Abstract
Indeterminate pulmonary nodules (IPNs), which are nodules that cannot be classified as definitively benign or malignant at the time of detection, are now diagnosed on the order of millions per year. Management of IPNs remains heavily debated, and routine practice ultimately involves some balance of overall clinical risk assessment and additional diagnostic tests or procedures which may generate significant risk, cost, and worry. Biomarkers are biologically based tests or indicators capable of accurately characterizing the physiologic properties of homeostasis and disease that are not otherwise easily evaluated by the clinician. Accurate biomarkers thereby serve as reliable surrogates for biological aberrancy, and importantly for the field of diagnostics, can signal early pathology before it becomes clinically detectable. In the realm of IPNs, biomarker development seeks to address a growing need for noninvasive adjunct tools that can be leveraged clinically to add clarity where diagnostic uncertainty exists. Here, effective diagnostic biomarkers have the potential to hone clinical management, accelerate treatment when indicated, and curb added unnecessary diagnostics. In this review article, the authors highlight the role for biomarkers in the diagnosis of IPNs, outline the methodology for successful biomarker development, and discuss contemporary IPN biomarker research and the remaining challenges and future directions for the field.
Keywords: Biomarkers, indeterminate pulmonary nodules (IPNs), lung cancer, Early Detection Research Network (EDRN)
Introduction
Lung cancer remains the leading cause of cancer mortality, accounting for up to 25% of all cancer-related deaths and annually claiming more lives than colon, breast, and prostate cancers combined (1). With over 2.2 million new cases diagnosed and 1.8 million attributable deaths in 2020 alone, lung cancer remains a major public health concern (2). Despite its prevalence, lung cancer has significantly lower survival than most cancers, with an overall 5-year survival rate of approximately 25% in the United States (3). Late diagnosis is responsible for much of these dismal statistics, and therefore monumental research and clinical efforts have sought to accelerate disease detection. The 2011 National Lung Screening Trial highlighted the utility of systematic lung cancer screening (4). However, this landmark study also illustrated a major limitation of image-based screening: the false positive finding. Over 96% of positive screening results in the low-dose computed tomography (CT) group were ultimately not malignant, underscoring the significant potential for additional costly, high risk, and anxiety-provoking diagnostics presented by indeterminate findings (4).
Indeterminate pulmonary nodules (IPNs), which are nodules that cannot be classified as definitively benign or malignant at the time of detection, are now diagnosed on the order of over 1.5 million annually in the United States and 95% ultimately prove to be benign (5). Beyond screening, IPNs are incidentally detected in up to 30% of CT scans obtained for other reasons (6,7). IPNs have been studied extensively, but their management has largely remained unchanged and is based on clinician best judgement given nodule size and characteristics, patient age, and history of smoking (8). Collectively, this translates into a dizzying epidemic of diagnostic uncertainty that is only growing.
As previously outlined by this team and others, the role for biomarkers in the diagnosis of IPNs is manifold and includes early detection of malignancy, enrichment of screening populations, and classification of IPNs as high- or low-risk (9-13). Focusing on 2020–2024, this review explores interval advances, contemporary perspectives, and remaining challenges in IPN biomarker science. We will illustrate the role for IPN biomarkers in clinical practice, outline the process for taking biomarkers from the bench to the bedside, discuss a select subset of biomarkers in development, and provide perspective on remaining barriers to implementation.
Methods
The PubMed online database was independently queried by two members of the research team. Keywords searched included “Biomarkers”, “Pulmonary Nodules”, “Lung Nodules”, “Indeterminate Pulmonary Nodules”, “IPNs”, “Lung Cancer”, “Early Detection”, and “Diagnosis”. Results were filtered to include only original research manuscripts published from 2020–2024 and available in English. Search results were further stratified by level of evidence, number of citations, and the impact factor of the publishing journal. Reviewers independently compiled lists of the most meaningful and significant publications, which were then compared to arrive at a consensus of select articles deemed to be representative of the most active and promising areas of research and suitable for inclusion here.
The making of a biomarker
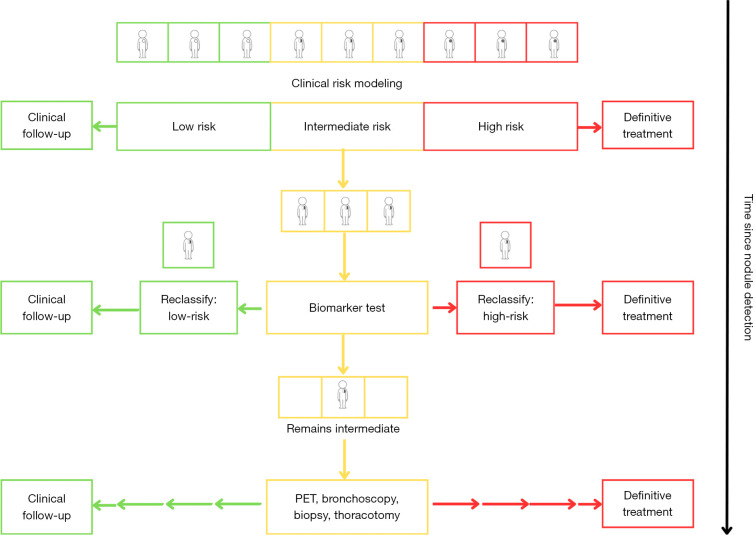
A thorough understanding of a biomarker’s role in clinical practice is fundamental to successful biomarker science. The ideal biomarker adds discriminatory power, aids in decision-making where diagnostic uncertainty exists, and ultimately changes clinical management rather than simply reinforcing it. Current IPN management typically defaults to an “opt-in” model, meaning IPN detection is usually followed by some combination of additional imaging, tests, or invasive procedures. Biomarkers add an “opt-out” pathway to this paradigm, as accurate “rule-out” biomarkers decrease pretest probability and reclassify intermediate findings as low risk to curb unnecessary diagnostics when the likelihood of malignancy is low (14,15). Conversely, accurate “rule-in” biomarkers reclassify intermediate findings as high risk to expedite the escalation of care when malignancy is likely (Figure 1).
Figure 1.
Risk classification framework for the clinical utility of a discriminatory biomarker for indeterminate pulmonary nodules. PET, positron emission tomography.
Since its inception by the National Cancer Institute in 2008, the Early Detection Research Network (EDRN) has laid the path to successful biomarker development and created a systematic five-phase framework for biomarker development which now serves as the field’s benchmark (16,17). Phases I and II comprise the exploratory studies for biomarker discovery, and the subsequent early validation and measurement of the biomarker’s discriminatory power, respectively. Phase III focuses on a biomarker’s ability to identify preclinical disease by testing it against longitudinally collected research samples. Finally, Phases IV and V consist of the prospective screening and large-scale population studies required to validate a candidate biomarker’s potential impact on its targeted population. Additional guidance published by the American Thoracic Society provides practical insight on biomarker assessment (18). As the authors discuss, the calculus behind any biomarker’s use necessarily involves some trade-off between its sensitivity and specificity, and corresponding false positive and negative rates, to arrive at a judgment about the acceptable harm/benefit ratio of its use.
Bottom line—biomarker development must be systematic and demonstrative of real clinical impact. A biomarker must be accurate, reproducible, risk-averse, cost-effective, and scalable to the broadest possible setting. Perhaps most importantly, a truly useful biomarker should add information that changes clinical management rather than simply corroborating decisions that would already be made in its absence. In the end, the role for biomarkers hinges on efficiency—biomarkers should expedite necessary follow-up and treatment, and reduce extraneous diagnostics that add unproductive costs, risks, and worry.
Protein-based biomarkers
Proteins have been extensively explored as biomarkers since they serve as a principal functional unit of biological activity and can be readily tested. As noninvasive surrogates for cellular activity, proteins can be leveraged to detect differential expression patterns and footprints at the molecular level. In the realm of lung cancer, this has been demonstrated by detection of tumor associated antigens, autoantibodies, activation of the complement cascade, and high throughput and omic-level analysis of expression profiles (19-22). Attractive features of most protein-based biomarker assays include their relatively low costs and potential for minimally invasive sampling, which could expedite large-scale deployment and enable longitudinal sampling for tumor evolution or recurrence. The success of single-protein biomarkers like prostate-specific antigen in prostate cancer has enticed investigation into correlates for lung cancer, but capturing similar success has proven elusive.
In 2021, Ostrin et al. applied a blood-based four marker protein panel previously validated for lung cancer risk to IPNs (23). This four-marker protein panel (4MP) consisted of immunoassays targeting three well-explored cancer proteins, carcinoembryonic antigen (CEA), cancer antigen 125 (CA-125), and cytokeratin-19 fragment (CYFRA 21-1), as well as one novel immunoassay to the cleaved propeptide of lung-specific surfactant protein B (pro-SFTPB). The 4MP was measured on two IPN cohorts from the University of Pittsburgh Medical Center and the University of Texas Southwestern. The 4MP was measured using the coefficients previously optimized for lung cancer risk determination, enabling continued validation for a new indication. The 4MP performed well in distinguishing benign from malignant nodules, with an area under the receiver operating characteristic curve (AUC) of 0.76 [95% confidence interval (CI): 0.69–0.82] in the Pittsburgh cohort. In this same cohort, nodule size alone performed well, with an AUC of 0.86, but nodule size combined with the 4MP yielded an increased AUC of 0.90. The 4MP improved sensitivity at a high specificity, raising sensitivity at 99% specificity from 14% for nodule size alone to 42% with the 4MP added. This was replicated in the Texas cohort, where the 4MP yielded an AUC of 0.87 (95% CI: 0.79–0.96).
Ajona et al. developed another protein biomarker model using complement derived fragment C4c in plasma in combination with two cancer-associated proteins, CYFRA 21-1 and C-reactive protein (CRP), and validated its performance in a population of 138 patients with incidentally found IPNs (24). Tumor growth is known to activate the complement cascade, a key component of the innate immune system, as a mechanism of host immune detection of cancer cells (25). Ajona and colleagues evaluated C4c, a detectable fragment released from the proteolysis of complement C4, as an adjunct to the more traditional cancer-associated proteins CYFRA 21-1 and CRP for malignancy prediction in IPNs. Their model performed well, with an AUC of 0.86 (95% CI: 0.80–0.92) and a specificity of 92% (24).
Beyond studies restricted to IPN populations, it is worth noting that extensive research aimed at characterizing protein signatures of lung cancers is ongoing and offers added potential for future application to IPNs. For example, in a prospective proteomic analysis of circulating inflammatory proteins, Dagnino et al. discovered an association between CUB domain-containing protein 1 (CDCP1) and lung cancer, then used gene expression analysis to illustrate a potential underlying biological mechanism for its role in carcinogenesis (25). In 2023, Dantas et al. performed a discovery screen via quantification of 45 cytokines and chemokines from tumor lysates and isogenic controls using an established murine model for lung cancer (26). Tissue inhibitor of metalloproteinase 1 (TIMP1), a regulatory protein for matrix metalloproteases which may facilitate extracellular matrix breakdown to aid in tumor cell invasion, was highly expressed in mice tumors and shown to be useful in differentiating human lung cancer cases. Additional studies have corroborated the promise of proteomic-level analysis for the early diagnosis of lung cancer using panels of tens or even hundreds of proteins, but again the potential for translation of such findings to IPN populations remains to be seen (27,28).
Circulating tumor cells (CTCs)
A term that often comes up in this domain, “liquid biopsy,” is commonly used to refer to the sampling of peripheral blood for circulating tumor material, such as CTCs and circulating tumor DNA (ctDNA), for the detection of occult malignancy (29). CTCs can be present in the peripheral blood even in early-stage lung tumors, and therefore their role as adjuncts in lung cancer diagnosis is under investigation (30). Akin to proteins, CTCs offer the advantage of minimally invasive sampling, but boast added specificity.
Mechanistically, CTC detection is commonly based on epithelial cell markers. For example, CellSearch® is an FDA-approved system for prostate, breast, and colorectal cancer diagnosis that uses antibody-based detection of epithelial cell adhesion molecule (EpCAM) and cytokeratin (31). In 2023, Wang et al. expanded on work showing that cell-surface glycoproteins called folate receptors (FRs) are highly expressed in non-small cell lung cancer (NSCLC) to study whether FR positivity could be applied to IPN discrimination (32). They used ligand-targeted polymerase chain reaction (PCR) for FR+ CTCs in blood samples from 898 patients with solitary nodules, 804 of whom were ultimately diagnosed with NSCLC. These were randomly divided into training and validation cohorts in a 2:1 ratio, which showed diagnostic AUCs of FR + CTC to be 0.650 (95% CI: 0.587–0.713) and 0.700 (95% CI: 0.603–0.796), respectively.
Beyond ligand-based PCR, fluorescence in situ hybridization (FISH) provides another popular technique for CTC detection. In 2022, Ma et al. used negative enrichment-fluorescence in situ hybridization (NE-FISH) technology to detect CTCs in patients with IPNs (33). Combining CTCs and protein biomarkers (CEA, CA-125, CYFRA 21-1, squamous cell carcinoma antigen) enabled the discrimination of benign and malignant IPNs with a sensitivity of 80.1% and AUC of 0.853 (95% CI: 0.800–0.897). Similarly in their 2021 publication, Feng et al. used 4-color FISH to identify circulating genetically abnormal cells (CACs) in a prospective study of 205 patients with IPNs ≤30 mm (34). Their 4-color FISH probes targeted instabilities in chromosomes 3 and 10 previously shown to occur in lung cancer, and demonstrated potential utility for lung cancer diagnosis in the training and validation cohorts (AUC 0.887 and 0.823, respectively, cutoff of ≥3 CACs). The authors proposed the superiority of CAC performance relative to several other commonly evaluated biomarkers for IPNs (CEA, squamous cell carcinoma antigen, neuron-specific enolase, pro-gastrin releasing peptide), though it should be noted the comparative values of these other biomarkers were assessed in isolation and not in combination as commonly done in studies evaluating their utility. Similar work by Katz et al. 2020 leveraged previous comparative genomic analysis of NSCLC tumors to develop FISH probes for two commonly identified gene copy variants, 10q22.3/CEP10 and 3p.22.1/3q29, to detect CTCs with up to 94% accuracy compared to biopsy (35). While the authors highlight the potential promise of these findings for IPNs, it should be noted that this study was performed on blood samples from subjects with and without cancer and validation in a true IPN cohort is outstanding.
CTCs can also be detected using targets other than epithelial biomarkers and chromosomal instability. Zhang et al. recently published on the use of a telomerase reverse transcriptase-based (TERT-based) technique for CTC detection in a population of 120 patients with IPNs size ≤20 mm (89 of whom ultimately received a pathologic diagnosis of cancer) (36). TERT is a catalytic subunit of the telomerase complex shown to play a key role in cancer cell survival by reactivating telomerase to bolster chromosomal stability (37). The authors showed TERT-based CTC detection provided high sensitivity and specificity for diagnosing malignancy in IPNs (sensitivity 85.4%, specificity 83.9%, detection threshold: 1 CTC), which improved even further when combined with CT imaging (sensitivity 89.9%, specificity 83.9%) (36).
Genetics and epigenetics
Specific genetic mutations and alterations in promoter methylation patterns can also be directly queried from the bloodstream to detect malignancy (38,39). While the sensitivity of assays targeting individual genetic or epigenetic changes may be limited, particularly in early-stage disease, whole-genome level analyses may overcome these obstacles (40). For instance, Mathios et al. applied machine learning to a genome-wide study of cell-free DNA (cfDNA) fragments in blood samples obtained from a prospective observational trial of 365 patients (LUCAS cohort) deemed high risk for lung cancer by IPNs on imaging or concerning symptoms and clinical history (41). In this group, the “DNA evaluation of fragments for early interception” (DELFI) approach used machine learning to analyze the size and distribution of cfDNA fragments to identify cancer cases with high fidelity (AUC 0.90; 95% CI: 0.86–0.94).
Analysis of tumor DNA expands beyond the bloodstream, as tumor-specific DNA has been detected in urine, saliva, sputum, pleural fluid, cerebrospinal fluid, and stool (42-44). In an observational nested case-control study of 101 patients with IPNs detected by CT imaging at The Johns Hopkins Hospital and the University of Illinois at Chicago, Liu and colleagues tested plasma and urine samples for promoter methylation in six cancer specific genes (CDO1, TAC1, HOXA7, SOX17, and ZFP42) (45). The presence of promoter methylation in three or more of these genes in both plasma and urine was used to predict malignancy in IPNs with a sensitivity of 73% and specificity of 92%. Additional studies have corroborated the efficacy of DNA methylation techniques in differentiating IPNs. For example, Chen et al. 2020 studied nanoparticle-based DNA extraction techniques to detect promoter methylation in eight lung cancer-specific genes (CDO1, TAC1, SOX17, HOXA7, HOXA9, GATA4, GATA5, PAX5) in 246 patients with CT-detected IPNs ≤30 mm (46). They found the three-gene combination of CDO1, SOX17, and HOXA7 yielded a sensitivity and specificity of 90% and 71% with an AUC of 0.88 (95% CI: 0.84–0.93) for lung cancer diagnosis. Gaga et al. recently published on their validation study of Lung EpiCheck®, which is a plasma-based six-methylation marker test for lung cancer that is currently under commercial development (47). While this study was not performed in an IPN population but rather in case-control European (179 cases, 137 controls) and Chinese (30 cases, 15 controls) validation cohorts, it highlights the capability and potential applicability of the PCR-based epigenetic product. The six-methylation marker panel performed well in overall lung cancer prediction in both validation sets, AUC 0.882 (95% CI: 0.846–0.918) and 0.899 (0.809–0.989), respectively.
MicroRNA (miRNAs)
miRNAs offer yet another avenue for the early detection of cellular aberrancy in IPNs. These non-coding, single-stranded molecules are key post-transcriptional regulators and contribute to the modulation of cellular growth, differentiation, and apoptosis (48,49). Thus, miRNA profiling focused on dysregulation of oncogenes and tumor suppressor genes has been the subject of abundant research for cancer detection, prognostication, and therapeutics (50). In their 2022 publication on miRNA carried by secreted lipid bilayer molecules called extracellular vesicles (EVs), Zheng et al. constructed a circulating small EV miRNA (CirsEV-miR) model to discriminate between benign and malignant IPNs (51). The authors profiled circulating small EV miRNAs in a training cohort of 47 patients with IPNs to develop a model based on the differential expression of five miRNAs and then tested their model in validation cohorts of IPNs patients. The CirsEV-miR model performed reasonably well in distinguishing benign from malignant nodules, even when nodule diameter was ≤10 mm (AUC 0.721, specificity 75.0% in the external validation group).
Beyond IPN specific populations, multiple recent reports on the use of miRNA for diagnosis in lung cancer populations in general have been published and provide additional support for the potential application of this biomarker modality to IPN discrimination. Ying et al. 2020 developed a five-miRNA panel for detecting early-stage NSCLC in a multicenter study using serum from 744 cases of NSCLC and 944 controls (52). Using real-time quantitative polymerase chain reaction (RT-qPCR), 35 candidate miRNA biomarkers were honed to a five-marker panel that showed an AUC of >0.90 across three independent validation cohorts. Zhong et al. similarly highlighted two miRNA molecules (miR-520c-3p, miR-1274b) that were elevated in NSCLC patients relative to healthy controls and patients with benign nodules (53). Their two-miRNA panel differentiated NSCLC and benign nodules with an AUC of 0.823 (95% CI: 0.730–0.915). Xue et al. 2020 identified a separate two-miRNA biomarker for NSCLC diagnosis consisting of miR-1228-3p and miR-181a-5p which showed a combined diagnostic AUC of 0.711 (95% CI: 0.593–0.828) for NSCLC (54). miRNA research has even suggested the possible role for the study of RNA modifications for lung cancer diagnosis, a realm akin to epigenetics in DNA work. Pei et al. showed that reversible methylation of the N6 position of adenosine in mRNA molecules (m6A) was significantly upregulated in NSCLC compared to healthy controls (0.085±0.033% vs. 0.056%±0.031%, P<0.001) and proposed its role as a potential diagnostic biomarker (55). Again, it remains to be seen if these findings could be extended to use in the diagnosis of IPNs.
Metabolomics and breathomics
Another intriguing area of work is “Metabolomics”, which broadly refers to the study of metabolic changes that occur at the cellular level with carcinogenesis. While the science behind these biomarkers is based in long-described metabolic strategies leveraged by cancer cells for survival and proliferation such as enhanced aerobic glycolysis, novel methods for detecting these changes early in disease have sparked research interest (56,57). For instance, Yao et al. described the use of ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS) to detect elevations in glycolytic metabolites associated with decreased tryptophan in sera for the differentiation of benign versus malignant pulmonary nodules (58). They used a 27-metabolite panel identified in a discovery cohort of 306 subjects to develop a discriminant model that achieved AUCs of 0.915 (sensitivity 86.7%, specificity 81.1%) and 0.945 (sensitivity 81.0%, specificity 97.9%) in internal (n=104) and external (n=111) validation cohorts, respectively. Outside the specific domain of IPNs, the metabolomic profiles of lung cancer patients have been studied as potentially useful diagnostic biomarkers for malignancy. For example, Qi et al. 2021 used high-resolution liquid chromatography-mass spectrometry (LC-MS) to show that a panel of metabolites could be applied to early cancer detection, and other recent publications on serum lipidomic biomarkers and machine learning have highlighted the potential of this field (59-61).
Similarly, “Breathomics” is the study of abnormal metabolic signatures created by malignancy in volatile compounds that diffuse into the alveoli and can be sampled from exhaled breath (62). This has been deemed a promising area of early lung cancer detection research, since the concentration of cancer-derived volatile organic compounds (VOCs) in exhaled breath has been shown to correlate with blood concentrations (63). Recent work in this domain has not been explicitly focused on IPN discrimination and instead has been largely descriptive, discovery phase work. Wang et al. 2022 investigated 28 previously identified candidate VOCs in a discovery cohort of 84 patients with lung cancer using high-pressure photon ionization time-of-flight mass spectrometry (HPPI-TOFMS) (64). They identified 16 candidate VOCs (mostly aldehydes and hydrocarbons) for lung cancer diagnosis and then performed external validation of the panel in 157 subjects with lung cancer and 368 healthy controls (AUC of 0.952, sensitivity 89.2%, specificity 89.1%). These metrics were similarly impressive when the diagnostic model included only the top eight VOCs (AUC 0.931, sensitivity 86.0%, specificity 87.2%). Still, the field of VOCs has been challenged by concerns over the reproducibility and variability of breath testing across different measurement methods, test environments, and patient factors, and it remains to be seen whether breathomics can provide value in IPNs specifically (65).
Microbiomics
There is increasing evidence showing that dysregulation of the lung microbiome may be linked to tumor formation, and that the sampling of compositional changes in microbiota may provide yet another realm of valuable markers for malignancy (66,67). While this is a rapidly evolving field and limited work in this domain has been performed on strictly IPN populations, growing research volume focused on microbiomic biomarkers in lung cancer suggests the potential for future application to nodule discrimination. The lower airways of patients with NSCLC have been shown to be enriched with taxa commonly associated with oral commensals, such as Veillonella, Prevotella, and Streptococcus, when compared with disease controls. Tsay et al. previously showed that the transcriptomic and microbiomic signatures of airway brushings from 85 IPN patients undergoing diagnostic bronchoscopy were significantly different between those ultimately diagnosed with benign versus malignant nodules (68). More specifically, the lower airways were enriched with oral taxa (Streptococcus and Veillonella) in malignancy, and these taxonomic signatures were associated with transcriptomic pathways known to be implicated with lung carcinogenesis, such as phosphoinositide 3-kinase (PI3K). These findings align with other prior work showing associations between enrichment with oral commensal microbiota and tumor protein 53 (TP53) mutated lung tumors, worse prognosis, and cancer progression (69,70).
Additional work by Shome et al. 2023 studied the distinct serum antimicrobial profiles of patients with benign and malignant pulmonary nodules (71). The authors profiled serum IgA and IgG antibodies against 901 microbial antigens from 27 bacteria and 29 viruses to construct an antibody panel capable of discriminating between benign and malignant nodules with an AUC of 0.80 (95% CI: 0.75–0.85). In 2020, Cheng et al. used 16S rRNA amplicon sequencing and principal coordinate analysis to identify significant differences in taxonomic profiles of lung microbiota in bronchoalveolar lavage samples of 54 patients presenting with suspicious nodules on CT (72). They developed a combination of ten microbial markers that showed an AUC of 0.791 (95% CI: 0.664–0.918) for discerning between benign disease and malignancy, which was boosted further to 0.845 (95% CI: 0.741–0.950) when combined with CEA, neuron-specific enolase (NSE), and CYFRA 21-1 levels. Collectively, these studies highlight the promise of a field that is growing at an exponential rate and whose potential contribution to biomarker science may be only beginning to be unveiled.
Radiomics
With the digitalization of clinical imaging and inception of machine learning and artificial intelligence, -omic level analysis of radiologic features and computer-assisted pattern recognition modeling for IPNs has been popularized. As the availability and capacity for the compilation of large volumes of imaging data continue to evolve, this field of “Radiomics” shows significant potential for “digital biomarkers” in IPN discrimination.
Work by Massion et al. 2020 used deep learning algorithms (Lung Cancer Prediction Convolutional Neural Network, “LCP-CNN”, by Optellum) for the successful reclassification of IPNs into low- and high-risk strata, and showed these can even outperform conventional clinical risk models (AUC 0.835, 95% CI: 0.754–0.907 versus AUC 0.781, 95% CI: 0.687–0.864) (73). Additional work by this group employed a prospective-specimen collection, retrospective-blinded evaluation (PRoBE) study design to apply these deep learning algorithms to dynamic data containing the temporal trends in IPN size and morphology that are seen clinically during longitudinal follow-up (74). In this study, Paez et al. showed that malignant and benign nodules appear to have distinct trends in LCP-CNN scores over time, with scores for benign nodules remaining largely stable versus scores for malignant nodules increasing over the course of follow-up. This highlights an important theory for clinically meaningful biomarkers in the sense that highly specific biomarker levels increase as disease burden progresses, and conversely, remain relatively stable in the absence of malignancy.
Additional work in this space by Baldwin et al. compared the discriminatory power of the LCP-CNN for IPNs with the highly validated Brock University Model, which was developed in the Pan-Canadian Early Detection of Lung Cancer Study and is recommended by the British Thoracic Society (75,76). In this retrospective validation study, the LCP-CNN score performed better at distinguishing benign and malignant pulmonary nodules than the Brock model (AUC 0.896, 95% CI: 0.876–0.915 vs. 0.868, 95% CI: 0.843–0.891). Moreover, the LCP-CNN had a false negative rate of 0.4% compared to that of the Brock model at 2.5% while maintaining comparable specificity. The authors concluded that the LCP-CNN had better discrimination and identified more benign disease without missing cancers, and therefore could guide future IPN management and reduce unnecessary CT surveillance.
As interest in radiomics continues to accelerate, there is growing evidence that the integration of machine learning and artificial intelligence models will be the field’s future (77-80). Recently, Prosper et al. outlined the role for quantitative, objective machine learning approaches for IPN classification beyond the historical standard of human semantic annotation (81). Nonetheless, and akin to other biomarker domains, radiomics will present new challenges as it continues to evolve. For instance, Xiao et al. showed that the performance of radiomic models across different available platforms may not be equivalent (82). In the discussion of their work comparing the performance of radiomic models between a commercial radiomic feature extractor (HealthMyne) and an open-source platform (PyRadiomics), the authors make the case for enhancing the transportability of models between radiomic platforms to enable direct performance comparisons and accelerate the collective progress of the field. A similar comparative analysis of eight statistical models for lung cancer prediction against nine cohorts representative of clinical uses showed that model performance was highly variable across clinical applications and no single model was a “clear winner” (83). These findings highlight the relative strengths and weaknesses of single models in different settings and underscore the need for comparative analyses that will help the field overcome the individual limitations of each.
Commercially available biomarkers
Currently, there are several commercially available tests for IPN discrimination that warrant discussion (Table 1). The Biodesix Nodify Lung® test for risk assessment of IPNs consists of two blood-based proteomic tests: Nodify CDT® and Nodify XL2®. The Nodify CDT® test measures levels of seven tumor associated autoantibodies in the blood, and the Nodify XL2® test is a proteomic integrated classifier (IC) test that measures the ratio of two proteins in the blood (LG3BP and C163A) in combination with several clinical and radiologic factors to determine which nodules are likely benign. The potential impact of these tests has been demonstrated in retrospective multicenter studies in which they reduced unnecessary invasive procedures without missing malignancy (84). Recently, Pritchett et al. published on the use of Nodify XL2® in the prospective multicenter ORACLE study, which aimed to assess the test’s ability to reduce invasive procedures (biopsies or surgeries) in patients with IPNs (85). In their propensity score matched analysis of 197 patients in the IC test group and 197 patients in the control group (usual clinical care), the authors found that patients in the IC test group were 74% less likely to undergo an invasive procedure (P<0.001). This corresponded to the test’s ability to reclassify 71 (36%) patients as low risk (probability of cancer <5%). Importantly, beyond the avoidance of unnecessary testing in low-risk IPNs, the proportion of patients with malignant nodules sent to surveillance was not statistically different between the IC test versus control groups. A separate large, prospective randomized clinical utility trial evaluating this IC test versus usual clinical management for IPNs is ongoing for further validation (NCT04171492).
Table 1. Selected commercially available biomarkers for pulmonary nodules and lung cancer.
| Product name | Classification | Biomarker candidate and assay | Source | Company |
|---|---|---|---|---|
| Nodify CDT® | Protein biomarker | Seven tumor associated autoantibodies; ELISA | Blood | Biodesix |
| Nodify XL2® | Integrated classifier (combination biomarker) | Ratio of two proteins (LG3BP, C163A), clinical and radiologic factors; MRM-MS | Blood | Biodesix |
| LCP-CNN | Radiomic biomarker | Convolutional neural network | Chest CT | Optellum |
| FirstLook | cfDNA | cfDNA fragmentomes; low-coverage WGS of cfDNA, machine learning | Blood | DELFI |
| Percepta® Nasal Swab | Genomic sequencing classifier | RNA; whole-transcriptome RNA sequencing | Nasal epithelial brushing | Veracyte |
| Percepta® GSC | Genomic sequencing classifier | RNA; whole-transcriptome RNA sequencing | Right bronchial brushing | Veracyte |
cfDNA, cell-free DNA; CT, computed tomography; DELFI, DNA evaluation of fragments for early interception; ELISA, enzyme-linked immunosorbent assay; MRM-MS, multiple reaction monitoring mass spectrometry; LCP-CNN, Lung Cancer Predictor Convolutional Neural Network; WGS, whole genome sequencing.
Additional blood-based commercial biomarker products include DELFI’s FirstLook test, which uses whole genome sequencing and machine learning to analyze patterns of DNA fragments (“fragmentomics”) to determine the likelihood of detecting lung cancer through screening. Other available products include the previously described LCP-CNN produced by Optellum for radiomic classification of lung nodules, as well as the transcriptome RNA sequencing-based classifiers developed by Veracyte for use on nasal epithelial (Percepta® Nasal Swab) or bronchial brushings (Percepta® GSC). Still, no singular commercial biomarker has been uniformly integrated into clinical practice, and the need for a universally accepted adjunct to the standard of care persists.
Combination biomarkers
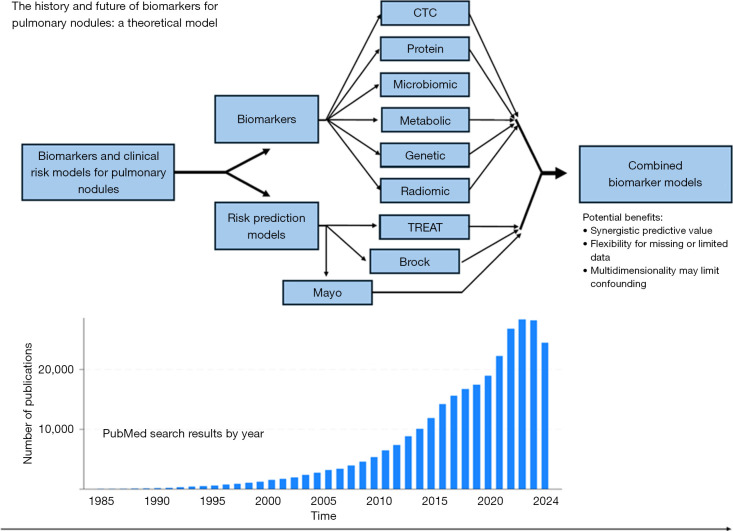
Combination biomarker models harness the attributes of multidimensional data including clinical factors, radiologic features, and molecular biomarker signals to optimize predictive performance. As illustrated above, the demand for useful IPN biomarkers has precipitated exponential growth in related research output. However, with this growth have followed divergent avenues for candidate discovery which could detract from actualizable clinical applicability as the science becomes increasingly complex. Combination models present the prospect of supplementing available clinical and radiological variables with the best biomarkers to achieve a synergistic outcome that maximizes predictive capability and minimizes the drawbacks of individual components when used in isolation (Figure 2).
Figure 2.
Theoretical model for combining biomarker modalities and clinical risk models for the discrimination of indeterminate pulmonary nodules. CTC, circulating tumor cell.
Multiple groups, including this team, are actively investigating such frameworks for IPN risk modeling. He et al. described the combination of their previously published circulating tumor DNA methylation model (PulmoSeek™) with a machine-learning assisted clinical and imaging biomarker model (CIBM) to classify benign versus malignant IPNs (86). Their combination model, termed “PulmoSeek™ Plus,” demonstrated superior discriminatory performance when compared to either the CIBM or PulmoSeek™ alone (AUC 0.91, 95% CI: 0.88–0.94). Similarly, Fahrmann et al. 2022 performed a blinded validation study using the Prostate, Lung, Colorectal, Ovarian screening trial (PLCO) data to evaluate the combined use of a four-marker protein panel (4MP) and a well-validated clinical lung cancer risk prediction model (PLCOm2012) in risk assessment (87). They found that the 4MP alone tested in case sera collected one year prior to diagnosis achieved an AUC of 0.79 (95% CI: 0.77–0.82), which increased to 0.85 (95% CI: 0.82–0.88) when combined with the PLCOm2012 model. Moreover, this combined 4MP + PLCOm2012 model showed significant improvement in sensitivity and specificity when compared directly with the 2021 United States Preventive Services Task Force (USPSTF21) guidelines for screening eligibility. Further analyses showed that the 4MP could predict lethal lung cancer and found added value in longitudinal measurement of the 4MP (88,89).
Kammer et al. developed a combination model for IPNs using clinical variables, a high-sensitivity CYFRA 21-1 serum assay, and a radiomic signature (90). This combination model outperformed the Mayo clinical model for malignancy prediction in IPNs, with an improvement in the AUC of 0.124 (95% CI: 0.091–0.156). Thereafter, Marmor et al. added to these findings by showing that within a combined model of molecular biomarkers, radiomics, and clinical risk factors, the variables of age, radiomic score, and levels of CYFRA 21-1 and CEA had the greatest predictive potential for malignancy in IPNs (91). Once again, this combination model outperformed the Mayo model with respect to both diagnostic accuracy and the associated net IPN risk reclassification index. At present, there continues to be explosive growth in research on combination models—all of which synthesize some of the above technologies to similarly bolster their cumulative predictive value and collectively signal a probable future direction for the research field (92-96).
Future challenges and directions
Exponential growth in the pool of candidate biomarkers in the literature has yielded an inevitable degree of divergence in the field. The multitude of competing assays, molecular markers, and analytical models demands comparative analyses and integrative testing to foster the very best biomarkers. While this seems an obvious task, it is important to underscore that the many biomarker studies being published often differ dramatically in phase of biomarker evaluation, as highlighted even in the select group of studies reviewed here. As highlighted previously, the EDRN’s systematic five-phase schema helps frame the lens by which individual biomarker studies are critically examined. A prerequisite understanding of whether a biomarker study is early phase, such as phase I and II studies focused on discovery and early evaluation of discriminatory power, or late phase, such as phase III and IV studies geared toward validation, is essential for accurate evaluation of a biomarker’s merit and readiness for clinical use. Comparative valuations of candidate biomarkers are therefore contingent on ensuring equivalency of the items being assessed. Indeed, a major obstacle for the field at large has been the translation of the success metrics promised by early-phase studies to external validation and population-level evidence. To this end, the studies discussed above have been tabulated for summative reference and annotated with EDRN Phase assignments. For additional clarity, studies performed on IPN populations specifically have been listed separately from those performed on lung cancer in general (Tables 2,3, respectively).
Table 2. Selected studies on biomarkers for the diagnosis of indeterminate pulmonary nodules, 2020–2024 (in studies with both training and validation cohorts, only validation performance metrics are displayed).
| Study | EDRN phase | Biomarker and assay | Study population (n) (cancer/benign nodules) |
Sens/Spec (%/%) | AUC | |
|---|---|---|---|---|---|---|
| Training | Validation | |||||
| Protein biomarkers | ||||||
| Ostrin 2021 (23) | II | pro-SFTPB, CA125, CYFRA 21-1, CEA; Luminex-based panel | – | 100/100; 30/30 | 42/99†; 26/95† | 0.76; 0.87 |
| Ajona 2021 (24) | I/II | C4c, CRP, CYFRA 21-1; ELISA and Luminex-based panel | 39/39 | 76/62 | 70/92 | 0.86 |
| CTCs | ||||||
| Wang 2023 (32) | I/II | Folate receptor positive CTCs; LT-PCR | 529/70 | 275/24 | 69.1/70.8 | 0.70 |
| Ma 2022 (33) | II | CTCs combined with CEA, CA125, CYFRA 21-1, SCC; FISH, ECLIA | 171/53 | – | 80.1/– | 0.85 |
| Feng 2021 (34) | II | Circulating genetically abnormal cells, CEA, ProGRP, SCC, CYFRA 21-1; FISH, ECLIA | 89/23 | 79/14 | 86.1/78.6 | 0.82 |
| Zhang 2021 (36) | II | TERT-based CTCs; flow cytometry imaging; FISH | 89/31 | – | 85.4/83.9 | 0.84 |
| Genetics and epigenetics | ||||||
| Liu 2020 (45) | II | Promoter methylation in plasma and urine CDO1, TAC1, HOXA7, HOXA9, SOX17, ZFP42 (at least 3); optimized methylation on beads, qMSP | 74/27 | – | 73.0/92.0 | 0.72 |
| Chen 2020 (46) | II | Promoter methylation in plasma CDO1, TAC1, SOX17, HOXA7, HOXA9, GATA4, GATA5, and PAX5; nanoparticle-based DNA extraction, qMSP | 163/83 | – | 90.0‡/71.0‡ | 0.88‡ |
| MicroRNA | ||||||
| Zheng 2022 (51) | I/II | CirsEV-miR model: miR-30c-5p, miR-30e-5p, miR-500a-3p, miR-125a-5p, miR-99a-5p; RT-qPCR | 30/17 | 38/24; 79/20 | – | 0.76; 0.78 |
| Zhong 2021 (53) | I/II | miR-520c-3p and miR-1274b; RT-qPCR | 159/31 | – | – | 0.82 |
| Metabolomics | ||||||
| Yao 2023 (58) | I/II | 27-metabolite panel; UPLC-HRMS | 136/170 | 30/74; 63/48 | 86.7/81.1; 81.0/97.9 | 0.915; 0.945 |
| Microbiomics | ||||||
| Shome 2023 (71) | I/II | Anti-microbial antibodies; microbial antigen array | 127/170 | – | 43/90 | 0.80 |
| Cheng 2020 (72) | I/II | Phylum TM7 and 6 genera (c:TM7-3, Capnocytophaga, Sediminibacterium, Gemmiger, Blautia and Oscillospira); 16S rRNA gene sequencing | 32/22 | – | – | 0.79 |
| Radiomics | ||||||
| Massion 2020 (73) | II/III | Chest CT; LCP-CNN model | 932/14,761 | 64/52; 63/400 | 98.4/44.2; 96.8/64.3 | 0.84; 0.92 |
| Baldwin 2020 (75) | II/III | Chest CT; LCP-CNN, Brock University model | – | 234/958 | 99.6/28.0 | 0.90 |
| Hunter 2022 (77) | I/II | Chest CT; LN-RPV | 366/220 | 158/94 | 84.0/39.0 | 0.75 |
| Liu 2020 (78) | I/II | Chest CT; radiomics nomogram | 449/113 | 207/56 | – | 0.81 |
| Yang 2024 (80) | I/II | Chest CT, nodule morphological characteristics; MIFNN | 85/297 | 33/68 | 80.9/85.1 | 0.84 |
| Combination biomarkers | ||||||
| He 2023 (86) | I/II | cfDNA methylation (PulmoSeek™), CIBM; PulmoSeek™ Plus | 214/44 | 209/74 | 98.0/50.0 | 0.91 |
| Kammer 2020 (90) | I/II | CYFRA 21-1, clinical variables, CT scan, Mayo risk score, radiomic score | 119/51 | 40/59; 50/49; 48/40 | 80.0/82.0 | 0.94; 0.76; 0.84 |
| Marmor 2023 (91) | I/II | CA-125, SCC, CEA, HE4, ferritin, ProGRP, CYFRA 21-1, NSE, hs-CRP, clinical variables, CT scan, Mayo risk score, radiomic score | 217/121 | – | 88.9/49.6 | 0.76 |
| Lastwika 2023 (93) | I/II | Large-scale microarray analysis, semantic features, radiomics; PSR | 69/66 | 71/78 | –/95.0 | 0.90 |
| Ling 2021 (94) | II | 7-autoantibody panel (p53, GAGE7, PGP9.5, CAGE, MAGEA1, SOX2, and GBU4-5), Mayo Clinic model | 193/118 | – | 93.5/58.0§; 91.4/72.8¶ | 0.80§; 0.95¶ |
| Wang 2020 (95) | I/II | 7-autoantibody panel (p53, GAGE7, PGP9.5, CAGE, MAGEA1, SOX2, GBI4-5), clinical variables; Nomogram with Scoring Table | 78/22 | 129/39 | 86.1/69.2 | 0.86 |
| Wang 2022 (96) | I/II | SMF, CEA; MP-NN | 643/214 | 315/106 | 81.8/56.9 | 0.78 |
†, Sens/spec when combined with nodule size; ‡, for combination of CDO1, SOX17, HOXA7; §, benign vs. stage I–II cancer; ¶, benign vs. stage III–IV cancer. CA125, cancer antigen 125; CEA, carcinoembryonic antigen; CIBM, combined clinical and imaging biomarker; CirsEV-miR, circulating sEV miRNA; CRP, C-reactive protein; CT, computed tomography; CTCs, circulating tumor cells; CYFRA 21-1, cytokeratin-19 fragment; ECLIA, electrochemiluminescence assay; EDRN, Early Detection Research Network; ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in situ hybridization; LCP-CNN, Lung Cancer Predictor Convolutional Neural Network; LN-RPV, large-nodule radiomics predictive vector; LT-PCR, ligand-targeted PCR; MIFNN, Multimodal Integrated Feature Neural Network; MP-NN, metabolic fingerprints with protein tumor marker neural network; NSE, neuron-specific enolase; ProGRP, Pro-gastrin-releasing peptide; pro-SFTPB, propeptide of lung-specific surfactant protein B; PSR, Plasma, Semantic, Radiomic model; qMSP, quantitative methylation-specific PCR; RT-qPCR, reverse transcriptase quantitative polymerase chain reaction; SCC, squamous cell carcinoma antigen; SMF, serum metabolic fingerprints; TERT, telomerase reverse transcriptase; UPLC-HRMS, ultra-performance liquid chromatography-high resolution mass spectrometry.
Table 3. Selected studies on biomarkers for the diagnosis of lung cancer, 2020–2024 (in studies with both training and validation cohorts, only validation performance metrics are displayed).
| Study | EDRN phase | Biomarker and assay | Study population (n) (case/control) | Sens/Spec (%/%) | AUC | |
|---|---|---|---|---|---|---|
| Training | Validation | |||||
| Protein biomarkers | ||||||
| Dagnino 2021 (25) | I/II | CDCP1; Olink® multiplex assay | 323/325 | 225/225 | – | 0.65 |
| Dantas 2023 (26) | I/II | TIMP1; Luminex-based panel TIMP1, RNA-sequencing | Mouse model | 122/39 | 79.5/64.1 | 0.78 |
| Lung Cancer Cohort Consortium (LC3) (27) | I | Olink® Proteomics protein panel | 731/731 | – | – | – |
| Davies 2023 (28) | I/II | Olink® Proteomics protein panel | 198/369 | 392/5,500 | – | 0.75 |
| CTCs | ||||||
| Katz 2020 (35) | I/II | CTCs, SP-A1, SP-A2, 3p22.1, 10q22.3; FISH | 61/57 | 46/43 | 80.4/100 | 0.98 |
| Genetics and epigenetics | ||||||
| Mathios 2021 (41) | I/II | cfDNA fragmentation profiles, CEA; clinical data, CT imaging; next-generation sequencing | 129/236 | 46/385 | – | – |
| Gaga 2021 (47) | I/II | Lung EpiCheck® (six cfDNA markers); PCR | 102/265 | 179/137; 30/15 | 87.2/64.2; 76.7/93.3 | 0.88; 0.90 |
| MicroRNA | ||||||
| Ying 2020 (52) | I/II | let-7a-5p, miR-375, miR-1-3p, miR-1291, miR-214-3p; RT-qPCR | 523/523 | 120/117; 67/273; 34/31 | 81.3/90.7 | 0.97; 0.92; 0.92 |
| Xue 2020 (54) | I | miR-1228-3p and miR-181a-5p; RT-qPCR | 50/30 | – | – | 0.71 |
| Pei 2020 (55) | I | Leukocyte N6-methyladenosine; PCR | 119/74 | – | – | 0.74 |
| Metabolomics | ||||||
| Qi 2021 (59) | I/II | Palmitic acid, heptadecanoic acid, 4-oxoproline, tridecanoic acid, ornithine; LC-MS | 64/50 | 34/25 | – | 0.87 |
| Noreldeen 2020 (60) | I/II | FA (20:4), FA (22:0), LPE (20:4); UHPLC-Q-TOF/MS, machine learning | 39/46 | 25/17 | 100/100 | 1.00 |
| Xie 2021 (61) | I | L-kynurenine, proline, spermidine, amino-hippuric acid, palmitoyl-l-carnitine, taurine; LC-MS, machine learning | 110/43 | – | 98.1/100 | 0.99 |
| Breathomics | ||||||
| Wang 2022 (64) | I/II | 16 VOC-panel of aldehydes, hydrocarbons, ketones, carboxylic acids, furan; HPPI-TOFMS | 84/0 | 157/368 | 89.2/89.1 | 0.95 |
| Radiomics | ||||||
| Ueda 2021 (79) | I/II | Chest radiographs; AI-based computer-assisted detection software | 59/253 | – | 60/96 | 0.90† |
| Combination biomarkers | ||||||
| Fahrmann 2022 (87) | III | 4MP (Pro-SFTPB, CA125, CEA, CYFRA 21-1); Luminex bead-based immunoassay, PLCOm2012 | – | 552/2,193 | 83.5/69.3 | 0.85 |
†, accuracy. AI, artificial intelligence; CA125, cancer antigen 125; CEA, carcinoembryonic antigen; cfDNA, cell-free DNA; CT, computed tomography; CTCs, circulating tumor cells; CYFRA 21-1, cytokeratin-19 fragment; FA, fatty acid; FISH, fluorescence in situ hybridization; HPPI-TOFMS, high-pressure photon ionization time-of-flight mass spectrometry; LC-MS, liquid chromatography-mass spectrometry; LPE, lysophosphatidylethanolamine; PCR, polymerase chain reaction; pro-SFTPB, propeptide of lung-specific surfactant protein B; RT-qPCR, reverse transcriptase quantitative polymerase chain reaction; UHPLC-Q-TOF/MS, ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry; VOC, volatile organic compound.
Another paramount challenge in IPN biomarker research is the heterogenous nature of lung cancer. Given this, it seems logical that the best diagnostic IPN biomarkers will also be multidimensional and considerate of the specific applications, strengths, and limitations of the individual components included. Ultimately, there may be no single “magic bullet”, but rather a mosaic tool kit comprised of eclectic ammunition designed to combat the multifaceted and diverse characteristics of lung nodules and cancer.
Finally, the domain of biomarker science carries with it a difficult lesson to be learned: a good scientific tool does not always translate into a good clinical tool. In this group’s own several decades of experience with biomarker development, our team has come to appreciate this pivotal principal—while statistical measures of diagnostic accuracy are reaching unprecedented levels for biomarker candidates, simplicity and affordability will remain key ingredients to the recipe of success for biomarker models. To span the diversity of demographic, geographic, and socio-economic factors that influence IPN decision making, biomarkers must be generalizable, affordable, and functionally scalable and practical to implement. The harmonious balance between these considerations and the model’s performance attributes remains a delicate art.
Conclusions
The breadth of research reviewed here highlights the field’s promise and provides reason to believe that we are closer than ever before to answering the urgent clinical need for effective IPN biomarkers. Obstacles persist, but so too does the tremendous rigor and commitment of the research field to advancing the needle forward. Combined biomarker models and the seemingly boundless potential of machine learning have yielded promising results and instilled new hope for the future. The true test of greatness will lie in the synthesis of the very best of a continually growing number of biomarker candidates, only hinted at above, into a scalable, cost-effective, reproducible, and risk-averse model that demonstrates tangible change in everyday clinical decisions. Informed by experience and invigorated by innovation, the field marches forward.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fayez Kheir) for the series “Advances in Interventional Pulmonary” published in Journal of Thoracic Disease. The article has undergone external peer review.
Funding: This work was supported by grants Clinical Utility of a Combined Biomarker Approach to Diagnose Lung Cancer (No. 5U01CA152662-13) and Surgical Oncology Training Grant (No. 5T32CA106183-20).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2010/coif). The special series “Advances in Interventional Pulmonary” was commissioned by the editorial office without any funding or sponsorship. M.N.K. reports consulting fees from Biodesix unrelated to the current work. E.J.O. received grants from the National Cancer Institute Early Detection Research Network. He was supported by the GRAIL, Inc. Scientific Advisory Board 12/2023 (relationship ended). He also has Intellectual property and patent pending on the 4-protein biomarker panel for lung cancer early detection. S.A.D. is an Executive Committee member of the American Thoracic Society Thoracic Oncology Assembly. S.A.D. and E.L.G. work on this team for this project, supported by grant No. 5U01CA152662-13. The authors have no other conflicts of interest to declare.
References
- 1.World Health Organization. Cancer fact sheets, lung. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer, accessed August, 2024.
- 2.American Thoracic Society. Lung Cancer Fact Sheet, 2021. Available online: https://www.thoracic.org/about/global-public-health/firs/resources/world-lung-cancer-day-fact-sheet-2021.pdf, accessed August, 2024.
- 3.American Lung Association. State of Lung Cancer, 2023. Available online: https://www.lung.org/research/state-of-lung-cancer/key-findings, accessed August, 2024.
- 4.National Lung Screening Trial Research Team ; Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudkerk M, Liu S, Heuvelmans MA, et al. Lung cancer LDCT screening and mortality reduction - evidence, pitfalls and future perspectives. Nat Rev Clin Oncol 2021;18:135-51. 10.1038/s41571-020-00432-6 [DOI] [PubMed] [Google Scholar]
- 6.Mazzone PJ, Lam L. Evaluating the Patient With a Pulmonary Nodule: A Review. JAMA 2022;327:264-73. 10.1001/jama.2021.24287 [DOI] [PubMed] [Google Scholar]
- 7.Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. 10.1164/rccm.201505-0990OC [DOI] [PubMed] [Google Scholar]
- 8.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. 10.1056/NEJMcp012290 [DOI] [PubMed] [Google Scholar]
- 9.Ostrin EJ, Sidransky D, Spira A, et al. Biomarkers for Lung Cancer Screening and Detection. Cancer Epidemiol Biomarkers Prev 2020;29:2411-5. 10.1158/1055-9965.EPI-20-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seijo LM, Peled N, Ajona D, et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol 2019;14:343-57. 10.1016/j.jtho.2018.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kammer MN, Massion PP. Noninvasive biomarkers for lung cancer diagnosis, where do we stand? J Thorac Dis 2020;12:3317-30. 10.21037/jtd-2019-ndt-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmor HN, Zorn JT, Deppen SA, et al. Biomarkers in Lung Cancer Screening: a Narrative Review. Curr Chall Thorac Surg 2023;5:5. 10.21037/ccts-20-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paez R, Kammer MN, Tanner NT, et al. Update on Biomarkers for the Stratification of Indeterminate Pulmonary Nodules. Chest 2023;164:1028-41. 10.1016/j.chest.2023.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr KF, Brown MD, Marsh TL, et al. Assessing the Clinical Impact of Risk Models for Opting Out of Treatment. Med Decis Making 2019;39:86-90. 10.1177/0272989X18819479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deppen SA, Grogan EL. Using Clinical Risk Models for Lung Nodule Classification. Semin Thorac Cardiovasc Surg 2015;27:30-5. 10.1053/j.semtcvs.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Early Detection Research: Investigating in Translational Research on Biomarkers of Early Cancer and Cancer Risk. Fourth Report. US Department of Health and Human Services, National Institutes of Health. NIH Publication, 07-6135 (2008). Available online: https://prevention.cancer.gov/sites/default/files/2023-02/edrn_4th-report_200801.pdf
- 17.Moncada V, Srivastava S. Biomarkers in oncology research and treatment: early detection research network: a collaborative approach. Biomark Med 2008;2:181-95. 10.2217/17520363.2.2.181 [DOI] [PubMed] [Google Scholar]
- 18.Mazzone PJ, Sears CR, Arenberg DA, et al. Evaluating Molecular Biomarkers for the Early Detection of Lung Cancer: When Is a Biomarker Ready for Clinical Use? An Official American Thoracic Society Policy Statement. Am J Respir Crit Care Med 2017;196:e15-29. 10.1164/rccm.201708-1678ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Yang Y, Zhu Y, et al. Blood protein biomarkers in lung cancer. Cancer Lett 2022;551:215886. 10.1016/j.canlet.2022.215886 [DOI] [PubMed] [Google Scholar]
- 20.Broodman I, Lindemans J, van Sten J, et al. Serum Protein Markers for the Early Detection of Lung Cancer: A Focus on Autoantibodies. J Proteome Res 2017;16:3-13. 10.1021/acs.jproteome.6b00559 [DOI] [PubMed] [Google Scholar]
- 21.Ajona D, Pajares MJ, Corrales L, et al. Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer. J Natl Cancer Inst 2013;105:1385-93. 10.1093/jnci/djt205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vachani A, Pass HI, Rom WN, et al. Validation of a multiprotein plasma classifier to identify benign lung nodules. J Thorac Oncol 2015;10:629-37. 10.1097/JTO.0000000000000447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrin EJ, Bantis LE, Wilson DO, et al. Contribution of a Blood-Based Protein Biomarker Panel to the Classification of Indeterminate Pulmonary Nodules. J Thorac Oncol 2021;16:228-36. 10.1016/j.jtho.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajona D, Remirez A, Sainz C, et al. A model based on the quantification of complement C4c, CYFRA 21-1 and CRP exhibits high specificity for the early diagnosis of lung cancer. Transl Res 2021;233:77-91. 10.1016/j.trsl.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagnino S, Bodinier B, Guida F, et al. Prospective Identification of Elevated Circulating CDCP1 in Patients Years before Onset of Lung Cancer. Cancer Res 2021;81:3738-48. 10.1158/0008-5472.CAN-20-3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dantas E, Murthy A, Ahmed T, et al. TIMP1 is an early biomarker for detection and prognosis of lung cancer. Clin Transl Med 2023;13:e1391. 10.1002/ctm2.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The blood proteome of imminent lung cancer diagnosis. Nat Commun 2023;14:3042. 10.1038/s41467-023-37979-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies MPA, Sato T, Ashoor H, et al. Plasma protein biomarkers for early prediction of lung cancer. EBioMedicine 2023;93:104686. 10.1016/j.ebiom.2023.104686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol Sci 2019;40:172-86. 10.1016/j.tips.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 30.Fiorelli A, Accardo M, Carelli E, et al. Circulating Tumor Cells in Diagnosing Lung Cancer: Clinical and Morphologic Analysis. Ann Thorac Surg 2015;99:1899-905. 10.1016/j.athoracsur.2014.11.049 [DOI] [PubMed] [Google Scholar]
- 31.Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol 2016;10:395-407. 10.1016/j.molonc.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Li P, Fei X, et al. A combined diagnostic model based on circulating tumor cell in patients with solitary pulmonary nodules. J Gene Med 2023;25:e3529. 10.1002/jgm.3529 [DOI] [PubMed] [Google Scholar]
- 33.Ma G, Yang D, Li Y, et al. Combined measurement of circulating tumor cell counts and serum tumor marker levels enhances the screening efficiency for malignant versus benign pulmonary nodules. Thorac Cancer 2022;13:3393-401. 10.1111/1759-7714.14702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng M, Ye X, Chen B, et al. Detection of circulating genetically abnormal cells using 4-color fluorescence in situ hybridization for the early detection of lung cancer. J Cancer Res Clin Oncol 2021;147:2397-405. 10.1007/s00432-021-03517-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz RL, Zaidi TM, Pujara D, et al. Identification of circulating tumor cells using 4-color fluorescence in situ hybridization: Validation of a noninvasive aid for ruling out lung cancer in patients with low-dose computed tomography-detected lung nodules. Cancer Cytopathol 2020;128:553-62. 10.1002/cncy.22278 [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Duan X, Zhang Z, et al. Combination of CT and telomerase+ circulating tumor cells improves diagnosis of small pulmonary nodules. JCI Insight 2021;6:e148182. 10.1172/jci.insight.148182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dratwa M, Wysoczańska B, Łacina P, et al. TERT-Regulation and Roles in Cancer Formation. Front Immunol 2020;11:589929. 10.3389/fimmu.2020.589929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phallen J, Sausen M, Adleff V, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med 2017;9:eaan2415. 10.1126/scitranslmed.aan2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446-51. 10.1038/nature22364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018;563:579-83. 10.1038/s41586-018-0703-0 [DOI] [PubMed] [Google Scholar]
- 41.Mathios D, Johansen JS, Cristiano S, et al. Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat Commun 2021;12:5060. 10.1038/s41467-021-24994-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cristiano S, Leal A, Phallen J, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019;570:385-9. 10.1038/s41586-019-1272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng M, Chen C, Hulbert A, et al. Non-blood circulating tumor DNA detection in cancer. Oncotarget 2017;8:69162-73. 10.18632/oncotarget.19942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel KM, Tsui DW. The translational potential of circulating tumour DNA in oncology. Clin Biochem 2015;48:957-61. 10.1016/j.clinbiochem.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Ricarte Filho J, Mallisetty A, et al. Detection of Promoter DNA Methylation in Urine and Plasma Aids the Detection of Non-Small Cell Lung Cancer. Clin Cancer Res 2020;26:4339-48. 10.1158/1078-0432.CCR-19-2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C, Huang X, Yin W, et al. Ultrasensitive DNA hypermethylation detection using plasma for early detection of NSCLC: a study in Chinese patients with very small nodules. Clin Epigenetics 2020;12:39. 10.1186/s13148-020-00828-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaga M, Chorostowska-Wynimko J, Horváth I, et al. Validation of Lung EpiCheck, a novel methylation-based blood assay, for the detection of lung cancer in European and Chinese high-risk individuals. Eur Respir J 2021;57:2002682. 10.1183/13993003.02682-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochem Soc Trans 2008;36:1224-31. 10.1042/BST0361224 [DOI] [PubMed] [Google Scholar]
- 49.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucci P. The Role of microRNAs in Cancer: Functions, Biomarkers and Therapeutics. Cancers (Basel) 2022;14:872. 10.3390/cancers14040872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng D, Zhu Y, Zhang J, et al. Identification and evaluation of circulating small extracellular vesicle microRNAs as diagnostic biomarkers for patients with indeterminate pulmonary nodules. J Nanobiotechnology 2022;20:172. 10.1186/s12951-022-01366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying L, Du L, Zou R, et al. Development of a serum miRNA panel for detection of early stage non-small cell lung cancer. Proc Natl Acad Sci U S A 2020;117:25036-42. 10.1073/pnas.2006212117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong Y, Ding X, Bian Y, et al. Discovery and validation of extracellular vesicle-associated miRNAs as noninvasive detection biomarkers for early-stage non-small-cell lung cancer. Mol Oncol 2021;15:2439-52. 10.1002/1878-0261.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue WX, Zhang MY, Li Rui, et al. Serum miR-1228-3p and miR-181a-5p as Noninvasive Biomarkers for Non-Small Cell Lung Cancer Diagnosis and Prognosis. Biomed Res Int 2020;2020:9601876. 10.1155/2020/9601876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pei Y, Lou X, Li K, et al. Peripheral Blood Leukocyte N6-methyladenosine is a Noninvasive Biomarker for Non-small-cell Lung Carcinoma. Onco Targets Ther 2020;13:11913-21. 10.2147/OTT.S267344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WARBURG O. On the origin of cancer cells. Science 1956;123:309-14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 57.Bamji-Stocke S, van Berkel V, Miller DM, et al. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics 2018;14:81. 10.1007/s11306-018-1376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao Y, Wang X, Guan J, et al. Metabolomic differentiation of benign vs malignant pulmonary nodules with high specificity via high-resolution mass spectrometry analysis of patient sera. Nat Commun 2023;14:2339. 10.1038/s41467-023-37875-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qi SA, Wu Q, Chen Z, et al. High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci Rep 2021;11:11805. 10.1038/s41598-021-91276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noreldeen HAA, Du L, Li W, et al. Serum lipidomic biomarkers for non-small cell lung cancer in nonsmoking female patients. J Pharm Biomed Anal 2020;185:113220. 10.1016/j.jpba.2020.113220 [DOI] [PubMed] [Google Scholar]
- 61.Xie Y, Meng WY, Li RZ, et al. Early lung cancer diagnostic biomarker discovery by machine learning methods. Transl Oncol 2021;14:100907. 10.1016/j.tranon.2020.100907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanna GB, Boshier PR, Markar SR, et al. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:e182815. 10.1001/jamaoncol.2018.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campanella A, De Summa S, Tommasi S. Exhaled breath condensate biomarkers for lung cancer. J Breath Res 2019;13:044002. 10.1088/1752-7163/ab2f9f [DOI] [PubMed] [Google Scholar]
- 64.Wang P, Huang Q, Meng S, et al. Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study. EClinicalMedicine 2022;47:101384. 10.1016/j.eclinm.2022.101384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanna GB, Boshier PR, Markar SR, et al. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:e182815. 10.1001/jamaoncol.2018.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SH, Sung JY, Yong D, et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 2016;102:89-95. 10.1016/j.lungcan.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 67.Segal LN, Clemente JC, Tsay JC, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 2016;1:16031. 10.1038/nmicrobiol.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsay JJ, Wu BG, Badri MH, et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am J Respir Crit Care Med 2018;198:1188-98. 10.1164/rccm.201710-2118OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greathouse KL, White JR, Vargas AJ, et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol 2018;19:123. 10.1186/s13059-018-1501-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsay JJ, Wu BG, Sulaiman I, et al. Lower Airway Dysbiosis Affects Lung Cancer Progression. Cancer Discov 2021;11:293-307. 10.1158/2159-8290.CD-20-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shome M, Gao W, Engelbrektson A, et al. Comparative Microbiomics Analysis of Antimicrobial Antibody Response between Patients with Lung Cancer and Control Subjects with Benign Pulmonary Nodules. Cancer Epidemiol Biomarkers Prev 2023;32:496-504. 10.1158/1055-9965.EPI-22-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng C, Wang Z, Wang J, et al. Characterization of the lung microbiome and exploration of potential bacterial biomarkers for lung cancer. Transl Lung Cancer Res 2020;9:693-704. 10.21037/tlcr-19-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Massion PP, Antic S, Ather S, et al. Assessing the Accuracy of a Deep Learning Method to Risk Stratify Indeterminate Pulmonary Nodules. Am J Respir Crit Care Med 2020;202:241-9. 10.1164/rccm.201903-0505OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paez R, Kammer MN, Balar A, et al. Longitudinal lung cancer prediction convolutional neural network model improves the classification of indeterminate pulmonary nodules. Sci Rep 2023;13:6157. 10.1038/s41598-023-33098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baldwin DR, Gustafson J, Pickup L, et al. External validation of a convolutional neural network artificial intelligence tool to predict malignancy in pulmonary nodules. Thorax 2020;75:306-12. 10.1136/thoraxjnl-2019-214104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. 10.1056/NEJMoa1214726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunter B, Chen M, Ratnakumar P, et al. A radiomics-based decision support tool improves lung cancer diagnosis in combination with the Herder score in large lung nodules. EBioMedicine 2022;86:104344. 10.1016/j.ebiom.2022.104344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu A, Wang Z, Yang Y, et al. Preoperative diagnosis of malignant pulmonary nodules in lung cancer screening with a radiomics nomogram. Cancer Commun (Lond) 2020;40:16-24. 10.1002/cac2.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ueda D, Yamamoto A, Shimazaki A, et al. Artificial intelligence-supported lung cancer detection by multi-institutional readers with multi-vendor chest radiographs: a retrospective clinical validation study. BMC Cancer 2021;21:1120. 10.1186/s12885-021-08847-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang R, Zhang Y, Li W, et al. Development and external validation of a multimodal integrated feature neural network (MIFNN) for the diagnosis of malignancy in small pulmonary nodules (≤10 mm). Biomed Phys Eng Express 2024. doi: . 10.1088/2057-1976/ad449a [DOI] [PubMed] [Google Scholar]
- 81.Prosper AE, Kammer MN, Maldonado F, et al. Expanding Role of Advanced Image Analysis in CT-detected Indeterminate Pulmonary Nodules and Early Lung Cancer Characterization. Radiology 2023;309:e222904. 10.1148/radiol.222904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao D, Kammer MN, Chen H, et al. Assessing the transportability of radiomic models for lung cancer diagnosis: commercial vs. open-source feature extractors. Transl Lung Cancer Res 2024;13:1907-17. 10.21037/tlcr-24-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li TZ, Xu K, Krishnan A, et al. Performance of Lung Cancer Prediction Models for Screening-detected, Incidental, and Biopsied Pulmonary Nodules. Radiol Artif Intell 2025;7:e230506. 10.1148/ryai.230506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kheir F, Uribe JP, Cedeno J, et al. Impact of an integrated classifier using biomarkers, clinical and imaging factors on clinical decisions making for lung nodules. J Thorac Dis 2023;15:3557-67. 10.21037/jtd-23-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pritchett MA, Sigal B, Bowling MR, et al. Assessing a biomarker's ability to reduce invasive procedures in patients with benign lung nodules: Results from the ORACLE study. PLoS One 2023;18:e0287409. 10.1371/journal.pone.0287409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He J, Wang B, Tao J, et al. Accurate classification of pulmonary nodules by a combined model of clinical, imaging, and cell-free DNA methylation biomarkers: a model development and external validation study. Lancet Digit Health 2023;5:e647-56. 10.1016/S2589-7500(23)00125-5 [DOI] [PubMed] [Google Scholar]
- 87.Fahrmann JF, Marsh T, Irajizad E, et al. Blood-Based Biomarker Panel for Personalized Lung Cancer Risk Assessment. J Clin Oncol 2022;40:876-83. 10.1200/JCO.21.01460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song L, Irajizad E, Rundle A, et al. Validation of a Blood-Based Protein Biomarker Panel for a Risk Assessment of Lethal Lung Cancer in the Physicians' Health Study. Cancers (Basel) 2024;16:2070. 10.3390/cancers16112070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Irajizad E, Fahrmann JF, Toumazis I, et al. Biomarker trajectory for earlier detection of lung cancer. EBioMedicine 2024;108:105377. 10.1016/j.ebiom.2024.105377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kammer MN, Lakhani DA, Balar AB, et al. Integrated Biomarkers for the Management of Indeterminate Pulmonary Nodules. Am J Respir Crit Care Med 2021;204:1306-16. 10.1164/rccm.202012-4438OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marmor HN, Jackson L, Gawel S, et al. Improving malignancy risk prediction of indeterminate pulmonary nodules with imaging features and biomarkers. Clin Chim Acta 2022;534:106-14. 10.1016/j.cca.2022.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Irajizad E, Fahrmann JF, Marsh T, et al. Mortality Benefit of a Blood-Based Biomarker Panel for Lung Cancer on the Basis of the Prostate, Lung, Colorectal, and Ovarian Cohort. J Clin Oncol 2023;41:4360-8. 10.1200/JCO.22.02424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lastwika KJ, Wu W, Zhang Y, et al. Multi-Omic Biomarkers Improve Indeterminate Pulmonary Nodule Malignancy Risk Assessment. Cancers (Basel) 2023;15:3418. 10.3390/cancers15133418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ling Z, Chen J, Wen Z, et al. The Value of a Seven-Autoantibody Panel Combined with the Mayo Model in the Differential Diagnosis of Pulmonary Nodules. Dis Markers 2021;2021:6677823. 10.1155/2021/6677823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang W, Zhuang R, Ma H, et al. The diagnostic value of a seven-autoantibody panel and a nomogram with a scoring table for predicting the risk of non-small-cell lung cancer. Cancer Sci 2020;111:1699-710. 10.1111/cas.14371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Zhang M, Pan X, et al. Integrative Serum Metabolic Fingerprints Based Multi-Modal Platforms for Lung Adenocarcinoma Early Detection and Pulmonary Nodule Classification. Adv Sci (Weinh) 2022;9:e2203786. 10.1002/advs.202203786 [DOI] [PMC free article] [PubMed] [Google Scholar]