Abstract
Endothelial cells (ECs) line the mammalian vascular system and respond to the hemodynamic stimulus of fluid shear stress, the frictional force produced by blood flow. When ECs are exposed to shear stress, one of the fastest responses is an increase of K+ conductance, which suggests that ion channels are involved in the early shear stress response. Here we show that an applied shear stress induces a K+ ion current in cells expressing the endothelial Kir2.1 channel. This ion current shares the properties of the shear-induced current found in ECs. In addition, the shear current induction can be specifically prevented by tyrosine kinase inhibition. Our findings identify the Kir2.1 channel as an early component of the endothelial shear response mechanism.
The response of endothelial cells (ECs) to their hemodynamic environment is crucial for the regulation of vascular tone and health, and is directly implicated in the formation of atherosclerotic lesions in large vessels. Endothelial responses to shear stress include changes in morphology (1), gene regulation (2), ion currents (3–6), cellular messenger systems (6, 7), and release of vascular factors (8, 9), but the mechanism by which ECs sense shear is not yet understood.
A variety of ionic conductances are observed in electrophysiological studies of arterial ECs (10–14). The major endothelial voltage-gated current has properties typical of a K+ inward rectifier current (13, 14): it is strongly inward rectifying with a reversal potential near the K+ equilibrium potential, its amplitude is a function of the extracellular K+ concentration, and it is blocked by extracellular Ba2+. Initiation of laminar shear stress (LSS) on ECs rapidly results in a K+ current (3, 4), which has inward rectifying properties, including block by Ba2+. This shear-induced endothelial current is rapidly activated, its amplitude varies with the magnitude of the imposed shear stress and remains constant throughout an applied steady shear, and it is fully reversible on cessation of flow. At hyperpolarized potentials the inward currents are large, but at depolarized voltages the outward currents are relatively small. The molecular nature of this shear-induced current is unknown.
We cloned a Kir2.1 K+ ion channel from bovine aortic ECs (11). Expression of this Kir2.1 clone in Xenopus laevis oocytes resulted in an inward rectifying K+ current (IK) that has properties similar to the major voltage-gated current present in large-vessel ECs (13). The similarity of the cloned Kir2.1 currents to EC shear-stress currents suggests that Kir2.1 may play a role in endothelial shear-stress response. Further support for this hypothesis is given by work showing that the Kir2.1 gene is necessary for endothelial vascular responses in the cerebral artery (15). To test this hypothesis, we examined Kir2.1 currents for response to shear.
Materials and Methods
Flow Apparatus.
The experimental apparatus used to deliver the fluid-shearing flow was designed to ensure laminar flow across the oocyte surface. The pipette (1.5 mm i.d.) was such that the inner diameter of the exit was 1.5 times the diameter of the oocyte (1.0 mm), and the position of the pipette tip was maintained to be within 0.5–1.0 diameter from the oocyte to minimize the divergence of the flow before it reached the oocyte. Placement of a pipette (1.5 mm i.d.) at a distance of less than one pipette-opening diameter away from the cell produces exposure to laminar flow (16). The Reynolds number Re was determined to be between 14 and 80, which is well within the required range for laminar flow of submerged jets (16), with the relation Re = (θϖD)/λ (where θ = fluid density, ϖ = average freestream velocity, D = sphere diameter, and λ = viscosity of water). Visual observations verified that the flow field was smooth and encompassed the oocyte. Flow was gravity driven, and the flow rate was varied by adjustment of hydrostatic pressure. A computer controlled the flow-switch valve during voltage-clamp protocols. The approximate average shear stress on the oocyte surface because of flow was calculated on the basis of the velocity profiles for a laminar flow field around a sphere (17). For Reynolds numbers Re in the range of 14–80, the coefficient of drag on a sphere is Cd ≈ 1. The relationship used to calculate LSS is Fdrag = 1/2ρAϖ2Cd (where A = cross-sectional area of sphere and ρ = water density). The LSS = (Fdrag/surface area of oocyte).
Molecular Biology and Cell Culture.
Kir2.1 cDNA (11) and all mutant constructions were transcribed for in vitro injection experiments. B4H cells were prepared as stable transfectants of HEK-293 cells by using Lipofectamine and pcDNA3.1zeo(+) with the Kir2.1 clone in the NotI–HindIII sites. Kir2.1 deletion mutation Δ382 was prepared by insertion of a termination codon at residue 382 and removal of the downstream 47 codons 383–427. Kir2.1 deletion mutant Δ402 was made by insertion of a termination codon at residue 402 and deletion of codons for residues 403–427. Kir2.1 was digested with PflmI and BsaBI; the fragment was isolated and cloned, and site-directed mutagenesis was performed to mutate Y242 to an F residue. A BstEII and NheI digestion fragment of Kir2.1 was used for site-directed mutation of Y366 to an F. Mutated fragments were sequenced and reinserted into the appropriate restriction-digested Kir2.1. All mutations were confirmed by DNA sequencing. Site-directed mutations of Kir2.1 were made by using QuickChange Site Directed Mutagenesis (Stratagene).
Voltage Clamping.
cDNA clones were transcribed in vitro; the capped RNA was injected into defolliculated Xenopus laevis stage V–VI oocytes. Oocytes were injected with either Kir2.1 cRNA (27 ng) or Kv1.1 cRNA (4 ng). They were two-microelectrode voltage clamped after 2 days. Electrophysiology recordings were made by using a Dagan TEV200 and analyzed with PCLAMP software (18). Electrodes were 1–3 MΩ. IKS amplitudes are reported as the averages ± SEM. Extracellular bath solution was ND38, composed of 38 mM NaCl, 60 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes (pH 7.5), and 0.3 mM niflumic acid. Where necessary, KCl was substituted for NaCl to maintain osmolarity of solutions containing different K+ concentrations. The bath solution was constantly exchanged, and shear stress was applied by using the apparatus shown (Fig. 1A). Membrane capacitance was determined (18). Oocytes were incubated overnight in normal buffer with one of the following added to the buffer before clamping (18): 1 mM gadolinium (19), 1 mM amiloride (20), 0.5 mM H-7, 0.5 mM dibutyryl-cAMP, or 0.9 mM dibutyryl-cGMP. Ca2+ levels were reduced (21). Neomycin was used at 5 mM (22). Genistein and diadiazein were injected to a final cytoplasmic concentration of 75 μM (23). Phenylarsine oxide was used at 10 μM (24). B4H cells were cultivated (25) with zeocin (0.2 mg/ml). For patch-clamp electrophysiology (26), cells were plated on 35-mm Petri dishes (precoated with poly-D-lysine) at a density of ≈104 cells per dish. Cells were suitable for recordings for 2–3 days after plating. The pipettes had resistances <2 MΩ, and approximately 75% of the access resistance (range, 3–4 MΩ) was canceled electronically. Extracellular solutions were composed of 5 mM NaCl, 150 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose, and 5 mM Hepes, pH 7.4. Internal solutions for filling pipettes were composed of 134 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM EGTA, and 10 mM Hepes, pH 7.4.
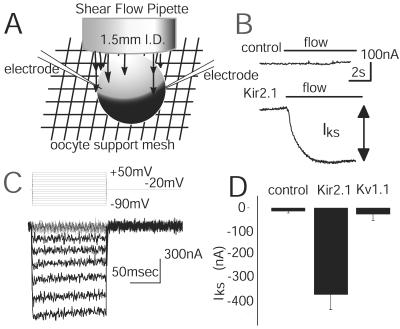
Figure 1.
LSS induces currents in oocytes expressing the Kir2.1 channel. (A) Experimental system showing an oocyte with two microelectrodes and the flow pipette in position to apply the LSS. (B) Upper trace, current recorded from a control oocyte, not expressing Kir2.1, when a steady flow is applied, holding potential −40 mV; lower trace, current from an oocyte expressing Kir2.1. The bar indicates the onset and duration of the flow. (C) The shear-induced current is visualized by subtracting the current traces recorded during flow from traces recorded in no-flow conditions. The oocyte was held at −20 mV with pulses from +50 to −90 mV in 10-mV steps. The difference current shown here is for 8.6 dynes/cm2 shear force. (D) The average amplitudes of IKS caused by an applied LSS of 8.6 dynes/cm2 at −40 mV in a bath of ND38 for control oocytes (n = 6), oocytes expressing the Kv1.1 channel (n = 7), and oocytes expressing the Kir2.1 channel (n = 8).
Data are shown as mean ± SEM. Statistical significance was calculated with ANOVA followed by Dunnett's test or Student's t test where appropriate. P < 0.05 was considered statistically significant.
Results
Expression of a Shear-Sensitive Current in Oocytes Expressing Kir2.1.
Voltage-clamped Xenopus oocytes were exposed to shear stress by laminar fluid flow (16) from a vertical pipette (17) (Fig. 1A). A voltage-clamped control cell displays very little current change in response to the onset of flow (Fig. 1B). However, for an oocyte expressing the Kir2.1 channel, and held at −40 mV, the onset of flow increased the inward current (Fig. 1B). The difference between current amplitude observed during zero-flow conditions (IK) and current amplitude generated during applied flow is called IKS (K+ shear current). IKS amplitude was greatest at hyperpolarizing membrane potentials (Fig. 1C). If the flow was introduced elsewhere into the chamber (i.e., not directly on the cell), then no shear response was observed (data not shown). The solutions in the bath and perfusate were identical, bath level did not alter the shear response, pipette movement did not give rise to IKS, and high extracellular K+ concentration precluded a K+-washout effect.
A group of oocytes expressing Kir2.1 (−4,361 ± 659 nA at −40 mV, n = 8) exhibit IKS (−370 ± 42 nA) (Fig. 1D). Oocytes isolated from the same donors, but not injected with Kir2.1 RNA transcript, displayed very little shear-induced current (−14 ± 7.2 nA, n = 6). To determine whether oocyte expression of another K+ channel results in currents similar to IKS, a mouse Kv1.1 clone (18) was tested. Oocytes expressing Kv1.1 (−5,498 ± 150 nA, n = 8) were tested for shear-induced current at −40 mV, but only small currents (similar to controls) were observed (−28 ± 25 nA, n = 8). Because the Kv1.1 channel is active at depolarizing potentials, measurements were also made at +20 mV, where this channel carries large outward currents (4,529 ± 740 nA, n = 4); still very little IKS-like current was observed (18 ± 28 nA, n = 4). We conclude that IKS observed when cells expressing Kir2.1 are exposed to LSS is not a generic feature of K+ channels.
Properties of the Shear-Induced Current.
The characteristics of the shear-induced current of Kir2.1 are displayed in Fig. 2. The IKS/voltage (I/V) relation (Fig. 2A) exhibits large inward currents at potentials more negative than the K+ reversal potential (Erev = −30 mV), and smaller outward currents when the applied voltage is elevated above the reversal potential. These properties were also reported for the I/V relation of the shear-induced current in ECs (3). The voltage dependence of IKS varies with external K+ concentration (data not shown). Ionic selectivity of IKS was determined by measuring the reversal potential at different extracellular K+ concentrations. The reversal potential shifts 55 mV per 10-fold change in [K+]o, as predicted by the Nernst equation, which indicates IKS is carried predominately by K+ ions. The possibility that chloride conductance contributes to IKS was tested by using the chloride channel inhibitors niflumic acid (0.3 mM) and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (1 mM); they had no effect (data not shown).
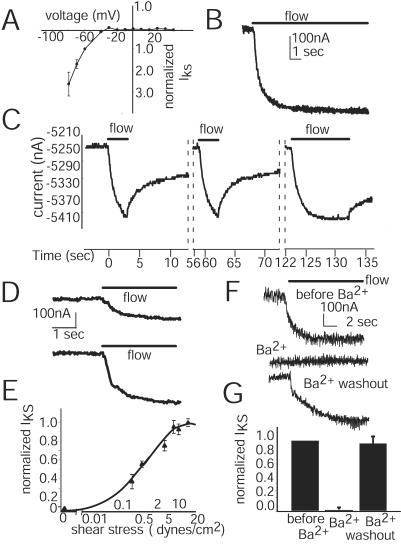
Figure 2.
IKS properties. (A) The IKS I/V relation for oocytes expressing Kir2.1 (external K+ = 60 mM) at a LSS of 8.6 dynes/cm2. Cells were held at −40 mV, and voltage pulses were 10-mV steps ranging from +50 to −90 mV for 100-msec duration. Currents shown are normalized (n = 9). (B) IKS attains a steady amplitude during long (12 sec) applied flows of 8.6 dynes/cm2 when cells are held at −40 mV. (C) Recovery from repeated shear applications. The flow (8.6 dynes/cm2) was applied at time 0 as indicated by the bar. Total cell current is shown. The oocyte was continuously clamped (−40 mV) through the experiment. Currents are not shown during the long recovery times between the dotted lines. (D) IKS induced by LSS of 3.7 dynes/cm2 (top trace) and 8.6 dynes/cm2 (lower trace). Both flows were applied for 8 sec, and the oocyte recovered original IK amplitude between flow applications. Cells were held at −40 mV. (E) Relation of IKS amplitude to the LSS magnitude. IKS amplitudes were normalized to the maximum shear response of each oocyte. Each oocyte (n = 6) was exposed to the entire range of stress, and response for each shear was averaged (bars = ±SEM) with holding potential of −40 mV. (F) Reversible inhibition of IKS by extracellular Ba2+. IKS currents induced in Kir2.1-injected oocytes by an applied LSS of 14.8 dynes/cm2 at a voltage of −40 mV in ND38. Current traces for a typical Kir2.1-injected oocyte are shown in the top trace. For the same oocyte, addition of 1 mM Ba2+ to the bath solution completely eliminates IKS when flow is applied again, but IKS recovers after removal of Ba2+ from bath solution. (G) Average amplitude of IKS for Kir2.1-injected oocytes (n = 7) in response to flow, after addition of 1 mM Ba2+, and after removal of Ba2+. Conditions were as in F.
The endothelial IKS varies with time and shear force magnitudes. Flows of a duration longer than 12 sec result in steady levels of current induction (Fig. 2B). Short-duration flow (<2 sec) is not long enough to achieve a steady current level even though IKS onset occurs rapidly (Fig. 2C). Flow cessation results in decrease of IKS, but recovery to preshear current levels is much slower than the rate of induction (Fig. 2C). Successive shear applications result in IKS responses with magnitudes and activation kinetics similar to the initial response (Fig. 2C). Thus IKS in oocytes does not display flow desensitization. Recovery time to preflow current levels was found to vary (1–5 min) with oocyte donors. To determine the dependence of IKS on shear magnitude, various force levels were applied. Current amplitude was larger when the shear magnitude was increased (Fig. 2D). IKS maximum was between 10 and 28.5 dynes/cm2 (Fig. 2E). LSS required to achieve 50% of maximum IKS was 0.7 dynes/cm2, which is similar to LSS at the half-activation observed (3) in ECs, which is 0.75 dynes/cm2. The sensitivity of IKS to Ba2+ was tested; Ba2+ is a widely used inhibitor of inward rectifiers. A typical IKS (Fig. 2F, top trace) was inhibited by addition of Ba2+ to the bath (Fig. 2F, middle trace), and restored on Ba2+ removal (Fig. 2F, bottom trace). The average amplitude of IKS for oocytes subjected to a LSS of 8.6 dynes/cm2 was −272 ± 70 nA (Fig. 2G). After addition of Ba2+ to the bath solution, the amplitude of IKS was reduced to 0 (Fig. 2G), and when the Ba2+ was washed out, the amplitude rapidly recovered to 96 ± 7% of the original IKS (−232 ± 60 nA, n = 7). The sensitivity of IKS to Ba2+ is consistent with the premise that IKS arises from the Kir2.1 channel and is not caused by another phenomenon such as increased nonspecific current leakage caused by flow. In addition, IKS displays the same inhibition by Ba2+ as reported for EC IKS (3, 4).
The rate of IKS activation by shear was determined by measuring τact (time from start of flow to half-maximal IKS) at LSS of 1.2–14.8 dynes/cm2. The value of τact at a LSS of 3.7 dynes/cm2 (407 ± 45 ms, n = 9) was similar to the value at 8.57 dynes/cm2 (390 ± 91 ms, n = 5) or 14.8 dynes/cm2 (335 ± 66 ms, n = 5). At 1.2 dynes/cm2, τact was larger (720 ± 75 ms, n = 4), which is probably because the perfusion system equilibrates more slowly at low shear magnitudes (data not shown). The value of τact does not seem to vary with the magnitude of LSS. The rapid rate at which IKS is induced by flow is similar to the rapid rate at which EC IKS is induced (3, 4).
In summary, the IKS found in oocytes expressing Kir2.1 shares the characteristics of ion selectivity, I/V, desensitization, Ba2+ sensitivity, kinetics, and response to shear-force magnitude with those reported for the shear currents in bovine aortic ECs.
Expression of the IKS in HEK-293 Cells Transfected with Kir2.1.
To verify that the shear responsiveness of Kir2.1 is not limited to oocytes, we expressed the channel in human 293 cells. A stable transfectant of HEK-293 cells with Kir2.1, B4H, was found to express currents with waveforms (Fig. 3A) and I/V properties (Fig. 3B) consistent with those of Kir2.1. Cells were exposed to fluid flow delivered from a 1.5-mm-i.d. pipette placed at a 45° angle to the substrate. The bottom of the pipette was <1 mm from the cell, and the flow rate was 5.7 ml/min, so the flow was laminar at the pipette tip. This configuration produces a shearing flow over the cell, but does not ensure that the flow is laminar. In addition, the cell is so small (≈0.05–0.1 mm diameter) that it is not possible to position the pipette relative to the cell sufficiently accurately to estimate the fluid velocity at the cell and therefore the shear stress on the cell, which can be crudely estimated to be between 3 and 7 dynes/cm2.
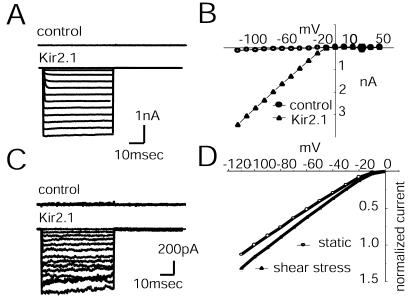
Figure 3.
IKS induced in HEK-293 cells. (A) B4H cells are a stable transfectant of the endothelial Kir2.1 clone into HEK-293 cells. Cell currents were recorded for potentials ranging from +50 to −120 mV. The upper traces labeled control are recorded from cells transfected with only the vector. The lower traces are recorded from Kir2.1-transfected cells. (B) I/V relation shows the inward rectification at hyperpolarizing potentials in B4H cells transfected with either vector (control) or Kir2.1 DNA. (C) The difference currents obtained by subtracting the total currents of cells subject to flow (2 dynes/cm2) from the currents under stationary conditions. The top trace is from a control HEK cell transfected with vector only; the lower traces are from a Kir2.1-transfected cell, B4H. (D) The I/V relation from B4H cells recorded with and without LSS (n = 7). The I/V is shown as normalized currents.
B4H cells exposed to this flow responded with an inward current (Fig. 3C, Kir2.1). Applying the same flow to cells transfected only with vector did not result in any IKS (Fig. 3 A and C, control). The average response was a current increase of 18% ± 3% (n = 7) at −100 mV (Fig. 3D). Results from previous work with bovine aortic ECs (4), which were subject to applied LSS of 5 dynes/cm2 in a flow chamber, showed a 40% current increase at −140 mV. Thus, the B4H clone displays an IKS of the same order of magnitude as found in the bovine aortic ECs. The difference in IKS is certainly affected by the differences in experimental methods and may also be caused by differences in cell geometry and B4H cellular components.
Shear-Induced Current Mechanisms.
To explore the mechanism and determine whether IKS is influenced by second messenger systems known to respond to LSS in ECs, IKS was measured in oocytes exposed to reagents that activate or inhibit cellular components (Fig. 4). The cell capacitance was measured to determine whether LSS induces oocyte membrane changes. The membrane capacitance of oocytes exposed to LSS (8.5 dynes/cm2) was normalized to membrane capacitance during nonshear conditions and was found to be equivalent (100% ± 2%, n = 10). Therefore, the cell membrane surface area is not appreciably altered by LSS.
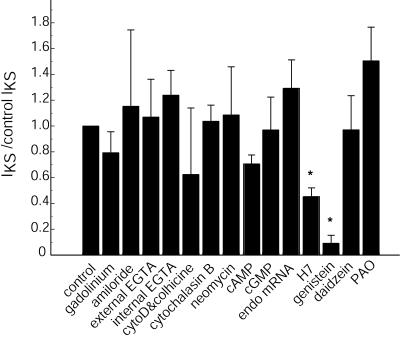
Figure 4.
Effects of pharmacological reagents on IKS. Results are normalized to the IKS of control oocytes expressing Kir2.1 and exposed to LSS of 8.6 dynes/cm2. The controls for each experimental condition were from the same donor and were measured under identical conditions (see Materials and Methods). Oocytes exposed to gadolinium or amiloride, inhibitors of stretch-activated channels, still display IKS current. To reduce or eliminate any Ca2+ elevation caused by shear, EGTA was added to the perfusion solution and injected into oocytes, but without effect. Microtubule or microfilament involvement was tested by cytochalasin D, cytochalasin B, or colchicine. An inhibitor of inositol phosphate release, neomycin, had no significant effect on IKS. To determine whether some unknown factor was encoded in endothelial mRNA, mRNA (20 ng) was coinjected into oocytes, but this coinjection did not significantly alter IKS amplitude. Activation of protein kinases A and G did not alter IKS amplitude. Inhibition of protein kinase by H7 did result in a reduction of IKS amplitude. Phosphotyrosine kinase (PYK) inhibition by genistein significantly reduced IKS amplitude when compared with the inactive analog daidzein (P < 0.05). The tyrosine phosphatase inhibitor phenylarsine oxide (PAO) did not inhibit IKS. Each bar represents the average ratio, with error bar SEM (n = 6–20). Asterisks indicate a significant difference between the control and experimental IKS amplitude (*, P < 0.05).
Gadinolium and amiloride inhibit mechanosensitive ion channels (19, 20). Their failure to alter IKS (Fig. 4) indicates that these types of mechanosensitive channels are not involved. The Kir3.1 K+ channel has been reported to display mechanosensitivity (27) because of membrane stretch. To determine whether membrane stretch was involved in the LSS response, IKS was measured in oocytes exposed to hypoosmotic stress. IKS in normal osmotic conditions (−411 ± 91 nA, n = 5) was similar to IKS under hypoosmotic conditions (−389 ± 85 nA, n = 5). The Kir2.1 IK at −70 mV was also measured under normal osmotic conditions (−3,297 ± 1,282 nA, n = 5) and was found to be similar to the current under hypoosmotic conditions (−3,420 ± 417 nA, n = 5). Therefore, membrane stretch induced by hypoosmotic stress did not alter IKS or IK. The lack of effect of osmotic stress on the Kir2.1 IK current has been reported (27).
Cellular Ca2+ levels increase during shear and alter EC IKS (12–14). Removal of intra- and extracellular Ca2+ by internal and external application of EGTA, respectively, had no significant impact on IKS.
Cytoskeletal components may be involved in mechanosensitive channels and shear responses (19). Kir2.1 channels have been found to link to the cytoskeleton (28). To determine whether the cytoskeleton acts as a transducer of force, IKS was measured in cells exposed to various inhibitors. Neither the microfilament inhibitor cytochalasin B nor the more potent cytochalasin D had an effect on IKS (Fig. 4). To test further the possible role of cytoskeletal protein interaction with Kir2.1, deletion mutants (Δ382 and Δ402) were tested and found to express IKS. These Kir2.1 mutations lack the postsynaptic density protein, disc-large, zonulin-1 (PDZ) domain shown to interact with the postsynaptic density protein/synapse-associated protein (PSD/SAP) family (28–30). The microtubule inhibitor colchicine had no effect.
Shear has been reported to alter phospholipase C and inositol phosphate levels (12, 22). However, neomycin, an inhibitor of both inositol phospholipid turnover and phospholipase C, did not result in any large changes in the shear response (Fig. 4).
LSS activates a variety of EC protein kinases (7). Kir2.1 contains a potential protein kinase A (PKA) phosphorylation site; thus, PKA activity may change IKS. PKA activation (by cAMP) reduced IKS to 71%, but protein kinase G stimulation (by cGMP) did not greatly alter IKS (Fig. 4). Conversely, the general kinase inhibitor H7 did result in a reduction of IKS to 45% of that of the controls (Fig. 4). If reducing the level of kinase activity reduces IKS, then removal of the PKA phosphorylation site on the channel would affect IKS. We tested a truncated deletion mutant of Kir2.1, Δ382, lacking the PKA phosphorylation site, but found the shear response intact (data not shown). If the effects of kinase activity on IKS are due to actions on other cell components, then the addition of endothelial mRNA encoding such components may alter IKS. This idea was tested by coexpressing endothelial mRNA with Kir2.1 mRNA. Coexpression resulted in higher IKS levels (129% ± 22%) (Fig. 4), which could be consistent with the mRNA enhancing the shear response. The observed IKS elevation was not caused by more Kir2.1 channel being expressed from EC mRNA, because the channel expression level from the EC mRNA was extremely low. Therefore, other components may be involved in IKS. To determine whether phosphotyrosine kinase (PYK) activity is required for IKS induction, the kinase inhibitor genistein was injected, which produced a reduction (91% ± 6%) of IKS (Fig. 4). The inactive analog of genistein, daidzein (Fig. 4), did not inhibit IKS (97% ± 25%). Because PYK activity may be required for IKS, tyrosine phosphatase activity should also have an effect on IKS. The tyrosine phosphatase inhibitor phenylarsine oxide was used to treat cells before measuring IKS. The phosphatase inhibitor treatment resulted in an increase in IKS (151% ± 26%) (Fig. 4). This result suggests that tyrosine phosphorylation may be necessary to generate IKS. In summary, inhibition or activation of second messenger systems did not result in significant (P < 0.05) changes in the IKS amplitude; only kinase inhibition with H7 or genistein produced significant IKS inhibition.
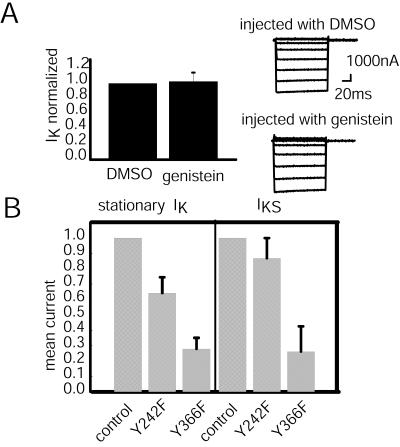
To determine whether genistein also inhibits the Kir2.1 inward stationary current, we examined the current expression in control (DMSO-injected) and genistein-injected cells (Fig. 5A). The currents found in the DMSO and genistein cells were indistinguishable (1.0 vs. 1.02) (Fig. 5A Right), which indicates that this amount of genistein does not inhibit the Kir2.1 inward stationary current, IK. Therefore, genistein can selectively separate IKS from the stationary IK by specific inhibition of IKS.
Figure 5.
Effects of PYK inhibition and phosphorylation site mutations on Kir2.1 currents. (A) Oocytes expressing Kir2.1 recorded at −60 mV. The amplitude of IK from DMSO-injected oocytes (1.0, n = 6) was used to normalize the currents from genistein-injected oocytes (1.02 ± 0.12, n = 8). The current traces on the right are representative voltage-clamp traces of oocytes expressing Kir2.1 currents, which were injected with DMSO or genistein and were held at −40 mV and clamped from +50 mV to −100 mV. (B) Mutations of the Kir2.1 PYK phosphorylation sites were made for Y242F or Y366F. The means of stationary IK are compared for mutant Kir2.1 channels and normal channels. Current amplitudes were recorded at −60 mV for LSS of 8.6 dynes/cm2 and were normalized by the average current amplitudes of controls (n = 8).
Tyrosine Phosphorylation Site Mutations.
Although these experiments clearly indicate that PYK activity is necessary for IKS induction, they do not identify the target of kinase activity. Two possibilities exist. First, the simplest mechanism would be a shear-activated kinase that directly phosphorylates the channel. Second, the kinase could act on some other protein(s), which then interacts with the channel, enabling the LSS response. To determine which mechanism is used, we mutated the two canonical PYK sites in the Kir2.1 channel at Y242 and Y366. Both mutants resulted in reduced levels of stationary current as compared with the control (Fig. 5B). At −60 mV the ratio of the Y242F current to that of control was 0.64 ± 0.1 (n = 6), and for Y366F the ratio was 0.28 ± 0.07 (n = 8). Shear induction of Y242F was very similar to control levels (0.87 ± 0.13, n = 6); so the PYK site Y242 is not necessary for IKS response. Shear-induced current from mutant Y366F only expressed 0.26 ± 0.16 (n = 6) of control levels, but Y366F also expresses a reduced amount of IK. Therefore, the PYK site Y366 influences both stationary and IKS, but the Y366F mutant still exhibits IKS. Taken as a whole, the data do not prove that a PYK is at an earlier step in LSS signal transduction, but they do show that the channel requires kinase activity for shear response. Furthermore, mutation of PYK channel sites failed to prevent IKS expression. Therefore, the required kinase activity could be acting on an unrecognized site on the channel or on another protein.
Discussion
In this report we show that the endothelial K+ ion channel Kir2.1 responds to LSS. The properties of the shear-induced Kir2.1 current are similar to the observed EC shear-induced current. The shear response of Kir2.1 is consistent with endothelial sensing of hemodynamic LSS. Physiologically, ECs undergo membrane hyperpolarization in response to LSS (31). The hyperpolarization precedes release of vascular factors such as NO and endothelin. Membrane potential changes also influence the level of cytosolic Ca2+ (6) and subsequent gene expression (32). Extracellular K+ has also been suggested as being important for vasodilatation (33). Elevation of external K+ would shift the inward rectifier properties to increase the Kir2.1 shear response. The loss of Kir2.1 resulted in loss of K+-mediated vasodilatation (15). The Kir2.1 channel is therefore a good candidate for being an early and necessary element in the endothelial LSS response.
Our results identify the molecular identity of a LSS-activated K+ channel. The results also suggest a mechanism for LSS-induced regulation. PYK activity is required for the channel to be LSS sensitive. IKS activation is prevented by PYK inhibition but does not change the regular Kir2.1 IK. Therefore, the LSS-induced current can be completely separated from the regular Kir2.1 current. Results with Kir2.1 mutations indicate PYK does not act on known phosphorylation sites. PYK may act on other channel sites or another protein. Such a hypothesized accessory protein could then interact with the Kir2.1 channel to facilitate the response to shear. The rapid response to shear implies that any accessory protein would need to be in close proximity to the channel protein and kinase. Tyrosine phosphatase activity would dephosphorylate the kinase site on the channel or accessory protein, thereby returning the channel to a shear-unresponsive state. Phosphatase inhibition would keep the channel in a shear-responsive state. The identities of other sites or potential accessory proteins are unknown, but Kir2.1 channels are known to interact with cytoskeleton proteins and to undergo regulation by kinases.
Previous work has shown that Kir2.1 is regulated by phosphorylation. Specifically, Kir2.1 can be modulated by PKA (34, 35), protein kinase C (34, 36), receptor-activated PYKs (24, 37, 38), or phosphatases (24, 37, 38). The site of PYK modulation was proposed to be Y242 (38). Recently, Tong et al. (24) also found this site to be important for PYK regulation of the Kir2.1 channel and involved in down-regulation by way of internalization. They also concluded that PYK activity is not acting directly on the channel. The internalization mechanism proposed (24) is most likely different, however, because we observed no oocyte capacitance changes and no decrease in current levels, and the time scales were different. Tyrosine phosphatase inhibitors reduce the Kir2.1 currents in RBL and tsa cells (38). However, the results reported here and in earlier studies (24, 38) do not indicate any inhibition of Kir2.1 stationary currents in oocytes by phosphatase inhibitors. Also, we did not observe genistein inhibition of IK, perhaps because phosphorylation activity levels differ between cell types. Recent work in signal transduction of LSS has focused on PYK activity. We found that genistein inhibits the LSS response just as LSS-induced kinases are inhibited in ECs (39). This work establishes a link between LSS signal transduction by kinase activation and by ion channels.
Inward rectifier function and localization are altered by nonchannel proteins. KATP channels are functionally altered by actin binding in myocytes (40). But IKS was not altered by actin disruption. G protein-coupled inward rectifier channels are bound and modified by integrins (41). Inward rectifiers also modify neuroblastoma cell integrin signaling (42). Endothelial integrins are involved in LSS signal transduction (39). Integrin PYK activity could be involved in LSS regulation of Kir2.1, but Kir2.1 lacks the integrin protein interaction motif of RGD. In neurons, the chapsyns or MAGUK protein family bind and modulate Kir channels (28–30). However, the conserved postsynaptic density protein, disc-large, zonulin-1 (PDZ)-binding domain recognition sequence of Kir2.1 can be excluded from LSS involvement on the basis of our studies of deletion mutant expression. In nonneuronal cells, the scaffolding proteins AKAP79 (43) and SAP97 (44) associate with Kir2.1. These associations are also controlled by PKA activity. PYKs also associate with SAP97 (45). The proposed accessory protein could be a scaffolding-type protein that also exhibits regulation by protein kinases and organizes channels as found in the T-tubule system (44). In synaptic membranes, scaffolding proteins bind and organize ion channels, receptors, kinases, and enzymes such as nitric oxide synthase. We speculate that the rich signaling of ECs also could use scaffolding proteins to keep channels, receptors, kinases, and even nitric oxide synthase in close proximity for rapid signal transduction.
The mechanisms used for signal transduction of mechanical stimuli are not understood in most biological systems. This report presents evidence that a Kir2.1 K+ ion channel can act as a LSS mechanotransducer, a significant and unique addition to the known properties of inward-rectifying K+ channels. Because ECs express Kir2.1, and the endothelial shear current response is very similar to the shear response displayed by the Kir2.1 channel, we suggest that endothelial shear current is caused by modulation of the Kir2.1 channel.
Acknowledgments
We thank W. Lindsley for help and discussion of LSS. S.F. was supported in part by a National Institutes of Health Training Grant. This work was supported in part by grants from the National Science Foundation and the Whitaker Foundation (to A.H.).
Abbreviations
- EC
endothelial cell
- LSS
laminar shear stress
- IKS, shear current
PYK, phosphotyrosine kinase
- IK
Kir2.1 stationary K+ current
- PKA
protein kinase A
References
- 1.Dewey CF, Jr, Bussolari S R, Gimbrone MA, Jr, Davies P F. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 2.Ohno M, Cooke J P, Dzau V J, Gibbons G H. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen S P, Claphan D E, Davies P F. Nature (London) 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs E R, Cheliakine C, Gebremedhin D, Birks E K, Davies P F, Harder D R. Pflügers Arch Eur J Physiol. 1995;431:129–131. doi: 10.1007/BF00374386. [DOI] [PubMed] [Google Scholar]
- 5.Barakat A I, Leaver E V, Pappone P A, Davies P F. Circ Res. 1999;85:820–828. doi: 10.1161/01.res.85.9.820. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer J, Distler A, Haasw W, Gogelein H. Proc Natl Acad Sci USA. 1994;91:2367–2371. doi: 10.1073/pnas.91.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng H, Peterson T E, Berk S C. Circ Res. 1995;77:869–879. doi: 10.1161/01.res.77.5.869. [DOI] [PubMed] [Google Scholar]
- 8.Rubanyi G M, Romero J C, Vanhoutte P M. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 9.Cooke J P, Rossitch E, Jr, Andon N A, Loscaizo J, Dzau V J. J Clin Invest. 1991;88:1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilius B, Viana F, Droogmans G. Annu Rev Physiol. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Forsyth S E, Hoger A, Hoger J H. FEBS Lett. 1997;409:277–282. doi: 10.1016/s0014-5793(97)00514-0. [DOI] [PubMed] [Google Scholar]
- 12.Luckhoff A, Clapham D E. Nature (London) 1992;355:365–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Schini W, Stoeckel H. Pflügers Arch Eur J Physiol. 1987;410:385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- 14.Colden-Stanfield M, Schilling W P, Ritchie A K, Eskin S G, Navarro L T, Kunze D L. Circ Res. 1987;61:632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- 15.Zaritsky J J, Eckman D M, Wellman G C, Nelson M T, Schwarz T L. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 16.McNaughton K J, Sinclair C G. J Fluid Mech. 1966;25:367–375. [Google Scholar]
- 17.Schlichting H. Boundary-Layer Theory. New York: McGraw-Hill; 1979. [Google Scholar]
- 18.Hoger J H, Walter A E, Vance D, Yu L, Davidson N, Lester H A. Neuron. 1991;6:227–236. doi: 10.1016/0896-6273(91)90358-7. [DOI] [PubMed] [Google Scholar]
- 19.Yang X C, Sachs F. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 20.Hong K, Driscoll M. Nature (London) 1994;367:470–473. doi: 10.1038/367470a0. [DOI] [PubMed] [Google Scholar]
- 21.Capco D G, Bement W M. Methods Cell Biol. 1991;36:249–270. doi: 10.1016/s0091-679x(08)60281-2. [DOI] [PubMed] [Google Scholar]
- 22.Prasad A R S, Logan S A, Nerem R M, Schwartz C J, Sprague E A. Circ Res. 1992;72:827–836. doi: 10.1161/01.res.72.4.827. [DOI] [PubMed] [Google Scholar]
- 23.Huang X Y, Morielli A D, Peralta E G. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 24.Tong Y, Brandt G S, Li M, Shapovalov G, Slimko E, Karschin A, Dougherty D E, Lester H A. J Gen Physiol. 2001;117:103–118. doi: 10.1085/jgp.117.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdoorn T A, Draguhn A, Ymer S, Seeburg P H, Sakmann B. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- 26.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 27.Ji S, John S A, Lu Y, Weiss J N. J Biol Chem. 1998;273:1324–1328. doi: 10.1074/jbc.273.3.1324. [DOI] [PubMed] [Google Scholar]
- 28.Cohen N A, Brenman J E, Snyder S H, Bredt D S. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 29.Nehring R B, Wischmeyer E, Döring F, Veh R W, Sheng M, Karschin A. J Neurosci. 2000;20:156–162. doi: 10.1523/JNEUROSCI.20-01-00156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horio Y, Hibine H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. J Biol Chem. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- 31.Davies P F. Physiol Rev. 1995;75:519–562. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnick N, Gimbrone M A. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 33.Edwards G, Dora K A, Gardener M J, Garland C J, Weston A H. Nature (London) 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 34.Fakler B, Brändle U, Glowatzki E, Zenner H-P, Ruppersberg J P. Neuron. 1994;13:1413–1420. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 35.Wischmeyer E, Karschin A. Proc Natl Acad Sci USA. 1996;93:5819–5823. doi: 10.1073/pnas.93.12.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones S V P. Mol Pharmacol. 1996;49:662–667. [PubMed] [Google Scholar]
- 37.Ruppersberg J P, Fakler B. Neuropharmacology. 1996;35:887–893. doi: 10.1016/0028-3908(96)00092-5. [DOI] [PubMed] [Google Scholar]
- 38.Wischmeyer E, Döring F, Karschin A. J Biol Chem. 1998;273:34063–34068. doi: 10.1074/jbc.273.51.34063. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T, Peterson T E, Kovach N L, Berk B C. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 40.Mazzanti M, Assandri R, Ferroni A, DiFrancesco D. FASEB J. 1996;10:357–361. doi: 10.1096/fasebj.10.2.8641571. [DOI] [PubMed] [Google Scholar]
- 41.McPhee J C, Dang Y L, Davidson N, Lester H A. J Biol Chem. 1998;273:34696–34702. doi: 10.1074/jbc.273.52.34696. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi L, Arcangeli A, Bartolini P, Mugnai G, Wanke E, Olivotto M. Biochem Biophys Res Commun. 1995;210:823–829. doi: 10.1006/bbrc.1995.1732. [DOI] [PubMed] [Google Scholar]
- 43.Dart C, Leyland M L. J Biol Chem. 2001;276:20499–20505. doi: 10.1074/jbc.M101425200. [DOI] [PubMed] [Google Scholar]
- 44.Leonoudakis D, Mailliard W S, Wingerd K L, Cleff D O, Vandenberg C A. J Cell Sci. 2000;114:987–998. doi: 10.1242/jcs.114.5.987. [DOI] [PubMed] [Google Scholar]
- 45.Hanada T, Lin L H, Chandy K G, Oh S S, Chishti A H. J Biol Chem. 1997;272:26899–26904. doi: 10.1074/jbc.272.43.26899. [DOI] [PubMed] [Google Scholar]