Abstract
Pituitary ablation (hypophysectomy) in rats was previously reported to cause a precipitous change in the relative abundance of two alternative splice variants of the “BK”- or “Maxi K”-encoding Slo gene in adrenal chromaffin cells. Inclusion of the optional “STREX” exon (STRess axis-regulated EXon) in a C-terminal splice site was reduced, in preference to the variant lacking an insert at this site. Adrenocorticotropic hormone (ACTH) injections prevented the drop in STREX inclusion, implicating stress-axis function, as opposed to other pituitary functions. Because ACTH promotes synthesis and release of glucocorticoids (corticosterone or cortisol, depending on species), we hypothesized that glucocorticoids applied directly would promote STREX inclusion. Contrary to predictions, we report that direct application of glucocorticoids to bovine cells in vitro decreased STREX inclusion. This effect was blocked by the glucocorticoid receptor antagonist RU38486. As with glucocorticoids, synthesis and release of the adrenal androgen dehydroepiandrosterone (DHEA) increases in response to stress or elevated ACTH levels in some species. We report that direct application of DHEA increased expression of the STREX variant in cultured bovine cells. Two other androgens, androstenedione and testosterone, had similar effects. We hypothesize that Slo splicing in adrenal chromaffin cells in vivo is differentially regulated by the integrative, concentration- and time-dependent actions of glucocorticoids and androgens, with potentially important ramifications for stress-evoked catecholamine secretion.
Alternative splicing gives rise to ion-channel variety that underlies diversity in the intrinsic firing properties of excitable cells. In adrenal chromaffin cells, calcium- and voltage-dependent BK channels are encoded by the Slo gene. Two splice variants at splice site 5 in Slo (Fig. 1B) occur in chromaffin cells, and these exhibit distinct functional properties when characterized in heterologous expression systems (1–3). Thus Slo channels with the optional STREX exon (STREX channels) activate faster and at more negative voltages and deactivate more slowly than those with no extra exon at this site (ZERO channels). Although BK-channel function is multifaceted, the facilitated activation of STREX-type Slo channels enhances repetitive firing in rat chromaffin cells by minimizing action potential duration and speeding recovery of Na channels from inactivation during the brief afterhyperpolarization (4).
Figure 1.
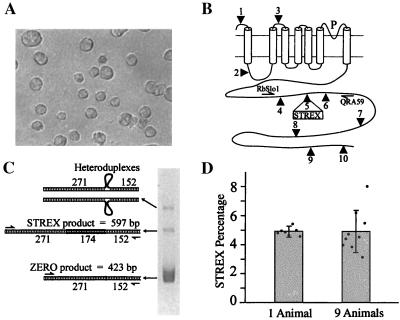
Reverse transcription (RT)-PCR amplification of splice variants of the Slo gene from bovine chromaffin cells in dissociated cell culture. (A) Typical chromaffin cells after 3 days in culture. (B) Schematic of the Slo protein showing alternative splice sites. Cylinders represent transmembrane helices, and the long C terminus, which contains the calcium-binding domain, is intracellular. By using the primers RbSlo1 and QRA59 on total RNA from cultured cells, PCR products were amplified from splice variants with and without the 174-bp STREX exon at splice site 5. No inserts were found at sites 4 or 6. (C) Products were run on a 3.5% agarose gel. Sizes are shown for double-stranded homoduplexed products from ZERO (lacking the STREX exon) and STREX variants. The uppermost band represents the two possible heteroduplexes resulting from annealing of STREX and ZERO strands, as discussed in the text. (D) The number of RT-PCR product strands that include the STREX exon, expressed as a percentage of the total (STREX + ZERO products). Note that greater variation is observed in measures of cells from 9 individual animals than in measures of 7 cell samples from a single animal. Error bars represent standard deviations.
Although there are many other examples of channel-alternative splicing, little is known about mechanisms regulating the decision. The incorporation of STREX into Slo transcripts has been shown in rats to decrease substantially after hypophysectomy. This phenomenon can be effectively prevented by injecting adrenocorticotropin (ACTH) (3). In classic hypothalamic–pituitary–adrenal axis (HPA) function, perceived stress triggers the release of corticotropin-releasing hormone (CRH) and/or arginine vasopressin (AVP) from the hypothalamus, which stimulates ACTH secretion from corticotropes of the anterior pituitary. ACTH is not thought to act directly on adrenomedullary chromaffin cells, but it has been shown to stimulate glucocorticoid secretion from steroidogenic cells in the adrenal cortex. Glucocorticoids, especially corticosterone in rats and cortisol in bovines (together abbreviated CORT), act directly on chromaffin cells through “genomic” mechanisms to control phenotypic features, e.g., spherical shape and phenylethanolamine N-methyltransferase (PNMT) expression (5, 6). Moreover, dexamethasone (DEX), a CORT analog, has been shown to regulate alternative splicing of the insulin-receptor mRNA (7). In recognition of this cascade, it was postulated that CORT was responsible for the presumably indirect effect of ACTH on STREX splicing in the rat (3). Attempts to test this hypothesis by using CORT implants in place of ACTH injections failed, but were considered inconclusive, in view of the normally very high local exposure of adrenal medullary cells to corticosteroids, a situation that is difficult or impossible to replicate with systemically introduced steroids. Thus, to test for direct regulation of Slo splicing by adrenal hormones and other factors, we established a model system by using primary culture of dissociated bovine chromaffin cells, in the absence of cortical tissue. Our results using this system suggest that CORT negatively affects STREX inclusion. A number of studies have shown that release of dehydroepiandrosterone (DHEA), like CORT, is increased by behavioral stress and ACTH (8–14); thus we tested for regulation of Slo splicing by DHEA, and several other steroids, alone and in combination with CORT.
Materials and Methods
Chromaffin Cell Preparation and Culture.
Bovine adrenal glands were transported from the slaughterhouse (Cudlin's, Newfield, NY) to the lab on ice within 30 min after removal. The glands were perfused with buffer I (118 mM NaCl/3.3 mM KCl/1 mM NaH2PO4/1 mM MgSO4⋅7H2O/10 mM glucose/25 mM Hepes, pH 7.4) followed by buffer II (buffer I plus 1× MEM vitamins, 1× MEM amino acids, 0.11g/liter sodium pyruvate, and 2 mM l-glutamine). After 1 h of collagenase perfusion (Roche Molecular Biochemicals, type B, 100 mg/100 ml of buffer II plus 0.0975% BSA), the gland was sliced open and medullary tissue was removed for a second collagenase treatment. During the whole process 95% oxygen/5% carbon dioxide was bubbled into the solution. After the second digestion, cells were filtered through a 70-μm cell strainer (Falcon), centrifuged at 500 × g for 10 min, and counted. The substrate of sterile 35-mm Falcon dishes was coated with Vitrogen (0.53 mg/ml, 400 μl per dish; Cohesion Technologies, Palo Alto, CA). Cells were then resuspended in RPMI 1640 (GIBCO/BRL) supplemented with 10% horse serum, 5% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and nystatin (100 units/ml). Approximately 106 cells were plated in each dish. After 24 h, the culture medium was changed to serum-free defined medium (RPMI 1640), to which steroids, other drugs, or vehicle was added.
RNA Extraction, Reverse Transcription (RT)-PCR, and Data Analysis.
We used PCR primers targeting constitutive exons and bracketing splice site 5 (Fig. 1; see also refs. 15–17) in the Slo gene to simultaneously amplify from two splice-variant transcripts occurring in RNA isolated from cultured chromaffin cells. The same primers have been used on RNA extracted from acutely dissected and homogenized rat and bovine adrenal medullary tissue to coamplify products from Slo variants, as described (3, 17). Total RNA was harvested by using the Qiagen RNeasy kit. RNA from each dish was quantified with a spectrophotometer, and 1.7 μg was added to each 20-μl reverse-transcription reaction [50 mM Tris⋅HCl (pH 8.3)/75 mM KCl/3 mM MgCl2/10 mM DTT/1 mM dNTP/200 units of Superscript II reverse transcriptase (GIBCO/BRL)/20 units of RNase-Out (Invitrogen)/10 μM oligo(dT)]. RT product (1.5 μl) was transferred to each 30-μl PCR [5 mM KCl/1 mM Tris⋅HCl (pH 9.0)/0.01% Triton X-100/0.16 mM each dNTP/1.25 mM MgCl2/0.375 μM each primer (QRA59, 5′-CACATTGGAGTCCATGTTGTC-3′; RbSlo1, 5′-AGTGCCTTCGTGGGCTGTCCTTC-3′)/0.625 unit of Taq DNA polymerase]. After 3 min at 95°C, 30 cycles were run with 30-s steps at 95, 55, and 72°C. After a final denaturation step of 3 min, denatured reaction products were allowed to reanneal for 30 min at 72°C. Six microliters of product was then run on a 3.5% agarose gel for 90 min at 30 V/cm. The ethidium-bromide-stained gel was UV transilluminated and images were captured with a Cohu camera and an LG-3 digitizer, controlled by using modified NIH image software (Scion, Frederick, MD). As described (17), this protocol yields three bands, the bottom and middle of which are amplification products from the ZERO- and STREX-splice-variant transcripts, respectively, in double-stranded homoduplex form (Fig. 1). The top band is composed of the two heteroduplexes formed by indiscriminate reannealing between the STREX sense and ZERO antisense strands, and the converse pairing. This three-band pattern could be reproduced by mixing independently generated STREX and ZERO products and then denaturing, reannealing, and running as described above.
To estimate relative STREX and ZERO copy numbers from band intensities, the STREX band intensity was corrected for its greater length by multiplying by 0.709 (i.e., 423 bp/597 bp). Half of the intensity value of the heteroduplex band was then added to each homoduplex value. The heteroduplex value was not corrected for length because the single-stranded DNA of the STREX exon loop would be expected to have a very low affinity for ethidium bromide. Representative samples were also run on denaturing polyacrylamide gels [1× Tris/borate/EDTA buffer (TBE)/8% polyacrylamide/40% deionized formamide] to eliminate heteroduplex formation, as described in Mahmoud et al. (17). Results from these samples were consistent with results from agarose gel analysis. Estimates of the STREX percentage in PCR templates, as opposed to products, were made as described in ref. 17. Briefly, a series of template mixes with various STREX percentages was designed, PCRs were run from the template mixes, and the templates and their respective products were run side-by-side on a gel. Product percentages had a somewhat sigmoidal relationship to template percentages, reflecting amplification efficiency differences and a modest competitive advantage of the more abundant template in the dual-product reaction, even in the linear range of PCR (17).
Unless otherwise indicated, all data are expressed as mean ± SEM. For statistical analysis, the one sample t test and the Bonferroni correction were used (Minitab, State College, PA). P values less than 0.05 were considered to be statistically significant.
Results
Quantitative Measurement of the Relative Abundance of STREX and ZERO Variants.
Slo primers flanking splice-site-5 were used for PCR amplification of RT products generated from dissociated bovine-cell RNA (Fig. 1; see also ref. 15). Amplification consistently yielded just two products, which have been verified by subcloning and sequencing to be bovine orthologs of rat Slo variants, with and without the optional 174-bp STREX exon (the terms STREX and ZERO are also used to refer to the corresponding 423-bp and 597-bp PCR products). These products manifest as two homoduplex bands and a heteroduplex band on 3.5% agarose gels, but as two bands on denaturing PAGE gels, as described above. Neither careful sizing of PCR products on denaturing PAGE gels nor sequencing product samples revealed any evidence for optional exons at splice sites 4 and 6 in chromaffin cells, as is consistent with previous studies (3). Our goal was to quantify the coamplified STREX and ZERO products by comparing relative band intensities on gels. To represent relative copy number, STREX and ZERO homoduplex-band intensities were normalized by product length, and half the heteroduplex-band intensity was then added to each length-corrected homoduplex band, as detailed above. Data were then expressed as the percentage of the total PCR product composed of the STREX form. A detailed description of methods used to test the quantitative accuracy of this RT-PCR approach has been published (17).
Significant week-to-week variation in measured STREX percentages made it important to identify sources of variation (experiments in one week used cells from a single animal). We thus processed seven dishes of untreated cells from a single dissociated bovine medulla, and control samples from nine different animals collected over as many weeks, at the same time (Fig. 1D). The variation measured between the seven samples from one animal (mean ± SD = 4.89 ± 0.26%) was clearly less than that between control samples from nine animals (mean ± SD = 4.9 ± 1.44%). The week-to-week variation therefore reflects variation in the STREX percentage between individual animals. Thus, to better separate treatment-dependent variation from interindividual variation, experimental values were normalized by dividing by values obtained from parallel control dishes prepared from the same adrenal gland, except where indicated.
Data are presented here as the percentage of the PCR products that were of the STREX type. To get an estimate of STREX percentages in native mRNA transcripts from the relative intensities of gel bands in PCR products, corrections were made for length differences and differences in amplification efficiency of STREX and ZERO, as described in Materials and Methods. We estimate that for both the seven dishes from one animal and the nine different animals (above), STREX-transcript percentages were approximately 12% of total Slo transcripts.
Slo Splice Variant Ratios Change with Time in Serum-Free Medium.
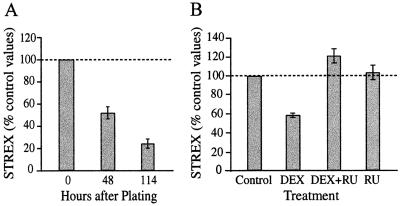
The STREX percentage showed a gradual decline with time in cell culture. Cells were plated in serum-containing medium to allow adhesion, and this medium was replaced with serum- and steroid-free medium after 24 h. By 48 and 114 h respectively, after plating, the mean percentage of STREX had dropped to 52% ± 5.51% and 24% ± 4.07% of initial values (n ≥ 6, P < 0.01) (Fig. 2A). The change in favor of ZERO under these culture conditions suggests a dependence on extrinsic factors for maintaining splice variant ratios. This observation is reminiscent of the decline in the STREX percentage observed after hypophysectomy in the rat (3). Because ACTH injection in the rat prevented the effect of hypophysectomy, we tested whether ACTH or glucocorticoids could act directly to influence Slo splicing. ACTH applied to bovine chromaffin cells in vitro had no effect on STREX percentages (0.8 unit/ml, n = 3).
Figure 2.
(A) The relative abundance of Slo splice variants in bovine chromaffin cells changes with time in culture. Total RNA was harvested 0, 48, or 114 h after plating. Serum-containing medium was replaced with serum-free medium (RPMI 1640, GIBCO/BRL) 24 h after plating. Mean ± SEM values for STREX as a percentage of the total Slo copy number (STREX + ZERO) in the RT-PCR product, after normalization against parallel control samples. STREX percentages at 48 and 114 h were significantly lower than that at 0 h (n ≥ 6, P < 0.01). (B) Dexamethasone (DEX) decreased the STREX percentage measured by RT-PCR at 24 h after addition. DEX (20 μM), RU38486 (RU; 25 μM), DEX plus RU38486, or vehicle alone (DMSO, 0.1%), were added at 24 h after plating, when the medium was changed to serum-free medium. Cells treated with DEX alone exhibited significantly lower STREX percentages than control cells (P < 0.001, n = 35). RU38486, a glucocorticoid- and progesterone-receptor antagonist, had no effect by itself, but prevented the decrease caused by DEX addition (P = 0.045, n = 5).
Glucocorticoid Effects on the STREX Percentage.
In contrast to expectations, within 24 h after addition of the synthetic glucocorticoid DEX (20 μM in serum-free medium), the STREX percentage had decreased to 58.7% of parallel, vehicle-treated controls (n = 35, P < 0.001 dishes, Fig. 2B). The natural glucocorticoid, cortisol, gave similar results (20 μM, n = 4, P < 0.001; see Fig. 5). Classical biological responses to corticosteroids are mediated by either the glucocorticoid receptor (GR) or the mineralocorticoid receptor (MR) (18). Spironolactone (50 μM), a MR receptor antagonist, did not prevent the drop in STREX induced by DEX. RU38486 is a high-affinity antagonist of both the GR and the progesterone receptor (PR), with a dissociation constant (Kd) of approximately 10−9 M (19). Concurrent application of RU38486, a glucocorticoid receptor antagonist, did block the accelerated decline in STREX seen with DEX alone (Fig. 2B). Progesterone had no effect on Slo splicing (20 μM, n = 8, Fig. 5). These observations are consistent with the hypothesis that RU38486 and DEX act selectively through the GR.
Figure 5.
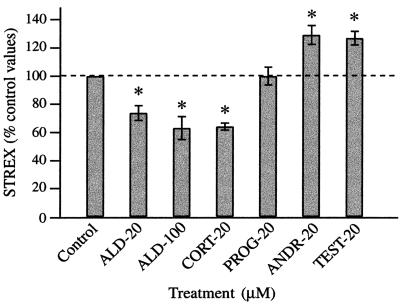
Other steroids also influence Slo alternative splicing. Steroids were added 24 h after plating, and cells were harvested 24 h later. Asterisks indicate significance (P ≤ 0.006). Aldosterone (ALD-20, ALD-100, concentrations in μM) was less potent, but had effects similar to those of cortisol (CORT-20). Progesterone (PROG-20) had no effect. Androgens, androstenedione (ANDR-20) and testosterone (TEST-20), had effects similar to those of DHEA.
The effect of DEX (20 μM) was significant within 14 h after application (see Fig. 4A, n = 5, P = 0.03). The STREX level had not detectably decreased after 5 h, however.
Figure 4.
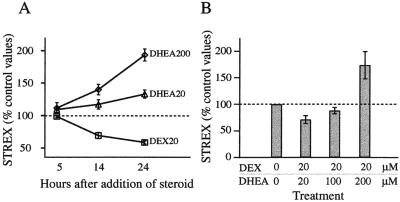
(A) Time course of DEX and DHEA effects on Slo splicing. DEX (20 μM), DHEA (20 μM or 200 μM), or vehicle alone (DMSO, 0.1%) was added 24 h after plating. Cells were harvested at various times after the addition of steroids. All values were normalized by the STREX percentage in parallel controls. By 14 h after addition, both DEX- and DHEA-treated cells showed significant differences from control cells (n = 5, P = 0.003 and n = 4, P = 0.02, respectively). (B) DEX and DHEA added simultaneously to culture dishes counteracted each other's effects on Slo splicing in vitro. The time course of drug treatment procedure was as described for Fig. 2B. DEX20/DHEA100 was significantly different from DEX20 alone (n = 9, P = 0.01), but not from controls.
Effects of DHEA on the STREX Percentage.
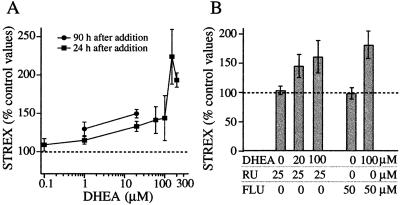
The observation that glucocorticoids affected Slo splicing in the direction opposite of that predicted from the combined hypophysectomy and ACTH injection experiment in rats (3) suggested that other factors may mediate the effect. DHEA was considered a good candidate for two reasons: (i) it is an adrenal corticosteroid produced and secreted by cells in close proximity to chromaffin cells, and (ii) its synthesis is promoted by stress and ACTH exposure. Our results demonstrate that by 24 h after addition, DHEA (20 μM) had increased the STREX percentage to 133% ± 5.2% of that in parallel controls (Fig. 3A, n = 21, P < 0.001). Simultaneous exposure to the GR antagonist RU38486 (25 μM) did not prevent the effect of 20 μM DHEA (Fig. 3B; STREX was 145% ± 19.7% of controls).
Figure 3.
(A) DHEA increases the relative abundance of STREX measured 24 h after addition. DHEA or vehicle alone (DMSO, 0.1%) was added at 24 h after plating, as in Fig. 2B. DHEA (1 μM) applied for 90 h, but not 24 h, was significantly different from controls, as were all time points at concentrations higher than 1 μM. STREX at 200 μM DHEA was significantly higher than at 20 μM. (B) The effect of DHEA (20 or 100 μM) was not blocked by the GR antagonist RU38486 (RU; 25 μM) or the androgen-receptor antagonist flutamide (Flu; 50 μM).
The response to 24-h treatment with DHEA exhibited a rough dose dependence; mean STREX values increased progressively between 1 and 150 μM DHEA, as illustrated in Fig. 3A. All concentrations were significantly different from controls except 100 nM and 1 μM. The effect of 200 μM DHEA treatment was significantly greater than that of 20 μM (193.39% ± 9.26%, n = 34, P < 0.001). Measured after 90 h of exposure, 1 μM DHEA was sufficient to raise STREX percentages significantly to 130% ± 8.9% of controls (Fig. 3A, n = 4, P = 0.044). After 90 h in 20 μM DHEA, STREX levels were 150% ± 5.5% of controls (n = 4, P = 0.003). The ability of DHEA to raise STREX levels was not blocked by either 25 μM RU38486, the GR and progesterone receptor antagonist, or by 50 μM flutamide, a specific antagonist of the best-characterized genomic androgen receptor (Fig. 3B).
As observed with DEX application, a significant change in STREX levels was first observed 14 h after DHEA addition (Fig. 4A), by which time STREX had increased to 139% ± 8.1% of controls, P = 0.016. No difference from controls was seen after 5 h in 20 μM DHEA.
Glucocorticoid and DHEA Had Opposing Effects on Slo Splicing.
When both DEX (20 μM) and DHEA (100 μM) were applied to cells at the same time, STREX levels after 24 h were 88.2% ± 6.21% of controls. This value was significantly higher than with 20 μM DEX applied alone (58.7% ± 2.44%, n = 9, P = 0.001, Fig. 4B), and was not significantly different from vehicle-treated controls. If the DEX was washed out after 24 h and replaced with vehicle alone for an additional 66 h, the STREX percentages remained low compared with parallel controls (50.4% ± 2.4%, n = 5). Similarly, for cells treated with 100 μM DHEA for 24 h and vehicle for 66 h, the STREX percentage was high (161.1% ± 22.7%, n = 7). Sequential application of the two steroids in either order gave results indistinguishable from controls (DHEA for 24 h followed by DEX for 66 h, and the converse sequence, respectively, yielded 103% ± 16.1%, and 92.5% ± 12.9%, n = 4 each). Thus, the effects of both steroids were persistent, and effects of the two steroids were additive whether exposure was simultaneous or sequential.
Other Steroids.
To determine whether other steroids known to occur in adrenals might also affect Slo splicing, several steroids were tested. Two androgens, androstenedione and testosterone (20 μM), significantly elevated STREX at 24 h to levels similar to those observed with DHEA (Fig. 5). Progesterone had no effect on STREX levels at 20 μM. Aldosterone, which has been referred to as a weak glucocorticoid (20), reduced STREX levels at 20 μM (n = 8, P = 0.002), and at 100 μM (n = 6, P = 0.006). This effect was similar to that of 20 μM cortisol (n = 4, P = 0.001).
Discussion
Bovine chromaffin cells dissociated and cultured in serum-free medium exhibit a gradual decrease in the relative abundance of STREX relative to ZERO transcripts of the Slo gene. This change is reminiscent of the drop in STREX that was precipitated by hypophysectomy in rats (3), and adds support to the idea that extrinsic factors influence splicing of the gene. In rats, ACTH injections with hypophysectomy prevented the drop in STREX, suggesting that the endocrine-stress axis was a positive factor in the inclusion of this exon. ACTH effects on adrenal medullary cells are typically mediated by their effects on steroidogenic adrenocortical cells. Thus, the first hypothesis arising from this study suggests that corticosterone, release of which is promoted by ACTH, mediates the effects of ACTH on Slo splicing in rat chromaffin cells. Extrapolating to dissociated bovine chromaffin cells in the absence of cortical cells, we predicted that cortisol, and DEX, would favor STREX over ZERO transcripts. Surprisingly, these glucocorticoids accelerated the decline in STREX levels seen in cell culture. This effect was prevented by coapplication of RU38486, a specific antagonist of both GR and the progesterone receptor (19). RU38486 has a very low affinity for the androgen receptor and no affinity for either mineralocorticoid or estradiol receptors. ACTH had no direct effect on chromaffin cells. Like CORT, the synthesis and release of another, much less well-understood steroid, DHEA, is stimulated by pituitary release of ACTH (8, 10–14). We found that application of androgens DHEA, testosterone, and androstenedione to bovine cells increased STREX levels in vitro, whereas CORT decreased STREX levels. Based on these results, we hypothesize that multiple steroid hormones, including glucocorticoids and adrenal androgens, complexly regulate STREX splicing and chromaffin cell excitability in vivo.
In many experiments described here we used what in some contexts might be considered pharmacological, or nonphysiological, concentrations of exogenous steroids (i.e., >10 μM). However, we report that 1 μM DHEA proved sufficient to significantly increase the relative abundance of STREX after a 90-h exposure. The effect of 20 μM also increased between 24 and 90 h, further indicating that the effect is cumulative, and that the cells do not desensitize on that time scale. Furthermore, steroid concentrations reported in the literature as effective in different contexts vary over a wide range. While 1 μM DEX measurably alters calcium-channel expression in chromaffin cells after 48 h (21, 22), acute effects of several steroids on catecholamine secretion are half-maximal at concentrations around 10 μM (23). It is also important to note that concentrations of steroids to which chromaffin cells are normally exposed, although difficult to measure, are much higher than systemic serum levels. Assuming a spatially uniform distribution throughout the whole adrenal, the CORT content measured by extraction from rat adrenals would give concentrations on the order of 100 μM (3). Steroids are not stored where they originate, and the blood supply to the adrenal medulla is largely derived from cortical sinus effluent, thus it is reasonable to suggest that chromaffin cells may be exposed to concentrations well in excess of 20 μM. Finally, it is also worth noting that the tissue-level assay used here amounts to averaging the responses of cells that may respond heterogeneously to the steroids, and thus will necessarily underestimate responses of any more sensitive subpopulations. Both steroid-receptor expression and steroid responses are heterogeneous in chromaffin cells. Whether the steroid effects described here are physiologically relevant in vivo requires further investigation. At the same time, we raise the possibility that Slo splicing in mature bovine cells may not be as dramatically responsive to steroid manipulations as one might anticipate from the rat hypophysectomy experiments. Possible explanations include the need for other steroidal or nonsteroidal cofactors, including neural input, the artificial nature and limited time of cell-culture conditions, and intrinsic limits to plasticity of Slo splicing that reflect genetic and epigenetic factors, including expression of splicing factors. The rat hypophysectomy experiments were done at 5 to 6 weeks of age (before puberty), whereas bovine cells were from mature animals. The possibility that splicing is more plastic earlier in life requires further investigation.
The negative effect of CORT on STREX inclusion was surprising. Not only was CORT a logical intermediary between ACTH and chromaffin cells, but circumstantial clues suggested a positive link. Thus, rat hypophysectomy sharply reduced CORT levels concurrently with the decline in STREX (3). Furthermore, bovine STREX levels are roughly half those of rats, and bovine serum cortisol levels are roughly 1/10 of rat corticosterone levels (episodic and circadian oscillations notwithstanding), and ACTH levels are at least 5-fold lower (24–28). In addition, there is a developmental correlate in rats, with a decline of both CORT and STREX during the postnatal stress-hyporesponsive period, and a roughly concurrent recovery of both thereafter (unpublished data). Among possible explanations for the paradoxical results with CORT are differences related to species or developmental age, and artifacts related to cell culture, including the absence of neural input, and many possible differences in the chemical environment.
The observation that DHEA can affect splicing is intriguing. Like glucocorticoids, the synthesis of DHEA responds rapidly to ACTH (8–14) and appears to fluctuate episodically and diurnally, typically in parallel with CORT (29). Bovine and rat adrenals produce DHEA (13, 30, 31), and synthesis would be expected to drop with hypophysectomy, although this issue needs to be revisited in rats (32, 33). The biological significance of adrenal DHEA is still largely a mystery. Reputed beneficial effects of DHEA on, for example, memory, libido, sense of well being, and maintenance of bone, skin, and muscle health in humans remain controversial, but are intriguing when contrasted with the negative health consequences of chronically elevated glucocorticoid levels (34–39). DHEA and CORT can have opposing actions on immune, cardiovascular, and other cell functions (40–45). In the nervous system, DHEA, like estradiol, appears to protect hippocampal neurons from glutamate excitotoxity and oxidative stress, whereas glucocorticoids are themselves toxic, and increase vulnerability to other toxins (45–48).
Importantly, CORT and DHEA responses to ACTH and various stressors are not always tightly coupled, but can differ greatly in time course and amplitude (8, 10, 12–14, 31, 33, 49). Although no comprehensive synthesis is yet possible, the studies suggest that steroid metabolism may shift in favor of glucocorticoid rather than adrenal androgen synthesis under conditions involving chronic psychological stress, chronic pituitary hypofunction, adrenal atrophy resulting from exogenous glucocorticoid treatment, chronic illness, and normal aging. If CORT and DHEA represent different endocrine or resource allocation strategies, this is likely to be reflected in the ratio of STREX and ZERO splice variants of Slo, and in catecholamine secretory function. Our observations are thus consistent with the notion that glucocorticoids (and aldosterone) and adrenal androgens (including DHEA) represent components of fundamentally different strategies for coping with, or recuperating from, stressors or threats of different nature, duration, or intensity. They represent one molecular level at which these hormones may interact to complexly tune chromaffin cell functional properties.
How might steroids affect Slo splicing? Glucocorticoids can act through intracellular steroid-receptors GR and mineralocorticoid receptor, which function as regulators of transcription. Blocking of the DEX effect by RU38486 (a GR and progesterone receptor antagonist), and the lack of a progesterone effect, suggests GR involvement. Because significant changes were first seen at 14 h, a change in transcription, e.g., of a splice-regulatory factor, could be involved. However, RU38486 can block some rapid, nongenomic effects of CORT (43), and GR can work through protein–protein interactions (50). Moreover, there are many reports of rapid, nongenomic effects of steroids, with downstream sequelae that can be slowly developing, and indirectly genomic. Neuroactive steroids can have rapid and selective effects on GABAA chloride channels, NMDA-gated channels, nicotinic acetylcholine receptors, 5-HT3 receptors, glycine receptors, σ receptors, and KCa channel β-subunits (51–56). DHEA has been reported to promote maturation of neocortical neurons by influencing Ca2+ entry through NMDA receptors (57). On chromaffin cells, DHEA and glucocorticoids have been shown to alter catecholamine secretion or Ca2+-channel functional expression on acute and chronic time scales (21–23, 58, 59). Importantly, the STREX/ZERO splicing decision has been shown to be modulated in a pituitary cell line by calcium-calmodulin kinase (CaM kinase IV), which interacts with specific exonic and intronic sequences by means of unidentified splicing factors (60). Thus, steroid effects on [Ca2+]i through ligand-gated channels and/or voltage-gated Ca channels could be involved.
Hormone-mediated changes in Slo splicing are virtually certain to affect chromaffin cell output. BK channels can be pro- or anti-excitatory in different cells, and perhaps even in a single cell under different circumstances. In chromaffin cells, BK channels can facilitate repetitive firing (1, 4, 61). The STREX exon has been shown to facilitate voltage- and Ca2+-dependent activation of Slo channels in heterologous expression systems (1–3). Facilitated activation of BK channels will favor rapid action potential repolarization and rapid recovery from a refractory state caused by Na-channel inactivation. The reduced excitability of chromaffin cells from hypophysectomized rats as compared with normal rats supports a proexcitatory role for the STREX exon (4). While its significance in chromaffin cells is not known, STREX also alters the regulatory profile of Slo channels; protein kinase A-dependent phosphorylation of a site within the STREX exon apparently shifts the direction of the BK channel's response to phosphorylation from an increase to a decrease in opening (62).
In summary, the present studies provide support for the hypothesis that adrenal androgens and glucocorticoids (and, presumably, also stress-related experiences) differentially modulate Slo splicing to fine-tune chromaffin cell excitability and catecholamine secretory responses. Although it is too early to draw any conclusions, it is interesting to speculate that the differential modulation by androgens and glucocorticoids might differentially prepare individuals for active and passive coping styles (63). Whatever the significance, this hormonal modulation is likely to have important ramifications for normal and pathological function of cardiovascular and other stress-responsive tissues.
Acknowledgments
We thank Cudlin's for supplying bovine adrenals and Drs. Jason McClean, Oliver Lung, Peter Lovell, Sahar Mahmoud, Oindrila Chatterjee, and Jonathan King for helpful comments on the manuscript and technical support. We also thank Lori Kwan and Dr. Gregor Dernick for technical support. This work was supported by National Institutes of Health Grant RO1 NS40790.
Abbreviations
- ACTH
adrenocorticotropic hormone
- STREX exon
stress axis-regulated exon
- DEX
dexamethasone
- ZERO channel
no extra exon at this site
- CORT
corticosterone and/or cortisol
- RT
reverse transcription
- DHEA
dehydroepiandrosterone
- GR
glucocorticoid receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Saito M, Nelson C, Salkoff L, Lingle C J. J Biol Chem. 1997;272:11710–11717. doi: 10.1074/jbc.272.18.11710. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan K, Michael T H, Jiang G J, Hiel H, Fuchs P A. Science. 1999;283:215–217. doi: 10.1126/science.283.5399.215. [DOI] [PubMed] [Google Scholar]
- 3.Xie J, McCobb D P. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 4.Lovell P V, McCobb D P. J Neurosci. 2001;21:3429–3442. doi: 10.1523/JNEUROSCI.21-10-03429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross M E, Evinger M J, Hyman S E, Carroll J M, Mucke L, Comb M, Reis D J, Joh T H, Goodman H M. J Neurosci. 1990;10:520–530. doi: 10.1523/JNEUROSCI.10-02-00520.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson D J. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- 7.Kosaki A, Webster N J. J Biol Chem. 1993;268:21990–21996. [PubMed] [Google Scholar]
- 8.Cutler G B, Jr, Davis S E, Johnsonbaugh R E, Loriaux D L. J Clin Endocrinol Metab. 1979;49:604–609. doi: 10.1210/jcem-49-4-604. [DOI] [PubMed] [Google Scholar]
- 9.Arvat E, Di Vito L, Lanfranco F, Maccario M, Baffoni C, Rossetto R, Aimaretti G, Camanni F, Ghigo E. J Clin Endocrinol Metab. 2000;85:3141–3146. doi: 10.1210/jcem.85.9.6784. [DOI] [PubMed] [Google Scholar]
- 10.Albertson B D, Hobson W C, Burnett B S, Turner P T, Clark R V, Schiebinger R J, Loriaux D L, Cutler G B., Jr J Clin Endocrinol Metab. 1984;59:13–18. doi: 10.1210/jcem-59-1-13. [DOI] [PubMed] [Google Scholar]
- 11.Parker L N. Endocrinol Metab Clin North Am. 1991;20:401–421. [PubMed] [Google Scholar]
- 12.Griffing G T, Allen J, Pratt H, Melby J C. Metabolism. 1985;34:631–636. doi: 10.1016/0026-0495(85)90090-3. [DOI] [PubMed] [Google Scholar]
- 13.Hung T T, LeMaire W J. J Steroid Biochem. 1988;29:721–726. doi: 10.1016/0022-4731(88)90174-4. [DOI] [PubMed] [Google Scholar]
- 14.Oberbeck R, Benschop R J, Jacobs R, Hosch W, Jetschmann J U, Schurmeyer T H, Schmidt R E, Schedlowski M. J Endocrinol Invest. 1998;21:148–153. doi: 10.1007/BF03347293. [DOI] [PubMed] [Google Scholar]
- 15.Meera P, Wallner M, Toro L. In: Potassium Channels in Cardiovascular Biology. Archer S L, Rusch N J, editors. New York: Kluwer Academic; 2001. pp. 49–70. [Google Scholar]
- 16.McCobb D. In: Molecular Insights into Ion Channel Biology in Health and Disease. Maue R A, editor. Amsterdam: Elsevier Science; 2002. , in press. [Google Scholar]
- 17.Mahmoud S F, Bezzerides A L, Riba R, Lai G-J, Lovell P V, Hara Y, McCobb D P. J Neurosci Methods. 2002;115:189–198. doi: 10.1016/s0165-0270(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 18.Rupprecht R, Arriza J L, Spengler D, Reul J M, Evans R M, Holsboer F, Damm K. Mol Endocrinol. 1993;7:597–603. doi: 10.1210/mend.7.4.8388999. [DOI] [PubMed] [Google Scholar]
- 19.Cadepond F, Ulmann A, Baulieu E E. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- 20.Meyer A S, Schmidt T J. J Steroid Biochem Mol Biol. 1997;62:97–105. doi: 10.1016/s0960-0760(97)00014-9. [DOI] [PubMed] [Google Scholar]
- 21.Fuller L Z, Lu C, McMahon D G, Lindemann M D, Jorgensen M S, Rau S W, Sisken J E, Jackson B A. J Neurosci Res. 1997;49:416–424. [PubMed] [Google Scholar]
- 22.Fuller L, Lu C, McMahon D, Alaudin E, Jorgensen M, Rau S, Sisken J, Jackson B. NeuroReport. 1997;8:1169–1172. doi: 10.1097/00001756-199703240-00022. [DOI] [PubMed] [Google Scholar]
- 23.Dar D E, Zinder O. Neuropharmacology. 1997;36:1783–1788. doi: 10.1016/s0028-3908(97)00150-0. [DOI] [PubMed] [Google Scholar]
- 24.el-Nouty F D, Elbanna I M, Johnson H D. J Dairy Sci. 1978;61:189–196. doi: 10.3168/jds.s0022-0302(78)83577-2. [DOI] [PubMed] [Google Scholar]
- 25.Veissier I, Le Neindre P. Reprod Nutr Dev. 1988;28:553–562. doi: 10.1051/rnd:19880402. [DOI] [PubMed] [Google Scholar]
- 26.Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- 27.Manzanares J, Corchero J, Fuentes J A. Brain Res. 1999;839:173–179. doi: 10.1016/s0006-8993(99)01756-4. [DOI] [PubMed] [Google Scholar]
- 28.Viau V, Chu A, Soriano L, Dallman M F. J Neurosci. 1999;19:6684–6693. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld R S, Hellman L, Roffwarg H, Weitzman E D, Fukushima D K, Gallagher T F. J Clin Endocrinol Metab. 1971;33:87–92. doi: 10.1210/jcem-33-1-87. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki T, Nawa K, Kominami S, Takemori S. Biochim Biophys Acta. 1992;1134:143–148. doi: 10.1016/0167-4889(92)90037-c. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi K, Nawata H, Kato K, Ibayashi H. Horm Metab Res. 1985;17:451–453. doi: 10.1055/s-2007-1013574. [DOI] [PubMed] [Google Scholar]
- 32.Milewich L, Axelrod L R. J Endocrinol. 1972;54:515–516. doi: 10.1677/joe.0.0540515. [DOI] [PubMed] [Google Scholar]
- 33.Odell W D, Parker L N. Endocr Res. 1984;10:617–630. doi: 10.1080/07435808409036520. [DOI] [PubMed] [Google Scholar]
- 34.Baulieu E E, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy M P, Latour F, et al. Proc Natl Acad Sci USA. 2000;97:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Cardounel A, Gursoy E, Anderson P, Kalimi M. Biochem Pharmacol. 2000;59:753–762. doi: 10.1016/s0006-2952(99)00385-8. [DOI] [PubMed] [Google Scholar]
- 36.McEwen B S. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 37.McEwen B S. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 38.McEwen B S. Prog Brain Res. 2000;122:25–34. doi: 10.1016/s0079-6123(08)62128-7. [DOI] [PubMed] [Google Scholar]
- 39.Kaminska M, Harris J, Gijsbers K, Dubrovsky B. Brain Res Bull. 2000;52:229–234. doi: 10.1016/s0361-9230(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 40.Blauer K L, Poth M, Rogers W M, Bernton E W. Endocrinology. 1991;129:3174–3179. doi: 10.1210/endo-129-6-3174. [DOI] [PubMed] [Google Scholar]
- 41.Browne E S, Porter J R, Correa G, Abadie J, Svec F. J Steroid Biochem Mol Biol. 1993;45:517–524. doi: 10.1016/0960-0760(93)90168-v. [DOI] [PubMed] [Google Scholar]
- 42.Araneo B A, Ryu S Y, Barton S, Daynes R A. J Surg Res. 1995;59:250–262. doi: 10.1006/jsre.1995.1162. [DOI] [PubMed] [Google Scholar]
- 43.Rong W, Wang W, Yuan W, Chen Y. Brain Res. 1999;815:51–59. doi: 10.1016/s0006-8993(98)01090-7. [DOI] [PubMed] [Google Scholar]
- 44.Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- 45.Kimonides V G, Spillantini M G, Sofroniew M V, Fawcett J W, Herbert J. Neuroscience. 1999;89:429–436. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- 46.Bastianetto S, Ramassamy C, Poirier J, Quirion R. Brain Res Mol Brain Res. 1999;66:35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 47.Kimonides V G, Khatibi N H, Svendsen C N, Sofroniew M V, Herbert J. Proc Natl Acad Sci USA. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardounel A, Regelson W, Kalimi M. Proc Soc Exp Biol Med. 1999;222:145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- 49.Parker L, Gral T, Perrigo V, Skowksy R. Metabolism. 1981;30:601–604. doi: 10.1016/0026-0495(81)90139-6. [DOI] [PubMed] [Google Scholar]
- 50.Ray A, Prefontaine K E. Proc Natl Acad Sci USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinder O, Dar D E. Acta Physiol Scand. 1999;167:181–188. doi: 10.1046/j.1365-201x.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 52.Falkenstein E, Tillmann H C, Christ M, Feuring M, Wehling M. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 53.Valverde M A, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde M I, Mann G E, Vergara C, Latorre R. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 54.Lupien S J, Nair N P, Briere S, Maheu F, Tu M T, Lemay M, McEwen B S, Meaney M J. Rev Neurosci. 1999;10:117–139. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- 55.Svec F, Porter J R. Proc Soc Exp Biol Med. 1998;218:174–191. doi: 10.3181/00379727-218-44285. [DOI] [PubMed] [Google Scholar]
- 56.Wehling M. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 57.Compagnone N A, Mellon S H. Proc Natl Acad Sci USA. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu P S, Lin M K, Hsieh H L. Neurosci Lett. 1996;204:181–184. doi: 10.1016/0304-3940(96)12350-8. [DOI] [PubMed] [Google Scholar]
- 59.Wagner P G, Jorgensen M S, Arden W A, Jackson B A. J Neurosci Res. 1999;57:643–650. [PubMed] [Google Scholar]
- 60.Xie J, Black D L. Nature (London) 2001;410:936–939. doi: 10.1038/35073593. [DOI] [PubMed] [Google Scholar]
- 61.Solaro C R, Prakriya M, Ding J P, Lingle C J. J Neurosci. 1995;15:6110–6123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian L, Duncan R R, Hammond M S, Coghill L S, Wen H, Rusinova R, Clark A G, Levitan I B, Shipston M J. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 63.Koolhaas J M, Korte S M, De Boer S F, Van Der Vegt B J, Van Reenen C G, Hopster H, De Jong I C, Ruis M A, Blokhuis H J. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]