Abstract
Nuclear acetyltransferases promote and deacetylases inhibit skeletal muscle-gene expression, suggesting the potential effectiveness of deacetylase inhibitors (DIs) in modulating skeletal myogenesis. Surprisingly, previous studies have indicated that DIs suppress myogenesis. The recent observations that histone deacetylases associate with the muscle-regulatory proteins MyoD and MEF2C only in undifferentiated myoblasts prompted us to evaluate the effect of DIs at distinct stages of the myogenic program. We found that exposure of established rodent and human muscle cells to distinct DIs has stage-specific effects. Exposure of undifferentiated skeletal myoblasts to DIs, followed by incubation in differentiation medium, enhanced the expression of muscle-specific reporters and increased the levels of endogenous muscle proteins, leading to a dramatic increase in the formation of multinucleated myotubes. By contrast, simultaneous exposure of muscle cells to differentiation medium and DIs inhibited the myogenic program. Likewise, embryos exposed in utero to nonteratogenic doses of DI at the early stages of somitic myogenesis (embryonic day 8.5) exhibited an increased number of somites and augmented expression of a muscle-specific transgene as well as endogenous muscle genes. The functional effects induced by DIs were mirrored by changes in the state of acetylation of histones present at a muscle-gene enhancer and of MyoD itself. These results represent the first evidence that DIs can enhance muscle differentiation and suggest the rationale for their use in manipulating adult and embryonic skeletal myogenesis.
Keywords: deacetylase inhibitors‖gene expression‖MyoD‖skeletal muscle differentiation‖embryos
The activity of MyoD is regulated by several independent but functionally coherent mechanisms (1). For instance, whereas recruitment of both chromatin remodeling complexes and p300/CBP and PCAF acetyltransferases onto muscle regulatory regions promotes MyoD-dependent transcription (2), histone deacetylases (HDACs) inhibit muscle gene expression (3, 4). An expected corollary to these observations is that inhibiting HDACs would enhance the activity of MyoD and other muscle regulatory factors (MRFs), thereby stimulating myogenesis. Surprisingly, previous studies have reported that exposure to the deacetylase inhibitor (DI) sodium butyrate inhibits muscle differentiation (5–7).
An explanation for these apparently conflicting results may be offered by the recent observation that HDAC members can be found in different complexes in myoblasts, when they associate with MRFs (3, 8, 9), and in myotubes, when they are either recruited by retinoblastoma tumor-suppressor protein (pRb) or excluded from the nucleus (3, 9). These considerations prompted us to evaluate the effects of DI at distinct stages of muscle differentiation. Our results demonstrate that when the exposure to DI is restricted to myoblasts—that is, when HDAC/MRF complexes are targeted—it enhances muscle-gene expression and leads to a dramatic increase in myotube formation, after subsequent incubation in a medium permissive for differentiation.
Materials and Methods
Cells, Plasmids, Transfections, and Luciferase (luc) Reporter Assay.
C2C12 skeletal muscle cells and human skeletal myoblasts were cultured in DMEM supplemented with 20% FBS (growth medium, GM) and induced to differentiate with DMEM supplemented with 2% horse serum and 1× insulin, transferrin, and selenium (differentiation medium, DM). C2C12 stable cell lines with integrated reporters are described in ref. 10. The muscle creatine kinase (MCK)-luc, 4RE-luc, E2F-luc, MLC1/3F-nLacZ, and Flag-HDAC1 plasmids are described elsewhere (11–14). Transient transfections were performed with FuGENE 6 transfection reagent. Luciferase values were corrected for protein concentration of the individual cell extracts.
Immunoblotting and Immunofluorescence.
For immunoblotting, total cell extracts were obtained after incubating the cells in standard lysis buffer, and Western blots were performed with an enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech) with the following Abs: anti-myosin heavy chain (MHC) (MF-20), anti-myogenin (FD5), and antitubulin E7 (Developmental Studies Hybridoma Bank, Univ. of Iowa, Iowa City, IA). For immunofluorescence, cells were stained with anti-MHC MF-20. Nuclei were visualized with 4′,6′-diamidino-2-phenylindole (DAPI). Alexa Fluor 488-conjugated secondary Abs (Molecular Probes) were used to reveal the primary Abs.
DI Treatment.
C2C12 and human skeletal myoblasts were exposed to sodium butyrate 5 mM (Sigma), trichostatin A (TSA) 50 nM (Upstate Biotechnology, Lake Placid, NY), or valproic acid (VPA) 10 mM (Sigma) for 24 h in GM. DIs were removed, and the cells were cultured in DM for at least 48 h before being analyzed. When indicated, the cells were exposed to DIs in DM for 48 h.
β-Galactosidase Assay.
Cells transfected with the MLC1/3F-nLacZ plasmid were processed for β-galactoside staining as described in ref. 10.
In Vivo Acetylation Assay.
The in vivo acetylation assay was performed as described in ref. 13.
Chromatin Immunoprecipitation (ChIP) Assay.
A ChIP assay was performed with the acetyl-histone H4 immunoprecipitation assay kit (Upstate Biotechnology) according to the manufacturer's instructions. PCR was performed on “input” DNA of different samples, and equivalent amounts of immunoprecipitated DNA were amplified by PCR with primers for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promoter and MCK enhancer (see supporting information, which is published on the PNAS web site, www.pnas.org).
Reverse Transcription (RT)-PCR.
C2C12 cells were treated with TSA (50 nM) for 16 h in GM and then switched to DM without TSA. Total RNA was isolated and RT-PCR was performed as described in supporting information.
Embryo Exposure to DIs.
MLC1/3F-nLacZ pregnant mice [embryonic day (E) 8.5] received i.p. injection of 15 μg of TSA at E8.5 and E9.5 or VPA as described (15). VPA was dissolved in distilled water and administered at doses of 6 mg per mouse (about 150 μg/ml) every 12 h at E8.5, E9, E9.5, and E10. VPA doses are in the therapeutic range in humans (50–150 μg/ml). Mice were killed at E10.5, and the embryos were observed under a dissecting stereomicroscope to evaluate their viability, presence of external malformations, and number of somites. Embryos were processed for β-galactosidase staining.
Results
The Effects of Deacetylase Inhibition on Skeletal Muscle Cells Depend on Their Differentiation Stage.
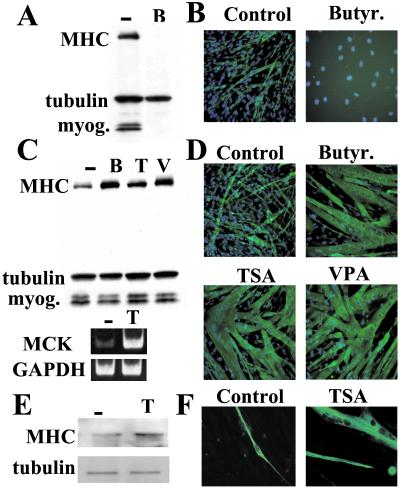
The DI sodium butyrate has been reported to inhibit myogenic differentiation (5–7). In those studies the cells were exposed to the DI when cultured in DM. In agreement with these findings, we observed that cells treated with sodium butyrate, when cultured in DM, failed to activate expression of both myogenin and MHC and to properly differentiate (Fig. 1 A and B). Numerous cells exposed to sodium butyrate in DM undergo a high frequency of apoptosis, as indicated by the reduced cell density (Fig. 1B and data not shown). It has recently been shown that HDAC1 associates with MyoD in undifferentiated skeletal myoblasts cultured in GM and is recruited by hypophosphorylated pRb to block E2F-dependent transcription in differentiated skeletal myotubes (9). Therefore, we speculated that exposure of skeletal myoblasts to DI during differentiation may impinge on the function of the HDAC1–pRb complex and hence adversely affect muscle-gene expression, by inducing sustained E2F activity, which is incompatible with the activation of the myogenic program (16). Indeed, DI exposure activates E2F-dependent transcription in cells cultured in DM but not in GM (see Fig. 2C). Thus, exposure to DI of either undifferentiated myoblasts or differentiating myocytes may have different outcomes. To test this hypothesis, we exposed undifferentiated skeletal muscle cells to sodium butyrate in GM for 24 h and then allowed them to differentiate in DM without sodium butyrate. With this protocol we sought to target HDACs only in undifferentiated myoblasts, when they are complexed to MRFs. Under these conditions, the cells up-regulated MHC expression and displayed a striking increased frequency of larger MHC-positive multinucleated cells as compared with untreated cells (Fig. 1 C and D). Transcription of the MCK, but not of the control GADPH gene, was enhanced when compared with untreated cells (Fig. 1C). MHC and MCK expression is restricted to late stages of muscle differentiation and requires the presence of functional pRb, whereas myogenin is activated at earlier stages and does not require pRb (17). Interestingly, the levels of myogenin were not affected by myoblast exposure to DI (Fig. 1C), suggesting that DIs selectively activate late muscle markers. Two other structurally unrelated DIs, TSA (18) and VPA [a short-chained fatty acid used as an anticonvulsant and mood stabilizer recently shown to inhibit histone deacetylases (19)], displayed effects undistinguishable from those exerted by sodium butyrate (Fig. 1 C and D). These results suggest that deacetylase inhibition—and not peculiar activities of the individual compounds—is likely to mediate their effects on myogenesis. Fluorescence-activated cell sorter (FACS) experiments showed that DI treatment did not significantly affect the normal accumulation of myoblasts in G0/G1 of the cell cycle once they were placed in DM (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). Another striking effect induced by DIs relates to their ability to increase the efficiency of myoblast fusion into MHC-positive myotubes. Although ∼15% of the total nuclei are present in elongated MHC-positive myotubes deriving from myoblasts not previously exposed to DIs, ∼75–80% of the total nuclei are detected in hypernucleated MHC-positive myotubes when myoblasts have been previously exposed to DIs (Fig. 1D and Table 1). The effect of DI exposure was verified further in primary human skeletal myocytes. Again, exposure of these cells to TSA (Fig. 1 E and F and Table 1), sodium butyrate, or VPA (data not shown) followed by incubation in DM enhanced the formation of MHC-positive multinucleated myotubes and increased the MHC expression levels. The same effect was also observed in rat L6 myocytes as well as in mouse-derived satellite cells (data not shown).
Figure 1.
DIs enhance muscle gene expression and myotube formation. (A) Subconfluent C2C12 cells were placed in DM and either simultaneously exposed to sodium butyrate (B) or incubated in DM for an additional 48 h. Total cellular extracts were obtained and analyzed by Western blotting for the presence of MHC, tubulin, and myogenin. (B) C2C12 cells were treated as described in A, and MHC was detected by immunofluorescence (green). Nuclei were stained with DAPI. (C) C2C12 cells cultured in GM were exposed to sodium butyrate (B), TSA (T), or VPA (V) for 24 h and then switched to DM for an additional 48 h in the absence of DI. The MHC levels were determined with the MF-20 Ab, and tubulin was visualized with the E7 Ab. Reverse transcription–PCR was performed on RNA obtained form cells treated with (T) or without (−) TSA as described above with specific primers for either MCK or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (D) C2C12 cells were treated as described in C, and MHC was detected by immunofluorescence. Nuclei were stained with DAPI. (E) Human skeletal myocytes (HSkMs) growing in GM were treated with TSA for 24 h and then switched to DM in the absence of TSA for an additional 48 h. Western blot analysis of MHC and tubulin expression in either untreated (Control) or TSA-treated (TSA) human skeletal myoblasts. (F) MHC was detected by immunofluorescence in HSkMs either untreated (Control) or exposed to TSA. Nuclei were stained with DAPI.
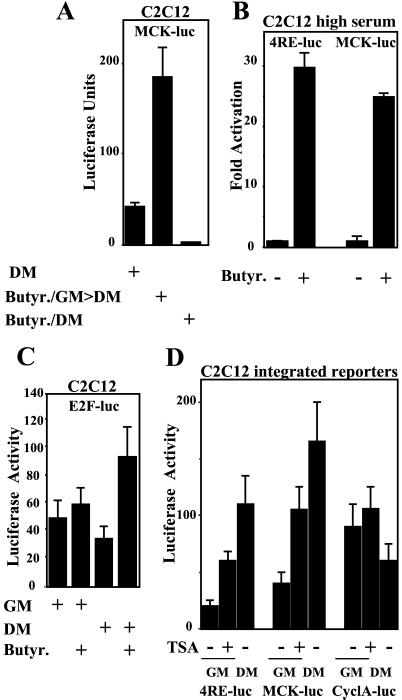
Figure 2.
DIs activate muscle-specific transcription in skeletal muscle cells. (A) The activation of MCK-luc was monitored after transient transfection in C2C12 cells. Luc activity was measured in untreated cells after incubation for 48 h in DM. Alternatively, C2C12 cells were exposed to sodium butyrate for 24 h in GM and then switched to DM without sodium butyrate (Butyr/GM>DM) for 48 h or simultaneously treated with sodium butyrate when cultured in DM (Butyr/DM) before being analyzed for luc activity. (B) Myoblasts transfected with either MCK- or 4RE-luc were exposed to sodium butyrate in GM. Luc activity was determined after 24 h whereas myoblasts were still cultured in GM and no signs of morphological differentiation were evident. (C) The transcriptional activity of an E2F-responsive construct was evaluated either in the absence or presence of sodium butyrate in GM and DM conditions, as described in A. (D) The activity of three integrated reporters (4RE-, MCK-, and CyclA- luc) in C2C12 cells was monitored after exposure (+) or not (−) to TSA for 48 h in GM. The reporter activity was also analyzed in cells differentiated in the absence of TSA (DM).
Table 1.
DIs augment the percentage of multinucleated skeletal myotubes
| Total nuclei (DAPI) | Nuclei/MHC-positive cells | |
|---|---|---|
| C2C12 cells | ||
| Untreated | ||
| Ex. 1 | 240 | 33 (13.7%) |
| Ex. 2 | 250 | 42 (16.8%) |
| Sodium butyrate | ||
| Ex. 1 | 250 | 210 (84.0%) |
| Ex. 2 | 274 | 230 (83.9%) |
| TSA | ||
| Ex. 1 | 266 | 200 (75.1%) |
| Ex. 2 | 320 | 210 (65.6%) |
| VPA | ||
| Ex. 1 | 187 | 153 (81.8%) |
| Ex. 2 | 240 | 187 (77.9%) |
| Human skeletal myoblasts | ||
| Untreated | 98 | 12 (12.2%) |
| Sodium butyrate | 111 | 43 (38.7%) |
| TSA | 117 | 56 (47.8%) |
| VPA | 106 | 45 (42.4%) |
C2C12 cells or human skeletal myoblasts were exposed to sodium butyrate, TSA, VPA, or left untreated for 24 h in GM. The medium was replaced with DM without DI, and MHC expression was detected by immunofluorescent staining. Nuclei were visualized with DAPI. The values in parentheses indicate the percentage of nuclei detected in MHC-positive cells.
The results presented in this section illustrate a stage-specific effect of DIs on myogenic differentiation. They also underscore the specific activation of late-muscle genes by DIs and demonstrate that the positive effect exerted by DIs on muscle differentiation is not restricted to one specific myogenic cell lineage.
DIs Target the MRFs.
Activation of the myogenic program depends on the combinatorial activity of the myogenic basic helix–loop–helix (bHLH) and MEF2 factors (20), which are inhibited by HDACs in myoblasts (3, 9). Therefore, we hypothesized that deacetylase inhibition in myoblasts may promote the myogenic program by targeting the activity of these MRFs. To test this hypothesis, we made use of the MCK enhancer, which is regulated by the synergistic activity of the myogenic bHLH and MEF2 (20). The MCK-luc reporter was transiently transfected in skeletal myoblasts, which were subsequently exposed to DIs either in GM or DM. The results of these experiments are illustrated in Fig. 2A and indicate that DI treatment stimulates transcription of the MKC reporter solely when the DIs were applied to cells cultured in GM. In contrast, exposure to sodium butyrate of cells cultured in DM inhibited activation of the MCK enhancer (Fig. 2A). Importantly, exposure to sodium butyrate increased transcriptional activity of both MCK- and 4RE-luc reporters even in undifferentiated myoblasts cultured in high serum (Fig. 2B). Similar results were obtained with TSA (data not shown). These findings are in agreement with the behavior of the endogenous genes MHC and MCK after DI treatment (Fig. 1). Because sodium butyrate and TSA target class I as well as class II HDACs, inhibition of members belonging to both families of deacetylases may mediate the prodifferentiation effect of TSA. Importantly, and in contrast to the behavior of muscle-specific reporters, transcription driven from an E2F-responsive construct was stimulated by butyrate only when cells were exposed in DM (Fig. 2C), consistent with the formation of the pRb–HDAC complex at this stage. To strengthen and extend our observations, we tested the effects of TSA on chromatin-integrated muscle-specific reporters. We used C2C12 clones stably transfected with the MCK-, 4RE-, and cyclinA-luc templates. The exposure to TSA led to 2–3-fold activation of MCK- and 4RE-luc in GM (Fig. 2D). On the contrary, serum-dependent transcription driven by an integrated cyclinA-luc construct was not stimulated by TSA treatment in GM (Fig. 2D).
These results indicate that exposure to DIs can activate muscle-specific reporters under conditions (i.e., high serum, GM) that are nonpermissive for myogenic transcription. However, the expression of endogenous differentiation markers—MHC and MCK—could not be detected at this stage (data not shown). This finding further indicates that the enhancement of myogenic differentiation promoted by DI treatment in myoblasts requires their subsequent exposure to differentiation cues (e.g., DM culture).
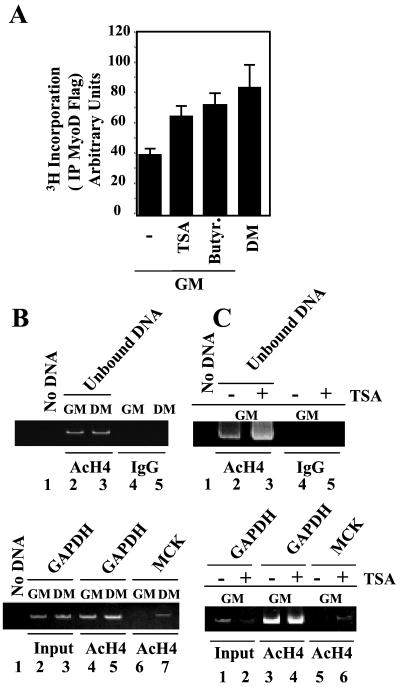
Acetylation of MyoD and Histones Surrounding MyoD-Binding Sites Correlates with the Functional Outcome of Deacetylase Inhibition.
Given that MyoD activity can be regulated by both acetyltransferases and deacetylases, a possible target of the DIs could be the acetylation status of MyoD (13) and of the histones surrounding MyoD-binding sites within muscle-specific enhancer/promoters. We first evaluated the acetylation of MyoD by [3H]acetate incorporation in myoblasts cultured either in the absence or presence of DIs. As shown in Fig. 3A, exposure to TSA or sodium butyrate stimulated the incorporation of [3H]acetate into a Flag-tagged MyoD transiently transfected in myoblasts cultured in GM. The levels of MyoD acetylation induced by DI exposure were comparable to those detected after placing the cells in DM. Exposure to TSA did not interfere with the formation of the MyoD–HDAC1 complex (data not shown), suggesting that TSA-mediated hyperacetylation of MyoD is obtained mainly through inhibition of the enzymatic activity of HDAC1 and not through its displacement from MyoD. To start analyzing the effects of TSA on histone acetylation, we first performed a ChIP assay with an Ab against hyperacetylated H4 histones to evaluate the acetylation of the histones surrounding the MyoD-binding sites of the MCK enhancer. As shown in Fig. 3B, the histones located on the MCK enhancer are hypoacetylated in undifferentiated myoblasts (Lower, lane 6) and become hyperacetylated after differentiation (lane 7), in agreement with the kinetics of the MCK transcriptional activation (see Fig. 1C). Exposure of undifferentiated myoblasts to TSA in GM resulted in histone hyperacetylation of the MCK enhancer before incubation in DM (Fig. 3C Lower, compare lanes 5 and 6). Given the importance of histone hyperacetylation in the activation of muscle-specific gene expression (1, 3, 4), it is possible that the anticipated hyperacetylation of the MCK enhancer by DI exposure in myoblasts accounts for the enhanced activation of MCK transcription after subsequent incubation in DM.
Figure 3.
Exposure of undifferentiated myoblasts to DIs results in MyoD and histone hyperacetylation. (A) MyoD acetylation in undifferentiated C2C12 myoblasts (GM) untreated (−) or treated with either sodium butyrate or TSA and in differentiated myotubes (DM) was measured by H3 incorporation of exogenously transfected Flag-tagged MyoD after metabolic labeling with H3 sodium acetate. (B) C2C12 myoblasts (GM) and myotubes (DM for 48 h) were formaldehyde cross-linked and processed for ChIP assay with Abs against acetyl-histone 4 (AcH4) or IgG (as control). PCR was performed with primers for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promoter and MCK enhancer as described in Materials and Methods. (C) Myoblasts cultured in GM and simultaneously exposed or not to TSA for 36 h were processed for ChIP as described in B.
TSA and VPA Anticipate Somitogenesis and Activate Muscle Transcription in Developing Embryos.
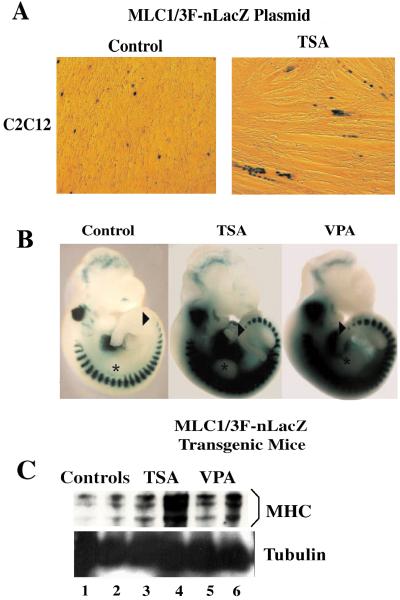
To investigate whether the effects of deacetylase inhibition observed in cultured cells could be recapitulated in the animal, we decided to monitor transcription directed by the myosin light chain 1/3F regulatory regions-lacZ (MLC 1/3F-nlacZ) transgene (12) in developing embryos exposed to either TSA or VPA. Before embarking on this analysis, we ascertained that the MLC3F-lacZ-nls construct was responsive to DI treatment in cultured cells. C2C12 cells were transfected with the MLC3F-lacZ-nls (nuclear localization signal) construct and then exposed to either TSA (Fig. 4A) or VPA (data not shown) at the myoblast stage for 24 h, before incubation in DM for additional 36 h. Fig. 4A shows that TSA-treated cells (Right) displayed an increased number of β-galactosidase-positive nuclei when compared with untreated cells (Left).
Figure 4.
Embryos exposed to DIs display anticipated somitogenesis and increased muscle gene expression. (A) MLC1/3F-nLacZ plasmid reporter construct was transfected in C2C12 cells. Cells were then exposed to TSA (50 nM) in GM and subsequently switched to DM for 36 h. Cells were fixed and then stained to detect β-galactosidase activity. Note that the differentiation extent after transfection is typically delayed. (B) E10.5 embryos derived from MLC1/3F-nLacZ transgenic mice previously exposed to either TSA or VPA treatment (see Materials and Methods) were stained for 1 h at 30°C to detect β-galactosidase activity. TSA- and VPA-treated embryos showed higher levels of MLC-lacZ transgene expression and numbers of somites expressing MLC1/3F-nLacZ than control embryos. Arrows indicate the last differentiated somite, which is close to segmental plate in treated embryos. Asterisks indicate the forelimb bud. (C) Western blot analysis of total extracts derived from total embryos exposed to either TSA or VPA was performed with anti-MHC (Upper) and antitubulin (Lower) Abs.
Previous work has shown that embryos from mice treated with TSA at E8.5 and killed at E9.5 during somitogenesis are modestly but consistently larger than control embryos and exhibited an increased (+2 to +6) number of somites (21). This finding correlates with increased acetylation of histone H4 and the number of somites expressing the myogenic factor Myf-5 (21). Because MyoD is not expressed at E9.5, we investigated the in vivo effect of TSA and VPA administered i.p. to pregnant mice (at a concentration of 0.5–1 mg/kg and 200 mg/kg, respectively) at postimplantation stages (E8.5) and killed at E10.5, when MyoD expression is clearly detectable in the myotomes (22). The overall expression of the MLC3F-lacZ-nls transgene was enhanced in somites of DI-treated as compared with untreated embryos (Fig. 4B). To confirm that exposure to TSA and VPA increases the expression of endogenous muscle-specific genes during embryonal myogenesis, we also evaluated the levels of MHC expression in DI-treated vs. untreated embryos. Exposure to TSA and VPA increased the expression of MHC, whereas the levels of tubulin remained constant, once again indicating a specific effect of these DIs on muscle-specific transcription (Fig. 4C). Moreover, the number of MLC3F-lacZ-nls-expressing somites (revealed in blue by β-galactosidase staining) were consistently increased in TSA- and VPA-treated embryos (see Table 3, which is published as supporting information on the PNAS web site, www.pnas.org). Notably, the increased number of somites at this stage did not result in any obvious malformations during postnatal development (see Table 4, which is published as supporting information on the PNAS web site, www.pnas.org), indicating that the somitogenesis was only transiently accelerated.
Together, these results indicate that DI treatment during the postimplantation stage, when critical morphogenetic events occur, results in accelerated formation of somites and increased expression of differentiation markers, as observed in cultured cells.
Discussion
The results reported here show that deacetylase inhibition has different outcomes on muscle transcription and differentiation depending on the stages of cell differentiation at which inhibitors are applied. When the DIs sodium butyrate, TSA, and VPA are transiently applied to undifferentiated myoblasts and then removed, once cells are induced to differentiate, they all enhance muscle gene expression and differentiation. This phenomenon correlates with hyperacetylation of MyoD, of histones surrounding the MCK enhancer, and with transcriptional activation of muscle-specific genes. On the contrary, if applied to muscle cells induced to differentiate by incubation in DM, sodium butyrate and VPA prevent their differentiation into myotubes. Interestingly, TSA can stimulate muscle-gene expression, albeit with much reduced efficacy, even when applied to confluent C2C12 cells cultured in DM (data not shown), suggesting that TSA may have overlapping yet distinct targets from sodium butyrate and VPA.
The morphological changes induced by DIs in myoblasts include an increased myoblast fusion into multinucleated myotubes (Fig. 1 and Table 1). Thus, one possibility is that DI treatment may modulate the expression of molecules that allow the recruitment of differentiation-defective myoblasts by cells that are competent to differentiate, thereby enhancing their fusion into multinucleated myotubes. Alternatively, persistence of MRF–HDAC complexes in differentiation-defective myoblasts may prevent these cells from executing the myogenic program, and DIs would promote their differentiation by removing the differentiation block imposed by HDACs. It is of interest that DIs selectively increase the expression of genes that are expressed at late stages of muscle differentiation (MHC and MCK) and whose expression requires the concerted activity of MRFs and pRb (17). Instead, expression of the early myogenic marker myogenin—whose expression does not require pRb (17)—is not affected by deacetylase inhibition (Fig. 1A). These findings indicate a promoter-specific effect of DIs. They also suggest an effect of DIs on regulators of late myogenesis, such as pRb. Beside its well established role in mediating cell-cycle arrest, pRb has been recently implicated in signaling MyoD-dependent activation of the MEF2 transactivation domain (23). Indeed, we have shown that the MyoD-MEF2 synergism is interrupted by HDAC1 (13). It is tempting to speculate that DI treatment may relieve a block imposed by HDACs on an as yet to be defined function of pRb necessary to activate the MRF network. Nonetheless, given the differentiation-defective phenotype of pRb−/− myocytes, the failure to rescue such phenotype with DIs (P.L.P., S.I. and V.S., unpublished data) underscores the concept that the mechanism underlying deficient myogenesis in pRb−/− cells is multifaced.
The hyperacetylation of MyoD and histones surrounding the MCK enhancer in myoblasts exposed to DIs is likely to account for the enhanced activity of MyoD and increased MCK expression, after the subsequent exposure to DM. However, in DIs exposed myoblasts the expression of MCK and other differentiation genes (e.g., MHC) was not detectable, despite the increased histone acetylation, until the cells were exposed to differentiation cues (DM). This finding suggests that histone hyperacetylation induced by DIs is not sufficient by itself to stimulate transcription; rather it provides an activatory signal that confers an advantage in the activation of transcription on specific stimuli, such as the differentiation cues provided by DM for muscle-specific promoter/enhancers.
Our experiments conducted in developing embryos indicate an increased somitogenesis and increased β-galactosidase staining after exposure to nonteratogenic doses of TSA and VPA. However, these experiments do not discriminate whether the increased β-galactosidase staining reflects enhanced expression of the transgene, a more global acceleration of myogenesis, or both. It is important to stress that myogenesis in somites occurs in the presence of high concentrations of growth factors such as fibroblast growth factor and Wnts (24) and hence the effect of DIs should be conceptually similar to that observed in cultures exposed to high serum. Steinbac et al. (25) have reported that early administration of TSA to pregastrula frog embryos blocks MyoD induction and reduces muscle differentiation. Although we have not experimentally addressed this apparent discrepancy, it is worth noting that induction and maintenance of MyoD activity is differentially controlled in Xenopus and mouse. Moreover, the route of TSA administration chosen by Steinbac et al. differs from ours, making it impractical to calculate the actual dose of TSA delivered to the embryos. The observations reported here represent the first example of stage-specific modulation of the myogenic program by agents that affect the balance between two emerging classes of transcriptional regulators—the acetyltransferases and the deacetylases. We suggest that our observations may provide important insights in designing the rationale for therapeutic approaches aimed at correcting pathological situations during embryonic development, inducing experimental muscle hypertrophy, and stimulating muscle regeneration in adult myogenesis. Remarkably, injury-mediated muscle regeneration by satellite and muscle stem cells entails a sequence of stages reminiscent of the multistep differentiation of cultured myoblasts, including initial activation and proliferation of myogenic precursors, followed by their fusion into myotubes. In this respect, the specific effect of DIs during the proliferative growth of myoblasts can be of particular relevance and suggests a rationale for their application in enhancing muscle regeneration.
Supplementary Material
Acknowledgments
We acknowledge Dr. Cristina Montagna for help with artwork. This work was supported by grants from the Muscular Dystrophy Association (to P.L.P.), the European Community and the Associazione Italiana per la Ricerca sul Cancro (Italy) (to C.N. and G.C.), and from Telethon (to G.C.). P.L.P. is supported by Telethon-Italy and is an Assistant Telethon Scientist.
Abbreviations
- DI
deacetylase inhibitors
- DM
differentiation medium
- GM
growth medium
- HDAC
histone deacetylase
- luc
luciferase
- MCK
muscle creatine kinase
- MHC
myosin heavy chain
- MRF
muscle regulatory factor
- pRb
retinoblastoma tumor-suppressor protein
- En
embryonic day n
- TSA
trichostatin A
- VPA
valproic acid
- ChIP
chromatin immunoprecipitation
- DAPI
4′,6′-diamidino-2-phenylindole
References
- 1.Puri P L, Sartorelli V. J Cell Physiol. 2000;185:155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Puri P L, Sartorelli V, Yang X J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y, Graessmann A, Nakatani Y, Levrero M. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 3.McKinsey T A, Zhang C L, Olson E N. Curr Opin Genet Dev. 2001;11:497–504. doi: 10.1016/s0959-437x(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 4.Sartorelli V, Puri P L. Front Biosci. 2001;6:D1024–D1047. doi: 10.2741/sartorel. [DOI] [PubMed] [Google Scholar]
- 5.Blau H M, Epstein C J. Cell. 1979;17:95–108. doi: 10.1016/0092-8674(79)90298-8. [DOI] [PubMed] [Google Scholar]
- 6.Fiszman M Y, Montarras D, Wright W, Gros F. Exp Cell Res. 1980;126:31–37. doi: 10.1016/0014-4827(80)90467-x. [DOI] [PubMed] [Google Scholar]
- 7.Johnston L A, Tapscott S J, Eisen H. Mol Cell Biol. 1992;12:5123–5130. doi: 10.1128/mcb.12.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mal A, Sturniolo M, Schiltz R L, Ghosh M K, Harter M L. EMBO J. 2001;20:1739–1753. doi: 10.1093/emboj/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puri P L, Iezzi S, Stiegler P, Chen T T, Schiltz R L, Muscat G E, Giordano A, Kedes L, Wang J Y, Sartorelli V. Mol Cell. 2001;8:885–897. doi: 10.1016/s1097-2765(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Woodring P J, Bhakta K S, Tamura K, Wen F, Feramisco J R, Karin M, Wang J Y, Puri P L. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helin K, Wu C L, Fattaey A R, Lees J A, Dynlacht B D, Ngwu C, Harlow E. Genes Dev. 1993;7:1850–1861. doi: 10.1101/gad.7.10.1850. [DOI] [PubMed] [Google Scholar]
- 12.Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartorelli V, Puri P L, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang J Y, Kedes L. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- 14.Taunton J, Hassig C A, Schreiber S L. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 15.Faiella A, Wernig M, Consalez G G, Hostick U, Hofmann C, Hustert E, Boncinelli E, Balling R, Nadeau J H. Hum Mol Genet. 2000;9:227–236. doi: 10.1093/hmg/9.2.227. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Helin K, Jin P, Nadal-Ginard B. Cell Growth Differ. 1995;6:1299–1306. [PubMed] [Google Scholar]
- 17.Novitch B G, Mulligan G J, Jacks T, Lassar A B. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida M, Horinouchi S, Beppu T. BioEssays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 19.Phiel C J, Zhang F, Huang E Y, Guenther M G, Lazar M A, Klein P S. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 20.Molkentin J D, Black B L, Martin J F, Olson E N. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 21.Nervi C, Borello U, Fazi F, Buffa V, Pelicci P G, Cossu G. Cancer Res. 2001;61:1247–1249. [PubMed] [Google Scholar]
- 22.Sassoon D, Lyons G, Wright W E, Lin V, Lassar A, Weintraub H, Buckingham M. Nature (London) 1989;341:303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- 23.Novitch B G, Spicer D B, Kim P S, Cheung W L, Lassar A B. Curr Biol. 1999;9:449–459. doi: 10.1016/s0960-9822(99)80210-3. [DOI] [PubMed] [Google Scholar]
- 24.Bailey P, Holowacz T, Lassar A B. Curr Opin Cell Biol. 2001;13:679–689. doi: 10.1016/s0955-0674(00)00271-4. [DOI] [PubMed] [Google Scholar]
- 25.Steinbac O C, Wolffe A P, Rupp R A. Biol Chem. 2000;381:1013–1016. doi: 10.1515/BC.2000.124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.