valve prolapse and prompt imaging screening. In a review of 215 Italian athletes with MVP, those with associated moderate to severe mitral regurgitation and ventricular arrhythmias (defined as either nonsustained ventricular tachycardia, ≥1 ventricular couplets, ≥3 PVCs on exercise testing, or ≥1,000 PVCs per 24 hours) more frequently developed the need for mitral valve surgery.18 Sports cardiologists and electrophysiologists can provide experienced consultation and counseling regarding the sports career implications and close the tactical athlete’s knowledge gap in understanding the risk of sudden cardiac arrest and future high-risk physical activity. Furthermore, a sports cardiologist is equipped with transitioning various athletes with a prior cardiac arrest to different physical activities that would be safer and gradually introduced with appropriate surveillance and monitoring. A sports cardiologist would be able to transition motivated athletes of various backgrounds to different exercise forms using cardiopulmonary exercise testing data, wearable technology, ambulatory monitoring, cardiac rehabilitation, graduated exercise prescriptions, as well as occupational and physical therapy consultations. This case also highlights the role of a sports cardiologist in understanding organizational guidelines and policies that direct return-to-duty actions in different athletes.
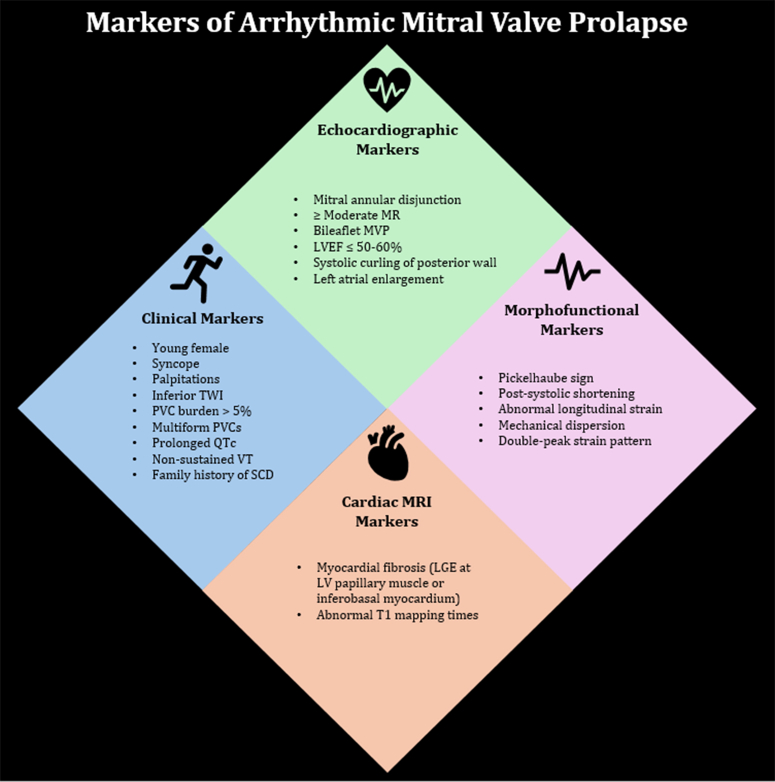
Figure 5.
Clinical, echocardiographic, cardiac magnetic resonance imaging, and morphofunctional markers of arrhythmic mitral valve prolapse. TWI = T-wave inversions; PVC = premature ventricular contraction; QTc = corrected QT interval; VT = ventricular tachycardia; SCD = sudden cardiac death; MR = mitral regurgitation; MVP = mitral valve prolapse; LVEF = left ventricular ejection fraction; LGE = late gadolinium enhancement.
This case report manuscript adhered to the CARE (cAse REport) guidelines.
Conclusions
This case of mitral annular disjunction and PVC-induced ventricular fibrillation in an active-duty helicopter pilot during a nighttime training exercise highlights risks that are not isolated only to the tactical athlete, but also extend to other individuals and broader communities they serve and support through their occupation. Accordingly, further discussion regarding more extensive cardiovascular screening for tactical athletic and military populations with high-risk occupations is warranted.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Air Force, the Department of the Army or the Department of Defense or the U.S. Government.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Faletra F.F., Leo L.A., Paiocchi V.L., et al. Morphology of mitral annular disjunction in mitral valve prolapse. J Am Soc Echocardiogr. 2022;35:176–186. doi: 10.1016/j.echo.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Eckart R.E., Scoville S.L., Campbell C.L., et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Eckart R.E., Shry E.A., Burke A.P., et al. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Smallman D.P., Webber B.J., Mazuchowski E.L., et al. Sudden cardiac death associated with physical exertion in the U.S. military, 2005-2010. Br J Sports Med. 2016;50(2):118–123. doi: 10.1136/bjsports-2015-094900. [DOI] [PubMed] [Google Scholar]
- 5.Magee C., Haigney M.C. Cardiovascular screening in the U.S. Military: time to reconsider the electrocardiogram. Milit Med. 2020;185(7-8):e1039–e1045. doi: 10.1093/milmed/usaa002. [DOI] [PubMed] [Google Scholar]
- 6.Murphy C., Saperstein A., Klusewith S., et al. Effective cardiovascular screening of military recruits requires an electrocardiogram. J Am Coll Cardiol. 2022;79(Suppl9):1638. [Google Scholar]
- 7.Strader J.R., Harrell T.W., Adair A., et al. Efficacy of echocardiographic screening of pilot applicants. Aviat Space Environ Med. 2008;79(5):514–517. doi: 10.3357/asem.2156.2008. [DOI] [PubMed] [Google Scholar]
- 8.Maron B.J., Shirani J., Poliac L.C., et al. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276(3):199–204. doi: 10.1001/jama.1996.03540030033028. [DOI] [PubMed] [Google Scholar]
- 9.Van der Bijl P., Stassen J., Haugaa K., et al. Mitral annular disjunction in the context of mitral valve prolapse: identifying the at-risk patient. JACC Cardiovasc Imaging. 2024;17(10):1229–1245. doi: 10.1016/j.jcmg.2024.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Basso C., Perazzolo Marra M., Rizzo S., et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 11.Basso C., Iliceto S., Thiene G., et al. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140(11):952–964. doi: 10.1161/CIRCULATIONAHA.118.034075. [DOI] [PubMed] [Google Scholar]
- 12.Osswald S.S., Gaffney F.A., Kruyer W.B., et al. Military aviators with mitral valve prolapse: long-term follow-up and aeromedical endpoints. Aviat Space Environ Med. 2007;78(9):845–851. [PubMed] [Google Scholar]
- 13.U.S. Department of the Air Force United States Air Force Aerospace Medicine Waiver Guide Compendium: Mitral, Tricuspid, and Pulmonic Valve Disorders. 2024. https://www.afrl.af.mil/Portals/90/Documents/711/USAFSAM/Air%20Force%20Waiver%20Guide%20Compendium.pdf
- 14.U.S. Code of Federal Regulations Title 14: Aeronautics and Space. Part 67: Medical Standards and Certification. 1996. https://www.ecfr.gov/current/title-14/chapter-I/subchapter-D
- 15.U.S. Department of the Army Standards of Medical Fitness: Army Regulation 40-501. 2019. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN37720-AR_40-501-002-WEB-4.pdf
- 16.U.S. Department of the Navy U.S. Navy Aeromedical Reference and Waiver Guide. 2025. https://www.med.navy.mil/Navy-Medicine-Operational-Training-Command/Naval-Aerospace-Medical-Institute/Aeromedical-Reference-and-Waiver-Guide/
- 17.Lancellotti P., Go Y.Y. New kid on the block: double-peak strain pattern in arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2023;16(4) doi: 10.1161/CIRCIMAGING.123.015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caselli S., Mango F., Clark J., et al. Prevalence and clinical outcome of athletes with mitral valve prolapse. Circulation. 2018;137(19):2080–2082. doi: 10.1161/CIRCULATIONAHA.117.033395. [DOI] [PubMed] [Google Scholar]