Abstract
Objective
Quetiapine has been used for treating patients with depression; however, drug-drug interactions (DDIs) deeply limit its individualized administration. This study explored DDIs and initial dose recommendation of quetiapine in depression patients based on real-world data.
Methods
Sixty-four real-world depression patients were used to investigate the effects of drug combinations on quetiapine using a non-linear mixed effect model (NONMEM).
Results
In the final model, paroxetine and fluvoxamine were included as covariates, which significantly affected the clearance rate of quetiapine, with ratios of about 1.00:0.54:0.48:0.26 in patients with depression who were not accompanied by paroxetine or fluvoxamine, patients with depression who were accompanied by paroxetine, patients with depression who were accompanied by fluvoxamine, and patients with depression who were accompanied by paroxetine and fluvoxamine. Furthermore, the initial dose optimizations of quetiapine were 20 and 16 mg/kg/day for depression patients not accompanied by paroxetine or fluvoxamine who weighted 40–80, and 80–120 kg, respectively. The initial dose of quetiapine was 8 mg/kg/day for depression patients accompanied by paroxetine who weighted 40–120 kg. The initial dose of quetiapine was 8 mg/kg/day for depression patients accompanied by fluvoxamine, who weighted 40–120 kg. The initial dose optimization of quetiapine was 4 mg/kg/day for depression patients accompanied by paroxetine and fluvoxamine who weighted 40–120 kg.
Conclusion
Our study explored DDIs and initial dose recommendation of quetiapine in depression patients from the real world, and the initial dose optimization of quetiapine was recommended based on the interaction with paroxetine or fluvoxamine.
Keywords: drug-drug interactions, initial dose recommendation, quetiapine, depression patients, real world
Graphical Abstract
Introduction
Depression is a mood disorder and demonstrates significant epidemiological features, notably a high prevalence and recurrence rates. It seriously affects physical and mental health, social communication, occupational ability, and physical activity.1,2 Epidemiological data indicate a progressive annual increase in depression diagnosis across global populations.3–8 The core mechanism of depression includes the imbalance of various neurotransmitter systems, involving the abnormal signaling of monoamine transmitters such as 5-hydroxytryptamine (5-HT) and dopamine (DA), which affect emotional regulation and cognitive function. Clinical management strategies are primarily determined by symptom severity. Mild cases typically respond to psychoeducational interventions, self-management techniques, and psychotherapeutic approaches. In moderate-to-severe cases, pharmacotherapy with antidepressant medications is the primary treatment method.9–11
As an antagonist of several neurotransmitter receptors in the brain, quetiapine is an atypical antipsychotic medication used for treating all schizophrenia variants. Quetiapine regulates neurotransmitter balance and improves mood and cognitive function by antagonizing dopamine D2 and 5-HT2A receptors as well as reducing the emotional symptoms associated with schizophrenia.12,13 Evidence supports the role of quetiapine in depression management.14–16 However, in the course of treatment for depression, multiple concomitant medications are often used.
Drug-drug interactions (DDIs) are the complex effects of two or more drugs taken by patients simultaneously or within a certain period. These interactions may manifest as either enhanced therapeutic outcomes with reduced adverse effects or conversely diminished efficacy, accompanied by unintended toxicity.17,18 Enhanced effects include increased efficacy and increased toxicity, whereas diminished effects include decreased efficacy and decreased toxicity.19,20 Optimal clinical management of polypharmacy requires strategic utilization of pharmacological characteristics. The clinical practice process should maximize therapeutic benefits while minimizing adverse reactions by carefully considering the DDIs mechanisms. This approach enhances medication safety and ensures treatment efficacy.20,21 On the basis of the principle of occurrence, DDIs can be divided into pharmacodynamic and pharmacokinetic interactions. Pharmacodynamic interactions produce four distinct outcomes: irrelevant, synergistic, additive, and antagonistic. Pharmacokinetic interactions primarily arise from drug-mediated alterations in drug absorption, distribution, metabolism, and excretion.22
As quetiapine is metabolized by hepatic enzymes, DDIs may affect its metabolism, consequently influencing quetiapine concentrations that directly correlate with therapeutic outcomes and safety assessments.18 Quetiapine is primarily metabolized by CYP3A4 and CYP2D6,23–26 and their inhibition/induction significantly alters drug levels. The therapeutic window of quetiapine in patients was 100–500 ng/mL,27 where subtherapeutic levels risk treatment failure, while supratherapeutic levels increase sedation/QT prolongation risks.
Generally speaking, in the evaluation of DDIs, it is important to check the DDIs database, their reporting, nature, and other features of DDIs. This will provide a systematic way to assess DDIs and their relevance in a clinical scope.28,29 DDIs may affect the pharmacokinetics of quetiapine, which in turn affects its concentration, ultimately leading to differences in the real-world need for quetiapine dose for clinical treatment. Population pharmacokinetic (PPK) is a quantitative pharmacological method for studying DDIs and formulating individualized dose plans.30 Several studies have been conducted on dose recommendation.31–37 Thus, this study aimed to explore DDIs and initial dose recommendation of quetiapine in real-world patients with depression via PPK.
Methods
Data Collection
Depression patients treated by quetiapine who were recruited from Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University between March 2021 and January 2024 were included. Inclusion criteria: (a) depression patients, (b) quetiapine treatment, (c) therapeutic drug monitoring for quetiapine. Exclusion criteria: depression patients with missing clinical medical record data. Quetiapine concentrations, relevant medical information of the corresponding patients were collected from real-world clinical practice records. The above research was approved by the Research Ethics Committee of the Xuzhou Oriental Hospital affiliated to Xuzhou Medical University, where the requirement for written informed consent could be waived since the data were collected retrospectively without patient identifiers. This study adhered to the Declaration of Helsinki.
Modeling
A quetiapine PPK model in depression patients was established via non-linear mixed effect modeling (NONMEM), where CL/F, V/F, and Ka (fixed at 1.46/h38) were included.
The inter-individual variability was demonstrated in Formula (i):
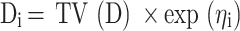 |
(i) |
Di represents the individual parameters. TV(D) shows typical individual parameters. ηi exhibited a symmetrical distribution.
The random residual variability was demonstrated in Formula (ii):
 |
(ii) |
Gi represents the observed concentrations. Ji demonstrated individual predicted concentrations. ε1, ε2 exhibited symmetrical distributions.
Relationship between weight and pharmacokinetic parameters was demonstrated in Formula (iii):
 |
(iii) |
Ki denotes the ith parameters. where Mi represents the ith individual’s weight. Mstd had a 70 kg standard weight and Kstd had typical individual parameters. O was an allometric coefficients of 0.75 and 1 for CL/F and V/F, respectively.39
Continuous and categorical covariate models are shown in Formulae (iv) and (v), respectively.
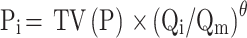 |
(iv) |
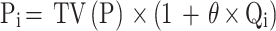 |
(v) |
where Pi is individual parameters. The TV(P) demonstrated typical individual parameters. θ demonstrated parameters for being estimated. where Qi represents the covariates of ith individual. Qm represents the median for covariates.
Covariate analysis of the quetiapine PPK model was performed using a stepwise method. The objective function value (OFV) variation was covariate inclusion criteria, among which OFV decrease > 3.84 (P < 0.05) was defined as the inclusion standard, and OFV increase > 6.63 (P < 0.01) was defined as the exclusion standard.
Model Evaluation
Visualization way and bootstrap were used to evaluate the final quetiapine PPK model.
Simulation
The Monte Carlo method was used to simulate quetiapine concentrations in patients with depression, and the therapeutic window of quetiapine in these patients was 100–500 ng/mL.27 In addition, paroxetine and fluvoxamine, which significantly affected the clearance rate of quetiapine in depression patients, were included as covariates. To determine whether paroxetine or fluvoxamine were co-administered, we simulated four different cases: (a) patients with depression who were not accompanied by paroxetine or fluvoxamine; (b) patients with depression who were accompanied by paroxetine; (c) patients with depression who were accompanied by fluvoxamine; and (d) patients with depression who were accompanied by paroxetine and fluvoxamine. Each case simulated thousand virtual patients with depression, with five different weight groups (40, 60, 80, 100, and 120 kg) and eight different dose groups (1, 4, 8, 12, 16, 20, 24, and 28 mg/kg/day). The probability of attaining a therapeutic concentration range was chosen as the evaluation criterion, and the upper limit of the therapeutic concentration indicated that when the concentration of the drug in the blood reached an upper limit, it may reduce the tolerance of the drug, increase the adverse reactions, or the therapeutic effect would no longer increase or even decrease. Therefore, we evaluated the probability of exceeding the ceiling of the therapeutic concentration (500 ng/mL) at thousand simulated concentrations as a secondary evaluation index.
Results
Patient Information
Sixty-four depression patients treated with quetiapine were included for analysis, where 24 men and 40 women, whose ages were 23.29 ± 13.79 years old, weights were 65.35 ± 16.63 kg. Relevant medical information is shown in Tables 1 and 2, respectively.
Table 1.
Demographic Data of Patients with Depression (n = 64)
| Characteristic | Mean ± SD | Median (Minimum-Maximum) |
|---|---|---|
| Gender (men/women) | 24/40 | / |
| Age (years) | 23.29 ± 13.79 | 17.05 (12.05–65.29) |
| Weight (kg) | 65.35 ± 16.63 | 65.00 (39.00–117.00) |
| Albumin (g/L) | 41.80 ± 3.01 | 41.75 (35.80–49.50) |
| Globulin (g/L) | 25.62 ± 2.67 | 25.70 (18.90–32.80) |
| Alanine transaminase (IU/L) | 31.58 ± 30.04 | 22.50 (5.00–190.00) |
| Aspartate transaminase (IU/L) | 24.79 ± 15.47 | 19.00 (12.00–94.00) |
| Creatinine (μmol/L) | 59.98 ± 12.63 | 60.50 (34.00–92.00) |
| Urea (mmol/L) | 4.10 ± 1.06 | 4.06 (1.62–7.06) |
| Total protein (g/L) | 67.42 ± 3.92 | 67.05 (57.70–75.70) |
| Total cholesterol (mmol/L) | 4.51 ± 0.90 | 4.34 (2.69–7.04) |
| Triglyceride (mmol/L) | 1.81 ± 0.97 | 1.61 (0.54–6.23) |
| Direct bilirubin (μmol/L) | 1.92 ± 0.95 | 1.75 (0.50–5.90) |
| Total bilibrubin (μmol/L) | 6.81 ± 2.96 | 6.05 (2.00–19.60) |
| Hematocrit (%) | 39.34 ± 4.26 | 38.30 (31.40–47.90) |
| Hemoglobin (g/L) | 130.51 ± 16.01 | 127.00 (104.00–164.00) |
| Mean corpuscular hemoglobin (pg) | 29.46 ± 1.72 | 29.40 (24.90–33.00) |
| Mean corpuscular hemoglobin concentration (g/L) | 331.34 ± 9.43 | 331.00 (310.00–351.00) |
Table 2.
Drug Combination in Patients with Depression (n = 64)
| Drug | N |
|---|---|
| Alprazolam tablets | 7 |
| Atorvastatin calcium tablets | 1 |
| Chlorpromazine hydrochloride tablets | 1 |
| Clonazepam tablets | 29 |
| Fluvoxamine maleate tablets | 4 |
| Haloperidol injection | 2 |
| Lamotrigine tablets | 1 |
| Lithium carbonate sustained-release tablets | 19 |
| Lorazepam tablets | 6 |
| Metformin hydrochloride tablets | 1 |
| Metoprolol succinate sustained-release tablets | 1 |
| Olanzapine tables | 1 |
| Paroxetine hydrochloride tablets | 5 |
| Propranolol hydrochloride tablets | 4 |
| Sertraline hydrochloride tables | 34 |
| Silymarin capsules | 2 |
| Sodium valproate sustained-release tablets | 12 |
| Sodium valproate tablets | 5 |
| Trihexyphenidyl hydrochloride tablets | 2 |
| Venlafaxine hydrochloride sustained-release capsules | 3 |
| Zopiclone tablets | 3 |
Note: N, number of patients receiving concomitant medications.
Modeling
The drug interaction evaluation process for quetiapine in depression patients was presented in Table S1. Paroxetine and fluvoxamine were included as covariates, which significantly affected the clearance rate of quetiapine, with ratios of about 1.00:0.54:0.48:0.26 in patients with depression who were not accompanied by paroxetine or fluvoxamine, patients with depression who were accompanied by paroxetine, patients with depression who were accompanied by fluvoxamine, and patients with depression who were accompanied by paroxetine and fluvoxamine. PPK model was as follows:
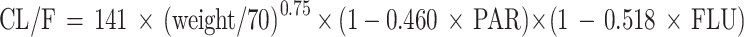 |
(vi) |
 |
(vii) |
PAR was administered as paroxetine, and FLU as fluvoxamine. When patients with depression received paroxetine or fluvoxamine, PAR or FLU was 1; If not, PAR or FLU was 0.
Evaluation
Figure 1 showed a visual evaluation of the quetiapine PPK model in patients with depression. Figure 2 showed the individual plots. Table 3 showed bootstrap validation. The above results indicated that final quetiapine PPK model was accurate and reliable.
Figure 1.
Model evaluation. (A) Observations vs population predictions. (B) Observations vs individual predictions. (C) absolute value of weighted residuals of individual (│iWRES│) vs individual predictions. (D) Weighted residuals vs time. (E) Quantiles of weighted residuals vs quantiles of normal. (F) Density vs weighted residuals. (G) Visual predictive check (VPC) of model, *P < 0.05.
Figure 2.
Individual plots. ID: patient ID number. Gray dot: DV, measured concentration. Red line or red dot (when the patient had only one concentration point): IPRED, Individual predictive value. Blue line or blue dot (when the patient had only one concentration point): PRED, Population predictive value.
Table 3.
Parameter Estimates of Quetiapine Final Model and Bootstrap Validation in Patients with Depression
| Parameter | Estimate | SE (%) | Bootstrap | Bias (%) | ||
|---|---|---|---|---|---|---|
| Median | 90% Confidence Interval | |||||
| CL/F (L/h) | 141 | 19.7 | 118 | [10, 166] | −16.31 | |
| V/F (L) | 1460 | 53.5 | 1060 | [23, 2468] | −27.40 | |
| Ka (h−1) | 1.46 (fixed) | – | – | – | – | |
| θPAR | −0.460 | 29.8 | −0.322 | [−0.637, −0.090] | −30.00 | |
| θFLU | −0.518 | 43.4 | −0.350 | [−0.748, −0.034] | −32.43 | |
| ωCL/F | 0.322 | 28.1 | 0.198 | [0.058, 0.401] | −38.51 | |
| σ1 | 0.365 | 18.7 | 0.358 | [0.236, 0.462] | −1.92 | |
| σ2 | 15.199 | 50.2 | 12.787 | [0.080, 31.819] | −15.87 | |
Notes: A 90% confidence interval is displayed as the 5th and 95th percentiles of the bootstrap estimates. CL/F, apparent oral clearance (L/h); V/F, apparent volume of distribution (L); Ka, absorption rate constant (h−1); θPAR, θFLU are the coefficients of paroxetine and fluvoxamine, respectively; ωCL/F, inter-individual variability of CL/F; σ1, residual variability, proportional error; σ2, residual variability, additive error; bias, prediction error; bias = (median-estimate)/estimate × 100%.
Recommended Dose
We simulated four different cases: (a) patients with depression who were not accompanied by paroxetine or fluvoxamine, (b) patients with depression who were accompanied by paroxetine, (c) patients with depression who were accompanied by fluvoxamine, and (d) patients with depression who were accompanied by paroxetine and fluvoxamine, as shown in Figures 3–6, respectively. The probability of attaining a therapeutic concentration range of quetiapine in depression patients was shown in Figure 7. Figure 7A–D represent patients with depression who were not accompanied by paroxetine or fluvoxamine, patients with depression who were accompanied by paroxetine, patients with depression who were accompanied by fluvoxamine, and patients with depression who were accompanied by paroxetine and fluvoxamine, respectively.
Figure 3.
The simulated quetiapine concentrations of patients with depression who were not accompanied with paroxetine or fluvoxamine. (A) Patients with depression weighted 40 kg. (B) Patients with depression weighted 60 kg. (C) Patients with depression weighted 80 kg. (D) Patients with depression weighted 100 kg. (E) Patients with depression weighted 120 kg.
Figure 4.
The simulated quetiapine concentrations of patients with depression who were accompanied with paroxetine. (A) Patients with depression weighted 40 kg. (B) Patients with depression weighted 60 kg. (C) Patients with depression weighted 80 kg. (D) Patients with depression weighted 100 kg. (E) Patients with depression weighted 120 kg.
Figure 5.
The simulated quetiapine concentrations of patients with depression who were accompanied with fluvoxamine. (A) Patients with depression weighted 40 kg. (B) Patients with depression weighted 60 kg. (C) Patients with depression weighted 80 kg. (D) Patients with depression weighted 100 kg. (E) Patients with depression weighted 120 kg.
Figure 6.
The simulated quetiapine concentrations of patients with depression who were accompanied with paroxetine and fluvoxamine. (A) Patients with depression weighted 40 kg. (B) Patients with depression weighted 60 kg. (C) Patients with depression weighted 80 kg. (D) Patients with depression weighted 100 kg. (E) Patients with depression weighted 120 kg.
Figure 7.
Probability to achieve the target concentrations of quetiapine in patients with depression. (A) Patients with depression who were not accompanied with paroxetine or fluvoxamine. (B) Patients with depression who were accompanied with paroxetine. (C) Patients with depression who were accompanied with fluvoxamine. (D) Patients with depression who were accompanied with paroxetine and fluvoxamine.
The initial dose recommendation of quetiapine in depression patients was shown in Table 4, in which the initial dose optimizations of quetiapine were 20 and 16 mg/kg/day for patients with depression not accompanied with paroxetine or fluvoxamine, who weighted 40–80, and 80–120 kg, respectively, and the probability of attaining a therapeutic concentration range for the doses of 20 and 16 mg/kg/day was 83.2–87.2%, 87.2–88.9%, respectively. The initial dose optimization of quetiapine was 8 mg/kg/day for depression patients accompanied by paroxetine, who weighted 40–120 kg, and the probability of attaining a therapeutic concentration range for a dose of 8 mg/kg/day was 92.6–97.1%. The initial dose optimization of quetiapine was 8 mg/kg/day for depression patients accompanied by fluvoxamine, who weighted 40–120 kg, and the probability of attaining a therapeutic concentration range for a dose of 8 mg/kg/day was 94.4–97.0%. The initial dose optimization of quetiapine was 4 mg/kg/day for depression patients accompanied by paroxetine and fluvoxamine, who weighted 40–120 kg, and the probability of attaining a therapeutic concentration range for a dose of 4 mg/kg/day was 98.9–99.8%.
Table 4.
Initial Dose Recommendation of Quetiapine in Patients with Depression
| Without Paroxetine | With Paroxetine | ||||||
|---|---|---|---|---|---|---|---|
| Without fluvoxamine | Without fluvoxamine | ||||||
| Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) | Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) |
| [40–80) | 20 | 83.2–87.2 | 2.8–7.5 | [40–120] | 8 | 92.6–97.1 | 0.2–2.3 |
| [80–120] | 16 | 87.2–88.9 | 2.6–5.2 | ||||
| With fluvoxamine | With fluvoxamine | ||||||
| Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) | Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) |
| [40–120] | 8 | 94.4–97.0 | 0.8–5.1 | [40–120] | 4 | 98.9–99.8 | 0 |
Secondary Evaluation Index
The probability of more than ceiling of the therapeutic concentration (500 ng/mL) at thousand simulated concentrations was used as a secondary evaluation index, as shown in Figure 8, where Figure 8A–D show patients with depression who were not accompanied by paroxetine or fluvoxamine, patients with depression who were accompanied with paroxetine, patients with depression who were accompanied with fluvoxamine, and patients with depression who were accompanied with paroxetine and fluvoxamine, respectively. For patients with depression who were not accompanied by paroxetine or fluvoxamine, the probability of exceeding ceiling of the therapeutic concentration was 2.8–7.5% and 2.6–5.2% for the initial dose optimization of quetiapine at 20 and 16 mg/kg/day, respectively. For patients with depression who were accompanied with paroxetine, the probability to more than ceiling of the therapeutic concentration was 0.2–2.3% for the initial dose optimization of quetiapine of 8 mg/kg/day. For patients with depression who were accompanied with fluvoxamine, the probability to more than ceiling of the therapeutic concentration was 0.8–5.1% for the initial dose optimization of quetiapine of 8 mg/kg/day. For patients with depression accompanied by paroxetine and fluvoxamine, the probability of exceeding ceiling of the therapeutic concentration was 0 for the initial dose optimization of quetiapine at 4 mg/kg/day. The results were presented in Table 4.
Figure 8.
Probability to exceed the upper limit of the target concentrations of quetiapine in patients with depression. (A) Patients with depression who were not accompanied with paroxetine or fluvoxamine. (B) Patients with depression who were accompanied with paroxetine. (C) Patients with depression who were accompanied with fluvoxamine. (D) Patients with depression who were accompanied with paroxetine and fluvoxamine.
Discussion
In clinical practice, pharmacokinetic interactions are frequently a source of DDIs. These interactions occur primarily when perpetrator drugs alter the activity of metabolic enzymes or transporters responsible for victim drug processing, leading to variable pharmacokinetic characteristics.40–44 Serious DDIs can significantly affect real-world patient care and optimal dose recommendation.45,46
Therefore, we constructed a PPK model of quetiapine in patients with depression by integrating real-world combination therapy data. This quantitative pharmacological framework specifically examined the effects of DDIs on quetiapine metabolism in patients with depression. This study aimed to screen out potential combined administration information affecting quetiapine clearance and recommend a precise quetiapine administration regimen for patients with depression. This study relied on clinical records, and the feasibility of relying on clinical records for DDIs analysis has been demonstrated in previous researches.47–49
Sixty-four real-world patients with depression were used to investigate the effects of DDIs on quetiapine using NONMEM. Of course, since this study was derived from sparse data in the real world, the information density was insufficient, which would have a certain impact on the stability of the PPK model. This was an objective characteristic of sparse data in the real world. However, judging from the published research, this influence had a limited impact on our main results. For example, in our study, the CL/F of quetiapine in patients with depression was 141 L/h, similar to the CL/F of quetiapine in patients with schizophrenia.30 In addition, the allometric scaling method was adopted in this study. The fixed inclusion of body weight as a core physiological covariate enabled the PPK model to have both individualized accuracy and clinical practicability. It not only supported the scientific transformation from a fixed dose to a weight gradient dose but also provided a basis for dynamic dose adjustment for patients with special physiological states. This kind of research had been reported in a considerable number of relevant literatures.50–52
The combined medications analyzed in this study included alprazolam tablets, atorvastatin calcium tablets, chlorpromazine hydrochloride tablets, clonazepam tablets, fluvoxamine maleate tablets, haloperidol injection, lamotrigine tablets, lithium carbonate sustained-release tablets, lorazepam tablets, metformin hydrochloride tablets, metoprolol succinate sustained-release tablets, olanzapine tables, paroxetine hydrochloride tablets, propranolol hydrochloride tablets, sertraline hydrochloride tables, silymarin capsules, sodium valproate sustained-release tablets, sodium valproate tablets, trihexyphenidyl hydrochloride tablets, venlafaxine hydrochloride sustained-release capsules, zopiclone tablets.
The final PPK model identified paroxetine and fluvoxamine as significant covariates influencing quetiapine clearance in patients with depression. When patients with depression received paroxetine simultaneously, quetiapine clearance decreased by 46.0%, and when patients with depression received fluvoxamine, quetiapine clearance decreased by 51.8%. Decreased clearance can lead to the accumulation of quetiapine, and higher quetiapine concentrations can lead to consciousness and movement disorders, nervous function abnormalities, metabolic disorders, endocrine abnormalities, etc. This is mainly because quetiapine is primarily metabolized by CYP3A4 and CYP2D6,23–26 however paroxetine is an inhibitor of CYP2D653,54 and fluvoxamine is an inhibitor of CYP3A4.55–57 Furthermore, optimized initial doses of quetiapine are recommended in patients with depression. This study replenishes quetiapine DDIs in a real-world setting. When patients with depression take paroxetine or fluvoxamine simultaneously, individualized doses of quetiapine could be guided more accurately and conveniently, according to the study.
However, since the present study was a single-center retrospective and small sample size real-world study. The population consisted of patients with depression from China. In addition, the limited number of patients co-administered with paroxetine and fluvoxamine. Therefore, in future studies, we need to optimize the sampling design (such as dense sampling), enhance the integrity of collection, and increase the number of enrolled patients via multicenter prospective study.
Conclusion
Our study explored DDIs and initial dose optimization of quetiapine in patients with depression from the real world for the first time, and the initial dose optimization of quetiapine was recommended based on the interaction with paroxetine or fluvoxamine.
Funding Statement
This study was funded by The Xuzhou Special Fund for Promoting Scientific and Technological Innovation (no. KC23217, no. KC23254), and The Medical Research Project of Jiangsu Provincial Health Commission (no. Z2023010), Jiangsu Province Education Science Planning Project (no. C/2022/01/36), Xuzhou Medical University Labor Education Special Project (no. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (no. 2023JSETKT136), Xuzhou Medical University Research Topic of Higher Education Teaching Reform (no. Xjyzrd202304), and the Suzhou Applied Basic Research Science and Technology Innovation Project (no. SYWD2024258).
Data Sharing Statement
The de-identified participant data will be made available upon reasonable request to the corresponding author. Available data includes individual participant records that support the published findings, with appropriate measures in place to maintain participant confidentiality according to established research ethics guidelines.
Disclosure
Xiao Chen, Yue Zhang, Di Yin and Ying-Wei Jin are co-first authors. The authors have no conflict of interest to disclose.
References
- 1.Li R, Wang X, Luo L, Yuan Y. Identifying the most crucial factors associated with depression based on interpretable machine learning: a case study from CHARLS. Front Psychol. 2024;15:1392240. doi: 10.3389/fpsyg.2024.1392240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Psychogiou L, Navarro MC, Orri M, Côté SM, Ahun MN. Childhood and adolescent depression symptoms and young adult mental health and psychosocial outcomes. JAMA Network Open. 2024;7(8):e2425987. doi: 10.1001/jamanetworkopen.2024.25987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davydow DS, Fenger-Gron M, Ribe AR, et al. Depression and risk of hospitalisations and rehospitalisations for ambulatory care-sensitive conditions in Denmark: a population-based cohort study. BMJ Open. 2015;5(12):e009878. doi: 10.1136/bmjopen-2015-009878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- 5.Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holvast F, Massoudi B, Oude Voshaar RC, Verhaak PFM. Non-pharmacological treatment for depressed older patients in primary care: a systematic review and meta-analysis. PLoS One. 2017;12(9):e0184666. doi: 10.1371/journal.pone.0184666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleine-Budde K, Muller R, Kawohl W, Bramesfeld A, Moock J, Rossler W. The cost of depression - a cost analysis from a large database. J Affect Disord. 2013;147(1–3):137–143. doi: 10.1016/j.jad.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 8.Park SC, Oh HS, Oh DH, et al. Evidence-based, non-pharmacological treatment guideline for depression in Korea. J Korean Med Sci. 2014;29(1):12–22. doi: 10.3346/jkms.2014.29.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540–560. doi: 10.1177/0706743716659417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenouvel E, Tobias S, Muhlbauer V, et al. Antidepressants for treating depression among older adults with dementia: a systematic review and meta-analysis. Psychiatry Res. 2024;340:116114. doi: 10.1016/j.psychres.2024.116114 [DOI] [PubMed] [Google Scholar]
- 12.Han L, Gu JQ, Mao JH, Liu XQ, Jiao Z. Insights into the population pharmacokinetics and pharmacodynamics of quetiapine: a systematic review. Expert Rev Clin Pharmacol. 2024;17(1):57–72. doi: 10.1080/17512433.2023.2295428 [DOI] [PubMed] [Google Scholar]
- 13.Zakhary T, Ahmed I, Luttfi I, Montasser M. Quetiapine versus haloperidol in the management of hyperactive delirium: randomized controlled trial. Neurocrit Care. 2024;41(2):550–557. doi: 10.1007/s12028-024-01948-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Y, Zhang J, Yang L, et al. A machine learning model for predicting blood concentration of quetiapine in patients with schizophrenia and depression based on real-world data. Br J Clin Pharmacol. 2023;89(9):2714–2725. doi: 10.1111/bcp.15734 [DOI] [PubMed] [Google Scholar]
- 15.Hao Y, Zhang J, Yu J, et al. Predicting quetiapine dose in patients with depression using machine learning techniques based on real-world evidence. Ann Gen Psychiatry. 2024;23(1):5. doi: 10.1186/s12991-023-00483-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poyurovsky M, Weizman A. Beneficial effect of quetiapine on sleep, anxiety, depression and myalgia symptoms in a patient with Post-COVID-19 condition. J Clin Psychopharmacol. 2023;43(4):381–382. doi: 10.1097/JCP.0000000000001706 [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, Zhang Y, Deng Y, Liu S, Zhang W. A comprehensive review of computational methods for drug-drug interaction detection. IEEE/ACM Trans Comput Biol Bioinform. 2022;19(4):1968–1985. doi: 10.1109/TCBB.2021.3081268 [DOI] [PubMed] [Google Scholar]
- 18.Sampson MR, Cao KY, Gish PL, et al. Dosing recommendations for quetiapine when coadministered with HIV protease inhibitors. J Clin Pharmacol. 2019;59(4):500–509. doi: 10.1002/jcph.1345 [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Leng J, Liu Y. Deep learning for drug-drug interaction extraction from the literature: a review. Brief Bioinform. 2020;21(5):1609–1627. doi: 10.1093/bib/bbz087 [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Yin J, Zhang L, Zhang Y, Chen X. Drug-drug interaction prediction: databases, web servers and computational models. Brief Bioinform. 2023;25(1):bbad445. doi: 10.1093/bib/bbad445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kou K, Sun X, Li M, et al. Beneficial effects of Wuzhi Capsule on tacrolimus blood concentrations in liver transplant patients with different donor-recipient CYP3A5 genotypes. J Clin Pharm Ther. 2022;47(2):200–210. doi: 10.1111/jcpt.13533 [DOI] [PubMed] [Google Scholar]
- 22.Gill J, Moullet M, Martinsson A, et al. Comparing the applications of machine learning, PBPK, and population pharmacokinetic models in pharmacokinetic drug-drug interaction prediction. CPT Pharmacometrics Syst Pharmacol. 2022;11(12):1560–1568. doi: 10.1002/psp4.12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu TL, Fang LS, Liou JR, Dai JS, Chen YL. Determination of quetiapine and its metabolites in plasma by field-enhanced sample stacking. J Food Drug Anal. 2021;29(4):709–716. doi: 10.38212/2224-6614.3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohail MU, Khan A, Pflaum RM, Patel M, Moody MA. An atypical case of neuroleptic malignant syndrome associated with ciprofloxacin and quetiapine. Cureus. 2023;15(3):e36178. doi: 10.7759/cureus.36178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stauble CK, Lampert ML, Mikoteit T, Hatzinger M, Hersberger KE, Meyer Zu Schwabedissen HE. Severe adverse drug reactions to quetiapine in two patients carrying CYP2D6*4 variants: a case report. Int J Mol Sci. 2021;22(12):6480. doi: 10.3390/ijms22126480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau K, McArthur E, Jeyakumar N, et al. Adverse events with quetiapine and clarithromycin coprescription: a population-based retrospective cohort study. Health Sci Rep. 2023;6(6):e1375. doi: 10.1002/hsr2.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–2):9–62. doi: 10.1055/s-0043-116492 [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Contreras MC, Cerda B, Lopez-Roman FJ, Segarra I. Patients with dementia: prevalence and type of drug-drug interactions. Front Pharmacol. 2024;15:1472932. doi: 10.3389/fphar.2024.1472932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Terol A, Caraballo MO, Palma D, et al. Calidad estructural de las bases de datos de interacciones [Quality of interaction database management systems]. Farm Hosp. 2009;33(3):134–146. [PubMed] [Google Scholar]
- 30.Chen X, Zhang Y, Yin D, et al. Optimization of initial dosage of quetiapine in schizophrenic patients: effects of fluvoxamine or duloxetine coadministration. Front Pharmacol. 2024;15:1496043. doi: 10.3389/fphar.2024.1496043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Fang Q, Wu Z, et al. Population pharmacokinetics and dosage optimization of linezolid in Chinese older patients. Eur J Clin Pharmacol. 2024;80(9):1295–1304. doi: 10.1007/s00228-024-03702-9 [DOI] [PubMed] [Google Scholar]
- 32.Bai J, Wen A, Li Z, Li X, Duan M. Population pharmacokinetics and dosing optimisation of imipenem in critically ill patients. Eur J Hosp Pharm. 2024;31(5):434–439. doi: 10.1136/ejhpharm-2022-003403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leegwater E, Baidjoe L, Wilms EB, et al. Population pharmacokinetics of trimethoprim/sulfamethoxazole: dosage optimization for patients with renal insufficiency or receiving continuous renal replacement therapy. Clin Pharmacol Ther. 2024;117(1):184–192. doi: 10.1002/cpt.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang P, Liu W, Ying Y, et al. Population pharmacokinetics of nirmatrelvir in Chinese patients with COVID-19: therapeutic drug monitoring and dosing regimen selection in clinical practice. Int J Antimicrob Agents. 2024;64(2):107199. doi: 10.1016/j.ijantimicag.2024.107199 [DOI] [PubMed] [Google Scholar]
- 35.Sitaruno S, Chumin T, Ngamkitpamot Y, Boonchu W, Setthawatcharawanich S. Population pharmacokinetics and loading dose optimization of intravenous valproic acid in hospitalized Thai patients. J Clin Pharmacol. 2024;64(11):1343–1350. doi: 10.1002/jcph.6102 [DOI] [PubMed] [Google Scholar]
- 36.Shen X, Li X, Lu J, et al. Population pharmacokinetic analysis for dose regimen optimization of vancomycin in Southern Chinese children. CPT Pharmacometrics Syst Pharmacol. 2024;13(7):1201–1213. doi: 10.1002/psp4.13151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng J, Peng L, Wang Y, Li J, Tang L, Yu Y. Population pharmacokinetics and dose optimization of magnesium sulfate in Chinese preeclampsia population. BMC Pregnancy Childbirth. 2024;24(1):424. doi: 10.1186/s12884-024-06620-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng ZQ, Jin YW, Yin D, et al. Model-informed precision dosing of quetiapine in bipolar affective disorder patients: initial dose recommendation. Front Psychiatry. 2024;15:1497119. doi: 10.3389/fpsyt.2024.1497119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708 [DOI] [PubMed] [Google Scholar]
- 40.Maideen NMP, Al Rashid S. Paxlovid (Nirmatrelvir/Ritonavir)-induced tacrolimus toxicity in organ transplant recipients - a review on drug interactions involving CYP3A enzymes. Curr Drug Saf. 2024;20(3):291–302. doi: 10.2174/0115748863331165240821194206 [DOI] [PubMed] [Google Scholar]
- 41.Jin S, Paludetto MN, Kurkela M, et al. In vitro assessment of inhibitory effects of kinase inhibitors on CYP2C9, 3A and 1A2: prediction of drug-drug interaction risk with warfarin and direct oral anticoagulants. Eur J Pharm Sci. 2024;203:106884. doi: 10.1016/j.ejps.2024.106884 [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Shao W, Wang X, et al. Physiologically based pharmacokinetic models for predicting lamotrigine exposure and dose optimization in pediatric patients receiving combination therapy with carbamazepine or valproic acid. Pharmacotherapy. 2024;44(9):711–721. doi: 10.1002/phar.4603 [DOI] [PubMed] [Google Scholar]
- 43.Gaud N, Gogola D, Kowal-Chwast A, et al. Physiologically based pharmacokinetic modeling of CYP2C8 substrate rosiglitazone and its metabolite to predict metabolic drug-drug interaction. Drug Metab Pharmacokinet. 2024;57:101023. doi: 10.1016/j.dmpk.2024.101023 [DOI] [PubMed] [Google Scholar]
- 44.Cho CK, Kang P, Jang CG, et al. PBPK modeling to predict the pharmacokinetics of venlafaxine and its active metabolite in different CYP2D6 genotypes and drug-drug interactions with clarithromycin and paroxetine. Arch Pharm Res. 2024;47(5):481–504. doi: 10.1007/s12272-024-01495-0 [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Hu K, Shi HZ, et al. Initial dosage optimization of olanzapine in patients with bipolar disorder based on model-informed precision dosing: a study from the real world. Front Pharmacol. 2024;15:1444169. doi: 10.3389/fphar.2024.1444169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen W, Hu K, Shi HZ, et al. Effects of sex differences and combined use of clozapine on initial dosage optimization of valproic acid in patients with bipolar disorder. Curr Pharm Des. 2024;30(29):2290–2302. doi: 10.2174/0113816128323367240704095109 [DOI] [PubMed] [Google Scholar]
- 47.Yang Q, Wang Y, Wang X, et al. Drug-drug interaction between diltiazem and tacrolimus in relation to CYP3A5 genotype status in Chinese pediatric patients with nephrotic range proteinuria: a retrospective study. Front Pharmacol. 2024;15:1463595. doi: 10.3389/fphar.2024.1463595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, Jiang L, Hu K, et al. Drug-drug interaction and initial dosage optimization of aripiprazole in patients with schizophrenia based on population pharmacokinetics. Front Psychiatry. 2024;15:1377268. doi: 10.3389/fpsyt.2024.1377268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X, Zhang H, Liu L, et al. Pharmacokinetics of nirmatrelvir/ritonavir and the drug-drug interaction with calcineurin inhibitor in renal transplant recipients. Eur J Clin Pharmacol. 2024;80(8):1219–1227. doi: 10.1007/s00228-024-03691-9 [DOI] [PubMed] [Google Scholar]
- 50.Niu WJ, Sun T, Liu L, et al. Population pharmacokinetics and dosing regimen optimisation of lopinavir in Chinese adults infected with HIV. Basic Clin Pharmacol Toxicol. 2019;124(4):456–465. doi: 10.1111/bcpt.13154 [DOI] [PubMed] [Google Scholar]
- 51.Cai X, Song H, Jiao Z, et al. Population pharmacokinetics and dosing regimen optimization of tacrolimus in Chinese lung transplant recipients. Eur J Pharm Sci. 2020;152:105448. doi: 10.1016/j.ejps.2020.105448 [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Wang D, Zheng F, Zhai X, Xu H, Li Z. Population pharmacokinetics and initial dose optimization of tacrolimus in children with severe combined immunodeficiency undergoing hematopoietic stem cell transplantation. Front Pharmacol. 2022;13:869939. doi: 10.3389/fphar.2022.869939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapil RP, Friedman K, Cipriano A, et al. Effects of paroxetine, a CYP2D6 inhibitor, on the pharmacokinetic properties of hydrocodone after coadministration with a single-entity, once-daily, extended-release hydrocodoneTablet. Clin Ther. 2016;38(1):228–229. doi: 10.1016/j.clinthera.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Kosheleff AR, Adeojo LW, et al. Impact of paroxetine, a strong CYP2D6 inhibitor, on SPN-812 (Viloxazine Extended-Release) pharmacokinetics in healthy adults. Clin Pharmacol Drug Dev. 2021;10(11):1365–1374. doi: 10.1002/cpdd.948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britz H, Hanke N, Volz AK, et al. Physiologically-based pharmacokinetic models for CYP1A2 drug-drug interaction prediction: a modeling network of fluvoxamine, theophylline, caffeine, rifampicin, and midazolam. CPT Pharmacometrics Syst Pharmacol. 2019;8(5):296–307. doi: 10.1002/psp4.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huth F, Schiller H, Jin Y, et al. Novel Bruton’s Tyrosine Kinase inhibitor remibrutinib: drug-drug interaction potential as a victim of CYP3A4 inhibitors based on clinical data and PBPK modeling. Clin Transl Sci. 2022;15(1):118–129. doi: 10.1111/cts.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugahara H, Maebara C, Ohtani H, et al. Effect of smoking and CYP2D6 polymorphisms on the extent of fluvoxamine-alprazolam interaction in patients with psychosomatic disease. Eur J Clin Pharmacol. 2009;65(7):699–704. doi: 10.1007/s00228-009-0629-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The de-identified participant data will be made available upon reasonable request to the corresponding author. Available data includes individual participant records that support the published findings, with appropriate measures in place to maintain participant confidentiality according to established research ethics guidelines.











