Abstract
Understanding the interaction of nitric oxide (NO) with red blood cells (RBCs) is vital to elucidating the metabolic fate of NO in the vasculature. Because hemoglobin (Hb) is the most abundant intraerythrocytic protein and reacts rapidly with NO, the interaction of NO with Hb has been studied extensively. We and others have shown the NO reaction with RBCs is nearly 1,000-fold slower than the reaction with cell-free Hb. Because the reaction rate of NO with cell-free Hb and RBCs is quite different, we hypothesize that different reaction products evolve under locally high NO concentrations, which can be generated by bolus NO addition or NO inhalation. Here we use electron paramagnetic resonance to show that bolus NO addition to cell-free Hb solutions results in nitrosylhemoglobin [HbFe(II)NO] formation as a minor product through a MetHb-dependent pathway. Further, the RBC is shown to be more prone to form HbFe(II)NO under this heterogeneous condition compared with an equivalent free-Hb solution. In both cases, trapping MetHb with cyanide blocked the formation of HbFe(II)NO. We conclude that the formation of HbFe(II)NO is a heterogeneous phenomenon involving three successive reactions of NO with the same heme molecule. These results were supported further by mathematically modeling NO–Hb reactions and diffusion.
The reactions of nitric oxide (NO) with cell-free hemoglobin (Hb; unless otherwise noted, Hb denotes both oxygenated and deoxygenated Hb) and red blood cell (RBC)-encapsulated Hb have been investigated extensively for many years. The physiological importance of these reactions was established with the identification of NO as the endothelium-derived relaxation factor (1, 2). However, the NO interaction with RBC-encapsulated Hb is still a problem of considerable controversy. As NO is inactivated by oxyHb at an extremely high rate (k ≈107 M−1⋅s−1) (3–5), how is NO bioactivity preserved in the cardiovascular system? We have shown experimentally that an RBC-free zone near the vessel wall reduces the consumption of NO by RBCs (6). In addition, NO does not react with RBC-encapsulated Hb as rapidly as it does with cell-free Hb (7) because of an extracellular diffusion limitation (8) and a submembrane diffusion barrier consisting of the RBC cytoskeleton and other relatively NO-inert proteins such as MetHb (9, 10).
Concurrent with the diffusion barrier model of NO preservation, another model has been proposed that involves new chemistry to explain the preservation of NO bioactivity in the vasculature (11–13). One stipulation of this theory is that nitrosylhemoglobin [HbFe(II)NO] is produced from the cooperative binding of NO to the minor population of free heme on normoxic Hb (≈99% oxyHb and 1% deoxyHb), thereby avoiding degradation of NO to nitrate. The formation of HbFe(II)NO in high concentrations is contrary to the original findings of Doyle and Hoekstra (3) and a later paper by Eich et al. (4). More recently, the formation of HbFe(II)NO was investigated further by multiple laboratories (5, 14–18).
Given that RBC-encapsulated Hb reacts much more slowly with NO than cell-free Hb, it is plausible that the reaction products differ under heterogeneous conditions in which locally high NO concentrations exist. As NO participates in a variety of reactions in biological solutions, the local NO concentration may determine the reaction pathway and thus product distribution. Here we examine the reaction of NO with cell-free Hb and RBC-encapsulated Hb by use of electron paramagnetic resonance (EPR) and mathematical simulation. We demonstrate that HbFe(II)NO is formed through the reduction of MetHb in locally high NO regions, and that RBCs facilitate the formation of HbFe(II)NO under this condition.
Materials and Methods
Chemicals.
DEA/NO, 2-(N,N-diethylamino)diazenolate 2-oxide, was purchased from Alexis (San Diego). HPLC water, potassium cyanide, iodine, sodium hydroxide, and acetic acid were purchased from Fisher Scientific. Sephadex G-25, α-cellulose, microcrystalline cellulose, EDTA, Hepes, potassium iodide, and potassium ferricyanide were purchased from Sigma.
Preparation of oxyHb and RBCs.
Bovine blood was collected in a heparinized (10 units/ml) container. The plasma and buffy coat were removed after centrifugation at 800 × g for 10 min. The cells were resuspended and washed three times in a buffer containing 40 mM Hepes/120 mM NaCl/5 mM glucose at pH 7.4, 285 milliosmolar (mOsm). After each wash, the cells were centrifuged at 800 × g for 10 min. RBCs were purified by filtration through a mixture of α-cellulose and microcrystalline cellulose. All solutions for preparing proteins were at 4°C. Cell lysate was prepared by lysing purified RBCs with 5 mM sodium phosphate solution, pH 8.0, or freeze lysis and centrifuging at 22,000 × g, 4°C, for 30 min. When needed, oxyHb was isolated by passing the cell lysate through a Sephadex G-25 fine column.
NO Reaction with Free oxyHb and RBC.
NO gas (Matheson) was purified by bubbling through deoxygenated 5 M NaOH as described elsewhere (19) and entrapped in a sealed-glass gas-collection tube (Ace Glass). A saturated NO solution was prepared by bubbling the purified NO through a deoxygenated buffer consisting of 40 mM Hepes and 120 mM NaCl in a Hamilton air-tight syringe. Dilutions of the saturated NO solution were made as needed by adding deoxygenated buffer. DEA/NO was prepared in 5 mM Tris solution at 9.4 pH adjusted to 300 mOsm with NaCl. The absorbance of DEA/NO was measured at 250 nm, and the concentration was calculated with an extinction coefficient of 6.5 mM−1⋅cm−1 (20). To both free oxyHb solutions and RBC suspensions, sufficient volumes of NO containing solution were added for a final concentration of 100 μM NO (100 μM NO solution or 65 μM DEA/NO). Samples were put in liquid nitrogen (for EPR) or on ice (for chemiluminescence) within 1 min for NO solution additions or after 40 min for DEA/NO additions. NO bolus additions to aerobically sealed samples were performed both inside and outside a controlled anaerobic chamber (Plas-Labs, Lansing, MI). RBC solutions at 30% hematocrit were determined to be the easiest to introduce consistently at the same volume into the EPR tube.
EPR Detection of MetHb and HbFe(II)NO.
After the addition of NO and immediate mixing, 200 μl of each sample was introduced into 3-mm-i.d. clear fused-quartz tubes and plunged into liquid nitrogen. The EPR spectrum of each sample then was measured on a Bruker (Billerica, MA) EMX spectrometer in the X band at 77 K by using a suprasil liquid-nitrogen finger Dewar (Wilmad, Buena, NY) in a Bruker 4119HS-W1 high-sensitivity cavity. The high-sensitivity cavity has a measured signal-to-noise ratio approximately six times higher than a standard cavity. To measure both MetHb and HbFe(II)NO (oxyHb is EPR-silent) in the same scan, a sweep width of 3,995 G (1 G = 0.1 mT) centered at 2,474 G was taken. The spectrometer was operated at 9.35 GHz, 10.1-mW microwave power, 15-G modulation amplitude, 100-kHz modulation frequency, and 20.48-ms time constant. Under these conditions, the power was not saturated. The sweep time was 41.94 s while two or four scans were taken of each sample. Detailed spectra of HbFe(II)NO were taken under the same conditions as described above except for the following. A modulation amplitude of 4 G was used with an 81.92-ms time constant and a sweep width of 400 G centered at 3,288 G. Four or eight scans were taken. Both MetHb and HbFe(II)NO were quantified by double integration using WINEPR SYSTEM 2.11 (Bruker), and comparison with a calibration curve generated by measuring UV-visible standardized MetHb and HbFe(II)NO samples in the EPR instrument.
Chemiluminescent Detection of S-Nitrosohemoglobin (SNO-Hb).
RBCs were lysed with two volumes of 0.5 mM EDTA/10 mM sodium phosphate buffer, pH 7.2. Cytosol and membrane fractions were separated by centrifugation for 30 min at 16,000 × g, 4°C. SNO-Hb was detected according to Gladwin et al. (14). In short, the cytosol fraction was diluted (1:4) in a mixture of 0.2 M KCN/0.2 M K2Fe(CN)6/0.5 mM EDTA/2% Triton X-100 solution to oxidize any Fe(II)NO species. Samples were light-protected and incubated at room temperature for 30 min before purification of the Hb fraction in a Sephadex G-25 column. Purified Hb then was injected into a mixture of 50 mg of potassium iodide and one iodine crystal in 9 ml of 80% acetic acid to release NO from SNO-Hb. The released NO was detected via the chemiluminescent reaction with ozone by using a Sievers NOA 280 (Ionics Instruments, Boulder, CO).
Mathematical Modeling.
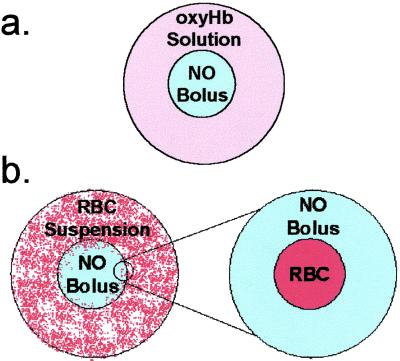
To simulate the reaction of a saturated NO bolus with cell-free Hb solutions, the bolus was assumed to be spherical in shape and surrounded by a similar sphere of Hb solution (Fig. 1a). Taking advantage of the symmetry, one fourth of the resultant geometry was modeled. A no-flux symmetry condition was imposed at the outside boundary of the Hb solution for all reactants. To simulate the RBC reaction with NO, the RBC was assumed to be spherical and surrounded by NO solution (Fig. 1b). For the RBC simulations, a permeability boundary condition was imposed at the RBC membrane (21). The reactions considered and relevant parameters are listed in Table 1. FEMLAB 2.0 (COMSOL, Los Angeles) on MATLAB 6 R12 (Mathworks, Natick, MA) was used to model the time- and space-dependent reactions of NO with RBCs and Hb. The geometry is available at http://www.seas.ucla.edu/∼liaoj/no.htm.
Figure 1.
Diffusion and reaction model of the reaction of a saturated NO bolus with a free-oxyHb solution and RBC suspension. (a) The reaction of a saturated NO bolus addition to a free-oxyHb solution was modeled as a sphere of NO solution enclosed by a sphere of oxyHb solution. (b) The addition of a saturated bolus to an RBC suspension was modeled by zooming in on a region where a single RBC is surrounded by the NO bolus. The RBC was modeled as a sphere enclosed by a sphere of saturated NO solution. For both the free-oxyHb and RBC reaction with a saturated NO bolus, the reactions considered and parameters are listed in Table 1.
Table 1.
Parameters used in the model of an NO bolus addition to cell-free Hb solution or RBC suspension
| Description | Parameter | Value |
|---|---|---|
| oxyHb–NO reaction (1) rate constant (4) | k1 | 3.0 × 107 M−1⋅s−1 |
| deoxyHb–NO reaction (2) rate constant (26) | k2 | 1.4 × 107 M−1⋅s−1 |
| MetHb–NO reaction (3) rate constant (4, 26) | k3 | 1.0 × 104 M−1⋅s−1 |
| Extracellular NO diffusivity (21) | DNO,ex | 2.6 × 10−9 m2⋅s−1 |
| Intracellular NO diffusivity (21) | DNO,in | 0.9 × 10−9 m2⋅s−1 |
| All Hb species diffusivity (27) | DHb | 7.4 × 10−11 m2⋅s−1 |
| RBC membrane permeability (21) | PRBC | 4.2 × 10−4 m⋅s−1 |
| Lipid membrane permeability (21) | Plipid | 0.9 m⋅s−1 |
| RBC effective radius (21) | a | 3.4 μm |
The RBC was assumed to have an intracellular concentration of 20 mM Hb.
Results
Products of the NO Reaction with oxyHb Depend on Local Concentrations.
In many in vitro experiments, a bolus of saturated (≈2 mM) NO solution is added to a sub-mM solution of oxyHb for a final NO concentration in physiologically relevant regimes (nM–μM). Yet, under physiological conditions the local concentration of NO generally does not exceed μM concentrations, let alone achieve mM concentrations. Given this situation, studies using bolus NO may skew conclusions applied to the physiological roles of NO because of transient and locally high NO concentrations that may lead to different reactions. To detect whether the reaction of NO with oxyHb depends on the local concentration of NO, a series of experiments was performed by using a saturated NO bolus, or DEA/NO, which was well mixed for the equal distribution of locally low concentrations of NO.
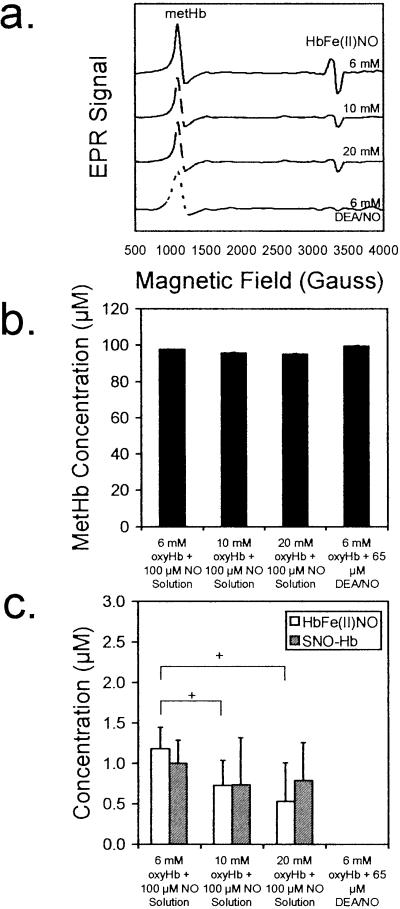
The addition of 100 μM (final concentration) NO as a saturated bolus to a series of oxyHb solutions resulted in the formation of MetHb, HbFe(II)NO, and SNO-Hb as also shown by others (12). Representative EPR spectra for the reaction of 100 μM NO solution with 6, 10, and 20 mM oxyHb are shown in Fig. 2a. In line with the classical model of NO reaction with oxyHb, MetHb was the primary product formed (Fig. 2b). HbFe(II)NO was formed also but in much smaller quantities and was attenuated by increasing the concentration of oxyHb (Fig. 2c). Addition of the NO bolus to an aerobically sealed sample in an aerobic or anaerobic environment had no effect on the HbFe(II)NO yield (data not shown). Increasing the concentration of oxyHb resulted in virtually no change in the formation of SNO-Hb (Fig. 2c). In contrast, by using 65 μM DEA/NO (each molecule of DEA/NO releases 1.5 molecules of NO) as the NO source, only MetHb was detected (Fig. 2). DEA/NO was determined not to destabilize HbFe(II)NO or SNO-Hb (data not shown). SNO-Hb was stable under our experimental conditions.
Figure 2.
The reaction of 100 μM NO with 6, 10, and 20 mM oxyHb results in the formation of primarily MetHb and smaller amounts of HbFe(II)NO and SNO-Hb when using a saturated NO solution (≈2 mM) as the NO source. Using 65 μM DEA/NO as the NO source results in MetHb formation exclusively. The concentrations of MetHb and HbFe(II)NO were measured by using EPR spectroscopy, whereas SNO-Hb was measured by using chemiluminescence. (a) A representative EPR spectrum is shown for the reaction of 100 μM NO from a saturated stock solution (final concentration) with each concentration of oxyHb (top three lines) and the reaction of 100 μM NO from 65 μM DEA/NO with 6 mM oxyHb (bottom line). (b) Regardless of the source of NO, MetHb was the primary reaction product. (c) HbFe(II)NO and SNO-Hb were formed in minor amounts. By using 65 μM DEA/NO, an NO donor that releases 1.5 mol of NO per mol of donor in the reaction with 6 mM oxyHb, only MetHb was detected. +, P < 0.05 compared with the 6 mM oxyHb reaction with 100 μM NO from a saturated stock solution (n = 8 for each set).
HbFe(II)NO Is Formed Through the Reduction of MetHb in Heterogeneous Solutions.
Although direct nitrosylation (the binding of NO to free heme) has been proposed as the primary mode of HbFe(II)NO formation under normoxic conditions (12), we propose another viable pathway for HbFe(II)NO formation after addition of NO as a bolus (Fig. 3a):
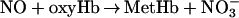 |
1 |
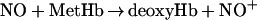 |
2 |
 |
3 |
According to this mechanism, NO first reacts with oxyHb at the boundary of the NO bolus to form MetHb. After local oxyHb is depleted, NO reduces locally available MetHb to deoxyHb and produces NO+, which is hydrolyzed to form N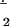 . deoxyHb can be nitrosylated to form HbFe(II)NO by binding with another NO molecule. In summary, three molecules of NO react with the same heme group successively to convert oxyHb to HbFe(II)NO. These reactions occur locally and transiently before the NO bolus is mixed completely.
. deoxyHb can be nitrosylated to form HbFe(II)NO by binding with another NO molecule. In summary, three molecules of NO react with the same heme group successively to convert oxyHb to HbFe(II)NO. These reactions occur locally and transiently before the NO bolus is mixed completely.
Figure 3.
(a) The formation of HbFe(II)NO (N) can proceed via two pathways: the reduction of MetHb (M) to deoxyHb (D), which then can be nitrosylated (binding of NO to the free heme) or direct binding of NO to existing deoxyHb. O, oxyHb. (b) Blocking the reduction of MetHb by adding 10 mM cyanide resulted in attenuation of HbFe(II)NO formation in the reaction of 100 μM saturated NO solution with 6 mM oxyHb (final concentrations) as measured by EPR. (c) No HbFe(II)NO was detected with the addition of 10 mM cyanide, while the concentration of SNO-Hb stayed relatively constant. +, P < 0.05 (n = 8).
To test this hypothesis, we added 10 mM cyanide into both the NO bolus and Hb solution to trap MetHb as it forms (22). Converting MetHb to cyano-MetHb prevents reduction to deoxyHb. As measured with EPR, 10 mM cyanide attenuated the formation of HbFe(II)NO below the detection limit (≈0.5 μM; Fig. 3 b and c). The presence of 10 mM cyanide was determined not to destabilize HbFe(II)NO under these experimental conditions (data not shown). This result directly implicates MetHb as an intermediary in the formation of HbFe(II)NO when a saturated NO bolus is used. However, even with 10 mM cyanide, the formation of SNO-Hb was evident when an NO bolus was added to an aerobic sample in an anaerobic environment (Fig. 3c), suggesting that SNO-Hb was not formed through the generation of NO+ from the reduction of MetHb. When NO solution was added in an aerobic environment, a sequential rise in SNO-Hb formation was evident (data not shown). Thus, it is possible that SNO-Hb is formed through N2O3 present in the NO stock solution. N2O3 formation after the addition of NO was not possible kinetically according to our mathematical simulation described below.
To support the mechanism further, we used a mathematical model to simulate the experimental condition. The model considers the NO bolus as a sphere dropped into an oxyHb solution (Fig. 1a). NO and all Hb species were allowed to diffuse out and into the bolus, respectively. The reactions (1–3) shown above are allowed to occur at any region according to the kinetic constants. The reaction of NO with dioxygen (k = 2.0 × 106 M−2⋅s−1) was slow, because this reaction is second order in NO and the local concentration of dioxygen was low, and whether it was included in the simulation did not alter the result.
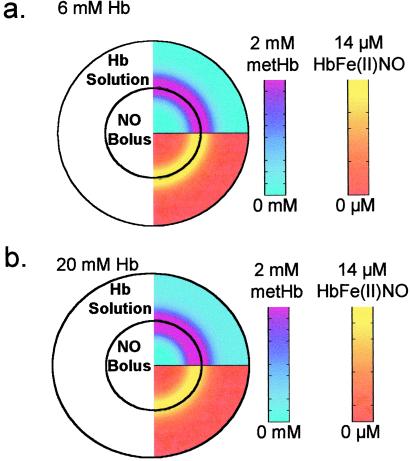
Simulating the reaction of a 2 mM NO bolus in a 6 mM free-oxyHb solution (both local concentrations) results in primarily MetHb and minor amounts of HbFe(II)NO formation (Table 2), which is consistent with our experimental observation. Further, HbFe(II)NO is formed only near the bolus where the concentration of MetHb is maximal and oxyHb and deoxyHb have been depleted (Fig. 4a). As the concentration of oxyHb solution increased, the local NO concentration at the bolus boundary decreased, as did HbFe(II)NO formation (Fig. 4b).
Table 2.
Results from the simulation of a 2 mM NO bolus with free or encapsulated Hb (Fig. 1 a and b, respectively)
| Model used | Simulation conditions | Parameters used | Concentration, μM
|
|
|---|---|---|---|---|
| MetHb | HbFe(II)NO | |||
| Bolus NO reaction with free Hb | Free Hb (6 mM) | As in Fig. 4 legend | 99.2 | 0.8 |
| Bolus NO reaction with free Hb | Free Hb (20 mM) | As in Fig. 4 legend | 99.3 | 0.7 |
| Bolus NO reaction with free Hb | Immobilized Hb (6 mM) | DHb = 0 m2⋅s−1 | 99.0 | 1.0 |
| Bolus NO reaction with RBC | RBC-encapsulated Hb* | P = 4.15 × 10−4 m⋅s−1 | 99.3 | 0.7 |
| Bolus NO reaction with RBC | RBC-encapsulated Hb† (assuming high membrane permeability) | P = 0.9 m⋅s−1 | 99.3 | 0.7 |
| Bolus NO reaction with RBC | Accelerated NO–heme adduct reaction‡ | k2 = 1.4 × 109 M−1⋅s−1 | 98.3 | 1.7 |
| Bolus NO reaction with RBC | Increased deoxyHb | [deoxyHb]0 = 2.9% | 99.0 | 1.0 |
| Bolus NO reaction with RBC Note: | NO interception by MetHb | MetHb sublayer | 98.4 | 1.6 |
Figure 4.
A mathematical model that accounts for both diffusion and reactions was used to simulate the reaction of 2 mM NO with 6 or 20 mM oxyHb (local concentrations). (a) oxyHb (6 mM). MetHb was formed rapidly near the bolus–Hb interface as shown in pink in the top half of the hemisphere. MetHb concentrations are indicated on the pink-to-blue color scale. The bottom half of the hemisphere shows that HbFe(II)NO also was formed (in yellow) near the NO–Hb interface, at which MetHb concentrations are high. HbFe(II)NO concentrations are indicated on the yellow-to-red scale. (b) Simulating the reaction of 20 mM oxyHb with 100 μM NO resulted in a similar situation as the 6 mM oxyHb reaction but with slightly attenuated HbFe(II)NO formation.
HbFe(II)NO Formation Enhanced but SNO-Hb Formation Diminished in RBC Suspensions.
Because the products of the NO reaction with cell-free Hb depend on the heterogeneity of the solution, it is possible that the reaction of a saturated NO bolus with an RBC suspension results in different products compared with the reaction with a cell-free Hb solution. To test this hypothesis, we added NO bolus from stock solutions of 2 or 0.5 mM to a 30% RBC suspension (equivalent to 6 mM heme in solution) for a final concentration of 100 μM NO. As a control, we added the same NO bolus to 6 mM cell-free Hb solution. The reaction of 100 μM NO with a 30% RBC suspension results in a greater formation of HbFe(II)NO (Fig. 5a) compared with a heme equivalent solution of cell-free oxyHb using a saturated NO solution as the NO source. Reducing the concentration of the NO solution from 2 to 0.5 mM NO had little effect on this outcome (Fig. 5a). However, when 10 mM cyanide was added to the 30% RBC suspension to block the reduction of MetHb, the formation of HbFe(II)NO was attenuated strongly (Fig. 5 a and b), suggesting the involvement of MetHb reduction in the reaction pathway. No SNO-Hb was detected in RBC samples (less than 50 nM), in contrast to the results from the free-oxyHb reaction of NO.
Figure 5.
The reaction of 100 μM NO (final concentration) with a 30% RBC suspension results in the formation of HbFe(II)NO. (a) Compared with a heme equivalent solution of oxyHb, the reaction of 100 μM NO with 30% RBCs results in greater formation of HbFe(II)NO when using a saturated NO solution (≈2 mM) as the NO source. When a stock solution of ≈0.5 mM NO was used in lieu of the saturated NO stock solution, slightly less HbFe(II)NO was formed in the RBC solution. Blocking the reduction of MetHb by adding 10 mM cyanide attenuated the formation of HbFe(II)NO. (b) A representative EPR spectra of the 100 μM saturated NO reaction with 30% RBCs with and without 10 mM cyanide. +, P < 0.05 (n = 8).
Interception of NO by MetHb May Explain HbFe(II)NO Formation in RBCs.
Because cyanide addition blocks the formation of HbFe(II)NO in both cell-free Hb solution and RBC suspensions, we suggest that the formation of HbFe(II)NO is through the same pathway. Then why does RBC-encapsulated Hb form more HbFe(II)NO than a cell-free Hb solution? As shown in Fig. 2, the reaction of a 2 mM NO bolus with 20 mM Hb, which is the intraerythrocytic Hb concentration, results in a slight decrease in HbFe(II)NO formation compared with a 6 mM Hb solution. Thus, it would be expected that if RBC-encapsulated Hb behaved similarly to cell-free Hb, HbFe(II)NO formation should be attenuated, because it is more difficult for three NO molecules to react with the same heme group in a highly concentrated Hb solution. Another explanation was needed. To make quantitative comparisons, mathematical models were used to test several scenarios. Although these mathematical models do not consider detailed mixing characteristics, they do provide insights that otherwise would be difficult to measure in an actual experiment.
RBCs are less mobile than cell-free Hb, thus an RBC suspension can be considered to be more heterogeneous than a cell-free Hb solution. This lower mobility may enhance HbFe(II)NO formation. To evaluate this scenario quantitatively, we used a model to simulate the NO bolus reaction with Hb solution with an Hb diffusivity of zero. As shown in Table 2, reduced Hb mobility increased HbFe(II)NO formation by ≈30%. However, this increase was insufficient to account for the greater than 2-fold increase in HbFe(II)NO formation in RBC suspension (Fig. 5a). We thus examined detailed differences between RBCs and cell-free Hb. The RBC reaction with an NO bolus was modeled by a single spherical RBC immersed in a locally high NO environment (near the bolus). NO was allowed to diffuse into the RBC and undergo reactions 1–3. Using the permeability calculated for the RBC previously (21), we show that HbFe(II)NO formation in this simulation was insufficient to explain the experimental data (Table 2). Increasing the membrane permeability to the value for a lipid bilayer did not result in an appreciable increase in HbFe(II)NO (Table 2).
Other scenarios in which HbFe(II)NO formation may occur include the accelerated NO–heme adduct formation (reaction 2) or increased deoxyHb content in RBCs. The former was suggested by Gow et al. (12), whereas the latter may occur because of lower oxygen affinity of Hb in the RBC because of 2,3-bisphosphoglycerate. In the RBC–NO bolus model, both scenarios enhanced the formation of HbFe(II)NO. However, HbFe(II)NO formation in these simulations cannot be abolished by MetHb trapping with cyanide, which is inconsistent with our experimental result. Thus, an alternative explanation that directly involved MetHb reduction was needed.
Previously we showed the importance of MetHb in regulating the entry of NO into the RBC (10). In that model, MetHb was proposed to be able to fill voids that may exist in the cytoskeleton to reduce NO entry. If this model is true, then it is likely that NO will encounter MetHb first as it enters the RBC. Using this idea, we modeled a shell of MetHb underneath the membrane that comprised 2% of the model radius. In this model, the enhanced formation of HbFe(II)NO through the reduction of MetHb was evident (Table 2).
Discussion
Although the mode of NO preservation in the vasculature is unclear, the RBC has been identified as being a primary participant (8–10, 13). Whether the reaction of NO with the RBC results in preservation by external or submembrane diffusion barriers, through adduction to the minor population of free-heme sites or through an undiscovered mechanism, is an area of intense research. Here we demonstrate that the products of the NO reaction with either cell-free Hb or RBC-encapsulated Hb depends on the local conditions of the reaction and suggest that inference from in vitro experiments using NO as a saturated bolus may lead to misleading conclusions for the role of NO in the vasculature.
When a bolus of saturated NO solution is used as the NO source, reactions occur under heterogeneous conditions. HbFe(II)NO formation was evident from EPR measurements and explained through mathematical modeling. More HbFe(II)NO was detected in the reaction of NO with RBC-encapsulated Hb than with cell-free Hb. Nevertheless, MetHb was the primary product when a bolus of NO was added and the exclusive product when NO was released homogeneously from DEA/NO. HbFe(II)NO formation was blocked by cyanide addition, supporting the MetHb-dependent pathway shown in reactions 1–3. The formation of HbFe(II)NO has been suggested to proceed through a cooperative effect as described by Gow et al. (12). Here, we evidenced no such effect, similar to observations by Huang et al. (16). One viable explanation for the discrepancy is that Gow et al. (12) used a low concentration of Hb, which increases the likelihood of local oxyHb depletion, which in turn increases HbFe(II)NO formation. The studies performed here and by Huang et al. (16) used higher concentrations of Hb, thus reducing the likelihood of local oxyHb depletion.
SNO-Hb was a minor product in the reaction of NO with Hb solutions. In the reactions of cell-free Hb with NO released from DEA/NO solution and the reaction of a bolus of NO solution with RBCs, SNO-Hb was undetectable. SNO-Hb formation in the bolus NO reaction with cell-free Hb was not blocked by cyanide, suggesting that it was formed from a MetHb-independent pathway. In addition, the concentration of SNO-Hb increased with each bolus addition when added in an aerobic environment, suggesting that contamination occurred, possibly through the syringe needle. Hence the most likely explanation for SNO-Hb formation is that N2O3 contaminated the NO stock solution. However, low concentrations of S-nitrosated species have been detected in vivo, suggesting that other pathways exist for S-nitrosation (14, 23).
In our hands, the reaction of NO with RBC resulted in greater formation of HbFe(II)NO compared with the reaction with cell-free Hb. Using cyanide-addition experiments, we conclude that MetHb reduction is involved in the enhanced formation of HbFe(II)NO in the RBC. To help explain why HbFe(II)NO formation is enhanced in RBC-encapsulated Hb, we used mathematical modeling to suggest possible scenarios in which the reaction of NO with Hb could result in greater HbFe(II)NO formation. From these results, we conclude that several factors in combination may be involved. (i) Because the mobility of the RBC is much lower than cell-free Hb, the RBC can be considered to be essentially stationary. Lowering the diffusivity of Hb resulted in enhanced HbFe(II)NO formation. (ii) Because the RBC possesses a diffusion barrier, whether it be external diffusion or a submembrane barrier, the consumption of NO is slowed, which allows the local concentration of NO to be sustained at higher concentrations, allowing the enhanced formation of MetHb and its eventual reduction and nitrosylation. (iii) MetHb attachment to the cytoskeleton has been observed (24). If MetHb were to block NO entry points in the cytoskeleton, then a molecule of NO would encounter MetHb before diffusing further into the RBC. Mathematical simulations suggest that the last scenario is most effective in explaining the data. In other simulations, increasing the adduct reaction rate, as suggested by Gow et al. (12), or increasing the deoxyHb content to 2% in the RBC also promotes HbFe(II)NO formation, but these mechanisms cannot be blocked by cyanide, contrary to our experimental data. Therefore, the existence of cytoskeleton-associated MetHb seems to be the most feasible explanation, although other possibilities may exist.
Recently, Huang et al. (15) showed that the reaction of a saturated NO bolus with oxyHb under normoxic conditions results in similar or greater concentrations of HbFe(II)NO compared with the reaction with RBC-encapsulated Hb. However, as the reaction products are formed in heterogeneous conditions, perturbations of these conditions such as the method of mixing, the concentration of Hb, or the manner by which NO solution is added could produce results different from those presented here.
The importance of understanding the metabolic fate and regulation of NO in the vasculature is of the utmost importance to develop therapeutic strategies based on NO biology. Here we demonstrated that under physiological conditions of low local NO, oxyHb is oxidized by NO to form MetHb. However, if the local concentration of NO were to increase to sufficiently high levels because of the induction of inducible NO synthase or from inhalation of NO under normoxic conditions, HbFe(II)NO formation may occur from the reduction of MetHb. Recently, the formation of HbFe(II)NO in concentrations observed here has been detected in blood by using NO inhalation therapy (14, 25). It is possible that some formation of HbFe(II)NO is through the reduction of MetHb, which exists in locally high concentrations either in paths of NO entry into the RBC or by newly formed through the oxidation of oxyHb near the membrane surface. These mechanisms also may be responsible for HbFe(II)NO formed because of high nitrosative stress.
Acknowledgments
We acknowledge Dr. Robert Taylor of the University of California (Los Angeles) Chemistry Department for his technical assistance with the EPR measurements. This work was funded by National Institutes of Health Grant R01 HL65741.
Abbreviations
- RBC
red blood cell
- HbFe(II)NO
nitrosylhemoglobin
- EPR
electron paramagnetic resonance
- DEA/NO
2-(N,N-diethylamino)diazenolate 2-oxide
- SNO-Hb
S-nitrosohemoglobin
Note Added in Proof.
Zhang and Hogg (28) have demonstrated artifacts from the addition of an NO bolus to myoglobulin solutions, which is relevant to the data presented here.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ignarro L J, Byrns R E, Buga G M, Wood K S. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 2.Palmer R M, Ferrige A G, Moncada S. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.Doyle M P, Hoekstra J W. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 4.Eich R F, Li T, Lemon D D, Doherty D H, Curry S R, Aitken J F, Mathews A J, Johnson K A, Smith R D, Phillips G N, Jr, Olson J S. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 5.Herold S, Exner M, Nauser T. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 6.Liao J C, Hein T W, Vaughn M W, Huang K T, Kuo L. Proc Natl Acad Sci USA. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster J R., Jr Proc Natl Acad Sci USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Miller M J, Joshi M S, Sadowska-Krowicka H, Clark D A, Lancaster J R., Jr J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 9.Vaughn M W, Huang K T, Kuo L, Liao J C. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 10.Huang K T, Han T H, Hyduke D R, Vaughn M W, Van Herle H, Hein T W, Zhang C, Kuo L, Liao J C. Proc Natl Acad Sci USA. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gow A J, Stamler J S. Nature (London) 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 12.Gow A J, Luchsinger B P, Pawloski J R, Singel D J, Stamler J S. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawloski J R, Hess D T, Stamler J S. Nature (London) 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 14.Gladwin M T, Ognibene F P, Pannell L K, Nichols J S, Pease-Fye M E, Shelhamer J H, Schechter A N. Proc Natl Acad Sci USA. 2000;97:9943–9948. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Hadimani S B, Rupon J W, Ballas S K, Kim-Shapiro D B, King S B. Biochemistry. 2002;41:2466–2474. doi: 10.1021/bi011470o. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Louderback J G, Goyal M, Azizi F, King S B, Kim-Shapiro D B. Biochim Biophys Acta. 2001;1568:252–260. doi: 10.1016/s0304-4165(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 17.Gladwin M T, Ognibene F P, Shelhamer J H, Pease-Fye M E, Noguchi C T, Rodgers G P, Schechter A N. Free Radical Res. 2001;35:175–180. doi: 10.1080/10715760100300721. [DOI] [PubMed] [Google Scholar]
- 18. Joshi, M. S., Ferguson, T. B., Jr., Han, T. H., Hyduke, D. R., Liao, J. C., Rassaf, T., Bryan, N., Feelisch, M. & Lancaster, J. R., Jr. (2002) Proc. Natl. Acad. Sci. USA99, in press. [DOI] [PMC free article] [PubMed]
- 19.Beckman J S, Wink D A, Crow J P. In: Methods in Nitric Oxide Research. Stamler J S, editor. New York: Wiley; 1996. pp. 61–70. [Google Scholar]
- 20.Keefer L K, Nims R W, Davies K M, Wink D A. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 21.Vaughn M W, Huang K T, Kuo L, Liao J C. Nitric Oxide. 2001;5:18–31. doi: 10.1006/niox.2000.0328. [DOI] [PubMed] [Google Scholar]
- 22.Ignarro L J, Fukuto J M, Griscavage J M, Rogers N E, Byrns R E. Proc Natl Acad Sci USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marley R, Patel R P, Orie N, Ceaser E, Darley-Usmar V, Moore K. Free Radical Biol Med. 2001;31:688–696. doi: 10.1016/s0891-5849(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 24.Giardina B, Scatena R, Clementi M E, Ramacci M T, Maccari F, Cerroni L, Condo S G. Adv Exp Med Biol. 1991;307:75–84. doi: 10.1007/978-1-4684-5985-2_7. [DOI] [PubMed] [Google Scholar]
- 25.Cannon R O, 3rd, Schechter A N, Panza J A, Ognibene F P, Pease-Fye M E, Waclawiw M A, Shelhamer J H, Gladwin M T. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharitonov V G, Bonaventura J, Sharma V S. In: Methods in Nitric Oxide Research. Stamler J S, editor. New York: Wiley; 1996. pp. 39–45. [Google Scholar]
- 27.Kreuzer F. Respir Physiol. 1970;9:1–30. doi: 10.1016/0034-5687(70)90002-2. [DOI] [PubMed] [Google Scholar]
- 28. Zhang, Y. & Hogg, N. (2002) Free Radical Biol. Med., in press.