Abstract
Background
Gastric cancer (GC) remains a major global health burden. High-salt diets are a key modifiable risk factor, promoting mucosal damage, chronic inflammation, and increased susceptibility to Helicobacter pylori infection. This study aimed to quantify the global, regional, and national GC burden attributable to high-salt diets and assess spatiotemporal trends and socio-demographic disparities from 1990 to 2021.
Methods
This study used data from the Global Burden of Disease (GBD) 2021 to assess the burden of GC attributable to high sodium intake. Mortality and disability-adjusted life years (DALYs) were estimated across 204 countries and territories from 1990 to 2021. Age-standardized mortality rate (ASMR) and age-standardized DALY rate (ASDR) were stratified by Socio-demographic Index (SDI) quintile and GBD region. Temporal trends were assessed using estimated annual percentage change (EAPC). Decomposition and inequality analyses were conducted to explore drivers of DALY changes and disparities across SDI levels.
Results
The global ASMR and ASDR for GC due to high salt intake decreased by 48.9% and 53.3%, respectively, from 1990 to 2021. In high-SDI regions, the ASMR decreased from 1.24 to 0.54 per 100,000 [EAPC =−2.72, 95% confidence interval (CI): −2.75, −2.70]. In contrast, in low-SDI regions, the ASMR and ASDR decreased by only 29.4% and 34.6%, respectively, with EAPCs of −1.14 (95% CI: −1.20, −1.08) and −1.41 (95% CI: −1.47, −1.36). East Asia showed the most notable decline, with the ASMR decreasing from 3.77 to 1.76 per 100,000 (EAPC =−2.54, 95% CI: −2.75, −2.33), and the ASDR dropping from 96.58 to 41.09 per 100,000 (EAPC =−2.88, 95% CI: −3.07, −2.69). while Sub-Saharan Africa exhibited limited progress.
Conclusions
While global efforts to reduce salt intake have yielded positive results, significant disparities were observed across regions, with high-SDI countries experiencing greater reductions compared to low-SDI regions. Therefore, future interventions should focus on salt reduction policies, improved dietary patterns, and enhanced screening programs, particularly in resource-limited settings.
Keywords: High-salt diets, gastric cancer (GC), global burden, Socio-demographic Index (SDI), health inequality
Highlight box.
Key findings
• From 1990 to 2021, the global age-standardized mortality rate (ASMR) and age-standardized disability-adjusted life years rate (ASDR) for gastric cancer (GC) attributable to high-salt diets declined by 48.9% and 53.3%, respectively.
• High-Socio-demographic Index (SDI) regions achieved the greatest reductions (ASMR: −56.4%; ASDR: −60.8%), while low-SDI regions experienced only modest declines (ASMR: −29.4%; ASDR: −34.6%), with some even reporting an increased GC burden.
• East Asia showed the most notable progress, whereas Sub-Saharan Africa exhibited limited improvement.
• Population aging remains a key driver of GC burden, offsetting epidemiological gains in some regions.
What is known and what is new?
• High-salt diets are a major modifiable risk factor for GC, particularly in East Asia, due to dietary habits and H. pylori infection.
• This study provides a comprehensive spatiotemporal analysis of GC burden due to high-salt intake, highlighting widening disparities between high- and low-SDI regions.
What is the implication, and what should change now?
• Strengthen global salt reduction policies, particularly in low-SDI regions.
• Expand H. pylori screening and eradication programs to reduce GC risk.
• Enhance GC screening and early detection efforts, particularly for aging populations.
• Implement public health education campaigns to raise awareness of the risks of high-salt diets.
Introduction
Gastric cancer (GC) remains one of the most prevalent malignant tumors worldwide (1). According to data from the Global Cancer Observatory (GLOBOCAN) 2022 and the Global Burden of Disease (GBD) Study 2021, over 1.23 million new GC cases were reported worldwide, comprising 4.9% of all newly diagnosed cancers (2,3). During the same period, GC-related mortality reached 954,373 cases, accounting for 6.8% of all cancer-related deaths (2,3). Although the global burden of GC has declined in recent years, substantial regional disparities remain. In developed countries, both incidence and mortality rates have significantly declined (4). However, nearly 70% of new GC cases are reported in developing countries, where it remains a major public health challenge (5).
Epidemiological studies have identified high-salt diets as a major modifiable risk factor for GC (6,7). Chronic excessive salt intake disrupts the gastric mucosal barrier, induces persistent inflammation, and increases susceptibility to Helicobacter pylori (H. pylori) infection, thereby elevating GC risk (8). Beyond promoting inflammation, high salt concentrations can directly damage the gastric mucosa, induce glandular epithelial hyperplasia, and accelerate intestinal metaplasia, all contributing to early-stage gastric carcinogenesis (9). A recent study suggested that high-salt diets may contribute to the rising incidence of early-onset GC in developed countries by accelerating gastric mucosal damage and carcinogenesis in younger populations (10).
Studies indicate that each 5-gram increase in daily dietary salt intake raises GC risk by 12% (10,11). Individuals with high salt consumption face a significantly greater GC risk compared to those with lower intake (11). Moreover, high-salt diets may enhance the expression of pro-inflammatory factors, alter gastric microbiota composition, and weaken the stomach’s protective mucus layer, collectively facilitating GC initiation and progression (10,12).
Previous studies have examined the global burden of GC. For example, the GBD 2017 and GBD 2019 studies analyzed GC incidence and mortality at both global and regional levels, revealing significant disparities across countries with varying socioeconomic development levels (13,14). However, most existing research has primarily focused on the overall GC burden, with limited systematic analysis of spatiotemporal variations attributable to high-salt diets. Moreover, trends in disease burden across countries with different Socio-demographic Index (SDI) levels remain insufficiently explored.
This study analyzes the global burden of GC attributable to high-salt diets from 1990 to 2021 using GBD 2021 data. It quantifies salt-related GC disease burden and explores trends across countries by SDI levels. The findings provide evidence to inform GC prevention strategies—such as salt reduction, dietary improvement, and screening—and support targeted public health interventions to promote global health equity. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-200/rc).
Methods
Data source
The data for this study were sourced from the GBD 2021 database, developed by the Institute for Health Metrics and Evaluation (IHME). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Institutional Review Board of the University of Washington reviewed and approved a waiver of informed consent for GBD studies (https://ghdx.healthdata.org/gbd-2021). This database offers comprehensive, multi-dimensional data on 204 countries and territories, encompassing mortality rates, disease burden, quality of life metrics, and risk factors for various diseases (15,16). All data were retrieved via the Global Health Data Exchange (GHDx) query tool, available at http://ghdx.healthdata.org/gbd-results-tool.
The identification of GC cases in this study was based on the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes. Specific coding details and the methodology for estimating cancer burden can be accessed at http://ghdx.healthdata.org/gbd-2021/code/cod-2. In the GBD 2021 study, dietary sodium intake was primarily estimated using 24-hour urinary excretion data and 24-hour dietary recall surveys. Additional data sources—such as food frequency questionnaires, household budget surveys, spot urine samples, and national food sales records—were adjusted to reflect 24-hour urinary sodium equivalents. High salt intake was defined as a 24-hour urinary sodium excretion exceeding 3 grams per day (g/day), a threshold used to assess the impact of high-salt diets on the burden of GC. This estimate was based on modeled population-level data rather than direct individual-level measurements (17-19).
This study included data on GC burden attributable to high salt consumption across all age groups. However, the GBD 2021 provides information only for individuals aged 25 years and older. The available metrics include the number of deaths, age-standardized mortality rate (ASMR), disability-adjusted life years (DALYs), and age-standardized DALYs rate (ASDR). For specific age groups (e.g., 25–29, 30–34, 35–39, and ≥95 years), GBD 2021 reports only crude rates. All data are accessible through the GBD Results Tool at http://ghdx.healthdata.org/gbd-results-tool.
Estimation of high sodium intake-attributed GC burden
The estimation methods employed in the GBD study have been extensively detailed in the relevant literature (15-19). In this study, spatiotemporal Gaussian process regression (ST-GPR), Cause of Death Ensemble model (CODEm) and disease modeling meta-regression (DisMod-MR) were utilized to model population salt intake levels across different age groups, sexes, and regions from 1990 to 2021. These methods were applied to estimate the impact of high salt consumption on the global burden of GC. Relative risk (RR) served as a key metric for evaluating the effect of varying salt intake levels on GC risk. RR values were derived from existing studies and meta-regression techniques across different exposure levels. By integrating salt intake exposure data with mortality estimates, the contribution of high-salt diets to GC burden was quantified and compared against the theoretical minimum risk exposure level (TMREL).
Furthermore, the population attributable fraction (PAF) was calculated by incorporating exposure levels, RR, and TMREL. This approach allowed for a more precise quantification of the impact of high salt intake on GC burden.
The standard equation for the PAF is as follows (19):
| [1] |
where PAFasgt was the PAF for GC burden being attributed to high sodium intake for age group a, gender s, location g, and year t. RRasg(x) was the RRs between exposure level x (from l to u) of sodium consumption and GC separated by age group a, gender s, and year t; Pasgt(x) was the proportion of the population exposed to sodium intake at the level x age group a, gender s, location g, and year t. TMRELas is the TMREL regarding age group a and gender s.
Deaths refer to the number of fatalities occurring within a certain population in the specific period. DALYs is a comprehensive measure that takes into account both the loss of life expectancy and the reduction in quality of life due to disease. The formula for calculating DALYs is: DALYs = YLL + YLD, where YLL (years of life lost) represents the years lost due to premature death, while YLD (years lived with disability)
measures the years of life lived with disability or reduced quality of life as a result of the disease. This combined approach allows for a more holistic understanding of the disease burden.
Estimated annual percentage change (EAPC)
The EAPC was utilized to assess trends in the GC burden attributable to high salt intake from 1990 to 2021. This metric is widely recognized as an indicator of changes in age-standardized rates (ASR) over time (20). The ASR was modeled using a regression equation: , where x stands for the calendar year. Then, EAPC could be obtained from the model: , and its 95% confidence intervals (CIs). Data on deaths, DALYs, ASMR, and ASDR were presented as absolute values accompanied by 95% uncertainty intervals (UIs), derived from the 2.5th and 97.5th percentiles of over 1,000 estimations. If both the upper limit of the EAPC and its 95% CI are negative, the rate is considered to be in decline. Conversely, if both the lower limit of the EAPC and its 95% CI are positive, the rate is considered to be increasing. If the 95% CI includes zero, the rate is deemed stable (21).
SDI
The SDI is a composite measure incorporating per capita income, total fertility rate (for individuals under 25 years old), and average years of education (for individuals aged 15 years and older). It serves as an indicator of a country’s overall social and economic development. Based on SDI values, countries are categorized into five levels: low, low-middle, middle, high-middle, and high (22). This classification enables the analysis of GC burden across different stages of socioeconomic development. The regional classification by SDI can be accessed from the IHME at https://ghdx.healthdata.org/search/site/SDI. To further examine the influence of SDI on disease burden, this study employs linear regression and Pearson correlation analysis to assess the relationship between SDI and ASDR. Additionally, a nonlinear regression model is applied to investigate the dynamic patterns of GC burden in relation to SDI, providing a more comprehensive understanding of how socioeconomic factors shape disease trends (19).
Statistical analyses
Additionally, decomposition analysis was performed to evaluate the contributions of population growth, population aging, and epidemiological changes to variations in GC DALYs. Population growth reflects changes in total population size, while population aging assesses the impact of shifts in age structure. Epidemiological changes, in contrast, represent burden variations that persist after adjusting for population size and age distribution, primarily influenced by advancements in medical interventions and screening programs (23).
To explore the relationship between DALYs and the SDI, frontier analysis was conducted using data envelopment analysis (DEA). This method identifies the theoretical optimal health burden (frontier value) at a given SDI level. The deviation of a country or region from the frontier value indicates the efficiency of health resource utilization—larger deviations suggest greater potential for improvement (24). The study also compared frontier shifts between 1990 and 2021 and examined efficiency differences across SDI classifications and geographic regions.
To assess health inequality, the study employed the slope index of inequality (SII) and the concentration index of inequality (CII). SII measures the absolute difference in DALYs between the highest and lowest SDI countries, while CII quantifies the relative disparity by analyzing the cumulative distribution of DALYs against SDI using a Lorenz curve approach. A positive CII value indicates that the disease burden is concentrated in high-SDI countries, whereas a negative value suggests a higher burden in low-SDI countries (24). All data processing and analyses were conducted using R Studio (version 4.2.1). Statistical significance was set at a P value <0.05. All models underwent rigorous testing to ensure the reliability and accuracy of the results.
Results
Global and regional trends in GC burden attributed to high salt intake
From 1990 to 2021, the global burden of GC attributable to high-salt diets declined substantially. The ASMR decreased from 1.74 per 100,000 in 1990 to 0.89 per 100,000 in 2021, with an EAPC of −2.26 (95% CI: −2.35, −2.18). Despite this decline, the absolute number of deaths rose from 67,844.54 to 75,661.15. Similarly, the ASDR dropped from 44.53 per 100,000 to 20.78 per 100,000 (EAPC =−2.56, 95% CI: −2.65, −2.47). However, the total number of DALYs showed only a modest reduction, from 1,845,616.80 to 1,804,591.52 (Tables 1,2).
Table 1. Age-standardized mortality and DALYs rates for high sodium intake-attributed gastric cancer from 1990 to 2021.
| Location | ASMR | ASDR | |||||
|---|---|---|---|---|---|---|---|
| 1990 (95% UI)† | 2021 (95% UI)† | 1990−2021 (EAPC) (95% CI) | 1990 (95% UI)† | 2021 (95% UI)† | 1990−2021 (EAPC) (95% CI) | ||
| Global | 1.74 (0.00, 8.74) | 0.89 (0.00, 4.37) | −2.26 (−2.35, −2.18) | 44.53 (0.00, 222.31) | 20.78 (0.00, 102.38) | −2.56 (−2.65, −2.47) | |
| SDI region | |||||||
| High SDI | 1.24 (0.00, 6.27) | 0.54 (0.00, 2.73) | −2.72 (−2.75, −2.70) | 29.89 (0.00, 151.09) | 11.60 (0.00, 58.09) | −3.11 (−3.14, −3.08) | |
| High-middle SDI | 2.44 (0.00, 12.41) | 1.18 (0.00, 5.79) | −2.43 (−2.57, −2.30) | 63.60 (0.00, 322.96) | 28.14 (0.00, 136.92) | −2.76 (−2.90, −2.63) | |
| Middle SDI | 2.30 (0.00, 11.48) | 1.11 (0.00, 5.43) | −2.47 (−2.60, −2.34) | 59.02 (0.00, 290.91) | 25.82 (0.00, 127.58) | −2.79 (−2.91, −2.67) | |
| Low-middle SDI | 0.82 (0.00, 4.11) | 0.59 (0.00, 3.00) | −0.95 (−1.00, −0.90) | 21.39 (0.00, 108.46) | 14.78 (0.00, 75.09) | −1.13 (−1.16, −1.09) | |
| Low SDI | 0.86 (0.00, 4.48) | 0.60 (0.00, 3.18) | −1.14 (−1.20, −1.08) | 22.47 (0.00, 116.97) | 14.71 (0.00, 78.41) | −1.41 (−1.47, −1.36) | |
| Geographic region | |||||||
| East Asia | 3.77 (0.00, 18.42) | 1.76 (0.00, 8.69) | −2.54 (−2.75, −2.33) | 96.58 (0.00, 469.07) | 41.09 (0.00, 206.63) | −2.88 (−3.07, −2.69) | |
| Southeast Asia | 0.91 (0.00, 4.61) | 0.56 (0.00, 2.89) | −1.71 (−1.78, −1.64) | 23.49 (0.00, 118.14) | 13.98 (0.00, 71.25) | −1.85 (−1.92, −1.78) | |
| Oceania | 1.36 (0.00, 7.12) | 1.06 (0.00, 5.52) | −0.83 (−0.89, −0.78) | 32.71 (0.00, 178.43) | 25.52 (0.00, 134.98) | −0.82 (−0.89, −0.76) | |
| Central Asia | 2.13 (0.00, 10.58) | 0.90 (0.00, 4.60) | −2.54 (−2.64, −2.43) | 57.97 (0.00, 288.43) | 23.08 (0.00, 119.44) | −2.80 (−2.88, −2.73) | |
| Central Europe | 1.54 (0.00, 7.69) | 0.71 (0.00, 3.49) | −2.58 (−2.67, −2.49) | 36.72 (0.00, 185.31) | 16.71 (0.00, 81.88) | −2.62 (−2.71, −2.53) | |
| Eastern Europe | 2.25 (0.00, 11.72) | 0.92 (0.00, 4.74) | −3.11 (−3.21, −3.01) | 61.63 (0.00, 320.72) | 23.54 (0.00, 119.38) | −3.40 (−3.52, −3.28) | |
| High-income Asia Pacific | 3.09 (0.00, 15.26) | 1.09 (0.00, 5.43) | −3.44 (−3.48, −3.39) | 74.62 (0.00, 365.66) | 22.57 (0.00, 112.13) | −3.92 (−3.97, −3.87) | |
| Australasia | 0.49 (0.00, 2.70) | 0.25 (0.00, 1.41) | −2.10 (−2.23, −1.98) | 11.25 (0.00, 61.37) | 5.72 (0.00, 31.14) | −2.16 (−2.27, −2.05) | |
| Western Europe | 1.04 (0.00, 5.47) | 0.41 (0.00, 2.10) | −3.00 (−3.10, −2.89) | 22.85 (0.00, 119.24) | 8.93 (0.00, 45.98) | −2.96 (−3.05, −2.87) | |
| Southern Latin America | 1.52 (0.00, 7.61) | 0.85 (0.00, 4.26) | −1.69 (−1.81, −1.58) | 35.00 (0.00, 176.91) | 19.43 (0.00, 97.04) | −1.73 (−1.85, −1.61) | |
| High-income North America | 0.40 (0.00, 2.10) | 0.23 (0.00, 1.17) | −1.88 (−1.92, −1.83) | 9.34 (0.00, 48.84) | 5.52 (0.00, 28.10) | −1.73 (−1.77, −1.68) | |
| Caribbean | 0.94 (0.00, 4.83) | 0.58 (0.00, 3.10) | −1.48 (−1.56, −1.40) | 21.93 (0.00, 114.63) | 14.24 (0.00, 76.67) | −1.31 (−1.41, −1.20) | |
| Andean Latin America | 2.67 (0.00, 13.48) | 1.71 (0.00, 8.65) | −1.64 (−1.80, −1.49) | 62.37 (0.00, 316.56) | 38.45 (0.00, 193.86) | −1.79 (−1.93, −1.64) | |
| Central Latin America | 1.70 (0.00, 8.53) | 0.93 (0.00, 4.83) | −2.18 (−2.26, −2.10) | 38.56 (0.00, 194.24) | 22.41 (0.00, 116.52) | −2.00 (−2.09, −1.92) | |
| Tropical Latin America | 1.58 (0.00, 7.95) | 0.78 (0.00, 4.01) | −2.36 (−2.42, −2.30) | 37.33 (0.00, 186.56) | 18.88 (0.00, 96.83) | −2.33 (−2.39, −2.27) | |
| North Africa and Middle East | 0.75 (0.00, 4.40) | 0.45 (0.00, 2.75) | −1.58 (−1.64, −1.51) | 19.66 (0.00, 114.77) | 11.00 (0.00, 65.92) | −1.83 (−1.90, −1.76) | |
| South Asia | 0.62 (0.00, 3.21) | 0.45 (0.00, 2.25) | −0.95 (−1.04, −0.86) | 17.02 (0.00, 87.29) | 11.45 (0.00, 57.60) | −1.19 (−1.27, −1.11) | |
| Central Sub-Saharan Africa | 0.68 (0.00, 4.03) | 0.51 (0.00, 2.99) | −0.96 (−1.00, −0.92) | 16.97 (0.00, 100.02) | 12.54 (0.00, 73.89) | −1.02 (−1.06, −0.98) | |
| Eastern Sub-Saharan Africa | 0.90 (0.00, 4.50) | 0.54 (0.00, 2.79) | −1.90 (−2.00, −1.81) | 23.37 (0.00, 118.12) | 12.94 (0.00, 67.73) | −2.21 (−2.32, −2.10) | |
| Southern Sub-Saharan Africa | 0.55 (0.00, 3.00) | 0.45 (0.00, 2.48) | −0.69 (−1.03, −0.35) | 14.62 (0.00, 78.11) | 11.52 (0.00, 62.18) | −0.75 (−1.10, −0.41) | |
| Western Sub-Saharan Africa | 0.57 (0.00, 3.06) | 0.49 (0.00, 2.54) | −0.28 (−0.37, −0.20) | 14.02 (0.00, 75.09) | 11.32 (0.00, 59.28) | −0.48 (−0.56, −0.39) | |
†, per 100,000 population. ASDR, age-standardized DALYs rate; ASMR, age-standardized mortality rate; CI, confidence interval; DALYs, disability-adjusted life years; EAPC, estimated annual percentage change; SDI, Socio-demographic Index; UI, uncertainty interval.
Table 2. Number of deaths and DALYs cases of high sodium intake-attributed gastric cancer.
| Location | Deaths number (95% UI) | DALYs number (95% UI) | |||
|---|---|---|---|---|---|
| 1990 | 2021 | 1990 | 2021 | ||
| Global | 67,844.54 (0.00 to 339,512.73) | 75,661.15 (0.00 to 372,194.01) | 1,845,616.80 (−0.03 to 9,206,157.73) | 1,804,591.52 (0.00 to 8,884,379.02) | |
| SDI region | |||||
| High SDI | 13,705.92 (0.00 to 69,402.89) | 12,208.66 (0.00 to 61,721.58) | 321,495.69 (0.00 to 1,625,677.68) | 230,573.68 (0.00 to 1,154,959.78) | |
| High-middle SDI | 24,047.50 (0.00 to 122,047.46) | 23,438.81 (0.00 to 114,552.68) | 653,552.16 (−0.02 to 3,320,830.23) | 551,396.41 (0.00 to 2,681,101.33) | |
| Middle SDI | 23,250.29 (0.00 to 115,302.52) | 28,816.28 (0.00 to 141,923.38) | 667,339.27 (−0.02 to 3,270,306.50) | 712,581.58 (0.00 to 3,527,047.08) | |
| Low-middle SDI | 4,880.69 (0.00 to 24,751.39) | 8,255.39 (0.00 to 41,815.52) | 145,052.73 (0.00 to 737,564.41) | 226,298.29 (0.00 to 1,148,264.79) | |
| Low SDI | 1,904.18 (0.00 to 9,916.48) | 2,893.77 (0.00 to 15,443.34) | 56,793.80 (0.00 to 295,505.22) | 82,603.09 (0.00 to 441,280.65) | |
| Geographic region | |||||
| East Asia | 31,815.60 (0.00 to 155,223.57) | 37,861.80 (0.00 to 188,112.18) | 910,165.99 (−0.04 to 4,421,005.46) | 906,420.39 (−0.01 to 4,574,157.79) | |
| Southeast Asia | 2,263.81 (0.00 to 11,384.92) | 3,571.60 (0.00 to 18,178.29) | 66,675.66 (0.00 to 336,540.35) | 97,670.36 (0.00 to 497,996.69) | |
| Oceania | 35.84 (0.00 to 195.58) | 72.09 (0.00 to 380.84) | 1,064.57 (0.00 to 5,908.38) | 2,143.71 (0.00 to 11,558.10) | |
| Central Asia | 998.02 (0.00 to 4,972.32) | 722.78 (0.00 to 3,724.68) | 28,789.80 (0.00 to 143,224.49) | 20,435.50 (0.00 to 105,882.27) | |
| Central Europe | 2,259.36 (0.00 to 11,339.28) | 1,600.00 (0.00 to 7,857.90) | 55,098.53 (0.00 to 277,908.93) | 34,860.24 (0.00 to 170,908.90) | |
| Eastern Europe | 6,322.64 (0.00 to 32,995.76) | 3,240.56 (0.00 to 16,644.57) | 173,732.78 (0.00 to 903,204.03) | 79,239.65 (0.00 to 402,533.45) | |
| High-income Asia Pacific | 6,059.35 (0.00 to 29,936.84) | 5,863.70 (0.00 to 29,532.38) | 151,937.98 (0.00 to 743,669.54) | 99,016.59 (0.00 to 495,285.28) | |
| Australasia | 113.13 (0.00 to 630.21) | 138.14 (0.00 to 787.55) | 2,602.89 (0.00 to 14,210.09) | 2,808.90 (0.00 to 15,482.52) | |
| Western Europe | 6,132.03 (0.00 to 32,392.14) | 4,049.66 (0.00 to 21,330.73) | 127,536.05 (0.00 to 667,811.00) | 76,170.59 (0.00 to 392,854.74) | |
| Southern Latin America | 685.50 (0.00 to 3,454.82) | 751.84 (0.00 to 3,766.66) | 16,277.62 (0.00 to 82,265.41) | 16,598.73 (0.00 to 82,996.48) | |
| High-income North America | 1,413.91 (0.00 to 7,465.54) | 1,481.69 (0.00 to 7,675.10) | 31,635.14 (0.00 to 165,388.25) | 32,630.83 (0.00 to 166,471.04) | |
| Caribbean | 236.21 (0.00 to 1,222.59) | 311.09 (0.00 to 1,670.65) | 5,804.55 (0.00 to 30,337.97) | 7,607.75 (0.00 to 40,990.47) | |
| Andean Latin America | 522.25 (0.00 to 2,644.79) | 988.32 (0.00 to 4,998.75) | 13,409.97 (0.00 to 68,083.61) | 23,127.69 (0.00 to 116,683.73) | |
| Central Latin America | 1,316.45 (0.00 to 6,624.97) | 2,295.17 (0.00 to 11,894.87) | 33,796.36 (0.00 to 170,527.82) | 57,296.69 (0.00 to 297,890.07) | |
| Tropical Latin America | 1,355.85 (0.00 to 6,802.21) | 1,988.04 (0.00 to 10,206.04) | 35,939.59 (0.00 to 179,651.92) | 49,223.00 (0.00 to 252,502.16) | |
| North Africa and Middle East | 1,257.34 (0.00 to 7,340.69) | 1,976.55 (0.00 to 11,965.72) | 37,024.39 (0.00 to 214,620.55) | 54,759.40 (0.00 to 325,675.88) | |
| South Asia | 3,630.87 (0.00 to 18,682.73) | 6,498.89 (0.00 to 32,699.54) | 112,571.19 (0.00 to 573,787.74) | 180,806.07 (0.00 to 908,078.08) | |
| Central Sub-Saharan Africa | 141.97 (0.00 to 838.20) | 262.04 (0.00 to 1,543.94) | 4,236.60 (0.00 to 24,980.08) | 7,863.51 (0.00 to 46,176.26) | |
| Eastern Sub-Saharan Africa | 657.50 (0.00 to 3,325.25) | 852.33 (0.00 to 4,457.26) | 19,716.43 (0.00 to 99,792.75) | 24,470.02 (0.00 to 129,514.73) | |
| Southern Sub-Saharan Africa | 148.19 (0.00 to 796.35) | 254.56 (0.00 to 1,387.35) | 4,431.39 (0.00 to 23,681.44) | 7,339.70 (0.00 to 39,115.11) | |
| Western Sub-Saharan Africa | 478.73 (0.00 to 2,558.73) | 880.31 (0.00 to 4,609.45) | 13,169.32 (0.00 to 70,651.20) | 24,102.24 (0.00 to 126,886.42) | |
DALYs, disability-adjusted life years; SDI, Socio-demographic Index; UI, uncertainty interval.
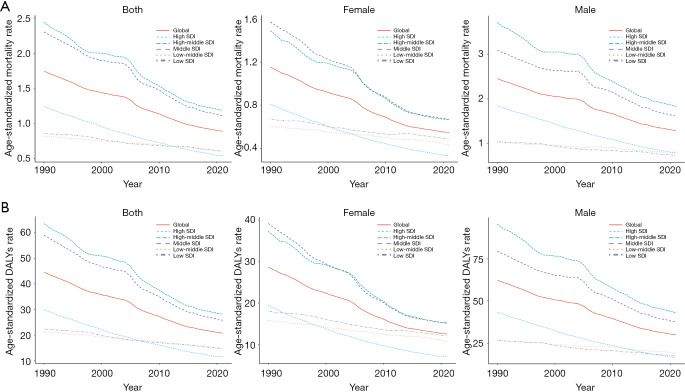
An SDI-stratified analysis revealed significant reductions in GC burden from 1990 to 2021, particularly in high-SDI regions. During this period, the ASMR and ASDR decreased by 56.4% and 60.8%, respectively. ASMR declined from 1.24 to 0.54 per 100,000 (EAPC =−2.72, 95% CI: −2.75, −2.70), while ASDR dropped from 29.89 to 11.60 per 100,000 (EAPC =−3.11, 95% CI: −3.14, −3.08) (Table 1, Figure 1, Figure S1). Over the same period, the total number of deaths in high-SDI regions fell from 13,705.92 to 12,208.66 (Tables 1,2, Figure 1A,1B), reflecting remarkable advancements in GC prevention and control.
Figure 1.
Trends in age-standardized rates of high sodium intake-attributed gastric cancer in SDI and GBD regions 1990–2021, by sex. (A) Age-standardized mortality rate; (B) age-standardized DALYs rate. DALYs, disability-adjusted life years; GBD, Global Burden of Disease; SDI, Socio-demographic Index.
In middle-SDI regions, ASMR decreased from 2.30 to 1.11 per 100,000 (EAPC =−2.47, 95% CI: −2.60, −2.34), while ASDR dropped from 59.02 to 25.82 per 100,000 (EAPC =−2.79, 95% CI: −2.91, −2.67), representing reductions of 51.7% and 55.9%, respectively. In contrast, lower-middle-SDI and low-SDI regions exhibited more modest declines in ASMR and ASDR, alongside increases in both mortality and DALYs. For instance, in lower-middle-SDI regions, ASMR and ASDR decreased by 27.9% and 31.0%, with EAPCs of −0.95 (95% CI: −1.00, −0.90) and −1.13 (95% CI: −1.16, −1.09), respectively. Likewise, in low-SDI regions, ASMR and ASDR fell by 29.4% and 34.6%, with EAPCs of −1.14 (95% CI: −1.20, −1.08) and −1.41 (95% CI: −1.47, −1.36) (Tables 1,2, Figure 1A,1B, Figure S2). However, the number of deaths in low-SDI regions increased from 1,904.18 to 2,893.77, while DALYs rose from 56,793.80 to 82,603.09 (Table 2).
A geographic analysis revealed that East Asia experienced the most substantial decline in GC burden. Between 1990 and 2021, the ASMR in this region decreased from 3.77 to 1.76 per 100,000, with an EAPC of −2.54 (95% CI: −2.75, −2.33). Likewise, the ASDR dropped from 96.58 to 41.09 per 100,000 (EAPC =−2.88, 95% CI: −3.07, −2.69) (Tables 1,2).
In contrast, while Sub-Saharan Africa exhibited a relatively low GC burden, the decline was modest. For instance, in Southern Sub-Saharan Africa, ASMR declined from 0.55 to 0.45 per 100,000, with an EAPC of just −0.69 (95% CI: −1.03, −0.35). Similarly, in Western Sub-Saharan Africa, ASMR decreased slightly from 0.57 to 0.49 per 100,000, with an EAPC of only −0.28 (95% CI: −0.37, −0.20) (Table 1).
Overall, high-SDI regions and specific areas such as East Asia exhibited the most notable reductions, whereas low-SDI regions, despite having a lower baseline burden, showed limited progress and, in some cases, even an increase.
Sex and age disparity of GC burden attributed to high salt intake
A gender-specific analysis from 1990 to 2021 revealed a significant decline in the GC burden attributable to high-salt diets in both males and females in high-SDI countries. The ASMR for males decreased from 1.84 to 0.80 per 100,000, while for females, it dropped from 0.81 to 0.33 per 100,000. Although low-SDI countries also experienced declines, the reductions were less substantial, with male ASMR decreasing from 1.04 to 0.72 per 100,000 and female ASMR from 0.67 to 0.48 per 100,000. Additionally, the decline in ASDR was more pronounced in high-income countries, where ASDR in males fell from 95.57 to 43.01 per 100,000, and in females from 37.32 to 15.25 per 100,000 (Figure 1A,1B, Tables S1,S2).
An age-group analysis further revealed that GC mortality linked to high-salt intake significantly increased among individuals aged 60–65 years, with mortality rates peaking in the 85–89 and 90–94 age groups. These results highlight that the burden of GC is predominantly concentrated in older populations. Among adults aged 25–49 years, ASDR attributable to high-salt GC was 0.17/100,000 and in the 50–64 year group, the ASDR increased to 0.97/100,000 for females (Figure S3, Table S3).
GC burden attributed to high salt intake in 204 countries and regions
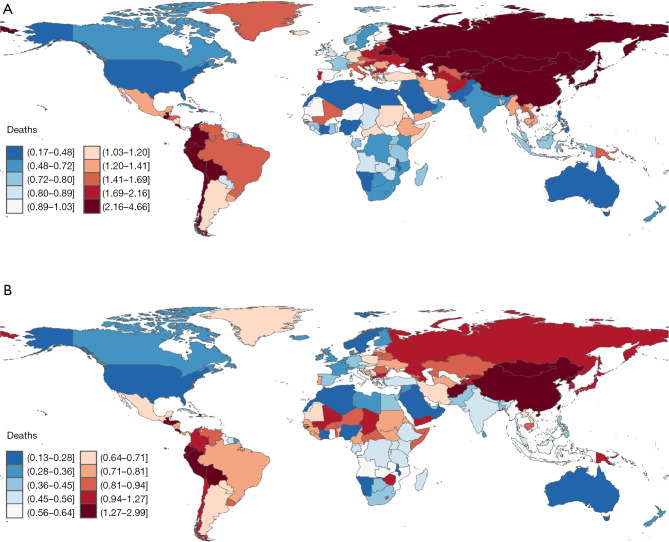
Between 1990 and 2021, the global ASMR of GC attributable to high-salt diets exhibited a general decline, though with substantial regional variations. East Asia, despite its relatively high mortality rate, experienced a pronounced downward trend. Within the region, Mongolia had the highest ASMR in 1990 at 4.52 per 100,000, which declined to 2.96 per 100,000 in 2021 (EAPC =−2.54, 95% CI: −2.75, −2.33). In China, ASMR decreased from 3.85 per 100,000 in 1990 to 1.78 per 100,000 in 2021 (EAPC =−2.72, 95% CI: −2.75, −2.70). North Korea also exhibited a significant decline, with ASMR falling from 2.30 to 1.80 per 100,000 (EAPC =−2.72, 95% CI: −2.74, −2.70). Japan showed a similar trend, with ASMR dropping from 2.81 per 100,000 in 1990 to 1.09 per 100,000 in 2021 (EAPC =−2.64, 95% CI: −2.75, −2.60) (Figure 2A,2B, Table S4).
Figure 2.
Age-standardized mortality rate of high sodium intake-attributed gastric cancer across 204 countries and territories in (A) 1990 and (B) 2021.
In Southeast Asia, for example, Indonesia’s ASMR fell from 0.80 per 100,000 in 1990 to 0.61 per 100,000 in 2021, while Malaysia’s ASMR decreased from 0.74 per 100,000 to 0.53 per 100,000. Conversely, mortality rates remained relatively stable in certain low-income countries such as Bangladesh, Nepal, and Pakistan. In South America, significant reductions in GC mortality were observed in Bolivia, Ecuador, and Peru, where ASMR decreased from 4.17, 2.55, and 2.33 per 100,000 in 1990 to 2.58, 1.50, and 1.60 per 100,000 in 2021, respectively (Figure 2A,2B, Table S3).
In 1990, the lowest mortality rates were recorded in Algeria (ASMR=0.32 per 100,000), Morocco (ASMR=0.17 per 100,000), Pakistan (ASMR=0.41 per 100,000), and Egypt (ASMR=0.24 per 100,000). By 2021, these countries continued to maintain low mortality rates, with some experiencing further declines. Algeria retained the lowest ASMR (0.18 per 100,000), while Morocco’s ASMR dropped further to 0.13 per 100,000. Additionally, the United Arab Emirates (ASMR=0.39 per 100,000) was also among the countries with the lowest mortality rates (Figure 2A,2B, Table S3).
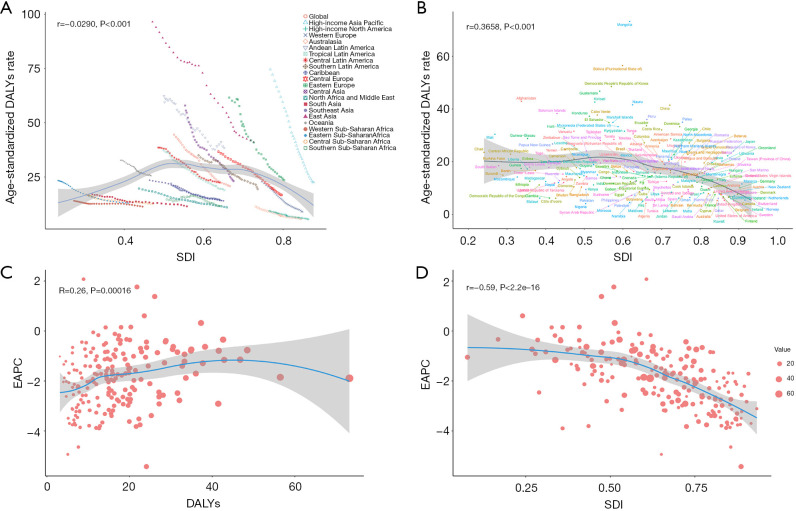
The correlation between GC burden attributed to high sodium intake and SDI
An analysis of 21 regions showed a weak inverse correlation between ASDR and SDI (r=−0.0290, P<0.001). High-SDI countries, such as those in Western Europe and North America, exhibited significantly lower ASDR, while middle-SDI countries, including East Asia and South America, had relatively higher ASDR. Moreover, as SDI decreased, ASDR also demonstrated a downward trend (Figure 3A).
Figure 3.
Correlations between ASDR, EAPC, DALYs, and SDI for gastric cancer attributed to high sodium intake. Correlation between ASDR and SDI across (A) 21 GBD regions and (B) 204 countries and territories; (C) correlation between EAPC and DALYs; (D) correlation between EAPC and SDI. ASDR, age-standardized DALYs rate; DALYs, disability-adjusted life years; EAPC, estimated annual percentage change; GBD, Global Burden of Disease; SDI, Socio-demographic Index.
An analysis of 204 countries identified a significant positive correlation between ASDR and SDI (r=0.3658, P<0.001), indicating that the GC burden is relatively higher in low-SDI countries, whereas the DALYs rate declines as SDI increases. Notably, Sub-Saharan African and South Asian countries, such as Afghanistan, Bangladesh, Niger, and Mali, exhibited higher ASDR. In contrast, high-SDI countries, including Switzerland, Iceland, and Canada, maintained consistently low ASDR levels. Although China, South Korea, and Japan have experienced a decline in the burden of high-salt diets, their GC burden remains significantly higher than that of other high-SDI countries. This underscores the trend that as socioeconomic development improves, the GC burden decreases substantially (Figure 3B).
An in-depth analysis of the relationship between EAPC, DALYs, and SDI revealed a weak positive correlation between EAPC and DALYs (r=0.26, P<0.001), indicating that regions with a higher GC DALY tend to experience a slower decline in mortality rates. Moreover, a significant negative correlation was found between EAPC and SDI (r=−0.59, P<0.001), suggesting that as SDI increases, the rate of decline in ASDR slows. These findings underscore the stark contrast in GC burden trends between high- and low-income countries: in low-SDI countries, the burden of GC has undergone more substantial changes, with a steeper decline in ASDR, whereas in high-SDI countries, the trend has remained relatively stable (Figure 3C,3D).
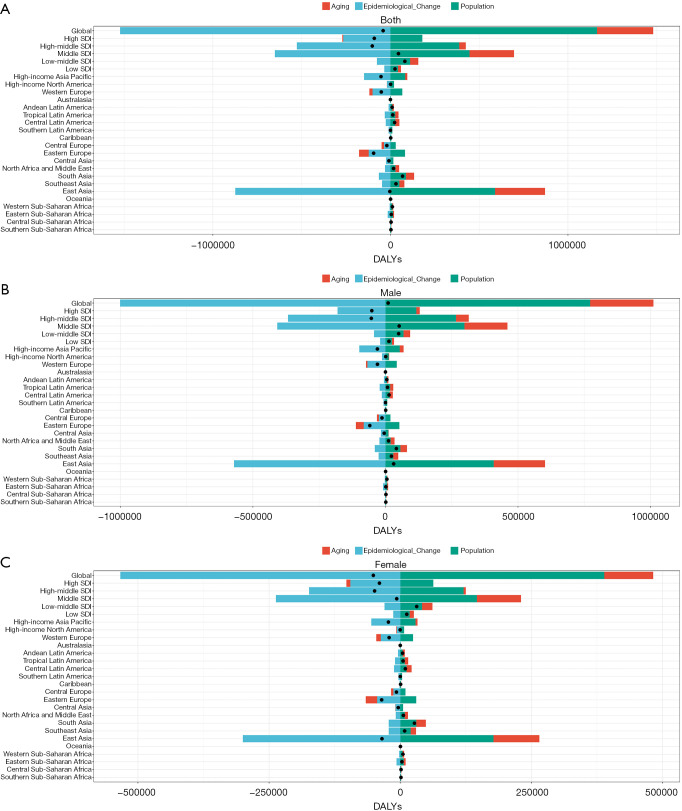
Decomposition analysis of DALYs
Globally, epidemiological changes have been the primary driver of the decline in DALYs due to GC, accounting for reductions of −1,000,075.45 DALYs in males and −533,579.23 DALYs in females. However, population growth (males: +771,978.83; females: +388,736.62) and aging (males: +238,328.58; females: +93,585.37) have contributed to an increased GC burden, partially offsetting the benefits of epidemiological improvements. As a result, the net change in DALYs was +10,231.96 for males, whereas females experienced a decrease of −51,257.24, underscoring a pronounced gender disparity in the shifting burden of GC (Figure 4A, Table S5).
Figure 4.
Decomposition analysis of DALYs attributed to high sodium intake-related gastric cancer across SDI and GBD regions from 1990 to 2021 in (A) both sexes; (B) males, and (C) females. Black dots represent the total change contributed by all three components. DALYs, disability-adjusted life years; GBD, Global Burden of Disease; SDI, Socio-demographic Index.
East Asia experienced the most significant decline in GC burden, with DALYs decreasing by −31,074.7 in males and −34,820.3 in females, primarily driven by epidemiological improvements (males: −570,799.24; females: −299,906.7). However, population growth (males: +408,033.97; females: +178,034.86) and aging (males: +193,839.97; females: +87,051.53) continued to exert a persistent impact on the disease burden in this region. Likewise, Eastern Europe (males: −59,064.32; females: −35,428.8) and the high-income Asia-Pacific region (males: −30,399.77; females: −22,521.62) exhibited substantial reductions. In contrast, GC burden increased in South Asia and low-income countries. Notably, South Asia recorded a rise in DALYs of +41,204.43 in males and +27,030.44 in females (Figure 4B, Table S6).
High-SDI countries saw the most pronounced reduction in GC burden (males: −51,343.77; females: −39,578.25), primarily driven by epidemiological improvements (males: −116,169.82; females: −63,061.07), with relatively minor effects from population growth and aging. Likewise, DALYs declined in high-middle SDI (males: −53,297.43; females: −48,858.33) and middle-SDI countries (males: −51,871.74; females: −6,629.43). By contrast, low-SDI and low-middle SDI countries experienced an increase in DALYs (low-SDI: males +13,363.93; females +12,445.36; low-middle SDI: males +49,793.16; females +31,452.4) (Figure 4C, Table S7).
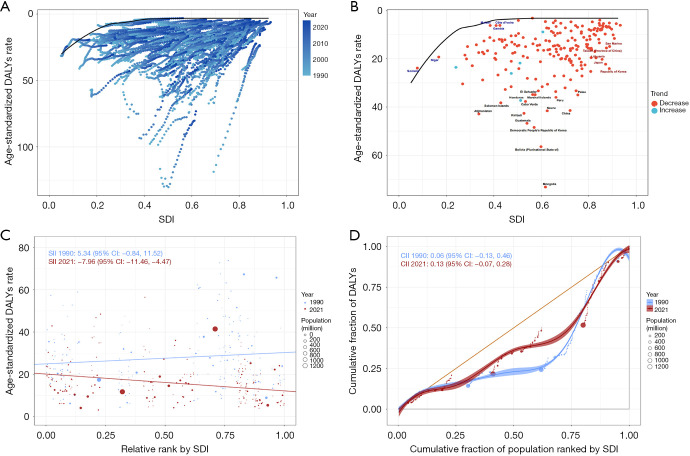
Frontier and inequality analysis of DALYs
High-SDI countries experienced a marked reduction in GC burden, whereas improvements in low-SDI countries were comparatively modest, with some nations even reporting an increase in GC burden (Figure 5A). A frontier analysis across 204 countries revealed that while most experienced a decline in DALY rates, Mongolia, Bolivia, and North Korea continued to bear a high GC burden. By comparison, although Japan, South Korea, and China showed a decline in GC DALY rates, their burden remained higher than that of many Western European and North American countries (Figure 5A,5B).
Figure 5.
Frontier and inequality analysis of DALYs attributed to high sodium intake-attributed gastric cancer in 204 countries and territories. (A,B) Frontier analysis; (C,D) inequality analysis. CI, confidence interval; CII, concentration index of inequality; DALYs, disability-adjusted life years; SDI, Socio-demographic Index; SII, slope index of inequality.
An in-depth analysis of inequality revealed widening disparities in DALY rates across countries with different SDI levels. Regression analysis showed that the negative correlation between DALY rates and SDI strengthened in 2021 (slope =−7.96, 95% CI: −11.46, −4.47), increasing notably from 5.34 in 1990 (95% CI: −0.84, 11.52), indicating a gradual shift of the GC burden toward low-SDI countries (Figure 5C). Additionally, the CII rose from 0.06 in 1990 (95% CI: −0.13, 0.46) to 0.13 in 2021 (95% CI: −0.07, 0.28), further supporting this redistribution of disease burden (Figure 5C,5D).
Discussion
This study, utilizing data from the GBD 2021 database, systematically evaluated the global burden of GC attributable to high-salt diets from 1990 to 2021 and investigated the impact of different SDI levels on disease burden dynamics. Key indicators, including ASMR, ASDR, and PAFs, were employed to assess disease burden, while decomposition and frontier analyses were conducted to quantify the spatiotemporal patterns and major driving factors of GC burden. Furthermore, a health inequality analysis was performed to examine disparities in GC burden across countries with varying SDI levels. The results demonstrated that the global ASMR and ASDR of GC due to high-salt diets decreased by 48.9% and 53.3%, respectively, highlighting a substantial reduction in GC mortality and disease burden worldwide. However, the magnitude of decline varied significantly across countries and regions.
Comparison with previous studies. A recent GBD-based analysis by Qin et al. [2025] similarly identified high sodium intake as a leading, modifiable contributor to the global gastric-cancer burden (3). Consistent with their report, our study reveals pronounced regional heterogeneity and a steady decline in high-salt-attributed GC mortality since 1990, while further providing sex- and SDI-specific PAFs and trends that were not examined previously.
The observed trend of a greater decline in high-SDI countries and a more modest reduction in low-SDI countries aligns with previous research, indicating that nations with higher socioeconomic development levels have achieved greater success in GC prevention and control (14,25). In high-SDI countries, ASMR and ASDR declined by 56.4% and 60.8%, respectively, and GC is no longer a prevalent malignancy in developed countries. This progress can be attributed to a range of public health interventions, including dietary improvements; enhanced GC screening—such as population-based biennial upper gastrointestinal endoscopy programmes for adults aged ≥40 years in countries like South Korea (26), as well as non-invasive ‘test-and-treat’ strategies using the urea breath test to detect and eradicate H. pylori in Western high-income countries—and the more efficient allocation of healthcare resources (27). In recent years, high-SDI countries have implemented various salt-reduction strategies as part of broader public health efforts. For instance, the United States has regulated the salt content in processed and fast foods to lower sodium intake. Singapore has focused on public education to encourage higher consumption of fruits and vegetables, thereby reducing reliance on salt. Additionally, Canada mandates sodium labeling on food packaging to help consumers make healthier choices, whereas Finland has promoted the use of the low-sodium mineral salt substitute “Pansalt®”, which contains 40% less sodium chloride than regular table salt and is now widely used to reformulate processed foods, thereby reducing overall dietary sodium exposure (28,29).
In comparison, ASMR and ASDR in low-SDI countries declined by only 29.4% and 34.6%, respectively, with some nations even experiencing an increase in disease burden, highlighting the ongoing health challenges in these regions. Potential contributing factors include the limited implementation of public health campaigns promoting healthy diets, low public awareness of the risks associated with high-salt intake, ingrained dietary habits, insufficient healthcare resources, and inadequate coverage of early GC screening, and potential under-reporting bias resulting from incomplete cancer-registry or vital-registration data (17,30).
Our findings indicate that East Asia accounts for 50% of the global GC burden attributed to high-salt intake, likely due to the region’s traditional dietary habits, which include the widespread consumption of pickled foods, soy sauce, and miso (31). In 2015, high-salt food consumption was responsible for 2.4% of all cancer cases in Japan (32). However, East Asia has demonstrated the most significant reduction in GC burden, largely attributed to the implementation of upper gastrointestinal endoscopic screening programs and systematic salt reduction policies. For example, Japan has established daily salt intake targets, recommending that men limit their intake to 7.5 grams and women to 6.5 grams to reduce the health risks associated with excessive salt consumption (33). Similarly, since 2007, China has implemented a range of salt reduction measures, including revising nutritional labeling standards for pre-packaged foods and strengthening nutrition and health education. According to the Report on the Nutrition and Chronic Disease Status of Chinese Residents [2015], the average daily household salt consumption in China was 10.5 grams in 2012, reflecting a decrease of 1.5 grams from 2002 (34). If global salt intake were reduced to the WHO-recommended 5 g/day, GC incidence and mortality rates would decline significantly, with the most pronounced impact observed in East Asia (11). Therefore, East Asian countries must further enhance salt reduction efforts to effectively mitigate the risk of GC associated with high-salt diets.
This study revealed that in 2021, the ASMR in males was approximately 2.5 times that in females, while the ASDR was about 2.8 times higher, indicating that men bear a significantly GC cancer burden from high-salt intake than women. This finding is consistent with previous research. The observed gender disparity likely arises from a combination of lifestyle and physiological factors. Men are more likely to consume high-salt and pickled foods, and they also have higher rates of smoking and alcohol consumption—both established risk factors for GC (35). Additionally, males exhibit a higher prevalence of H. pylori infection, further exacerbating their GC burden (36). In contrast, estrogen may confer a protective effect in females. Studies suggest that estrogen enhances the gastric mucosal barrier, reduces inflammation, and mitigates oxidative stress, thereby lowering the risk of GC in women (37). However, this protective effect appears to diminish after menopause. Our analysis showed that the age-specific mortality rates attributable to high-sodium intake increased markedly among females aged over 50 years.
Our decomposition analysis highlights population aging as a major contributor to the rising burden of GC DALYs, particularly in East Asia and South Asia, where demographic shifts have intensified the disease burden. Although epidemiological progress has reduced the overall disease burden, the effects of aging have resulted in an increase in absolute GC cases and mortality in some countries. This trend is closely linked to higher sodium intake among older adults, coupled with age-related declines in gastrointestinal and masticatory functions, which further restrict dietary diversity (38). As the global population continues to age, future GC prevention strategies should place greater emphasis on older adults by enhancing screening coverage, increasing public awareness, and promoting dietary interventions to mitigate the growing disease burden associated with population aging (39).
Notably, disparities in GC burden are widening, with the disease burden increasingly shifting towards low-SDI countries. The CII increased from 0.06 in 1990 to 0.13 in 2021, indicating that the global reduction in GC burden has been largely driven by improvements in high-SDI countries, while progress in low-SDI countries has remained sluggish. Low-SDI countries continue to face significant challenges, including food safety issues, entrenched traditional dietary habits, high consumption of processed high-salt foods, limited healthcare resources, and inadequate GC screening coverage (30). Furthermore, the lack of robust policies promoting healthy eating and low public awareness of the health risks associated with high-salt diets have further contributed to the slow decline in GC burden in these regions (40). A national-level assessment of salt reduction initiatives in India, Indonesia, Thailand, and Sri Lanka revealed that government-led policies—such as imposing limits on salt in processed foods and implementing public health education campaigns—have played a critical role in reducing per capita salt intake (41,42).
This study provides a comprehensive quantification of the long-term impact of high-salt diets on GC burden, elucidating the influence of epidemiological shifts, population aging, and socioeconomic development on disease trends. From a public health perspective, the findings emphasize the necessity of salt reduction strategies, H. pylori screening, early GC detection, and targeted health policy interventions, particularly in low-SDI countries.
Limitations
There are several limitations in this study. First, the accuracy of our findings may be affected by data constraints: the GBD database relies on country-reported data, and relatively weak disease surveillance systems in many low-SDI countries may lead to under- or overestimation of GC burden. Second, high-salt exposure in the present analysis is based on estimated dietary sodium intake at the population level, rather than direct individual-level measurements, which may introduce measurement bias (17-19). Third, although H. pylori infection is a well-established cofactor in gastric carcinogenesis, the GBD 2021 risk-factor framework does not provide harmonized, country- and year-specific estimates of H. pylori prevalence. As such, we could not formally assess any potential synergistic effect between high sodium intake and H. pylori infection on GC mortality (43).
Furthermore, the study did not account for other determinants of GC—such as overall dietary patterns, genetic predisposition, and environmental exposures—limiting a more comprehensive understanding of the high-salt-GC relationship (44,45). Finally, the absence of detailed national and regional data on dietary interventions constrained our ability to evaluate the impact of specific policy measures. Future research should integrate standardized H. pylori data, prospective cohort studies, and molecular investigations to substantiate and refine these findings.
Conclusions
This study systematically assessed the long-term impact of high-salt diets on the global GC burden from 1990 to 2021. The results indicate that while GC mortality and disease burden have significantly declined worldwide, improvements in low-SDI countries have been relatively slow, with the burden gradually shifting towards these regions. Future efforts should focus on salt intake regulation, H. pylori screening, and early GC detection, particularly in resource-limited settings, to mitigate disparities in disease burden and enhance global prevention strategies.
Supplementary
The article’s supplementary files as
Acknowledgments
We appreciate the high-quality data provided by the Global Burden of Disease Study 2021 collaborators. Additionally, we acknowledge the use of JD_GBDR (V2.22, Jingding Medical Technology Co., Ltd.) for generating the figures.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study utilized publicly available data from the Global Burden of Disease (GBD) database, which does not include confidential or personally identifiable information. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Institutional Review Board of the University of Washington reviewed and approved a waiver of informed consent for GBD studies (https://ghdx.healthdata.org/gbd-2021).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-200/rc
Funding: This research was funded by the General Project of Natural Science Foundation of Chongqing Science and Technology Bureau, China (grant No. CSTB2023NSCQ-MSX1037), and the Science and Technology Bureau of Yuzhong District of Chongqing, China (grant No. 20240125).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-200/coif). The authors have no conflicts of interest to declare.
References
- 1.Grad C, Grad S, Fărcaş RA, et al. Changing trends in the epidemiology of gastric cancer. Med Pharm Rep 2023;96:229-34. 10.15386/mpr-2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 3.Qin N, Fan Y, Yang T, et al. The burden of Gastric Cancer and possible risk factors from 1990 to 2021, and projections until 2035: findings from the Global Burden of Disease Study 2021. Biomark Res 2025;13:5. 10.1186/s40364-024-00720-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun DQ, Yang F, Li H, et al. Regional disparities in trends of global gastric cancer incidence and mortality from 1990 to 2019. Zhonghua Zhong Liu Za Zhi 2022;44:950-4. 10.3760/cma.j.cn112152-20220120-00049 [DOI] [PubMed] [Google Scholar]
- 5.Zhu M, Jin G. JPMoGCT, Medicine P. Genetics and Molecular Signature of Gastric Cancer. In: Wei J, Liu B. eds. Personalized Management of Gastric Cancer. Springer, Singapore; 2017:15-33. [Google Scholar]
- 6.Jin G, Lv J, Yang M, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol 2020;21:1378-86. 10.1016/S1470-2045(20)30460-5 [DOI] [PubMed] [Google Scholar]
- 7.Kronsteiner-Gicevic S, Thompson AS, Gaggl M, et al. Adding salt to food at table as an indicator of gastric cancer risk among adults: a prospective study. Gastric Cancer 2024;27:714-21. 10.1007/s10120-024-01502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balendra V, Amoroso C, Galassi B, et al. High-Salt Diet Exacerbates H. pylori Infection and Increases Gastric Cancer Risks. J Pers Med 2023;13:1325. 10.3390/jpm13091325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Y, Hou X, Fang Q, et al. High-salt diet decreases FOLFOX efficacy via gut bacterial tryptophan metabolism in colorectal cancer. Mol Med 2025;31:66. 10.1186/s10020-025-01122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurek M, Szewc M, Sitarz MZ, et al. Gastric Cancer: An Up-to-Date Review with New Insights into Early-Onset Gastric Cancer. Cancers (Basel) 2024;16:3163. 10.3390/cancers16183163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Chen L, Cheng J, et al. Effect of Dietary Salt Intake on Risk of Gastric Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies. Nutrients 2022;14:4260. 10.3390/nu14204260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang S, Xu J, Wan P, et al. Recent advances in the role of high-salt diet in anti- and pro-cancer progression. Front Immunol 2025;16:1542157. 10.3389/fimmu.2025.1542157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020;5:42-54. 10.1016/S2468-1253(19)30328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R. Burden of Stomach Cancer Incidence, Mortality, Disability-Adjusted Life Years, and Risk Factors in 204 Countries, 1990-2019: An Examination of Global Burden of Disease 2019. J Gastrointest Cancer 2024;55:787-99. 10.1007/s12029-023-01005-3 [DOI] [PubMed] [Google Scholar]
- 15.Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024;403:2162-203. 10.1016/S0140-6736(24)00933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Du J, Wu X, et al. Global burden attributable to high sodium intake from 1990 to 2019. Nutr Metab Cardiovasc Dis 2021;31:3314-21. 10.1016/j.numecd.2021.08.033 [DOI] [PubMed] [Google Scholar]
- 18.Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024;403:2133-61. 10.1016/S0140-6736(24)00757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Wang A, Yang S, et al. The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China. Nutrients 2023;15:5088. 10.3390/nu15245088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Y, Xie H, Cai F, et al. Global burden of multidrug-resistant tuberculosis in children and adolescents. Pediatr Res 2025. [Epub ahead of print]. doi: . 10.1038/s41390-025-03917-1 [DOI] [PubMed] [Google Scholar]
- 21.Hankey BF, Ries LA, Kosary CL, et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control 2000;11:31-5. 10.1023/a:1008953201688 [DOI] [PubMed] [Google Scholar]
- 22.Kuang Z, Wang J, Liu K, et al. Global, regional, and national burden of tracheal, bronchus, and lung cancer and its risk factors from 1990 to 2021: findings from the global burden of disease study 2021. EClinicalMedicine 2024;75:102804. 10.1016/j.eclinm.2024.102804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Pan L, Guo Q, et al. The impact of global, regional, and national population ageing on disability-adjusted life years and deaths associated with diabetes during 1990-2019: A global decomposition analysis. Diabetes Metab Syndr 2023;17:102791. 10.1016/j.dsx.2023.102791 [DOI] [PubMed] [Google Scholar]
- 24.Guan SY, Zheng JX, Feng XY, et al. Global burden due to modifiable risk factors for autoimmune diseases, 1990-2021: Temporal trends and socio-demographic inequalities. Autoimmun Rev 2024;23:103674. 10.1016/j.autrev.2024.103674 [DOI] [PubMed] [Google Scholar]
- 25.Nouri M, Zayeri F, Akbari ME, et al. Association between Gastric Cancer Mortality-to-Incidence Ratio and Human Development Index: Evidence from the Global Burden of Disease Study 2016. Arch Iran Med 2021;24:869-75. 10.34172/aim.2021.130 [DOI] [PubMed] [Google Scholar]
- 26.Luu XQ, Lee K, Jun JK, et al. Risk of upper gastrointestinal cancer and death in persons with negative screening results: results from the National Cancer Screening Program in South Korea. Gastric Cancer 2023;26:580-9. 10.1007/s10120-023-01387-0 [DOI] [PubMed] [Google Scholar]
- 27.García-Morales N, Pérez-Aísa Á, Fiorini G, et al. Helicobacter pylori Diagnostic Tests Used in Europe: Results of over 34,000 Patients from the European Registry on Helicobacter pylori Management. J Clin Med 2023;12:4363. 10.3390/jcm12134363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffo A, Carcea M, Moneta E, et al. Influence of different levels of sodium chloride and of a reduced-sodium salt substitute on volatiles formation and sensory quality of wheat bread. J Cereal Sci 2018;79:518-26. [Google Scholar]
- 29.Lee JJ, Mulligan C, Ahmed M, et al. Comparing Canada's 2018 proposed and 2022 final front-of-pack labelling regulations using generic food composition data and a nationally representative dietary intake survey. Public Health Nutr 2024;27:e223. 10.1017/S1368980024001496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa MP, Monjardino T, Santos CC, et al. Completeness Evaluation of Adult-Population-Based Cancer Registries: A Systematic Review. Cancers (Basel) 2025;17:1123. 10.3390/cancers17071123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong Y, Kim ES, Lee J, et al. Trends in sodium intake and major contributing food groups and dishes in Korea: the Korea National Health and Nutrition Examination Survey 2013-2017. Nutr Res Pract 2021;15:382-95. 10.4162/nrp.2021.15.3.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takasu A, Gotoda T, Suzuki S, et al. Daily Diet and Nutrition Risk Factors for Gastric Cancer Incidence in a Japanese Population. Gut Liver 2024;18:602-10. 10.5009/gnl230354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchihashi T. Dietary salt intake in Japan - past, present, and future. Hypertens Res 2022;45:748-57. 10.1038/s41440-022-00888-2 [DOI] [PubMed] [Google Scholar]
- 34.Hipgrave DB, Chang S, Li X, et al. Salt and Sodium Intake in China. JAMA 2016;315:703-5. 10.1001/jama.2015.15816 [DOI] [PubMed] [Google Scholar]
- 35.Xing Y, Hosaka H, Moki F, et al. Gender Differences in Patients with Gastric Adenocarcinoma. J Clin Med 2024;13:2524. 10.3390/jcm13092524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao Y, Zhou Y, Zhao L, et al. Sex differences in Helicobacter pylori infection and recurrence rate among 81,754 Chinese adults: a cross-sectional study. BMC Gastroenterol 2024;24:305. 10.1186/s12876-024-03404-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gan X, Dai G, Li Y, et al. Intricate roles of estrogen and estrogen receptors in digestive system cancers: a systematic review. Cancer Biol Med 2024;21:898-915. 10.20892/j.issn.2095-3941.2024.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo HX, Wang Q, Wang C, et al. Secular Trends in Gastric and Esophageal Cancer Attributable to Dietary Carcinogens From 1990 to 2019 and Projections Until 2044 in China: Population-Based Study. JMIR Public Health Surveill 2023;9:e48449. 10.2196/48449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Shan T, Zhang D, et al. Nowcasting and forecasting global aging and cancer burden: analysis of data from the GLOBOCAN and Global Burden of Disease Study. J Natl Cancer Cent 2024;4:223-32. 10.1016/j.jncc.2024.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contreras Navarro A, Gallagher K, Griffin S, et al. Systematic Review on the Impact of Salt-Reduction Initiatives by Socioeconomic Position to Address Health Inequalities in Adult Populations. Nutr Rev 2025;83:e1090-100. 10.1093/nutrit/nuae088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohan S, Dorairaj P, Krishnan A. Promoting population-wide salt reduction in South East Asia Region: current status and future directions. WHO South-East Asia Journal of Public Health 2013;17:72-9. [Google Scholar]
- 42.Suhendi S, Abdullah A, Shalihati F. The effectiveness of the salt policy in Indonesia. Jurnal Manajemen dan Agribisnis 2020. doi: 10.17358/jma.17.3.315 [DOI]
- 43.Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol 2023;20:338-49. 10.1038/s41571-023-00747-0 [DOI] [PubMed] [Google Scholar]
- 44.Pu K, Feng Y, Tang Q, et al. Review of dietary patterns and gastric cancer risk: epidemiology and biological evidence. Front Oncol 2024;14:1333623. 10.3389/fonc.2024.1333623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He P, Li X, Zou D, et al. Environmental factors inducing gastric cancer: insights into risk and prevention strategies. Discov Oncol 2025;16:25. 10.1007/s12672-025-01771-5 [DOI] [PMC free article] [PubMed] [Google Scholar]