Abstract
Background
The 5-year overall survival (OS) for stage IV pancreatic cancer is dismal despite aggressive systemic therapy. Stereotactic body radiation therapy (SBRT) involves delivering precise, highly conformal, and biologically effective doses of radiation via a linear accelerator to the tumor region. Clinical trials have shown improvement in OS and progression-free survival (PFS) with SBRT plus standard chemotherapy in oligometastatic (<5 metastatic lesions) solid tumors such as breast, lung, colorectal, and prostate cancers, when compared to chemotherapy alone. Factors predicting response to SBRT need to be further explored in oligometastatic pancreatic cancer (oPC). The study aims to assess the role of SBRT in the management of patients with oPC.
Methods
We conducted a retrospective cohort study of oPC patients treated at the Mayo Clinic during the period from January 2012 to January 2022, who underwent SBRT to at least one site, including the primary site and/or sites of metastases, received at least 4 months of chemotherapy, and had a minimum of 1-year follow-up. Pertinent data were collected from the electronic health records after institutional review board (IRB) approval. The response rates (RRs) were assessed using the RECIST v1.1 criteria, and the PFS and OS were calculated using the Kaplan-Meier method. Multivariate Cox regression was used to determine a statistically significant correlation between treatment and genomic characteristics with OS and PFS.
Results
Sixty-one patients with oPC were identified, among whom 38% were female. Eighty-seven percent were Caucasian, and 13% were other ethnicities (African American, Hispanic, and Asian). The median age was 66 years. Patients received gemcitabine (gem) or 5-fluorouracil (5-FU) based chemotherapy. Eight-five percent of patients received chemotherapy within 3 months of SBRT and the median follow-up time of 16 months. The RR was 25% in the primary lesion and 17% in metastatic lesions. SBRT to primary pancreas lesion correlated with higher OS [hazard ratio (HR): 0.27, 95% confidence interval (CI): 0.082–0.89, P=0.03] but showed no difference in PFS (HR: 0.97, P=0.95) when compared to SBRT to any other metastatic site. SBRT to liver metastases had no improvement in OS (P=0.92) or PFS (P=0.70) versus SBRT to other metastatic sites. The type of chemotherapy (gem vs. 5-FU based) alongside SBRT within 3 months did not influence OS (P=0.47) or PFS (P=0.62) in these patients. Among 30 patients who underwent circulating tumor deoxyribonucleic acid (ctDNA) testing, SMAD4 gene alteration correlated with significantly higher PFS (HR: 0.23, 95% CI: 0.065–0.87, P=0.03) but had no relation with OS (HR: 0.60, 95% CI: 0.18–2.03, P=0.41) compared to patients with undetectable SMAD4 alteration.
Conclusions
SBRT plus chemotherapy may have benefits in some patients with oPC. SBRT to primary pancreas lesion led to better OS compared with SBRT to other metastatic sites. SMAD4 alteration in ctDNA testing correlated with higher PFS in oPC patients who received SBRT. This implies a potential role for genomic biomarker-based patient selection in oPC. These findings are currently being studied in a randomized clinical trial evaluating SBRT plus chemotherapy in oPC (NCT04975516).
Keywords: Stereotactic body radiation therapy (SBRT), oligometastatic disease (OMD), pancreatic ductal adenocarcinoma (PDAC), circulating tumor deoxyribonucleic acid (ctDNA)
Highlight box.
Key findings
• Stereotactic body radiation therapy (SBRT) directed to the primary pancreatic tumor is associated with improved overall survival in patients with oligometastatic pancreatic cancer (oPC).
• Alterations in the SMAD4 gene were associated with prolonged progression-free survival.
What is known and what is new?
• SBRT is an established modality in the management of localized pancreatic cancer.
• This study provides novel evidence supporting the utility of SBRT in the treatment of oPC, extending its role beyond locoregional disease.
What is the implication, and what should change now?
• Selected patients with oPC may derive meaningful clinical benefit from SBRT and should be evaluated for its inclusion in multimodal treatment strategies.
Introduction
Pancreatic cancer (PC) is an extremely aggressive disease, with a 5-year relative survival of 12.8% for all stages combined and only 3.1% in the presence of distant metastasis. PC is ranked as the third leading cause of cancer-related deaths in the United States (1).
Surgery with chemotherapy (either neoadjuvant or adjuvant) is currently the main curative approach in resectable and borderline resectable PC (2). However, advanced radiotherapy techniques have garnered significant interest in PC, specifically in patients not eligible for surgery.
Stereotactic body radiation therapy (SBRT) is a form of external beam radiation that utilizes advanced radiation planning and delivery techniques to deliver highly conformal, highly targeted radiation using high doses per fraction with ≤5 fractions. SBRT aims to deliver ablative doses to target lesions in a short course and can enable less time away from systemic therapies. Several studies have demonstrated excellent local control with tolerable side effects in resectable, borderline resectable, and locally advanced pancreatic cancers (LAPC) (3,4) treated with SBRT.
Current treatment guidelines for patients with stage IV pancreatic ductal adenocarcinoma (PDAC) and good performance status include systemic therapy and clinical trials. Systemic treatment options include FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), modified FOLFIRINOX, NALIRIFOX (liposomal irinotecan, fluorouracil, leucovorin, and oxaliplatin), and gemcitabine plus albumin-bound paclitaxel, with or without subsequent chemoradiation. Additionally, patients with poor performance status can opt for palliative care (5). However, there are mounting data across various malignancies, including PC, suggesting that patients with limited volume metastatic disease (oligometastases) can benefit from local therapies aimed at all sites of metastasis in addition to standard systemic therapies (6-8). For example, the SABR-COMET phase II trial included patients with breast, lung, colorectal, and prostate cancers. The 5-year overall survival (OS) rate was 17.7% in the standard palliative radiotherapy arm [95% confidence interval (CI): 6% to 34%] vs. 42.3% in the stereotactic ablative radiotherapy (SABR) arm (95% CI: 28% to 56%, P=0.006) suggesting that SABR may improve survival outcomes in patients with limited metastatic disease. The EXTEND trial was a randomized phase II trial that compared oligometastatic pancreatic cancer (oPC) treated with systemic therapy with or without ablative radiotherapy to all sites of metastasis and showed improved disease-free survival with SBRT (8).
In this study, we aimed to further evaluate the role of SBRT in the management of oPC and explore the potential value of using circulating tumor DNA (ctDNA) in patient stratification. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-100/rc).
Methods
Data source
The data for this retrospective study were collected from the Mayo Clinic electronic health records after institutional review board (IRB) approval (IRB No. 23-001533). We retrieved all patients with metastatic PC from January 2012 to January 2022 using the Mayo Data Explorer (MDE) software. We included patients with the following criteria: males or females, 18 years or older, histologically or cytologically confirmed pancreatic adenocarcinoma, oligometastasis (1–5 lesions), measurable disease on computed tomography (CT) or magnetic resonance imaging (MRI) scans per RECIST v.1.1 criteria, at least one lesion treated with SBRT, chemotherapy for at least 4 months, an Eastern Cooperative Oncology Group (ECOG) score of 0–2 at the start of SBRT treatment, and a minimum of one year follow-up period. Lastly, we correlated disease progression and survival with the molecular profile from ctDNA testing. Due to the retrospective design of the study, the requirement for informed consent was waived by the institutional review board. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Statistical analysis
The OS and progression-free survival (PFS) were calculated from the start date of the first SBRT session to the date of death or disease progression, respectively. We used the Kaplan-Meier method to estimate the OS and PFS. Spearman rank correlation was used to determine if age, ECOG performance status, baseline CA19-9, and chemotherapy regimen were associated with outcomes.
The response rate (RR) of the SBRT-treated lesions, either primary or metastatic sites, was also reported. RR was defined as the proportion of lesions with a complete response (CR) or partial response (PR) to treatment based on the RECIST v1.1 criteria.
Results
Patient characteristics
A total of 220 patients with stage IV PC were reviewed. Sixty-one patients met the inclusion criteria and were included in the analysis (Table 1). Among these, 38% were females, 87% were Caucasian, and 13% were other ethnicities (African American, Hispanic, Asian, etc.). The median age was 66 years, and 70.5% of patients had CA19-9 <200 U/mL before receiving SBRT. Common sites of metastasis included 41 (67%) to the liver, 20 (33%) to the lymph nodes, 22 (36%) to the lungs, and 5 (8%) to the bones. Thirty-eight percent received gemcitabine-based chemotherapy, 34.4% received 5-fluorouracil-based chemotherapy, and 27.9% received other regimens such as capecitabine and pembrolizumab. Eight-five percent of patients received chemotherapy within 3 months of SBRT. The median radiation dose was 50 Gy (range, 20–60 Gy), and the median number of fractions delivered was 5 (range, 1–15 fractions).
Table 1. Demographics (n=61).
| Total number of patients | Value |
|---|---|
| Age (years) | 66 [48–83] |
| Gender | |
| Female | 23 [38] |
| Male | 38 [62] |
| Ethnicity | |
| White | 53 [87] |
| Others | 8 [13] |
| Primary tumor location | |
| Head | 46 [75.4] |
| Body/tail | 15 [24.6] |
| Sites of metastasis | |
| Liver | 41 [67] |
| LN | 20 [33] |
| Lung | 22 [36] |
| Bone | 5 [8] |
| CA19-9 at SBRT | |
| <200 U/mL | 43 [70.5] |
| ≥200 U/mL | 18 [29.5] |
| Chemotherapy regimen | |
| 5-FU-based | 21 [34.4] |
| Gemcitabine-based | 23 [37.7] |
| Others | 17 [27.9] |
| Radiation dose (Gy) | 50 [20–60] |
| No. of fractions | 5 [1–15] |
Data are expressed as median [range] or n [%]. 5-FU, 5-fluorouracil; LN, lymph node; SBRT, stereotactic body radiation therapy.
OS and PFS
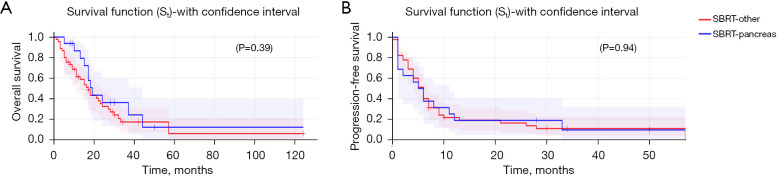
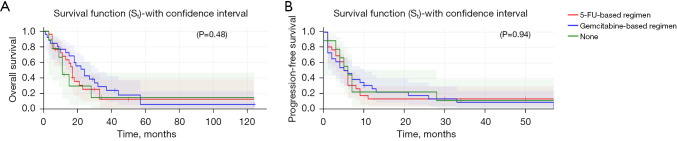
SBRT to primary pancreas lesion correlated with higher OS [hazard ratio (HR): 0.27, 95% CI: 0.082–0.89, P=0.032] (Figure 1A) but showed no difference in PFS (HR: 0.97, 95% CI: 0.4–2.3, P=0.95) (Figure 1B) when compared to SBRT to any other metastatic site. SBRT to liver metastases had no improvement in OS (P=0.92) or PFS (P=0.70) vs. SBRT to other metastatic sites. The type of chemotherapy (gemcitabine vs. 5-fluorouracil-based regimen vs. other) alongside SBRT within 3 months did not influence OS (P=0.47) (Figure 2A) or PFS (P=0.62) (Figure 2B) in these patients.
Figure 1.
OS (primary pancreas vs. other metastatic sites) (A) and PFS (primary pancreas vs. metastatic sites) (B) by SBRT site. OS, overall survival; PFS, progression-free survival; SBRT, stereotactic body radiation therapy.
Figure 2.
OS (A) and PFS (B) by chemotherapy regimen (5-FU based vs. gemcitabine based vs. other). 5-FU, 5-fluorouracil; OS, overall survival; PFS, progression-free survival.
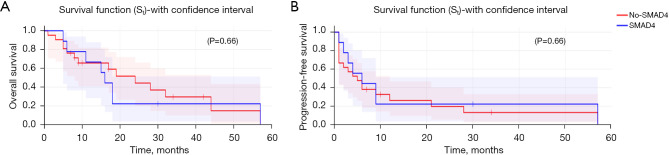
In our cohort, 30 patients underwent ctDNA testing. SMAD4 gene alteration did not correlate with OS (HR: 0.60, P=0.41) (Figure 3A) but correlated with significantly higher PFS (HR: 0.23, 95% CI: 0.065–0.87, P=0.03) (Figure 3B) compared to patients with undetectable SMAD4 alteration.
Figure 3.
OS (A) and PFS (B) with/without SMAD4 mutation. OS, overall survival; PFS, progression-free survival.
RR and disease control rate (DCR)
With a median follow-up time of 16 months, the RR of SBRT-targeted lesions was 25% in the pancreas and 17.6% in different metastatic lesions (Table 2). The DCR was 86.6% in the pancreas and 58.5% in different metastatic lesions. Thirty patients were evaluated for response in the primary pancreatic lesion, only one patient achieved CR after 4 months of SBRT plus gemcitabine/nab-paclitaxel, 3 patients achieved PR, 22 patients had stable disease (SD), and 4 patients had progression of disease (PD). Fifty-three patients were evaluated for response in metastatic lesions after treatment, 2 patients achieved CR in the radiated lesion, 7 patients achieved PR, 22 had SD, and 22 patients had PD (Figure 4).
Table 2. SBRT-target lesions (primary versus metastatic) and the RR.
| SBRT-target lesion [number of lesions treated] | RR (%) |
|---|---|
| Primary [16] | 25 |
| Metastatic [51] | 17.6 |
| Liver [28] | 14.2 |
| Lung [8] | 25 |
| Peritoneum [6] | 33.3 |
RR, response rate; SBRT, stereotactic body radiation therapy.
Figure 4.
Swimmer plot showing duration of treatment and best response in patients with oPC. Each line represents one patient, timing of SBRT, and associated chemotherapy regimen. (A) Response in patients with oPC and liver-only metastasis. (B) Response in patients with oPC and liver metastasis + at least one more site of metastasis. Each bar represents one subject in the study. *, lung, peritoneum, and bone. oPC, oligometastatic pancreatic cancer; SBRT, stereotactic body radiation therapy.
Discussion
More than 50% of cases of PC have metastatic disease at the time of diagnosis (9). Systemic therapy is currently the standard of care in stage IV PC. However, the evolution of systemic therapy is very slowly advancing. Therefore, the search for new strategies to fight stage IV PC beyond cytotoxic chemotherapy should be encouraged and implemented early in treatment. One of these strategies is radiotherapy in the form of SBRT, which has already shown promising results in LAPC and advanced colorectal cancer (10,11). In addition, SBRT has been proposed as an alternative to metastasectomy in pulmonary oligometastasis from hepatocellular carcinoma (12). Recently, the EXTEND trial reported results of a small phase II randomized study comparing oPC patients treated with chemotherapy with or without ablative radiotherapy to all sites of metastases (7). The study showed a nearly 8-month improvement in PFS with the addition of ablative radiotherapy and an improvement in median time to progression of a new lesion of 9 months.
The concept of oligometastatic disease (OMD) was first proposed by Hellman and Weichselbaum in 1995 (13). It was proposed as an intermediate stage between localized and widespread disease. Due to the growing interest in the use of metastasis-directed radiotherapy (MDRT) for the treatment of OMD, a clear and consistent definition was needed. Based on the recommendations from the European Society for Radiotherapy and Oncology (ESTRO) and the European Organisation for Research and Treatment of Cancer (EORTC), OMD was defined as 1–5 metastatic lesions where all metastatic lesions can be safely treated (14), and was classified into induced (has a history of poly metastasis) or genuine OMD, which has no history of polymetastasis. Genuine OMD can be further subdivided into de novo OMD (first presentation) and repeat OMD (with a prior history of OMD) (15). Patients with oligometastases have shown significantly better OS compared to patients with widespread metastasis in other cancer types, such as nasopharyngeal carcinoma (16).
A meta-analysis based on 12 studies, which comprised 943 patients with oligometastatic cancers who underwent SABR, aimed to assess safety and clinical benefit. This study’s most common primary sites were prostate, colorectal, breast, and lung cancers. Rates of acute and late grade 3 to 5 toxicities were less than 13% (17). None of the patients in our study developed grade 3 or higher toxicities. However, 8 patients (13%) developed grade 1 toxicities that included diarrhea, pain, and fatigue.
Few retrospective studies have been conducted on this patient population (18-22). We wanted to explore the role of SBRT in oPC further as a promising therapeutic option. Interestingly, we found that SBRT to the primary PC improved OS but not PFS, contrary to results from previous studies that showed improvement in both OS and PFS. This can be attributed to polyprogression, where patients transition from OMD to polymetastatic (>5 lesions), which strongly influences survival and predicts poor prognosis.
Few case reports have demonstrated a phenomenon termed the “abscopal effect”, in which local radiotherapy has an anti-tumor effect on distant lesions, especially when combined with immunotherapy (23,24). However, further studies are needed to understand this phenomenon in oPC treated with SBRT, as well as the feasibility of administering SBRT to all metastatic lesions and its effect on survival as compared to treating the primary pancreatic lesion only. Furthermore, the order in which radiotherapy is administered needs to be addressed in patients with OMD, either concurrently or sequentially.
Study limitations and reflection on previous studies
There is a lack of a universal definition for oPC, which makes it challenging to compare results from different studies. For example, some studies define OMD as three or less metastatic lesions, while other studies define it as two or less lesions that are smaller than 4 cm, and only in the lung or liver (25). Three ongoing prospective clinical trials are investigating surgical resection after neoadjuvant chemotherapy in oPC, including the CSPAC-1 (The Chinese Study Group for Pancreatic Cancer) trial, the HOLIPANC (hepatic oligometastatic adenocarcinoma of the pancreas) trial, and the ScanPan 1 trial (26,27).
To better prove the actual benefit of SBRT in oPC, a randomized controlled trial is required to compare SBRT + standard chemotherapy vs. chemotherapy alone in patients with oPC. A phase II trial is currently being conducted at the Mayo Clinic (NCT04975516) with the primary objective of PFS, and the secondary objectives are RR, OS, adverse events, and longitudinal assessment of circulating tumor cells and ctDNA.
In a previous study, SMAD4 gene alteration was associated with metastatic progression (30.0% vs. 14.5%; P=0.009) in patients with localized PC receiving first-line FOLFIRINOX (28). However, this association was not present in patients receiving gemcitabine/nab-paclitaxel. PC patients with SMAD4 gene inactivation in general had a shorter median OS of 11.5 vs. 14 months in patients with intact SMAD4 (HR: 1.92, 95% CI: 1.20–3.05, P=0.006) (29). In another interesting study, poly (ADP-ribose) polymerase (PARP) inhibitor olaparib combined with radiotherapy synergistically decreased the growth of SMAD4-deficient PC in vivo and in vitro (30).
Given this study’s limitations, including its retrospective nature and the small number of patients, results should be interpreted with caution. In addition, the Kaplan-Meier method provides unadjusted survival probabilities. Large prospective studies are needed to overcome these limitations (Table 3). In this study, we wanted to address the rising role of SBRT as a promising treatment strategy in OMD, specifically oPC.
Table 3. Ongoing studies using SBRT in oPC.
| NCT | Title | Phase | Primary objective | Arms | Status |
|---|---|---|---|---|---|
| NCT04975516 | Standard of Care Chemotherapy with or Without Stereotactic Body Radiation Therapy for Oligometastatic Pancreatic Cancer | II | PFS | Arm 1: SBRT once daily or every other day for 5 fractions and receive chemotherapy per standard of care | Recruiting |
| Arm 2: chemotherapy per standard of care | |||||
| NCT04498767 | Stereotactic Body Radiotherapy in Patients with Rare Oligometastatic Cancers (OligoRARE) | III | OS | Arm 1: standard of care + palliative RT | Recruiting |
| Arm 2: standard of care + SBRT | |||||
| NCT03862911 | Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic (1-3 Metastases) Cancer (SABR-COMET-3) | III | OS | Arm 1: palliative radiotherapy | Recruiting |
| Arm 2: SABR 35 Gy in 5 fractions daily | |||||
| NCT06593431 | Extending Outcomes for Pancreas Cancer Patients with Nominal Oligometastatic Disease (EXPAND) | III | PFS | Arm 1: metastasis-directed therapy | Not yet recruiting |
| Arm 2: systemic therapy alone |
NCT, National Clinical Trial; oPC, oligometastatic pancreatic cancer; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SABR, stereotactic ablative radiotherapy; SBRT, stereotactic body radiation therapy.
Conclusions
SBRT in combination with chemotherapy appears beneficial in selected patients with oPC. Notably, SBRT to the primary pancreatic lesion was associated with improved OS. SMAD4 alterations identified through ctDNA testing correlated with prolonged PFS, suggesting a potential role for radiogenomic-based treatment stratification. These findings support ongoing prospective studies evaluating the integration of SBRT with systemic therapy in this patient population.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The data for this retrospective study were collected from the Mayo Clinic electronic health records after institutional review board (IRB) approval (IRB No. 23-001533). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Due to the retrospective design of the study, the requirement for informed consent was waived by the institutional review board.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-100/rc
Funding: The study was supported by the K-12 grant program (K12CA090628, to H.B., who is a Paul Calabresi Scholar at the Mayo Clinic Cancer Center), and the National Institute of Minority Health and Health Disparity Career Development grant (K23MD017217, to N.H.T.).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-100/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-100/dss
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 2.Wu HY, Li JW, Li JZ, et al. Comprehensive multimodal management of borderline resectable pancreatic cancer: Current status and progress. World J Gastrointest Surg 2023;15:142-62. 10.4240/wjgs.v15.i2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oar A, Lee M, Le H, et al. Australasian Gastrointestinal Trials Group (AGITG) and Trans-Tasman Radiation Oncology Group (TROG) Guidelines for Pancreatic Stereotactic Body Radiation Therapy (SBRT). Pract Radiat Oncol 2020;10:e136-46. 10.1016/j.prro.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 4.Jung J, Yoon SM, Park JH, et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One 2019;14:e0214970 . 10.1371/journal.pone.0214970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:439-57. 10.6004/jnccn.2021.0017 [DOI] [PubMed] [Google Scholar]
- 6.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludmir EB, Sherry AD, Fellman BM, et al. Addition of Metastasis-Directed Therapy to Systemic Therapy for Oligometastatic Pancreatic Ductal Adenocarcinoma (EXTEND): A Multicenter, Randomized Phase II Trial. J Clin Oncol 2024;42:3795-805. 10.1200/JCO.24.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA 2021;326:851-62. 10.1001/jama.2021.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrelli F, Comito T, Barni S, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol 2018;129:427-34. 10.1016/j.radonc.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 11.Shouman MA, Fuchs F, Walter F, et al. Stereotactic body radiotherapy for pancreatic cancer - A systematic review of prospective data. Clin Transl Radiat Oncol 2024;45:100738 . 10.1016/j.ctro.2024.100738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin YS, Yun JK, Jung J, et al. Comparison of Metastasectomy and Stereotactic Body Radiation Therapy for Pulmonary Oligometastasis From Hepatocellular Carcinoma: A Propensity Score-Weighted Analysis. Int J Radiat Oncol Biol Phys 2025;121:432-41. 10.1016/j.ijrobp.2024.09.022 [DOI] [PubMed] [Google Scholar]
- 13.Milano MT, Biswas T, Simone CB, 2nd, et al. Oligometastases: history of a hypothesis. Ann Palliat Med 2021;10:5923-30. 10.21037/apm.2020.03.31 [DOI] [PubMed] [Google Scholar]
- 14.Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol 2020;148:157-66. 10.1016/j.radonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. 10.1016/S1470-2045(19)30718-1 [DOI] [PubMed] [Google Scholar]
- 16.Tian YH, Zou WH, Xiao WW, et al. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: A subset with better overall survival. Head Neck 2016;38:1152-7. 10.1002/hed.24345 [DOI] [PubMed] [Google Scholar]
- 17.Lehrer EJ, Singh R, Wang M, et al. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2021;7:92-106. 10.1001/jamaoncol.2020.6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang L, Ye Y, Feng Z, et al. Stereotactic body radiation therapy for the primary tumor and oligometastases versus the primary tumor alone in patients with metastatic pancreatic cancer. Radiat Oncol 2024;19:111 . 10.1186/s13014-024-02493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji X, Zhao Y, He C, et al. Clinical Effects of Stereotactic Body Radiation Therapy Targeting the Primary Tumor of Liver-Only Oligometastatic Pancreatic Cancer. Front Oncol 2021;11:659987 . 10.3389/fonc.2021.659987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elamir AM, Karalis JD, Sanford NN, et al. Ablative Radiation Therapy in Oligometastatic Pancreatic Cancer to Delay Polyprogression, Limit Chemotherapy, and Improve Outcomes. Int J Radiat Oncol Biol Phys 2022;114:792-802. 10.1016/j.ijrobp.2022.07.019 [DOI] [PubMed] [Google Scholar]
- 21.Gkika E, Adebahr S, Schimek-Jasch T, et al. Stereotactic body radiotherapy (SBRT) in recurrent or oligometastatic pancreatic cancer: Simultaneus intergrated protection (SIP) versus conventional SBRT A toxicity review of two different treatment approaches. J Clin Oncol 2016;34:e15692 . 10.1007/s00066-017-1099-8 [DOI] [PubMed] [Google Scholar]
- 22.Sourial F, Shamsesfandabadi P, Wegner RE, et al. Stereotactic Body Radiation Therapy for Liver Oligometastases in Pancreatic Cancer: Enhancing Precision and Outcomes. International Journal of Radiation Oncology, Biology, Physics 2024;120:e275. [Google Scholar]
- 23.Endo M, Fukuda Y, Okada K, et al. Abscopal Effect after Stereotactic Body Radiotherapy with Nivolumab for Lung Metastasis of Head and Neck Cancer: A Case Report. Case Rep Oncol 2023;16:1345-52. 10.1159/000534609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi F, Wang X, Teng F, et al. Abscopal effect of metastatic pancreatic cancer after local radiotherapy and granulocyte-macrophage colony-stimulating factor therapy. Cancer Biol Ther 2017;18:137-41. 10.1080/15384047.2016.1276133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandel P, Wallace MB, Stauffer J, et al. Survival of Patients with Oligometastatic Pancreatic Ductal Adenocarcinoma Treated with Combined Modality Treatment Including Surgical Resection: A Pilot Study. J Pancreat Cancer 2018;4:88-94. 10.1089/pancan.2018.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebauer F, Damanakis AI, Popp F, et al. Study protocol of an open-label, single arm phase II trial investigating the efficacy, safety and quality of life of neoadjuvant chemotherapy with liposomal irinotecan combined with Oxaliplatin and 5-fluorouracil/Folinic acid followed by curative surgical resection in patients with hepatic Oligometastatic adenocarcinoma of the pancreas (HOLIPANC). BMC Cancer 2021;21:1239 . 10.1186/s12885-021-08966-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei M, Shi S, Hua J, et al. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: protocol of a multicentre, prospective, randomised phase III control trial (CSPAC-1). BMJ Open 2019;9:e033452 . 10.1136/bmjopen-2019-033452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ecker BL, Tao AJ, Janssen QP, et al. Genomic Biomarkers Associated with Response to Induction Chemotherapy in Patients with Localized Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 2023;29:1368-74. 10.1158/1078-0432.CCR-22-3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674-9. 10.1158/1078-0432.CCR-09-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Yu T, Zhao Z, et al. SMAD4 Limits PARP1 dependent DNA Repair to Render Pancreatic Cancer Cells Sensitive to Radiotherapy. Cell Death Dis 2024;15:818 . 10.1038/s41419-024-07210-7 [DOI] [PMC free article] [PubMed] [Google Scholar]