Abstract
The nucleocapsid protein (NC) of HIV type 1 (HIV-1) is a nucleic acid chaperone that facilitates the rearrangement of nucleic acid secondary structure during reverse transcription. HIV-1 NC contains two CCHC-type zinc binding domains. Here, we use optical tweezers to stretch single λ-DNA molecules through the helix-to-coil transition in the presence of wild-type and several mutant forms of HIV-1 NC with altered zinc-finger domains. Although all forms of NC lowered the cooperativity of the DNA helix–coil transition, subtle changes in the zinc-finger structures reduced NC's effect on the transition. The change in cooperativity of the DNA helix–coil transition correlates strongly with in vitro nucleic acid chaperone activity measurements and in vivo HIV-1 replication studies using the same NC mutants. Moreover, Moloney murine leukemia virus NC, which contains a single zinc finger, had little effect on transition cooperativity. These results suggest that a specific two-zinc-finger architecture is required to destabilize nucleic acids for optimal chaperone activity during reverse transcription in complex retroviruses such as HIV-1.
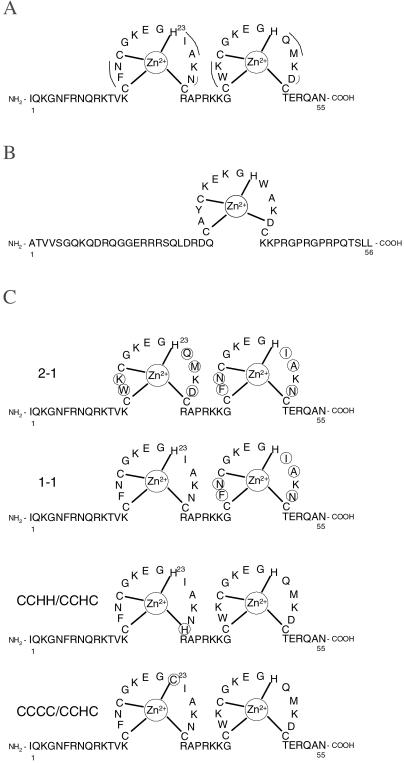
The nucleocapsid protein (NC) is a small highly basic nucleic acid-binding protein found in all orthoretroviruses (1). NC possesses nucleic acid chaperone activity, by which it facilitates the rearrangement of nucleic acids into conformations that are thermodynamically more stable than the original structure. To achieve such rearrangements, the base pairs of nucleic acid structures that are normally very stable must be broken, whereas other complementary structures must be formed (2, 3). All orthoretroviral proteins contain either one or two zinc-finger motifs of the form CCHC (4, 5). Two high-resolution NMR structures of HIV-1 NC bound to viral RNA stem–loop sequences indicate that the two zinc fingers (Fig. 1A) interact specifically with several purine bases in the RNA loops (6, 7). In contrast, Moloney murine leukemia virus (MLV) NC, which contains only one zinc finger (Fig. 1B), does not bind to isolated stem–loop structures of Moloney MLV genomic RNA with high affinity (8). Recent studies have shown that the zinc fingers of HIV-1 NC are required for at least some of its nucleic acid chaperone functions. For example, a mutant form of NC with both CCHC motifs changed to SSHS (SSHS NC) is unable to bind zinc and displays a severely reduced ability to facilitate the minus-strand transfer step in reverse transcription (9). In contrast, the single zinc finger of Moloney MLV NC is not required for minus-strand transfer in that system (10).
Figure 1.
(A) Amino acid sequence and two-zinc-finger structure of HIV-1 NC (pNL4–3 isolate, GenBank accession no. AF324493). Differences between the two zinc-finger motifs are indicated by a solid curved line. (B) Amino acid sequence and single zinc-finger structure of Moloney MLV NC (GenBank accession no. J02255). (C) HIV-1 NC mutants used in this study. In the 2–1 NC mutant, the sequences of the two zinc fingers are switched relative to wild-type NC, whereas in the 1–1 NC mutant, the first finger is repeated in place of the second. For the CCCC/CCHC mutant, residue His-23 is replaced with Cys, whereas the CCHH/CCHC mutant has Cys-28 replaced with His. In all cases, changes relative to wild-type NC are circled.
We recently used an optical tweezers instrument to measure the effect of NC on the overstretching transition of single λ-DNA molecules (11). We have shown that the overstretching force and thermal melting point of DNA exhibit similar trends as a function of pH, and that a model of the overstretching transition as force-induced melting (12) accurately describes the dependence of the overstretching force on pH (13) and temperature (14). Thus, this technique allows us to study the DNA helix–coil transition at very high resolution and at room temperature (15). The latter capability is particularly useful for studying protein–nucleic acid interactions, because temperature effects on protein properties do not complicate the analysis.
We have used the DNA stretching technique to investigate the capability of NC to alter the base pairing of single λ-DNA molecules at room temperature (11). We found that the helix–coil transition free energy (ΔG) of λ-DNA was significantly reduced in the presence of HIV-1 NC, whereas ΔG was actually increased in the presence of SSHS NC. Therefore, the deletion of the zinc-finger structures was detrimental to NC's helix destabilizing function. We also showed that only wild-type NC altered the cooperativity of the helix–coil transition, as evidenced by the observed change in slope of the DNA overstretching transition. On the basis of in vitro measurements of nucleic acid chaperone activity (9) and our single molecule stretching measurements, we proposed that there are two components to the nucleic acid chaperone activity of NC. First, the high charge density of NC causes electrostatic attraction between nucleic acids, allowing complementary double-stranded structures to interact more readily. Second, NC destabilizes the helical structure of DNA by altering the cooperativity of the helix–coil transition, such that thermal fluctuations are sufficient to melt small helical DNA sections. Precisely how NC's zinc fingers contribute to its chaperone function is unknown.
Materials and Methods
The dual-beam optical tweezers instrument used in this study consists of two counterpropagating diode lasers focused to a small spot inside a liquid flow cell. One 4.4-μm-diameter streptavidin-coated polystyrene bead (Bangs Laboratories, Fisher, IN) was held in the optical trap formed by the laser beams. Another streptavidin-coated bead was held on the end of a glass micropipette. To obtain force–extension measurements, a single double-stranded DNA molecule that had been labeled on the 3′ end of opposite strands with biotin was captured between the two beads (13). The DNA molecule was then stretched by moving the pipette and measuring the resulting calibrated force on the bead in the trap, as previously described (13, 14). The buffer used in this study for capturing DNA was 10 mM Hepes with 95 mM NaCl and 5 mM NaOH, pH 7.5.
The absolute extension of the molecule was estimated by measuring the distance between the centers of the two beads by using an image captured with a charge-coupled device camera. The change in position of the pipette was measured by using a feedback-compensated piezoelectric translation stage that is accurate to 5 nm (Melles Griot, Irvine, CA). The position measurement was converted to a measurement of the molecular extension by correcting for the trap stiffness, which was 62 ± 3 pN/μm. For the measurements reported here, the pipette was moved in 100-nm steps, and after each step the force was measured 100 times and averaged, thus averaging out contributions of thermal motion to the force measurement.
HIV-1 nucleocapsid protein (NC) used in these experiments was prepared as described (16). The preparation of HIV-1 NC mutants has also been described (17). After capturing a single DNA molecule in the tethering buffer, the molecule was stretched to verify that the usual force–extension curve was obtained. To measure the effect of the protein on this transition, 4–5 cell volumes of a buffer solution with a reduced NaCl concentration containing a fixed amount of NC was added to the experimental cell until the buffer surrounding the captured DNA molecule was completely exchanged.
Results and Discussion
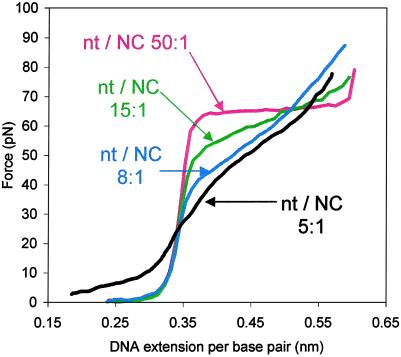
We report here the use of single DNA molecule stretching to investigate the requirement for NC's specific zinc-finger architecture in the nucleic acid chaperone activity of NC. Although the two zinc fingers of HIV-1 NC share a common CCHC motif, five of the amino acid residues present in the loops between the residues that coordinate the zinc atoms differ between the two motifs (Fig. 1A). These experiments were carried out by using various mutant forms of HIV-1 NC with changes in the zinc-finger domains (Fig. 1C). For wild-type NC, we used a constant concentration of protein (approximately 10 nM) and altered the ionic strength of the buffer to regulate the fraction of NC bound to the λ-DNA. The fraction of wild-type NC bound per DNA nucleotide was estimated as described (refs. 11 and 18). As previously reported, the change in the slope of the overstretching transition appears to saturate at a nucleotide/NC ratio of 8:1 (10 nM NC and 50 mM Na+), which is the critical binding density required for the observation of optimal nucleic acid chaperone activity (Fig. 2) (3). For comparison, we first measured the effect of NC mutants on DNA stretching under these conditions.
Figure 2.
DNA stretching curves in 10 mM Hepes, pH 7.5, containing 10 nM NC and 150 mM (red line), 75 mM (green line), 50 mM (blue line), and 25 mM [Na+] (black line). These concentrations correspond to an estimated 50:1, 15:1, 8:1, and 5:1 nucleotide/NC ratio, respectively (11), as indicated on the graph.
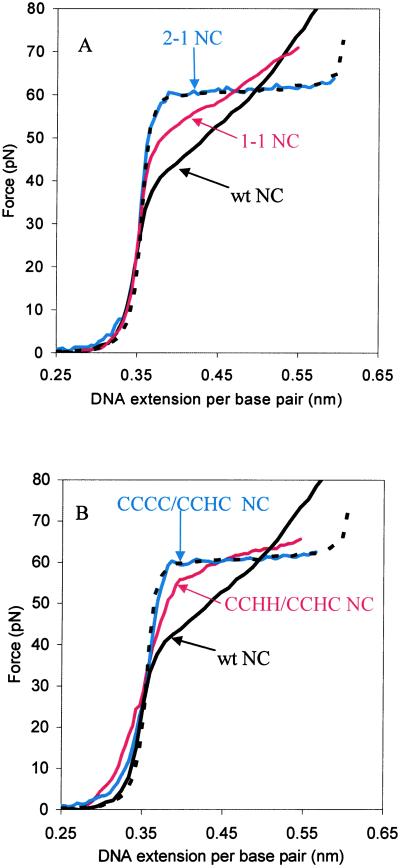
Representative force–extension curves for λ-DNA in the presence of the NC mutants are shown in Fig. 3. We first stretched DNA in the presence of a mutant in which the positions of the two fingers are switched (Fig. 1C, 2–1 NC). The results show that, at a concentration of 10 nM, the finger switch mutant has no effect on DNA overstretching (Fig. 3A). Both the slope and the plateau level of the overstretching transition are unchanged relative to an experiment carried out in the absence of protein. In contrast, when the second finger is simply replaced with the first finger (Fig. 1C, 1–1 NC), there is a clear effect on the stretching curve (Fig. 3A). The 1–1 mutant lowers the cooperativity, although the magnitude of the effect on DNA overstretching is reduced compared with wild-type NC. We conclude from these data that it is important to have the first finger in the N-terminal position for effective nucleic acid chaperone activity.
Figure 3.
DNA stretching curves in the presence of 10 nM wild-type and mutant HIV-1 NCs in 10 mM Hepes, 50 mM [Na+]. (A) Comparison of experiments conducted with the 2–1 NC finger switch mutant (blue), the 1–1 NC finger repeat mutant (red), and wild-type HIV-1 NC (solid black line). An experiment performed in the absence of NC is also shown (dashed black line). (B) Comparison of DNA stretching with CCCC/CCHC NC (blue), CCHH/CCHC NC (red), no NC (dashed black line), and wild-type NC (solid black line).
Having established the importance of the position of the first finger in NC's chaperone function, we next tested mutants with more subtle changes in the CCHC motif of the first finger. In one mutant tested, His-23 was replaced with Cys (Fig. 1C, CCCC/CCHC), and in a second variant, Cys-28 was replaced with His (Fig. 1C, CCHH/CCHC). CCCC zinc-finger motifs are found in steroid hormone receptor proteins, whereas CCHH motifs are found in transcription factors (19). As shown in Fig. 3B, binding of the CCCC/CCHC NC variant has no effect on the cooperativity of DNA overstretching when present at 10 nM concentration. The effect of CCHH/CCHC on the overstretching transition is also significantly reduced compared with wild-type HIV-1 NC at this concentration (Fig. 3B). An NMR structure of the Cys-23(13–64)NCp7 peptide indicates that the single His-23 to Cys change induces a change in the conformation of the N-terminal finger and increases the distance between both fingers (20). Although this mutant still binds zinc strongly, it has significantly reduced RNA-binding affinity (20). Thus, the altered behavior of the CCCC/CCHC variant in the DNA stretching experiment does not appear to be due to a significantly reduced zinc-binding affinity but may be due at least in part to reduced DNA binding (20).
These results can be quantified by fitting the force–extension behavior to the standard Zimm–Bragg model for a helix–coil transition (Table 1) (11, 21). In this fit, we use two adjustable parameters, ΔG and σ. The stability of the helix with respect to the coil form of DNA is determined by the free energy change (ΔG) from helix to coil state, given by the area between the stretching curves for double- and single-stranded DNA. The cooperativity is given by the parameter σ, which increases with the slope of the transition and as the cooperativity decreases. The average number of bases that must be simultaneously melted to nucleate a melted domain within a double-stranded structure is approximately σ−1/2 (22). Both decreases in ΔG and increases in σ indicate destabilization of nucleic acid secondary structure, because ΔG is the energy per base pair required to undergo the helix to coil transition, whereas σ determines the number of base pairs that must be simultaneously melted during the transition. The helix–coil transition free energy and cooperativity parameter for DNA stretching in the presence of wild-type HIV-1 NC and the mutants examined here are given in Table 1. We also show the percent chaperone activity, relative to wild-type HIV-1 NC, for each of these mutants, as measured in an in vitro minus-strand transfer assay, which was designed to mimic the first of two strand-transfer steps that occur during viral DNA synthesis (17). During minus-strand transfer, the newly synthesized minus-strand strong-stop DNA is annealed to a complementary region of the RNA genome located at the 3′ terminus. NC greatly stimulates the annealing step of minus-strand transfer (9, 23), which results in the formation of a long 98-nucleotide base-paired binary complex.
Table 1.
Parameters describing the helix–coil transition of single λ-DNA molecules in the presence of HIV-1 nucleocapsid protein zinc finger variants
| HIV-1NC | ΔG, kBT/bp | σ | Chaperone activity, %† |
|---|---|---|---|
| Wild-type | 1.4 ± 0.3 | 0.130 | 4+ |
| 1-1 | 1.7 ± 0.3 | 0.050 | 3+ |
| CCHH/CCHC | 1.8 ± 0.3 | 0.025 | 2+ |
| 2-1 | 1.8 ± 0.3 | 0.001 | <1+ |
| CCCC/CCHC | 1.8 ± 0.3 | 0.001 | <1+ |
| SSHS/SSHS | 2.7 ± 0.3* | 0.001* | 1+‡ |
Data were obtained at 10 nM NC concentration in 50 mM Na+, 10 mM Hepes, pH 7.5. The error in the free energy per base pair (ΔG) is estimated as the difference between ΔG determined by using wild-type NC single-stranded (ss)DNA stretching curves (11) and ssDNA stretching curves without NC (33). The free energy is reported in units of kBT per base pair, where kB is the Boltzmann constant and T = 293 K is room temperature (1 kBT = 581 cal/mol). The cooperativity parameter (σ) is defined as described in the text.
Data are from ref. 11.
Data are from ref. 17. Chaperone activity refers to in vitro synthesis of the minus-strand DNA transfer product. The values refer to percentage of wild-type activity: 4+, 75–100%; 3+, 50–75%; 2+, 25–50%; 1+, 1–25%.
Data are from ref. 9.
Although the total transition free energy does not change significantly for the new NC mutants tested here, the cooperativity parameter decreases relative to that of wild-type NC for all of the NC variants. For example, the cooperativity parameter for the 1–1 finger repeat mutant is about 40% of the value determined for wild-type NC. For the same mutant, the chaperone activity has been measured to be about 50–75% of that observed for wild-type NC (17). Similarly, the CCHH/CCHC variant's cooperativity parameter is 20% of the wild-type value, whereas the chaperone activity is measured to be 25–50% relative to wild-type (17). The rest of the mutations tested had even larger effects on NC's ability to change σ, resulting in no measurable change in cooperativity relative to an experiment carried out in the absence of NC. In particular, stretching experiments performed with both the 2–1 and the CCCC/CCHC variants resulted in an estimated cooperativity parameter of less than 1% of the wild-type value, whereas the chaperone activity is also less than 1% (17). Interestingly, the 1–1 mutant, which induces a cooperativity change closer to wild-type than any other mutant, is the only mutant tested that exhibits in vivo replication activity, suggesting a connection between the helix destabilization capability of NC and HIV-1 replication (17, 24, 25).
The transition cooperativity is a measure of the barrier to nucleation of melted domains of DNA. When the cooperativity is low, as it is in the presence of HIV-1 NC, thermal fluctuations cause the melting and reannealing of domains of DNA. This melting and reannealing are responsible for NC's ability to rearrange DNA secondary structure. Thus, a protein that is able to reduce the cooperativity should rearrange secondary structure more readily. Nucleic acid chaperone activity is believed to be an essential function of NC in vivo and, as summarized in Table 1, a recent study using the same mutants investigated here (17) indicated that even subtle alterations of the zinc-finger structure dramatically reduce NC's ability to facilitate minus-strand transfer. The excellent correlation between the effects of NC mutation on the helix–coil transition and the chaperone activity as measured by the minus-strand transfer assay strongly supports the hypothesis that the observed change in cooperativity is directly related to NC's chaperone function.
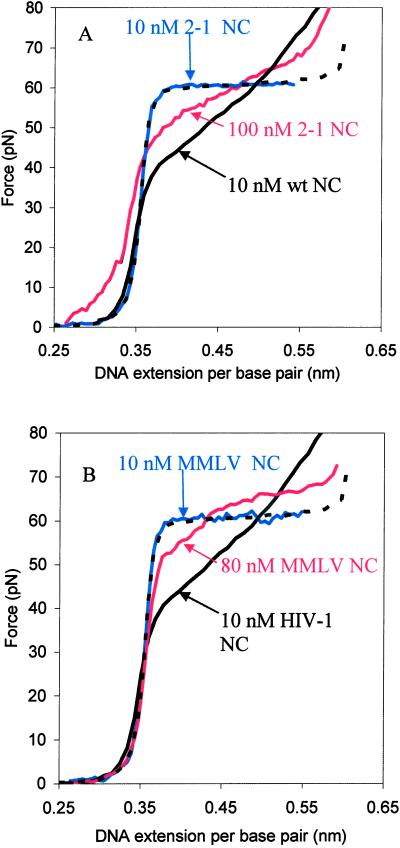
Under fixed binding conditions, the relative changes in cooperativity observed with the NC mutants tested parallel the in vitro chaperone activity (Table 1). We next carried out DNA stretching experiments by using increased concentrations of protein for mutants that showed no chaperone activity at 10 nM. The results for 2–1 NC are shown in Fig. 4A. As the protein concentration is increased from 10 to 100 nM, we observe a significant change in the slope of the overstretching transition. At 100 nM, the cooperativity observed in the presence of 2–1 NC resembles that of the 1–1 mutant at 10 nM concentration. The CCCC/CCHC mutant showed a slight change in cooperativity at protein concentrations greater than 50 nM (data not shown). Thus, all of the NC mutants tested that contained two zinc fingers were able to destabilize double-stranded DNA somewhat when present at higher concentrations. We also note that at high protein binding, a finite force is required to stretch double-stranded DNA even at extensions less than the B-form contour length of 0.34 nm/bp (Fig. 4A). This is a manifestation of aggregation forces due to the high charge of NC. These forces are also responsible in part for the nucleic acid chaperone activity of NC (11).
Figure 4.
(A) Concentration dependence of DNA overstretching in the presence of the HIV-1 NC (2–1) finger switch mutant. DNA stretching curves in 10 nM (blue line) and 100 nM (red line) 2–1 NC are shown, along with stretching curves in the absence of NC (dashed black line) and in the presence of wild-type NC (solid black line). (B) Comparison of DNA stretching with Moloney MLV NC and HIV-1 NC. DNA stretching curves in the presence of 10 nM (blue line) and 80 nM (red line) Moloney MLV NC are shown, along with stretching curves in the absence of NC (dashed black line) and in the presence of 10 nM HIV-1 NC (solid black line).
These results suggest that the change in the ability of NC to alter the helix–coil transition on subtle alteration of the zinc-finger architecture may be due to a decrease in the λ-DNA-binding affinity of the mutant forms of NC. This change in binding affinity may also contribute to the reduction in in vitro chaperone activity observed for these mutants (17). However, binding measurements of HIV-1 NC variants to poly(ɛA) showed that the binding constant of 2–1 NC was comparable to that of wild-type NC, whereas 1–1 NC had a binding constant 6 times greater than that of wild-type NC (26). In contrast, the ability of HIV-1 2–1 NC to alter the cooperativity of the DNA helix–coil transition is significantly reduced compared with either wild-type or 1–1 NC (Fig. 3A and Table 1). Thus, the effects observed on the transition cooperativity, and therefore on the chaperone activity, of NC are more likely to be due to alterations in the specific interactions between the zinc fingers and the DNA rather than to significantly altered binding affinity.
We have shown that the first finger appears to be more important for the chaperone activity of HIV-1 NC than the second finger. To directly probe the requirement, if any, for two zinc fingers, stretching experiments were carried out with Moloney MLV NC, which contains only one zinc-finger motif (Fig. 1B). When DNA is stretched in the presence of this protein at a concentration of 10 nM, the cooperativity of the helix–coil transition is not altered (Fig. 4B). Even at an 80 nM concentration, there is very little change in cooperativity (Fig. 4B). Moreover, the shape of the transition is altered in a manner that cannot be interpreted as a simple change in cooperativity. Thus, although further studies are needed to fully understand the changes induced by MLV NC at high protein concentration, this single zinc-finger variant clearly does not affect the cooperativity of the helix–coil transition under the specific conditions that lead to optimum chaperone activity in the presence of HIV-1 NC.
It is surprising that HIV-1 NC is more effective at destabilizing the DNA helix than Moloney MLV NC, because both proteins must act as nucleic acid chaperones during retroviral reverse transcription. However, both NC proteins have a high charge density, and it is known that this property is sufficient for facilitating nucleic acid rearrangements involving small numbers of base pairs, such as those involved in annealing tRNA to the primer-binding site of the genomic RNA (27). In contrast, a highly charged peptide cannot effectively facilitate minus-strand transfer in HIV-1 (9). This process involves annealing of the transactivation response (TAR) RNA, which is predicted to contain 24 base pairs, to the complementary DNA sequence, which can also form a stable secondary structure (23). In contrast, the only known secondary structural element that must be destabilized for Moloney MLV minus-strand transfer is much less stable than the HIV-1 TAR region (28). Therefore, whereas it is possible that the observed differences between the one- and two-zinc-finger proteins may be due to differences in nucleic acid binding affinities, it is reasonable to hypothesize that Moloney MLV NC does not require helix-destabilization capabilities to facilitate minus-strand transfer in vivo, whereas HIV-1 NC does. This prediction is supported by the DNA stretching studies reported here and by the inability of Moloney MLV NC to facilitate minus-strand transfer of the 98 nucleotide HIV-1 repeat (R) region (10).
By measuring the effect of NC protein variants on the helix–coil transition of single DNA molecules, we have demonstrated several important aspects of nucleic acid chaperone activity. The excellent correlation between the ability of mutants of HIV-1 NC to alter the cooperativity of the helix–coil transition and their chaperone activity, as measured by an in vitro minus-strand transfer assay (17), supports the conclusion that the change in transition cooperativity induced by NC binding indeed is responsible for the nucleic acid chaperone activity. Even subtle changes in the first zinc-finger architecture of NC impaired its ability to alter the helix–coil transition cooperativity. Nevertheless, all of the mutants examined that contained two zinc fingers were able to alter the transition to some extent when present at high concentrations. In contrast, Moloney MLV NC, which contains only one zinc finger, does not substantially alter the transition cooperativity, although at high concentrations it does appear to destabilize some portions of the DNA molecule in a manner that is not yet completely understood. Furthermore, SSHS NC, which lacks zinc-finger structures altogether, was unable to alter the cooperativity even at high protein binding (data not shown).
All retroviral RNA genomes contain a repeat region known as “R” located at opposite ends of the genome, which varies in length from 15 to 247 nucleotides (1). In reverse transcription of the RNA genome, the minus-strand strong-stop DNA that is initially formed contains a copy of the R region that must be annealed to the complementary region of RNA on the opposite end of the retroviral genome. Some simple retroviruses (see ref. 29 for definition) with relatively short R regions, such as Moloney MLV and other similar murine viruses, have NC proteins with only one zinc finger (30), whereas retroviruses with long repeat regions that form hairpin structures, such as HIV-1, HIV-2, and human T-lymphotrophic virus, type I HTLV 1 (31), contain NCs with two zinc fingers. The results reported here are consistent with the hypothesis that retroviruses with a long R region that can form stable secondary structure require an NC protein with two zinc fingers for optimal capability to destabilize nucleic acid structures, whereas simple retroviruses require only one zinc domain. However, because the zinc fingers of NC are also involved in packaging (30, 32) and other processes in the retroviral life cycle (1), simple retroviruses that possess two zinc fingers, e.g., Rous sarcoma virus, may have evolved this structure for these alternate functions.
Acknowledgments
We thank Donald G. Johnson, Bradley P. Kane, and Dr. Nick Lee for purification of wild-type and mutant NC proteins, and Ms. Carmen Silvers for preparation of biotin-labeled λ-DNA. We also thank Drs. Judith Levin and Jianhui Guo for helpful discussions and for sharing data before publication. Funding for this project was provided by National Institutes of Health Grant AI43231 (K.M.F.) and Northeastern University (M.C.W.). This work was also supported in part by the National Cancer Institute, Department of Health and Human Services, under Contract NO1-CO-12400 with SAIC Frederick, Incorporated (R.J.G.).
Abbreviations
- NC
nucleocapsid protein
- HIV-1
HIV type 1
- MLV
murine leukemia virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Negroni M, Buc H. Annu Rev Genet. 2001;35:275–302. doi: 10.1146/annurev.genet.35.102401.090551. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchihashi Z, Brown P O. J Virol. 1994;68:5863–5870. doi: 10.1128/jvi.68.9.5863-5870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rein A, Henderson L E, Levin J G. Trends Biochem Sci. 1998;23:297–301. doi: 10.1016/s0968-0004(98)01256-0. [DOI] [PubMed] [Google Scholar]
- 4.Berg J M. Science. 1986;232:485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- 5.Covey S N. Nucleic Acids Res. 1986;14:623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Guzman R N, Wu Z R, Stalling C C, Pappalardo L, Borer P N, Summers M F. Science. 1998;279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 7.Amarasinghe G K, Guzman R N D, Turner R B, Chancellor K J, Wu Z R, Summers M F. J Mol Biol. 2000;301:491–511. doi: 10.1006/jmbi.2000.3979. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza V, Melamed J, Habib D, Pullen K, Wallace K, Summers M F. J Mol Biol. 2001;314:217–232. doi: 10.1006/jmbi.2001.5139. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Wu T, Anderson J, Kane B F, Johnson D G, Gorelick R J, Henderson L E, Levin J G. J Virol. 2000;74:8980–8988. doi: 10.1128/jvi.74.19.8980-8988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allain B, Rascle J B, de Rocquigny H, Roques B, Darlix J L. J Mol Biol. 1998;277:225–235. doi: 10.1006/jmbi.1997.1596. [DOI] [PubMed] [Google Scholar]
- 11.Williams M C, Rouzina I, Wenner J R, Gorelick R J, Musier-Forsyth K, Bloomfield V A. Proc Natl Acad Sci USA. 2001;98:6121–6126. doi: 10.1073/pnas.101033198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouzina I, Bloomfield V A. Biophys J. 2001;80:882–893. doi: 10.1016/S0006-3495(01)76067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams M C, Wenner J R, Rouzina I, Bloomfield V A. Biophys J. 2001;80:874–881. doi: 10.1016/S0006-3495(01)76066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams M C, Wenner J R, Rouzina I, Bloomfield V A. Biophys J. 2001;80:1932–1939. doi: 10.1016/S0006-3495(01)76163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams M C, Rouzina I, Bloomfield V A. Acc Chem Res. 2002;35:159–166. doi: 10.1021/ar010045k. [DOI] [PubMed] [Google Scholar]
- 16.Lee B M, De Guzman R N, Turner B G, Tjandra N, Summers M F. J Mol Biol. 1998;279:633–649. doi: 10.1006/jmbi.1998.1766. [DOI] [PubMed] [Google Scholar]
- 17.Guo J, Wu T, Kane B F, Johnson D G, Henderson L E, Gorelick R J, Levin J G. J Virol. 2002;76:4370–4378. doi: 10.1128/JVI.76.9.4370-4378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouzina I, Bloomfield V A. Biophys Chem. 1997;64:139–155. doi: 10.1016/s0301-4622(96)02231-4. [DOI] [PubMed] [Google Scholar]
- 19.Berg J M, Shih Y. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 20.Déméné H, Dong C Z, Ottmann M, Rouyez M C, Jullian N, Morellet N, Mély Y, Darlix J L, Fournié-Zaluski M C, Saragosti S, et al. Biochemistry. 1994;33:11707–11716. doi: 10.1021/bi00205a006. [DOI] [PubMed] [Google Scholar]
- 21.Zimm B H. J Chem Phys. 1960;33:1349–1356. [Google Scholar]
- 22.Cantor C R, Schimmel P R. Biophysical Chemistry. New York: Freeman; 1980. [Google Scholar]
- 23.You J C, McHenry C S. J Biol Chem. 1994;269:31491–31495. [PubMed] [Google Scholar]
- 24.Gorelick R J, Chabot D J, Rein A, Henderson L E, Arthur L O. J Virol. 1993;67:4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelick R, Gagliardi T, Bosche W, Wiltrout T, Coren L, Chabot D, Lifson J, Henderson L, Arthur A. Virology. 1999;256:92–104. doi: 10.1006/viro.1999.9629. [DOI] [PubMed] [Google Scholar]
- 26.Urbaneja M A, Kane B P, Johnson D G, Gorelick R J, Henderson L E, Casas-Finet J R. J Mol Biol. 1999;287:59–75. doi: 10.1006/jmbi.1998.2521. [DOI] [PubMed] [Google Scholar]
- 27.Hargittai M R S, Mangla A T, Gorelick R J, Musier-Forsyth K. J Mol Biol. 2001;312:985–997. doi: 10.1006/jmbi.2001.5021. [DOI] [PubMed] [Google Scholar]
- 28.Trubetskoy A M, Okenquist S A, Lenz J. J Virol. 1999;73:3477–3483. doi: 10.1128/jvi.73.4.3477-3483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [PubMed] [Google Scholar]
- 30.Berkowitz R, Fisher J, Goff S P. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 31.Golinelli M P, Hughes S H. Virology. 2001;285:278–290. doi: 10.1006/viro.2001.0970. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Barklis E. J Virol. 1995;69:5716–5722. doi: 10.1128/jvi.69.9.5716-5722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith S B, Cui Y J, Bustamante C. Science. 1996;271:795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]