Massive irreparable rotator cuff tears (RCTs) pose a significant clinical challenge, often resulting in superior humeral head migration and severe functional limitations.24 Surgical management options, including arthroscopic débridement, biceps tenotomy, tendon transfers, superior capsular reconstruction, and reverse total shoulder arthroplasty, each have specific indications and limitations.1,13 For instance, reverse total shoulder arthroplasty effectively restores function and alleviates pain in patients with irreparable RCTs; however, its suitability for young, active individuals remains questionable due to concerns regarding implant longevity and associated complications.13 Tendon transfers yield acceptable functional outcomes but are technically demanding, with a postoperative course that may be too burdensome for elderly patients.6 Superior capsular reconstruction appears most appropriate for isolated irreparable supraspinatus tears, according to a recent systematic review,23 though the limited availability of high-quality comparative studies prevents definitive conclusions about its long-term efficacy. Biceps tenotomy, a relatively simple procedure, has been shown to relieve pain in select patients with irreparable RCTs, but long-term outcomes remain insufficiently documented.2
The InSpace balloon (Stryker, Kalamazoo, MI, USA), introduced as a minimally invasive alternative, is reported to restore humeral head positioning, reduce subacromial contact pressures, and is implanted arthroscopically with reduced technical complexity compared to reconstructive techniques.7,19,22
Based on current literature, the most suitable candidate for this procedure is an elderly individual with low functional demands who experiences refractory pain attributable to a supraspinatus tendon tear, exhibits minimal glenohumeral arthropathy, and maintains an intact subscapularis, having demonstrated inadequate response to conservative management.21,22 The subacromial spacer has further gained traction as a protective adjunct in the repair of massive yet reparable RCT; however, a recent comparative study has revealed that it does not significantly lower rerupture rates nor effectively reduce the number of tendons that sustain rerupture in these patients compared to those who undergo isolated cuff repair.11
So far, studies on the subacromial InSpace balloon spacer have provided inconclusive evidence regarding its efficacy and associated complications.4,17,18 A recent systematic review reported reoperation rates up to 33% and complication rates up to 19.6%, highlighting the uncertainty surrounding its outcomes.8 This is further corroborated by the (to our knowledge) only 2 available randomized controlled trials, which reached conflicting conclusions.10,21
We report a case of revision 4 months after cuff repair and balloon implantation with multiple “shattered glass-like” appearing fragments and foreign body–induced inflammation and cuff rerupture resulting in functional restriction and persistent pain.
Case report
The patient, a 58-year-old woman, underwent rotator cuff repair involving the supraspinatus and subscapularis tendons of her right shoulder 11 months ago at an external facility. Following a rerupture, a second surgery was performed 7.5 months later, involving reconstruction of the supraspinatus and infraspinatus tendons along with the placement of a subacromial InSpace balloon at the same facility. However, 4 weeks after the second procedure, she experienced worsening shoulder pain and progressive functional decline, prompting her to later seek consultation at our outpatient clinic. She reported increasing weakness and severe mobility restrictions, primarily an inability to actively raise her arm.
On physical examination, there were no generalized or local signs of infection. The shoulder exhibited well-healed surgical scars. The deltoid muscle demonstrated good activation across all movement planes, though overall function was significantly compromised. Severe range of motion restriction was observed, with pseudoparalysis during elevation. Despite the ability to maintain external rotation at 30°, abduction strength was markedly reduced. The external rotation lag test was negative, while the subscapularis test revealed only mild weakness with slight functional impairment. Magnetic resonance arthrography of the right shoulder confirmed a second rerupture of the supraspinatus and infraspinatus tendons (Fig. 1).
Figure 1.
Magnetic resonance arthrography of the right shoulder. T2-weighted fat-suppressed oblique coronal sequence (A) and T1-weighted fat-suppressed sagittal sequence (B) illustrate a complete second rerupture of the supraspinatus and infraspinatus tendons.
After discussing treatment options with the patient and considering her relatively young age we recommended another posterosuperior cuff reconstruction attempt with potential semitendinosus tendon autograft interposition (Strings technique).12 The surgery was performed 4 months after the last procedure during which the InSpace balloon was inserted.
Arthroscopic evaluation was performed with the patient in the beach-chair position under general anesthesia combined with an interscalene plexus block. The posterior portal was used to introduce a 4-mm 30° arthroscope for diagnostic evaluation. Intra-articular findings included fibrinous deposits and pannus formation around the subscapularis tendon and within the rotator interval, suggesting chronic inflammation. Early degenerative cartilage changes were observed, with grade 2 damage on the humeral head and milder involvement of the glenoid. Biopsy specimens were obtained, and a partial synovectomy with adhesiolysis in the rotator interval was performed. Macroscopically, findings suggested a potential low-grade infection.
Upon advancing the scope into the subacromial space through the lateral portal, extensive obliteration was noted, with multiple balloon fragments scattered throughout (Fig. 2, Supplementary Video 1). These remnants were carefully extracted using a shaver and grasping forceps (Fig. 3), including the residual valve of the balloon (Fig. 4, Supplementary Video 1). The subacromial space exhibited significant inflammatory changes, prompting additional tissue sampling for microbiological and histopathological analysis. A thorough débridement and additional adhesiolysis were performed. Examination of the rotator cuff confirmed an intact subscapularis and teres minor tendon as well as a complete retear of the supraspinatus and cranial infraspinatus tendons (Fig. 5). Despite tenolysis, the rotator cuff remained irreparable. The cuff tissue appeared highly inflamed and retracted, making immediate reconstruction with interposition grafting unfeasible due to the substantial inflammatory changes within the joint and the suspected presence of a low-grade infection.
Figure 2.
Arthroscopic view from the lateral portal showing a “shattered-glass” appearance of the subacromial space completely obliterated by numerous fragmented remnants of the InSpace balloon.
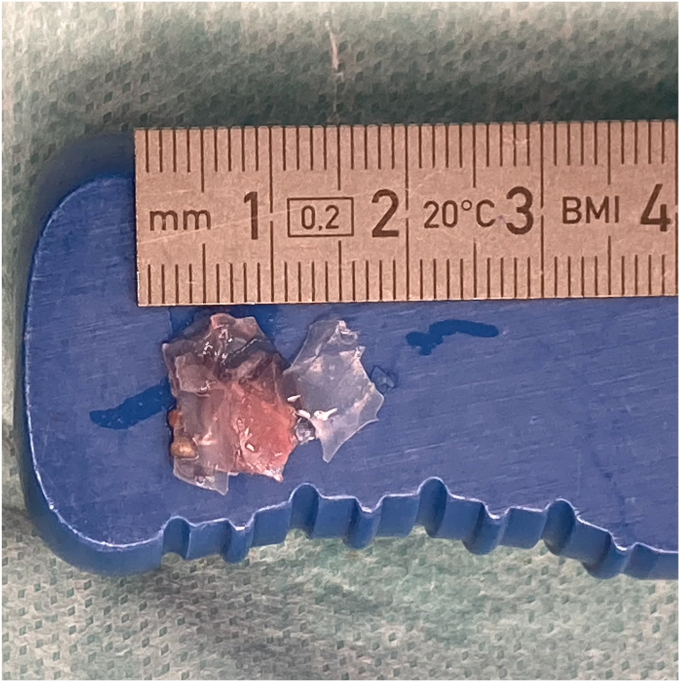
Figure 3.
Macroscopic appearance of the extracted ruptured InSpace balloon fragments.
Figure 4.
Arthroscopic visualization from the lateral portal highlighting the balloon's valve as a freely floating structure within the subacromial space.
Figure 5.
Condition of the posterosuperior rotator cuff tendons after extensive subacromial débridement of balloon fragments.
Microbiological analysis of the biopsy samples did not reveal bacterial infection. Histopathological examination identified a surface with up to 3 cell layers, underlain by a fibrotic stroma with extensive fibrin exudation and moderate chronic inflammation. The inflammatory infiltrate was composed primarily of eosinophils with sparse neutrophils (Fig. 6), without evidence of crystal deposition, cellular atypia, or malignancy. Gram (Brown-Brenn) staining showed no bacterial presence, while immunohistochemical analysis using CD15 staining highlighted focal neutrophilic accumulation of up to 25 neutrophils per high-power field (Fig. 7). The final histopathologic diagnosis confirmed foreign body–induced chronic inflammation, fibrosis, and eosinophilic infiltration, attributed to the retained balloon fragments.
Figure 6.
High-magnification histological view showing focal presence of neutrophilic granulocytes (highlighted in red circles) within the stromal tissue.
Figure 7.
Immunohistochemical analysis of the synovial membrane showing CD15 positivity (left). Gram (Brown-Brenn) staining reveals no evidence of bacterial presence (right).
Discussion
The InSpace balloon is a biodegradable poly L-lactide-co-ε-caprolactone spacer designed to undergo complete resorption within 12 months. Therefore, it is yet unclear how the device would provide long-term benefit. One theory suggests that the spacer temporarily replicates the biomechanical function of the native rotator cuff by lowering the humeral head, enabling the remaining stabilizers (pectoralis major, latissimus dorsi, and subscapularis) to compensate for the torn cuff immediately after surgery through improved force vectors facilitated by the adjusted humeral head position.4 Nevertheless, unsatisfactory outcomes have been reported as early as 16 months postoperatively.15 Further, the reported degradation timeline may not be consistently achieved, potentially contributing to adverse events. A rare case described by Calvo et al.3 documented a patient experiencing persistent pain 1-year postimplantation, attributed to failed device resorption, with histopathological analysis confirming a foreign body reaction.20
On the other hand, early biomechanical failure of InSpace balloon remains both underestimated and insufficiently investigated.5,9 In a case report detailing early rupture of a subacromial balloon spacer, Mangan and Shafritz9 described a patient who developed sudden-onset shoulder pain and restricted range of motion 3.5 weeks postimplantation; however, although the authors deemed a small-sized spacer appropriate during the index procedure, they inflated the balloon with 16 mL of saline, exceeding the manufacturer's recommended volume of 9-11 mL,16 which may have contributed to mechanical failure.
In the present case, the balloon was deployed as a protective adjunct during the first revision procedure to safeguard the cuff repair; however, the subsequent functional decline and persistent shoulder pain were likely consequences of balloon fragmentation, triggering a chronic inflammatory response, as confirmed by histopathological analysis in addition to the cuff rerupture. These findings are consistent with a similar case reported by Fury et al.,5 where balloon fragmentation resulted in synovial ulceration and diffuse chronic inflammation.
Even though the proposed mechanism of action of the subacromial balloon spacer appears plausible, imaging studies have not demonstrated any significant alterations in either the acromiohumeral distance or the Hamada classification.17,20 This lack of observable structural changes raises concerns regarding the purported stabilizing effect advocated by the implant's proponents. The only 2 randomized controlled trials identified by the authors on this topic reached conflicting conclusions, with 1 suggesting potential harm10 and the other reporting favorable outcomes,21 further casting doubt on the device's role in the management of irreparable RCTs. Moreover, the literature highlights a broad spectrum of complications linked to the device, with incidence rates reported as high as 19.6%.4,8,15,17,18
In a prospective 3-year follow-up study, Piekaar et al.14 concluded that balloon implantation significantly reduces pain and enhances functional daily activities. However, this improvement may be attributed to biceps tenotomy and tenodesis,2 which the authors systematically performed in their cohort, raising questions about the true independent efficacy of the balloon itself.
Last but not the least, Savarese and Romeo's 2012 paper16 characterized the subacromial balloon spacer as a palliative intervention, not a curative solution, specifically for elderly patients with significant comorbidities and irreparable RCTs where extensive surgical reconstruction is contraindicated.
Conclusion
We presented a case with multifragmented balloon remnants observed 4 months after index surgery along with an inflammatory response questioning the mechanical benefit and biological burden posed on the surrounding tissue by the resorptive processes after Inspace Balloon implantation.
Disclaimers
Funding: No funding was disclosed by the authors.
Conflicts of interest: Philipp Moroder serves as a consultant, receives royalties and fellowship support from Arthrex, is a shareholder and receives royalties from Alyve Medical, is a shareholder of Kairos Medical and Zurimed, is a patent co-applicant with Johnson & Johnson, and receives royalties from Medacta and Medi. The other authors, their immediate family, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Patient consent: Obtained.
Footnotes
Institutional review board approval was not required for this case study.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jseint.2025.04.008.
Supplementary Data
References
- 1.Bedi A., Dines J., Warren R.F., Dines D.M. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 2.Boileau P., Baqué F., Valerio L., Ahrens P., Chuinard C., Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89:747–757. doi: 10.2106/JBJS.E.01097. [DOI] [PubMed] [Google Scholar]
- 3.Calvo E., Valencia M., Merino-Garcia J.A., Luengo-Alonso G. Symptomatic foreign body reaction secondary to subacromial balloon spacer placement: a case report. J Shoulder Elbow Surg. 2020;29:e313–e316. doi: 10.1016/j.jse.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Deranlot J., Herisson O., Nourissat G., Zbili D., Werthel J.D., Vigan M., et al. Arthroscopic subacromial spacer implantation in patients with massive irreparable rotator cuff tears: clinical and radiographic results of 39 retrospectives cases. Arthroscopy. 2017;33:1639–1644. doi: 10.1016/j.arthro.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Fury M.S., Cirino C.M., White A.E., Bauer T.W., Taylor S.A. Rice-body synovitis, foreign body reaction, and rotator cuff failure after subacromial balloon spacer augmentation of a rotator cuff repair. JBJS Case Connect. 2023;13 doi: 10.2106/JBJS.CC.23.00009. [DOI] [PubMed] [Google Scholar]
- 6.Gerber C., Rahm S.A., Catanzaro S., Farshad M., Moor B.K. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2013;95:1920–1926. doi: 10.2106/JBJS.M.00122. [DOI] [PubMed] [Google Scholar]
- 7.Horneff J.G., Abboud J.A. Balloon arthroplasty: indications, technique, and European outcomes. Ann Joint. 2018;3:85. doi: 10.21037/aoj.2018.10.02. [DOI] [Google Scholar]
- 8.Levy K.H., White C.A., Pujari A., Patel A.V., Kator J.L., Parsons B.O., et al. Subacromial balloon spacer implantation is a promising alternative for patients with massive irreparable rotator cuff tears: a systematic review. Arthroscopy. 2024;40:162–173.e2. doi: 10.1016/j.arthro.2023.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Mangan J., Shafritz A. Symptomatic early rupture of the InSpace subacromial balloon spacer: a case report. HSS J. 2024 doi: 10.1177/15563316241257233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe A., Parsons H., Parsons N., Brown J., Fox J., Gemperlé Mannion E., et al. Subacromial balloon spacer for irreparable rotator cuff tears of the shoulder (START:REACTS): a group-sequential, double-blind, multicentre randomised controlled trial. Lancet. 2022;399:1954–1963. doi: 10.1016/S0140-6736(22)00652-3. [DOI] [PubMed] [Google Scholar]
- 11.Minarro J.C., Bassi C., Boltuch A., Urbano-Luque M., Buijze G.A., Lafosse L., et al. Subacromial balloon spacer does not reduce the retear rate for massive rotator cuff tears: a comparative study. Arthroscopy. 2024;40:242–248. doi: 10.1016/j.arthro.2023.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Moroder P., Akgün D., Siegert P., Thiele K., Plachel F. “Strings” (multiple tendon interposition autografts) for reconstruction of presumably irreparable rotator cuff tears. Arthrosc Tech. 2020;9:e459–e467. doi: 10.1016/j.eats.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh J.H., Park M.S., Rhee S.M. Treatment strategy for irreparable rotator cuff tears. Clin Orthop Surg. 2018;10:119. doi: 10.4055/cios.2018.10.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piekaar R.S.M., Bouman I.C.E., van Kampen P.M., van Eijk F., Huijsmans P.E. The subacromial balloon spacer for massive irreparable rotator cuff tears: approximately 3 years of prospective follow-up. Musculoskelet Surg. 2020;104:207–214. doi: 10.1007/s12306-019-00614-1. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz Ibán M.A., Lorente Moreno R., Ruiz Díaz R., Álvarez Sciamanna R., Paniagua Gonzalez A., Lorente Gómez A., et al. The absorbable subacromial spacer for irreparable posterosuperior cuff tears has inconsistent results. Knee Surg Sports Traumatol Arthrosc. 2018;26:3848–3854. doi: 10.1007/s00167-018-5083-3. [DOI] [PubMed] [Google Scholar]
- 16.Savarese E., Romeo R. New solution for massive, irreparable rotator cuff tears: the subacromial “biodegradable spacer”. Arthrosc Tech. 2012;1:e69–e74. doi: 10.1016/j.eats.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senekovic V., Poberaj B., Kovacic L., Mikek M., Adar E., Dekel A. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol. 2013;23:311–316. doi: 10.1007/s00590-012-0981-4. [DOI] [PubMed] [Google Scholar]
- 18.Senekovic V., Poberaj B., Kovacic L., Mikek M., Adar E., Markovitz E., et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg. 2017;137:95–103. doi: 10.1007/s00402-016-2603-9. [DOI] [PubMed] [Google Scholar]
- 19.Singh S., Reeves J., Langohr G.D.G., Johnson J.A., Athwal G.S. The subacromial balloon spacer versus superior capsular reconstruction in the treatment of irreparable rotator cuff tears: a biomechanical assessment. Arthroscopy. 2019;35:382–389. doi: 10.1016/j.arthro.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Singh S., Reeves J., Langohr G.D.G., Johnson J.A., Athwal G.S. The effect of the subacromial balloon spacer on humeral head translation in the treatment of massive, irreparable rotator cuff tears: a biomechanical assessment. J Shoulder Elbow Surg. 2019;28:1841–1847. doi: 10.1016/j.jse.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Verma N., Srikumaran U., Roden C.M., Rogusky E.J., Lapner P., Neill H., et al. InSpace implant compared with partial repair for the treatment of full-thickness massive rotator cuff tears. J Bone Joint Surg Am. 2022;104:1250–1262. doi: 10.2106/JBJS.21.00667. [DOI] [PubMed] [Google Scholar]
- 22.Viswanath A., Drew S. Subacromial balloon spacer – where are we now? J Clin Orthop Trauma. 2021;17:223–232. doi: 10.1016/j.jcot.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werthel J.-D., Vigan M., Schoch B., Lädermann A., Nourissat G., Conso C. Superior capsular reconstruction – a systematic review and meta-analysis. Orthop Traumatol Surg Res. 2021;107 doi: 10.1016/j.otsr.2021.103072. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H., et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.