Abstract
Fragile X syndrome, the most common inherited form of human mental retardation, is caused by mutations of the Fmr1 gene that encodes the fragile X mental retardation protein (FMRP). Biochemical evidence indicates that FMRP binds a subset of mRNAs and acts as a regulator of translation. However, the consequences of FMRP loss on neuronal function in mammals remain unknown. Here we show that a form of protein synthesis-dependent synaptic plasticity, long-term depression triggered by activation of metabotropic glutamate receptors, is selectively enhanced in the hippocampus of mutant mice lacking FMRP. This finding indicates that FMRP plays an important functional role in regulating activity-dependent synaptic plasticity in the brain and suggests new therapeutic approaches for fragile X syndrome.
Fragile X syndrome is a prevalent form of inherited mental retardation, occurring with a frequency of 1 in 4,000 males and 1 in 8,000 females. The syndrome is also characterized by developmental delay, hyperactivity, attention deficit disorder, and autistic-like behaviors (1). There is no effective treatment for fragile X syndrome.
The syndrome is typically caused by a repeat expansion mutation in the FMR1 gene that encodes FMRP, the fragile X mental retardation protein. FMRP is known to associate with translating polyribosomes and a subset of brain mRNAs and is believed to function as a regulator of protein synthesis (2–5). Involvement of FMRP in synaptic plasticity has long been suspected, because polyribosomes, FMR1 mRNA, and FMRP are all present in dendritic spines, the major site of synaptic transmission on cortical neurons (6). The Fmr1 null mutant (knockout) (Fmr1-KO) mouse, which has a behavioral phenotype consistent with fragile X syndrome, provided an opportunity to test this hypothesis. However, protein synthesis-dependent late-phase long-term synaptic potentiation (LTP) was found to be unaffected in the hippocampus of mutant mice (7, 8).
Reexamination of this issue was prompted by recent work showing that local synaptic control of protein synthesis is required for stable expression of a second form of hippocampal synaptic modification: long-term depression (LTD) triggered by activation of group 1 metabotropic glutamate receptors (mGluRs) (9–11). A role for FMRP is this form of synaptic plasticity was further suggested by the fact that FMRP is one of the proteins known to be synthesized in response to mGluR activation (6).
We report here that mGluR-dependent LTD (mGluR-LTD) is significantly altered in the hippocampus of Fmr1-KO mice. Rather than a deficit, however, we find that mGluR-LTD is augmented in the absence of FMRP. This finding is consistent with the recent discovery that FMRP normally functions as a negative regulator of translation (5, 12, 13). We propose that exaggerated LTD and/or mGluR function are responsible for aspects of the behavioral phenotype in fragile X syndrome, and that antagonists of group 1 mGluRs should be considered as possible therapeutic agents.
Materials and Methods
Hippocampal slices were prepared from postnatal day (P)21–30, C57BL/6 congenic Fmr1-KO mice and their wild-type (WT) littermates, as described (10). Slices were collected in ice-cold dissection buffer containing (in mM): sucrose, 212; KCl, 2.6; NaH2PO4, 1.25; NaHCO3, 26; MgCl2, 5; CaCl2, 0.5; and dextrose, 10. CA3 was removed immediately after sectioning. Slices recovered for 1–5 h at 30°C in artificial cerebrospinal fluid (ACSF) containing (in mM) NaCl, 124; KCl, 5; NaH2PO4, 1.25; NaHCO3, 26; MgCl2, 1; CaCl2, 2; and dextrose, 10, saturated with 95% O2, 5% CO2. For recording, slices were placed in a submersion recording chamber and perfused with 30°C ACSF at a rate of 2 ml/min.
Field potentials (FPs) were recorded with extracellular recording electrodes (1.0 MΩ) filled with ACSF and placed in stratum radiatum of area CA1. Synaptic responses were evoked by a 200-μsec current pulse to Schaffer collateral axons with a concentric bipolar tungsten stimulating electrode. Stable baseline responses were collected every 30 sec by using a stimulation intensity (10–30 μA) yielding 50–60% of the maximal response. mGluR-LTD was induced in the presence of the N-methyl-D-aspartate (NMDA) receptor (NMDAR) antagonist d-(-)-2-amino-5-phosphono-pentanoic acid (D-APV) (50 μM) by using paired-pulse low-frequency stimulation (PP-LFS) consisting of 900 pairs of stimuli (50-msec interstimulus interval) delivered at 1 Hz. NMDAR-LTD was induced by using 900 single pulses delivered at 1 Hz (14).
Waveforms were filtered at 2 kHz, acquired, and digitized at 10 kHz on a personal computer by using experimenter's workbench (DataWave Systems, Boulder, CO). The group data were analyzed as follows: (i) the initial slope of the FP for each experiment was expressed as percentages of the preconditioning or 3,5-dihydroxyphenylglycine (DHPG) baseline average (2), and the time scale in each experiment was converted to time from the onset of conditioning or DHPG. All experiments were performed blind to the genotype of the mice, determined after analysis of individual experiments. After genotyping, the time-matched normalized data were averaged across experiments and expressed in the text and figures as the means (± SEM). Significant differences between groups were determined by using an independent t test and the Komolgarov–Smirnov test.
R,S-DHPG and D-APV were purchased from Tocris (St. Louis, MO). All other chemicals were purchased from Sigma. DHPG was prepared as a 100× stock in H20, aliquoted, and stored at −20°C. Fresh stocks were made once per week. A 10× stock of D-APV was prepared in ACSF and stored at 4°C. These stocks were diluted in ACSF to achieve their final concentrations.
Results
Normal Synaptic Transmission in Fmr1-KO Mice.
Hippocampal slices were prepared from P21–30 C57BL/6 congenic Fmr1-KO mice and their WT littermates. Excitatory synaptic FPs evoked by stimulation of the Schaffer collaterals were recorded extracellularly from the stratum radiatum of area CA1. In all cases, the experimenters were blind to the genotype.
Previous studies have examined the properties of transmission at Schaffer collateral synapses in the CA1 region of hippocampus of these mutant mice. In terms of basal transmission, excitability, paired-pulse facilitation, early-phase LTP elicited with 100 Hz stimulation, and late-phase (protein synthesis-dependent) LTP induced with θ-burst stimulation, Fmr1-KO mice were indistinguishable from WT littermates (7, 8). It can be inferred from these findings that excitatory synaptic transmission mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and NMDARs and the state of inhibition are not appreciably affected by the absence of FMRP. Because of this extensive prior characterization, we did not examine these properties here. However, we did confirm that FP amplitudes in response to increasing stimulus current were not different between Fmr1-KO and WT littermates [F(1,350) = 0.358, P > 0.5], the maximum amplitude of FP from Fmr1-KO mice (1.73 ± 0.19 mV; n = 39 slices from 17 mice) was not different from WT (1.63 ± 0.16 mV; n = 36 slices from 18 mice; P = 0.36), and the stimulus currents used to evoke baseline responses were not different between groups (Fmr1-KO 22 ± 1 μA; WT 23 ± 2 μA).
mGluR-LTD Induced by Synaptic Stimulation Is Enhanced in Fmr1-KO Mice.
Paired-pulse stimulation repeated at 1 Hz for 15 min (PP-LFS) induces LTD that is independent of NMDARs and requires activation of group 1 mGluRs (9, 10, 15) and the rapid translation of preexisting mRNA (9). We therefore first examined the consequences of PP-LFS in slices from KO and WT animals.
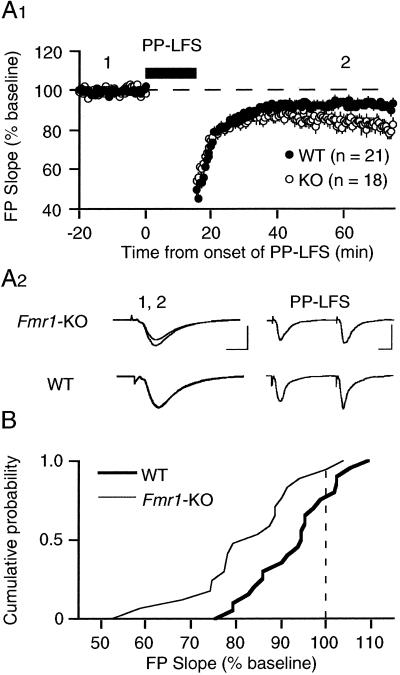
We found that PP-LFS (delivered in the presence of 50 μM D-APV to block NMDARs) produced a small but significant LTD in WT mice (93 ± 3% 60 min after PP-LFS; n = 21 slices from 10 mice; Fig. 1). The magnitude of LTD in these experiments is considerably less than what we have seen in previous studies using rats (9, 10). This difference is likely because of the species and the strain of mice used. This finding of reduced LTD in C57BL/6 mice was not entirely unexpected, as the magnitude of the NMDAR-dependent form of LTD is also typically less in these animals as compared with rats (personal observations). However, we were surprised to find that the magnitude of LTD induced with PP-LFS was significantly increased in slices prepared from KO animals (82 ± 3%; n = 18 slices from 8 mice; different from WT at P < 0.004; t test). The difference first emerged approximately 15 min after the tetanus; there was no indication that responses during or immediately after the PP-LFS were different in KO and WT animals (Fig. 1A). To further evaluate whether the distribution of depression values was different between KO and WT groups, a Kolmolgarov–Smirnov test was performed on the cumulative probability distribution, and this confirmed a significant difference (P < 0.05; Fig. 1B).
Figure 1.
Synaptic induction of mGluR-LTD using PP-LFS is significantly enhanced in hippocampus of Fmr1-KO mice as compared with WT controls. (A1) Average time course of the change in FPs after PP-LFS. LTD in KO animals measured 82 ± 3% of prePP-LFS baseline (n = 18 slices from 8 mice; open circles) as compared with 93 ± 2% in WT controls (n = 21 slices from 10 mice; filled circles; different at P = 0.004, t test). (A2) Representative FPs (2 min average) taken at the times indicated by the numbers on the graph. [Bars = 1 mV, 5 msec (1, 2) and 1 mV, 10 msec (PP-LFS).] (B) Cumulative probability distributions of FP slope values (% of baseline), measured 1 h after PP-LFS in individual slices from both KO and WT groups. The distribution in KO mice is significantly different from that in WT mice, as determined by Kolmolgarov–Smirnov test (P < 0.05). All experiments were performed blind, in the presence of the NMDAR antagonist D-APV (50 μM).
mGluR-LTD Induced by DHPG Is Enhanced in Fmr1-KO Mice.
Another way to induce mGluR LTD is to apply the selective group 1 mGluR agonist DHPG (16). The advantages of this approach are that more synapses are affected more uniformly, and that it circumvents the need for presynaptic activation. Previous work under the same conditions as our experiments has shown a dose-dependent induction of LTD after DHPG (50–100 μM, 5 min). Activation of mGluR5 is required for induction, and protein synthesis is required for stable expression, of DHPG-LTD (9, 10). LTD with PP-LFS and DHPG are also mutually occluding, suggesting they use the same saturable expression mechanism (10). Therefore, in an attempt to confirm that mGluR-LTD is increased in Fmr1-KO mice using an independent method, we performed another series of experiments using DHPG to induce plasticity.
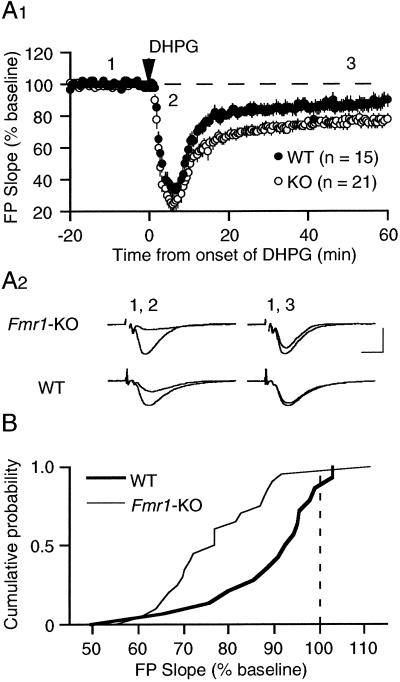
As in the previous study, experiments were performed in the presence of D-APV to eliminate the confound of NMDAR-dependent synaptic modifications. We used 100 μM DHPG (5 min) to induce a saturating level of LTD. The results showed, again, a significant enhancement of mGluR-LTD in slices from KO mice (Fig. 2). DHPG application to slices from Fmr1-KO mice resulted in depression of FP slope values to 77 ± 3% of preDHPG baseline (measured 60 min after DHPG application n = 21 slices from 9 mice). In comparison, DHPG-induced LTD was 88 ± 4% in WT mice (15 slices from 8 animals; P = 0.02; Fig. 2A). The Kolmolgarov–Smirnov test performed on the cumulative probability distribution confirmed the statistical significance of this difference (P < 0.05; Fig. 2B). Although the acute effect of DHPG on synaptic transmission also appeared to be slightly enhanced in Fmr1-KO slices, this difference was not statistically significant (maximal acute depression: WT: 36 ± 4%, KO: 26 ± 5% of preDHPG baseline values). Western blots of hippocampal homogenates also confirmed that mGluR5 levels are comparable in KO and WT mice (data not shown).
Figure 2.
Brief application of the mGluR agonist DHPG (5 min; 100 μM) induces greater LTD of synaptic responses in hippocampus of Fmr1-KO mice as compared with WT littermate controls. (A1) Plotted are average (±SEM) FP slope values over the time course of the experiment. In Fmr1-KO animals, the response 60 min after treatment was depressed to 77 ± 3% of preDHPG baseline (n = 21 slices from 9 mice; open circles); in interleaved WT controls, the response was depressed to 88 ± 4% of baseline (n = 15 slices from 8 mice; filled circles; different at P = 0.02; t test). (A2) Representative FPs (2 min average) taken at the times indicated by the numbers on the graph. (Bar = 1 mV; 5 msec.) (B) Cumulative probability distributions of FP slope values (% of baseline), measured 1 h after DHPG in individual slices from both KO and WT groups. The distribution in KO mice is significantly different from that in WT mice as determined by Kolmolgarov–Smirnov test (P < 0.05).
NMDAR-Dependent LTD Is Normal in Fmr1-KO Mice.
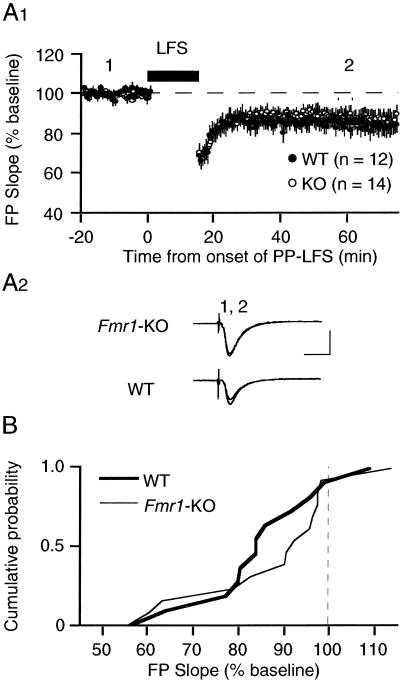
Two forms of homosynaptic LTD coexist at CA3–CA1 synapses: mGluR-LTD and a form that is triggered by activation of NMDARs (NMDAR-LTD) (17). NMDAR-LTD in hippocampal slices is independent of mGluR activation and protein synthesis but instead requires activation of postsynaptic protein phosphatases (9, 18–20). We examined NMDAR-LTD in Fmr1-KO mice to determine whether FMRP selectively regulates protein synthesis-dependent plasticity or LTD mechanisms in general. NMDAR-LTD was elicited by delivering 900 single pulses at 1 Hz (14). In contrast to mGluR-LTD, NMDAR-LTD was normal in Fmr1-KO mice (86 ± 4%; 14 slices from 8 animals) as compared with WT littermates (84 ± 4%; 12 slices from 4 animals; P = 0.6; Fig. 3). These results suggests that FMRP may specifically regulate mGluR- and protein synthesis-dependent plasticity.
Figure 3.
Synaptic induction of NMDAR-dependent LTD using a 1-Hz LFS protocol is comparable in Fmr1-KO mice and WT controls. (A1) Average time course of the change in FPs after LFS. LTD in KO animals measured 86 ± 4% of preLFS baseline (n = 14 slices from 8 mice; open circles) as compared with 84 ± 4% in WT controls (n = 12 slices from 4 mice; filled circles; P = 0.6, t test). (A2) Representative FPs (2 min average) taken at the times indicated by the numbers on the graph. (Bar = 1 mV, 10 msec.) (B) Cumulative probability distributions of FP slope values (% of baseline), measured 1 h after LFS in individual slices from both KO and WT groups. The distribution in KO mice is not significantly different from that in WT mice as determined by the Kolmolgarov–Smirnov test. All experiments were performed blind.
Discussion
Using two distinct induction protocols, we show that mGluR-dependent LTD is significantly increased in the hippocampus of animals lacking FMRP. The most straightforward hypothesis is that FMRP regulates LTD downstream of the mGluRs, likely at the level of mRNA translation.
Although many questions remain, we believe the implications of these data warrant sharing this finding without delay. If aspects of fragile X syndrome are related to exaggerated mGluR-dependent synaptic plasticity, drugs that inhibit group 1 mGluRs and/or LTD might be considered for the treatment of this disorder.
Involvement of FMRP in the Regulation of LTD.
Activation of postsynaptic group 1 mGluRs (primarily mGluR5), either by the selective agonist DHPG or by synaptically released glutamate, triggers LTD at Schaffer collateral synapses in area CA1 of the hippocampus. Recent evidence suggests that one expression mechanism for the LTD of synaptic transmission is the internalization of AMPA and NMDARs (11, 21). Both synaptic depression and glutamate receptor internalization can be initiated by mGluR activation without new protein synthesis, but the stable expression of the change fails to occur when mRNA translation (but not transcription) is inhibited (9, 11). The critical site of protein synthesis is the postsynaptic dendrite (9).
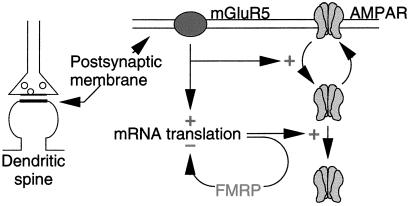
One mRNA that is known to be translated in response to postsynaptic group 1 mGluR activation encodes FMRP (6). Thus, the present experiments were designed to test the obvious hypothesis that the mGluR-dependent synthesis of FMRP plays a role in the stabilization of LTD. We were initially surprised to discover that LTD was actually enhanced in the absence of FMRP; however, this finding is consistent with a number of recent studies suggesting that FMRP can function as a negative regulator of mRNA translation (5, 12, 13). Taken together, the data are consistent with a model in which an increase in FMRP normally serves to limit expression of LTD by inhibiting mGluR-dependent translation of other synaptic mRNAs (Fig. 4). One message that is negatively regulated by FMRP encodes the microtubule associated protein MAP1b, which has been shown in Drosophila to regulate synaptic structure and function (5). Indeed, recent studies have shown an increase of MAP1b mRNA on polyribosomes in cells derived from fragile X patients, consistent with FMRP being a negative regulator of MAP1b translation (3). It will be of considerable interest to examine the role of MAP1b in the expression of hippocampal LTD.
Figure 4.
Model. Previous research has shown that activation of mGluR5 stimulates the internalization of AMPA receptors and NMDARs (not shown; ref. 11). The stable expression of this modification requires protein synthesis, which we propose is negatively regulated by FMRP synthesized in response to mGluR activation. Therefore, in the absence of FMRP, LTD magnitude is increased.
In addition to LTD triggered by activation of group 1 mGluRs, there is a second well-studied form of homosynaptic LTD that is induced by activating NMDARs (22). In hippocampal slices, expression of NMDAR-mediated LTD is not protein synthesis dependent for at least 1 h (9, 23) and does not occlude mGluR-mediated LTD (10, 17). Our finding of normal NMDAR-LTD in the Fmr1-KO mice supports the idea that these forms of LTD use distinct mechanisms. It is interesting to note that another form of NMDAR-dependent plasticity, LTP, is also unaffected in Fmr1-KO mice (7, 8). Taken together, the results suggest that FMRP may be selectively involved in synaptic modifications that are triggered in response to mGluR-stimulated protein synthesis.
Role of LTD and FMRP in Cortical Development.
It has been suggested that mechanisms of LTD and LTP normally work in concert to fine-tune patterns of synaptic connectivity during development (24, 25) and to store memories in the adult brain (26). One consequence of activating mGluRs in cultured hippocampal neurons is a long-term decrease in the surface expression of the ionotropic glutamate receptors that mediate synaptic transmission, possibly as a prelude to synapse elimination (11). Thus, in the absence of FMRP, enhanced LTD could interfere with the establishment and maintenance of strong synapses required for normal brain function.
In this context, it is noteworthy that dendritic spine development is slowed in the cerebral cortex of Fmr1-KO mice (27). Dendritic spines are the major targets of glutamatergic synapses in the cortex. Synapses are formed during development when long thin protospines emitted by pyramidal cell dendrites make contact with nearby axons (28). As the synapse stabilizes, the spines shorten and become fatter. An increased percentage of long thin dendritic processes, reminiscent of protospines, is a characteristic feature of cortical neurons in FMRP-deficient mice (27, 29) and affected humans (30, 31). It was previously suggested that this phenotype might be a consequence of reduced synapse elimination (6). We propose instead that the underlying defect may actually be enhanced activity- and mGluR-dependent synapse turnover, abnormally prolonging a state in which neurons are actively seeking new synaptic input. Consistent with this idea, it was reported very recently that hippocampal neurons in culture express significantly longer thinner spines after DHPG treatment. Like LTD, this effect of DHPG requires protein synthesis (32).
Treatment of Fragile X Syndrome.
The intriguing association of group 1 mGluRs and activity-dependent protein synthesis is not restricted to early postnatal development, the cerebral cortex, or LTD. mGluR- and protein synthesis-dependent LTD can still be elicited in hippocampus from mature animals, where it may contribute to memory storage, particularly during novel or stressful situations (33–35). Moreover, recent work has also shown that LTD in the cerebellum, long known to depend on group 1 mGluRs and believed to contribute to learning motor reflexes (36), also requires rapid translation of mRNA (37). Finally, there is evidence that mGluR-triggered protein synthesis in the hippocampus can reduce the threshold for synaptic potentiation (38) and trigger epileptiform activity (39, 40). It is conceivable that FMRP normally functions as a negative feedback regulator of all these physiological processes. In this context, it is interesting to note that the prominent features of fragile X syndrome also include heightened responses to novelty, compulsions, and seizures.
Taken together, the data lead us to hypothesize that fragile X mental retardation is a consequence of increased mGluR-dependent protein synthesis and/or LTD in the brain, both during early postnatal development and in adulthood. We have found that LTD magnitude increases with increasing activation of mGluR5 (10). It follows that titration of a competitive antagonist will produce a graded reduction in this mGluR- and protein synthesis-dependent response. Thus, our hypothesis prompts an obvious question: Could inhibitors of group 1 mGluR-mediated synaptic transmission be effective in the treatment of this disorder? Although additional studies are obviously required to test this hypothesis, these data point to a rational pharmaceutical approach for fragile X syndrome.
Acknowledgments
Special thanks to Dr. Stephanie Ceman for performing the Western blot analysis of mGluR5 and to Dr. Michael Tranfaglia and Katie Clapp for encouragement. This work was supported in part by grants from FRAXA to K.M.H. and from the National Institutes of Health to M.F.B and S.T.W., both investigators with the Howard Hughes Medical Institute.
Abbreviations
- LTP
long-term potentiation
- mGluR
group 1 metabotropic glutamate receptor
- LTD
long-term depression
- mGluR-LTD
mGluR-dependent LTD
- Pn
postnatal day n
- ACSF
artificial cerebrospinal fluid
- FP
field potential
- PP-LFS
paired-pulse low-frequency stimulation
- DHPG
3,5-dihydroxyphenylglycine
- D-APV
d-(-)-2-amino-5-phosphono-pentanoic acid
- Fmr1-KO
Fmr1 knockout
- WT
wild type
- NMDA
N-methyl-D-aspartate
- NMDAR
NMDA receptor
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
References
- 1.Jin P, Warren S T. Hum Mol Genet. 2000;9:901–908. doi: 10.1093/hmg/9.6.901. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Absher D, Eberhart D E, Brown V, Malter H E, Warren S T. Mol Cell. 1997;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- 3.Brown V, Jin P, Ceman S, Darnell J C, O'Donnell W T, Tenenbaum S A, Jin X, Feng Y, Wilkinson K D, Keene J D, et al. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J C, Jensen K B, Jin P, Brown V, Warren S T, Darnell R B. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y Q, Bailey A M, Mathies H J G, Renden R B, Smith M A, Speese S D, Rubin G M, Broadie K. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 6.Weiler I J, Greenough W T. Am J Med Genet. 1999;83:248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Godfraind J M, Reyniers E, De Boulle K, D'Hooge R, De Deyn P P, Bakker C E, Oostra B A, Kooy R F, Willems P J. Am J Med Genet. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Paradee W, Melikian H E, Rasmussen D L, Kenneson A, Conn P J, Warren S T. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 9.Huber K M, Kayser M S, Bear M F. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 10.Huber K M, Roder J C, Bear M F. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 11.Snyder E M, Philpot B D, Huber K M, Dong X, Fallon J R, Bear M F. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 12.Laggerbauer B, Ostareck D, Keidel E, Ostareck-Lederer A, Fischer U. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Zhang Y, Ku L, Wilkinson K D, Warren S T, Feng Y. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek S M, Bear M F. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp N, Bashir Z I. Neuropharmacology. 1999;38:495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 16.Palmer M J, Irving A J, Seabrook G R, Jane D E, Collingridge G L. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 17.Oliet S H, Malenka R C, Nicoll R A. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 18.Mulkey R M, Herron C E, Malenka R C. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- 19.Mulkey R M, Endo S, Shenolikar S, Malenka R C. Nature (London) 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 20.Sawtell N B, Huber K M, Roder J C, Bear M F. J Neurophysiol. 1999;82:3594–3597. doi: 10.1152/jn.1999.82.6.3594. [DOI] [PubMed] [Google Scholar]
- 21.Xiao M Y, Zhou Q, Nicoll R A. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Bear M F, Abraham W C. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 23.Kauderer B S, Kandel E R. Proc Natl Acad Sci USA. 2000;97:13342–13347. doi: 10.1073/pnas.97.24.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bear M F, Cooper L N, Ebner F F. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- 25.Bear M F, Rittenhouse C D. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Bear M F. Proc Natl Acad Sci USA. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimchinsky E A, Oberlander A M, Svoboda K. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dailey M E, Smith S J. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comery T A, Harris J B, Willems P J, Oostra B A, Irwin S A, Weiler I J, Greenough W T. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinton V J, Brown W T, Wisniewski K, Rudelli R D. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 31.Irwin S A, Patel B, Idupulapati M, Harris J B, Crisostomo R A, Larsen B P, Kooy F, Willems P J, Cras P, Kozlowski P B, et al. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Vanderklish P W, Edelman G M. Proc Natl Acad Sci USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot B D, Miyakawa T, Bear M F, Tonegawa S. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 34.Bear M F. Proc Natl Acad Sci USA. 1999;96:9457–9458. doi: 10.1073/pnas.96.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braunewell K H, Manahan-Vaughan D. Rev Neurosci. 2001;12:121–140. doi: 10.1515/revneuro.2001.12.2.121. [DOI] [PubMed] [Google Scholar]
- 36.Bear M F, Linden D J. In: Synapses. Cowan W M, Sudhoff T C, Stevens C F, editors. Baltimore: Johns Hopkins Univ. Press; 2001. pp. 455–517. [Google Scholar]
- 37.Karachot L, Shirai Y, Vigot R, Yamamori T, Ito M. J Neurophysiol. 2001;86:280–289. doi: 10.1152/jn.2001.86.1.280. [DOI] [PubMed] [Google Scholar]
- 38.Raymond C R, Thompson V L, Tate W P, Abraham W C. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merlin L R, Bergold P J, Wong R K. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- 40.Wong R K, Bianchi R, Taylor G W, Merlin L R. Adv Neurol. 1999;79:685–698. [PubMed] [Google Scholar]