Abstract
Background
In regions with a high prevalence of tuberculosis, it is crucial to accurately differentiate between tuberculosis pleural effusion (TPE) and parapneumonic effusion (PPE). The current study aimed to evaluate the potential role of the pleural fluid lactate dehydrogenase (LDH)/adenosine deaminase (ADA) ratio in differentiating between TPE and PPE.
Methods
In the first section, the pleural fluid LDH/ADA ratio was compared between 45 patients with TPE and 81 patients with PPE within our study population, and its diagnostic efficacy was assessed. In the second section, we conducted a meta-analysis incorporating six previous publications and the current study.
Results
In our study population, a cut-off value of 18.63 for the pleural fluid LDH/ADA ratio was established for diagnostic performance analysis. The area under the curve (AUC) to discriminate between TPE and PPE was 0.960 [95% confidence interval (CI): 0.902 to 0.989]. As for sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), positive predictive value and negative predictive value, they were 94.44% (95% CI: 81.3% to 99.3%), 84.85% (95% CI: 73.9% to 92.5%), 6.23 (95% CI: 3.50 to 11.09), 0.065 (95% CI: 0.017 to 0.25), 77.3% (95% CI: 65.6% to 85.8%), and 96.6% (95% CI: 87.9% to 99.1%), respectively. The diagnostic accuracy of the pleural fluid LDH/ADA ratio was further tested and confirmed in the subsequent meta-analysis. The meta-analysis demonstrated that the pooled sensitivity and specificity were 90% (95% CI: 82% to 94%) and 91% (95% CI: 84% to 95%), respectively.
Conclusions
The current study suggests that the pleural fluid LDH/ADA ratio is a useful marker for distinguishing TPE from PPE.
Keywords: Pleural fluid lactate dehydrogenase/adenosine deaminase ratio (pleural fluid LDH/ADA ratio), tuberculosis pleural effusion (TPE), parapneumonic effusion (PPE)
Highlight box.
Key findings
• The current study suggests that the pleural fluid lactate dehydrogenase (LDH)/adenosine deaminase (ADA) ratio is a useful marker for distinguishing tuberculosis pleural effusion (TPE) from parapneumonic effusion (PPE).
What is known and what is new?
• In regions with a high prevalence of tuberculosis, it is crucial to accurately differentiate between TPE and PPE. In recent years, the ratio of LDH to ADA has been utilized to differentiate between TPE and PPE with a high degree of diagnostic accuracy.
• In the present study, we aimed to assess the potential role of the pleural fluid LDH/ADA ratio in distinguishing between TPE and PPE within our cohort. A cut-off value of 18.63 for the pleural fluid LDH/ADA ratio was established for diagnostic performance analysis. The area under the curve to discriminate between TPE and PPE was 0.960 [95% confidence interval (CI): 0.902 to 0.989]. We also summarized the results from our own findings and those of others through a meta-analysis. The meta-analysis demonstrated that the pooled sensitivity and specificity were 90% (95% CI: 82% to 94%) and 91% (95% CI: 84% to 95%), respectively.
What is the implication, and what should change now?
• Where diagnostic challenges exist, the pleural fluid LDH/ADA ratio serves as a valuable diagnostic tool. Clinicians can initiate empirical anti-tuberculosis treatment while awaiting culture results, especially in young patients from regions with a high incidence of tuberculosis. On the other hand, in low-prevalence regions, this test may yield a lower positive predictive value due to a higher rate of false positives, thereby necessitating confirmatory testing, such as biopsy.
Introduction
Pleural effusion is a common respiratory condition caused by a variety of reasons, including microbial infections, heat failure, pulmonary embolism, and cancer (1). The clinical spectrum of pleural effusions caused by infections is broad, and the differential diagnosis among pleural effusions caused by different infections remains challenging. Tuberculosis pleural effusion (TPE) is one of the most frequent forms of extra-pulmonary tuberculous resulting from Mycobacterium tuberculosis infection (2,3). Although a definitive diagnosis requires microbiological detection via smear and culture, or the identification of caseating granulomas in pleural tissue, a comprehensive analysis of pleural fluid including biomarkers such as adenosine deaminase (ADA) and gamma interferon provides a noninvasive procedure with high diagnostic accuracy in clinical practice (4).
Although there were methods based on lymphocytic or neutrophilic predominance, and ADA levels in pleural fluid to discriminate TPE from parapneumonic effusion (PPE) (5,6), they are not always effective when one disease exhibits similar phenotypic similarities to the other (7-9). ADA is a promising and valuable biomarker for the diagnosis of TPE, however, its performance varies across regions with different tuberculosis prevalence rates (10-12). Additionally, a non-negligible proportion of PPE patients also exhibit high ADA levels exceeding 40 IU/L (13). In recent years, the ratio of lactate dehydrogenase (LDH) to ADA has been utilized to differentiate between TPE and PPE with a high degree of diagnostic accuracy, particularly in regions with a high prevalence of tuberculosis (14,15).
In the present study, we aimed to assess the potential role of the pleural fluid LDH/ADA ratio in distinguishing between TPE and PPE within our cohort, and we summarized the results from our own findings and those of others through a meta-analysis. We present this article in accordance with the STROBE and PRISMA reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2295/rc).
Methods
Study populations
We conducted a retrospective review of all patients diagnosed with TPE or PPE at the Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital from 2020 to 2023. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Ethics Committee of Beijing Chaoyang Hospital of Capital Medical University approved this study (No. 2018-11-21-12). The need for informed consent was waived by the ethics committee of Beijing Chaoyang Hospital of Capital Medical University because of the retrospective nature of the study.
Diagnostic criteria
TPE was diagnosed when either Ziehl-Neelsen staining or Löwenstein-Jensen culture of pleural fluid, sputum, or pleural biopsy specimens yielded positive results, or when granulomas were found in parietal pleural biopsy specimens. PPE was diagnosed when any effusion associated with bacterial pneumonia, lung abscess, or bronchiectasis; empyema was also included in this group.
Data collection
Demographic characteristics and laboratory test results were extracted from electronic medical records, including age, gender, smoking history, drinking history, symptom duration, comorbidity types, and pleural ADA and LDH. The ratio of pleural fluid LDH/ADA was calculated.
Statistical analysis
The optimal cut-off values were determined using Youden’s J statistic and sensitivity, specificity, and receiver operating characteristic (ROC) curve were examined to assess the diagnostic performance of the pleural fluid LDH/ADA ratio. The area under the curve (AUC) and reclassification parameters consisting of the net reclassification index (NRI) (16) and integrated discrimination improvement (IDI) (17) were also used to compare the diagnostic benefit of the LDH/ADA ratio over ADA alone. All significance tests were two-sided, with P<0.05 indicating statistically significant. Statistical analysis was performed using SPSS version 26 (IBM Corporation, Armonk, NY, USA), MedCalc version 9.3.1 (Med-Calc, Inc., Mariakerke, Belgium), and R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Meta-analysis
We conducted a systematical search of several medical databases, including Medline, Embase, and the Cochrane Library, up to June 2024. The search strategy was as follows: (“TPE” OR “tuberculosis pleural effusion” OR “tuberculous pleuritis” OR “PPE” OR “parapneumonic effusion” OR “infectious pleurisy” OR “pleural effusion”) AND (“Lactate dehydrogenase to adenosine deiminase ratio” OR “LDH to ADA” OR “LDH/ADA”).
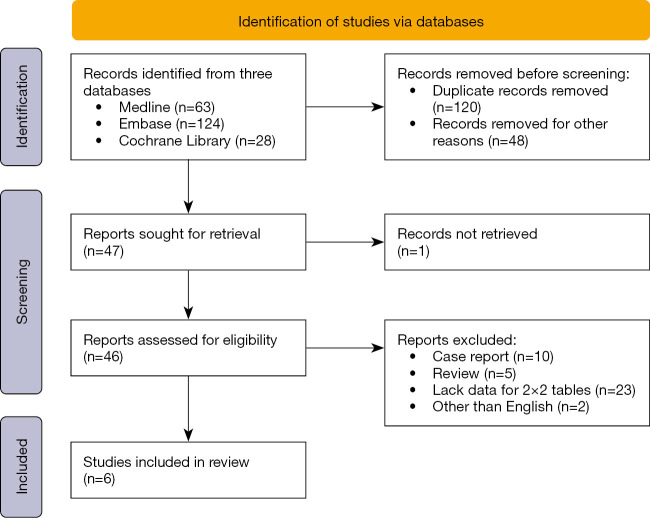
Studies were included if they were full-text and could provide accurate 2×2 data including true-positives, true-negatives, false-positives, and false-negatives. Only English articles were permitted for the full-text review and final analysis. The exclusion criteria for publications were as follows: articles in the form of reviews, chapters, guidelines, case reports, editorials, or letters; non-human studies; studies that lacked data for 2×2 tables. When the same sample group of individuals was analyzed in multiple publications, the results were accounted for only once.
The data retrieved from the studies encompassed participant characteristics, cut-off values, publication year, and methodological quality. Two reviewers scrutinized all related articles and an investigator made the final decision on the disagreements and examined whether any additional studies have been neglected.
The pooled weighted sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR), SROC curve, and meta-regression were performed using Stata version 9 (Stata Corporation, College Station, TX, USA) and Meta-DiSc for Windows (XI Cochrane Colloquium, Barcelona, Spain). Inconsistency was computed to indicate the variability caused by unobserved heterogeneity. Since publication bias is of concern for meta-analysis of diagnostic studies, we examined the potential presence of this bias using the Deeks funnel plot asymmetry test. A P value of less than 0.05 was considered to indicate a statistically significant result.
Results
Patient characteristics
In the current study, we retrospectively reviewed patients who were definitely diagnosed with TPE or PPE at the Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital from 2020 to 2023, as shown in Figure 1. A total of 126 patients were included, as depicted in Table 1. Among these patients, 45 were diagnosed with TPE, and 81 with PPE. The average age of the TPE patients was younger than that of the PPE patients in our cohort.
Figure 1.
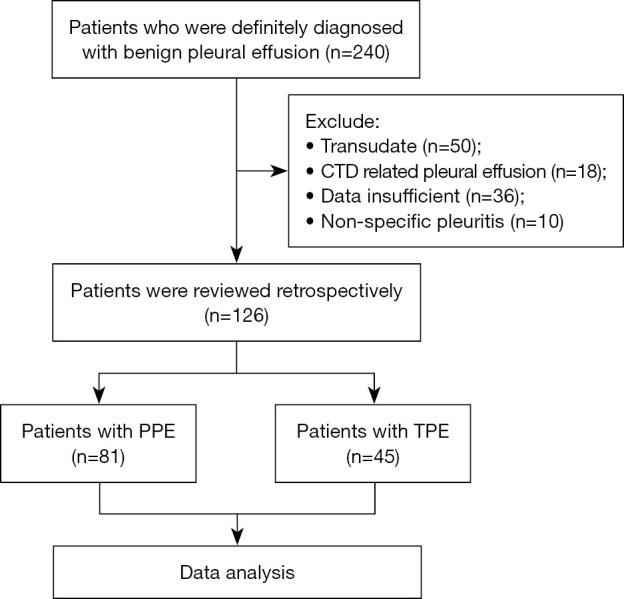
Flow diagram for our study population. CTD, connective tissue disease; PPE, parapneumonic effusion; TPE, tuberculosis pleural effusion.
Table 1. Comparison of clinical and laboratory findings between patients with TPE and PPE.
| Characteristics | All (n=126) | TPE (n=45) | PPE (n=81) | P value |
|---|---|---|---|---|
| Age (years) | 62 [17–91] | 39 [31–65] | 65 [53–75] | <0.001 |
| Male | 86 (68.3) | 21 (46.7) | 65 (80.2) | <0.001 |
| Smoking history | 0.006 | |||
| Current/former | 61 (48.4) | 14 (31.1) | 47 (58.0) | |
| No | 60 (47.6) | 30 (66.7) | 30 (37) | |
| NA | 5 (4.0) | 1 (2.2) | 4 (4.9) | |
| Drinking history | 0.04 | |||
| Current/former | 31 (24.6) | 6 (13.3) | 25 (30.9) | |
| No | 89 (70.6) | 38 (84.4) | 51 (63) | |
| NA | 6 (4.8) | 1 (2.2) | 5 (6.2) | |
| Symptom duration (days) | 14 [9–22] | 12 [8–17] | 17 [7–24] | 0.02 |
| Comorbidity types | ||||
| Hypertension | 20 (15.9) | 5 (11.1) | 15 (18.5) | 0.28 |
| Diabetes mellitus | 17 (13.5) | 3 (6.7) | 14 (17.3) | 0.10 |
| Cerebrovascular disease | 7 (5.6) | 0 (0.0) | 7 (8.6) | 0.04 |
| Coronary heart disease | 28 (22.2) | 4 (8.9) | 24 (29.6) | 0.007 |
| Pleural fluid biomarkers | ||||
| LDH (U/L) | 320.0 [174.0–565.8] | 341.5 [221.8–453.3] | 263.5 [157.0–777.3] | 0.23 |
| ADA (U/L) | 18.5 [9–38.3] | 39.5 [30.3–58.0] | 11.0 [5.0–20.0] | <0.001 |
| LDH/ADA ratio | 21.6 [10.3–38.7] | 7.9 [6.4–11.8] | 32.9 [21.5–52.5] | <0.001 |
Continuous variables are presented as median [interquartile range] and qualitative variables as n (%). ADA, adenosine deaminase; LDH, lactate dehydrogenase; NA, not available; PPE, parapneumonic effusion; TPE, tuberculosis pleural effusion.
In the study population, 46.7% of TPE patients were male, whereas the majority of PPE patients (80.2%) were male. Additionally, there were differences in smoking and drinking history between TPE and PPE patients. We also collected the concentrations of pleural fluid LDH, ADA, and the pleural fluid LDH/ADA ratio. The PPE group exhibited a higher incidence of cardiocerebrovascular comorbidities and prolonged symptom duration, which may be associated with their advanced age. We found that the pleural fluid ADA concentrations were significantly higher in patients with TPE than in those with PPE patients with values of 39.5 vs. 11.0 U/L. Although no significant differences in pleural fluid LDH levels were observed between the two groups, the pleural fluid LDH/ADA ratio was much lower in the TPE group compared to the PPE group (7.9 vs. 32.9).
Diagnostic values of pleural LDH, ADA, and the pleural fluid LDH/ADA in our study population
To evaluate the diagnostic accuracy of the pleural fluid LDH/ADA ratio in discrimination between TPE and PPE in our study population, AUC was calculated to be 0.960 (P<0.001, 95% CI: 0.902 to 0.989). In comparison, for ADA alone, the AUC was 0.889 (P<0.001, 95% CI: 0.815 to 0.941), as shown in Figure 2. The optimal threshold for the pleural fluid LDH/ADA ratio was determined to be 18.63, with a sensitivity of 94.44% (95% CI: 81.3% to 99.3%) and a specificity of 84.85% (95% CI: 73.9% to 92.5%), as depicted in Figure 3. The rule-out threshold with maximizing sensitivity was 27.00 whereas the rule-in threshold with maximizing specificity was 8.91. The PLR was 6.23 (95% CI: 3.50 to 11.09) and the NLR was 0.065 (95% CI: 0.017 to 0.25). The positive predictive value was 77.3% (95% CI: 65.6 to 85.8%) and the negative predictive value was 96.6% (95% CI: 87.9% to 99.1%). Compared with ADA alone, there was a significant increase in reclassification statistics when assessing with the pleural fluid LDH/ADA ratio through NRI 1.174 (P<0.001) and IDI 0.395 (P<0.001).
Figure 2.

Diagnostic accuracy of ADA alone and the pleural fluid LDH/ADA ratio in discrimination between TPE and PPE in our study population. ROC curves illustrated the diagnostic performance of ADA alone and the pleural fluid LDH/ADA ratio in our study population including 45 patients with TPE and 81 patients with PPE. ADA, adenosine deaminase; AUC, area under the curve; LDH, lactate dehydrogenase; PPE, parapneumonic effusion; ROC, receiver operating characteristic; TPE, tuberculosis pleural effusion.
Figure 3.
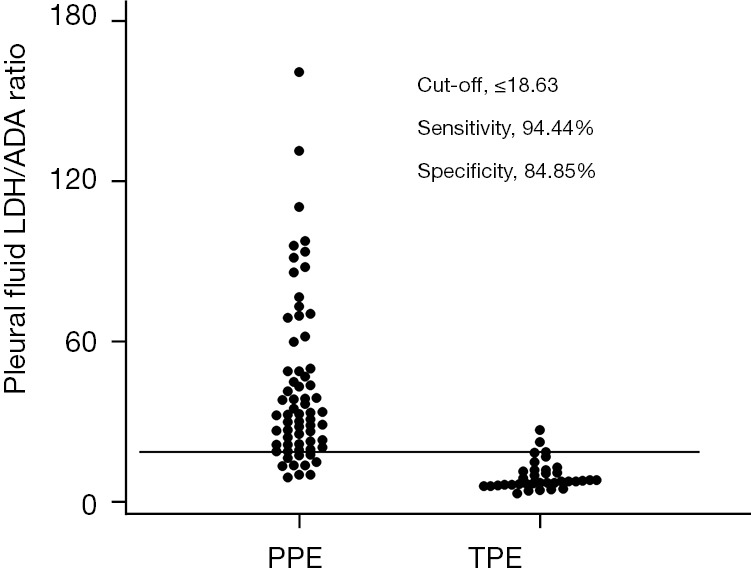
Diagnostic performance of the pleural fluid LDH/ADA in discrimination between TPE and PPE in our study population. A cut-off value of 18.63 for pleural fluid LDH/ADA exhibited high sensitivity and specificity for discrimination between TPE and PPE in our study population. ADA, adenosine deaminase; LDH, lactate dehydrogenase; PPE, parapneumonic effusion; TPE, tuberculosis pleural effusion.
Overall diagnostic accuracy of pleural fluid LDH/ADA ratio from meta-analysis
After conducting an independent and extensive review (Figure 4), we found that six publications reported on the pleural fluid LDH/ADA ratio for distinguishing between TPE and PPE (14,18-22). Along with the data from our current study, we integrated seven eligible studies to conduct a meta-analysis. The basic information of these studies is listed in Table 2. The average sample size of the seven included studies on pleural fluid LDH/ADA ratio was 212 (range, 93 to 518). A total of 1,480 subjects were included in the meta-analysis and 880 patients were TPE and 600 were PPE. Most of the studies were conducted in tuberculosis prevailing regions except for Spain.
Figure 4.
Flow diagram for meta-analysis.
Table 2. Characteristics of the included studies.
| Study | Year | Country | High-incidence setting | Age range (years) | Number of patients | Cut-off | Prospective | QUADAS score | |
|---|---|---|---|---|---|---|---|---|---|
| TPE | PPE | ||||||||
| Wang J | 2017 | China | Yes | 17–82 | 72 | 47 | 16.20 | No | 9 |
| Beukes A | 2021 | South Africa | Yes | Unknown | 160 | 4 | 12.50 | Yes | 12 |
| Vieira JL | 2021 | Brazil | Yes | ≤18 | 25 | 68 | 8.3 | No | 11 |
| Ho CY | 2022 | China | Yes | Unknown | 125 | 139 | 14.2 | No | 9 |
| Núñez-Jurado D | 2023 | Spain | No | Unknown | 41 | 155 | 10.95 | No | 10 |
| Zhao T | 2024 | China | Yes | Unknown | 412 | 106 | 24.32 | No | 12 |
| The current study | 2024 | China | Yes | ≥18 | 45 | 81 | 18.63 | No | 10 |
PPE, parapneumonic pleural effusion; QUADAS, quality assessment for studies of diagnostic accuracy; TPE, tuberculous pleural effusion.
The guidelines from the Quality Assessment of Diagnostic Accuracy Studies (QUADAS; with a maximum score of 14) (23) were utilized to evaluate the methodological quality. Table 2 indicated that the study design exhibited a high level of quality, and the diagnostic accuracy of the studies was commendable, as all achieved high QUADAS scores (≥9).
The forest plot illustrates the sensitivity and specificity of seven pleural fluid LDH/ADA assays in discriminating between TPE and PPE. The pooled sensitivity and specificity were 0.90 (95% CI: 0.82 to 0.94) and 0.91 (95% CI: 0.84 to 0.95), respectively (Figure 5A). The PLR and NLR were 9.54 (95% CI: 5.13 to 17.74) and 0.11 (95% CI: 0.06 to 0.21), respectively. The summary ROC (SROC) curve of the pleural fluid LDH/ADA ratio was very close to the expected upper left corner, indicating an AUC of 0.96 (95% CI: 0.94 to 0.97) (Figure 5B).
Figure 5.

Meta-analysis of overall diagnostic performance of the pleural fluid LDH/ADA ratio in discrimination between TPE and PPE. (A) The forest plots of estimates of sensitivity, specificity for the pleural fluid LDH/ADA ratio in discrimination between TPE and PPE in meta-analysis. The point estimates of sensitivity and specificity from each study are shown as solid circles. Error bars are 95% CI. (B) A SROC curve with 95% CI for the pleural fluid LDH/ADA ratio in discrimination between TPE and PPE in meta-analysis. Each solid circle represents one study in the meta-analysis. ADA, adenosine deaminase; AUC, area under the curve; CI, confidence interval; LDH, lactate dehydrogenase; PPE, parapneumonic effusion; SE, standard error; SROC, summary receiver operating characteristic; TPE, tuberculosis pleural effusion.
The post-test probabilities of TPE were investigated based on various pre-test probabilities and pleural fluid LDH/ADA ratio data. The investigation revealed that for a patient with 10% pre-test probability (a hypothetical low-risk) (2,24), and a positive pleural fluid LDH/ADA test, the estimated post-test probability was 43%. Conversely, a negative result excluded TPE with a 2% post-test probability (Table 3). For a patient with a pre-test probability of 25% or 50%, a positive test result for pleural fluid LDH/ADA increased the probability to 70% or 87%, respectively. Consequently, a negative fluid LDH/ADA result decreased the post-test probability of the disease to 4% or 12%, respectively.
Table 3. Post-test probability of TPE according to pre-test probability.
| Post-test probability | Pre-test probability | ||
|---|---|---|---|
| 10% | 25% | 50% | |
| With positive result (%) | 43 | 70 | 87 |
| With negative result (%) | 2 | 4 | 12 |
TPE, tuberculous pleural effusion.
Finally, we performed the funnel plots to assess publication bias. Although the results showed some asymmetry (Figure 6), the Deeks test was non-significant (P=0.92), suggesting the publication bias was not present.
Figure 6.
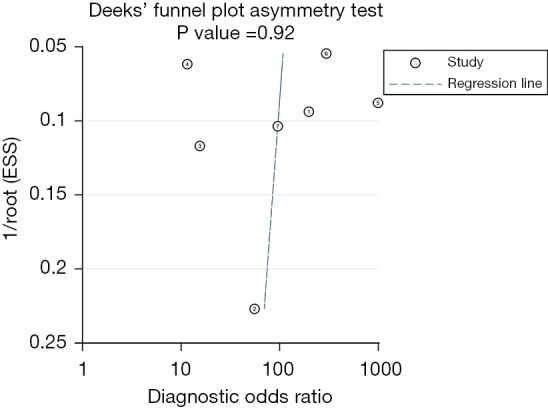
Deeks’ funnel graph for the assessment of potential publication bias for meta-analysis. The funnel graph plots the DOR against the standard error of the log of the DOR (an indicator of sample size). Each circle represents each study in the meta-analysis. The central dash line indicates the summary DOR. DOR, diagnostic odds ratio; ESS, effective sample size.
Discussion
TPE and PPE are common types of exudative pleural effusion in regions with a high prevalence of tuberculosis (25). Timely and appropriate therapy is crucial for reducing mortality and morbidity (7). Therefore, the development of a convenient and accurate diagnostic approach is of particular importance in these areas. To overcome the disadvantage of approaches based on predominant cell populations and ADA levels, researchers have found that the pleural fluid LDH/ADA ratio was a promising and useful biomarker for discrimination between TPE and PPE.
In our current study, we tested and confirmed the diagnostic performance of pleural fluid LDH/ADA ratios in discrimination between TPE and PPE within our cohort. We found that a pleural fluid LDH/ADA ratio ≤18.63 offered a sensitivity of 94.44%, specificity of 84.85%, a PLR of 6.23, and a NLR of 0.065 for identifying TPE. Subsequently, we summarized the existing results of diagnostic performance of pleural fluid LDH/ADA ratios from other studies and our own, providing more convinced evidence to support the notion that the pleural fluid LDH/ADA ratio is a valuable diagnostic biomarker for discriminating between TPE and PPE, with the pooled sensitivity and specificity of 90.0% and 91.0%, respectively. A NLR of 0.065 in our data suggests that if the pleural fluid LDH/ADA ratio is negative, the likelihood of the patient having TPE is very low. This could potentially prevent unnecessary invasive diagnostic procedures for patients in such cases.
The NRI and IDI were initially introduced to assess the added predictive value of a new marker, and have been widely used. In our cohort analysis, the NRI reached 1.174 (P<0.001), reflecting enhanced diagnostic discrimination capacity. Furthermore, there was a significant statistical difference between ADA and pleural fluid LDH/ADA with an IDI of 0.395 (P<0.001). Collectively, considering the three performance indicators (AUC, NRI, and IDI), they provide robust evidence for the diagnostic superiority of the pleural fluid LDH/ADA ratio over conventional ADA testing.
A key objective of meta-analysis is to explore the causes of heterogeneity rather than to calculate a single summary measure. In our study, we identified significant heterogeneity in sensitivity, specificity, PLR, and NLR among the analyzed studies. We found no evidence to indicate that studies of higher quality exhibited better test performance compared to those of lower quality. Furthermore, we noted that prospective design, cut-off values, and incidence settings did not significantly affect diagnostic accuracy. In the subgroup analysis that included studies from mainland China, heterogeneity was notably reduced, suggesting that the source of heterogeneity might be associated with regional prevalence differences.
The pre-test probability of TPE was considered to assisting in making clinical decisions based on the analysis of pleural fluid LDH/ADA ratio results from a meta-analysis. The results demonstrated that when the predicted probability of TPE is 10% (an assumptive low-risk), the overall post-test probability of positive pleural fluid LDH/ADA ratio results for TPE is 43.0%. However, in high prevalence settings, a positive pleural fluid LDH/ADA ratio result increases the probability to 87.0%, suggesting that the use of pleural fluid LDH/ADA ratio is not recommended in TPE non-prevalent region, and the diagnostic performance of pleural fluid LDH/ADA ratio is much better in TPE prevalent regions. Where diagnostic challenges exist, the pleural fluid LDH/ADA ratio serves as a valuable diagnostic tool. Clinicians can initiate empirical anti-tuberculosis treatment while awaiting culture results, especially in young patients from regions with a high incidence of tuberculosis. On the other hand, in low-prevalence regions, this test may yield a lower positive predictive value due to a higher rate of false positives, thereby necessitating confirmatory testing, such as biopsy.
There are several limitations in our study. Firstly, the analysis of the diagnostic performance of the pleural fluid LDH/ADA ratio in distinguishing between TPE and PPE was conducted as a retrospectively at a single-center, making it difficult to completely avoid selection bias. Consequently, future research should incorporate larger, multi-center samples to improve generalizability. Secondly, the number of available studies was limited, with only six, excluding the current study, and one of these studies included only four PPE specimens (18). The small sample size and the specific type of samples used may lead to selection bias and publication bias, despite the non-significant results of the Deeks funnel plot asymmetry test. Thirdly, different studies have used varying cut-off values to differentiate between TPE and PPE based on the optimal combination of sensitivity and specificity, which could also lead to biased outcomes. Fourthly, our current study only focused on the diagnostic performance of the pleural fluid LDH/ADA ratio in distinguishing between TPE and PPE, excluding other types of pleural effusion.
Conclusions
In summary, our current study has demonstrated that the pleural fluid LDH/ADA ratio serves as a beneficial biomarker for the discrimination between TPE and PPE in our cohort. Our meta-analysis further suggested that for differentiating between TPE and PPE, the pleural fluid LDH/ADA ratio exhibited a better performance in regions where TPE is prevalent. However, it is not recommended for use in non-prevalent regions. This indicator is cost-effective, has a short turnaround time, and is easily accessible. Compared to using ADA alone, it exhibits superior diagnostic performance. Clinicians can utilize this readily available marker to better distinguish between TPE and PPE.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Ethics Committee of Beijing Chaoyang Hospital of Capital Medical University approved this study (No. 2018-11-21-12). The need for informed consent was waived by the ethics committee of Beijing Chaoyang Hospital of Capital Medical University because of the retrospective nature of the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE and PRISMA reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2295/rc
Funding: This work was supported by the Reform and Development Program of Beijing Institute of Respiratory Medicine (Nos. Ggyfz202420 and Ggyfz202518).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2295/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2295/dss
References
- 1.Shen-Wagner J, Gamble C, MacGilvray P. Pleural Effusion: Diagnostic Approach in Adults. Am Fam Physician 2023;108:464-75. [PubMed] [Google Scholar]
- 2.Wang W, Zhou Q, Zhai K, et al. Diagnostic accuracy of interleukin 27 for tuberculous pleural effusion: two prospective studies and one meta-analysis. Thorax 2018;73:240-7. 10.1136/thoraxjnl-2016-209718 [DOI] [PubMed] [Google Scholar]
- 3.Hussein M, Thomas M, Al-Tikrity M, et al. Etiology of exudative pleural effusion among adults: differentiating between tuberculous and other causes, a multicenter prospective cohort study. IJID Reg 2024;12:100425. 10.1016/j.ijregi.2024.100425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 2019;24:962-71. 10.1111/resp.13673 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Zamalloa A, Taboada-Gomez J. Diagnostic accuracy of adenosine deaminase and lymphocyte proportion in pleural fluid for tuberculous pleurisy in different prevalence scenarios. PLoS One 2012;7:e38729. 10.1371/journal.pone.0038729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008;102:744-54. 10.1016/j.rmed.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Yoo SS, Lee SY, et al. Pleural fluid adenosine deaminase/serum C-reactive protein ratio for the differentiation of tuberculous and parapneumonic effusions with neutrophilic predominance and high adenosine deaminase levels. Infection 2017;45:59-65. 10.1007/s15010-016-0928-5 [DOI] [PubMed] [Google Scholar]
- 8.Lin MT, Wang JY, Yu CJ, et al. Mycobacterium tuberculosis and polymorphonuclear pleural effusion: incidence and clinical pointers. Respir Med 2009;103:820-6. 10.1016/j.rmed.2008.12.023 [DOI] [PubMed] [Google Scholar]
- 9.Ruan SY, Chuang YC, Wang JY, et al. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822-7. 10.1136/thoraxjnl-2011-201363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YC, Rogers JT, Rodriguez RM, et al. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest 2001;120:356-61. 10.1378/chest.120.2.356 [DOI] [PubMed] [Google Scholar]
- 11.Jiménez Castro D, Díaz Nuevo G, Pérez-Rodríguez E, et al. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. Eur Respir J 2003;21:220-4. 10.1183/09031936.03.00051603 [DOI] [PubMed] [Google Scholar]
- 12.Sivakumar P, Marples L, Breen R, et al. The diagnostic utility of pleural fluid adenosine deaminase for tuberculosis in a low prevalence area. Int J Tuberc Lung Dis 2017;21:697-701. 10.5588/ijtld.16.0803 [DOI] [PubMed] [Google Scholar]
- 13.Light RW. Update on tuberculous pleural effusion. Respirology 2010;15:451-8. 10.1111/j.1440-1843.2010.01723.x [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Liu J, Xie X, et al. The pleural fluid lactate dehydrogenase/adenosine deaminase ratio differentiates between tuberculous and parapneumonic pleural effusions. BMC Pulm Med 2017;17:168. 10.1186/s12890-017-0526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakiston M, Chiu W, Wong C, et al. Diagnostic Performance of Pleural Fluid Adenosine Deaminase for Tuberculous Pleural Effusion in a Low-Incidence Setting. J Clin Microbiol 2018;56:e00258-18. 10.1128/JCM.00258-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas LE, O'Brien EC, Piccini JP, et al. Application of net reclassification index to non-nested and point-based risk prediction models: a review. Eur Heart J 2019;40:1880-7. 10.1093/eurheartj/ehy345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157-72; discussion 207-12. 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 18.Beukes A, Shaw JA, Diacon AH, et al. The Utility of Pleural Fluid Lactate Dehydrogenase to Adenosine Deaminase Ratio in Pleural Tuberculosis. Respiration 2021;100:59-63. 10.1159/000509555 [DOI] [PubMed] [Google Scholar]
- 19.Vieira JL, Foschiera L, Ferreira ICS, et al. Performance of the quantification of adenosine deaminase and determination of the lactate dehydrogenase/adenosine deaminase ratio for the diagnosis of pleural tuberculosis in children and adolescents. J Bras Pneumol 2021;47:e20200558. 10.36416/1806-3756/e20200558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho CY, Tsai YH, Chang CC, et al. The role of pleural fluid lactate dehydrogenase-to-adenosine deaminase ratio in differentiating the etiology of pleural effusions. Chin J Physiol 2022;65:105-8. 10.4103/cjp.cjp_104_21 [DOI] [PubMed] [Google Scholar]
- 21.Núñez-Jurado D, Rodríguez-Martín I, Guerrero JM, et al. LDH/ADA ratio in pleural fluid for the diagnosis of infectious pleurisy. Clin Exp Med 2023;23:5201-13. 10.1007/s10238-023-01194-y [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, Zhang J, Zhang X, et al. Clinical significance of pleural fluid lactate dehydrogenase/adenosine deaminase ratio in the diagnosis of tuberculous pleural effusion. BMC Pulm Med 2024;24:241. 10.1186/s12890-024-03055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Yu YX, Wang XJ, et al. Diagnostic Accuracy of Interleukin-27 between Tuberculous Pleural Effusion and Malignant Pleural Effusion: A Meta-Analysis. Respiration 2018;95:469-77. 10.1159/000486963 [DOI] [PubMed] [Google Scholar]
- 25.Maji A, Maikap MK, Jash D, et al. Role of common investigations in aetiological evaluation of exudative pleural effusions. J Clin Diagn Res 2013;7:2223-6. 10.7860/JCDR/2013/6738.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]