Abstract
The study of the development of the mammary gland at the molecular level in the animal is difficult because of the complex tissue organization of the gland. We have previously developed an in vitro system for genetic analysis of mammary cell differentiation, based on the cell line LA7 clonally derived from a rat mammary adenocarcinoma. This cell line, after induction with DMSO, differentiates forming structures called domes. This process is under strict gene regulation, and we have previously identified several of the genes involved. In the present paper, we have defined the meaning of dome formation in relation to mammary development, by showing that treatment of LA7 cells with the lactogenic hormones hydrocortisone and prolactin induces dome formation; in the animal, these hormones precede and accompany milk production. Moreover, dome formation is accompanied by expression within the cells of the milk protein genes WDMN1 and β-casein, which are differentiation markers for the gland during pregnancy and lactation. We also show that two proteins, highly expressed in the mammary gland during lactation, HSP90-β and annexin I, are strongly expressed in DMSO-induced LA7 cells. Both proteins are essential in the formation of domes because when their synthesis is blocked by antisense RNA oligonucleotides, dome formation is abolished. Thus our in vitro system is a model for lobulo-alveolar development, and the genes identified in the pathway of dome formation are likely to be involved in the early differentiation steps occurring in the rat mammary gland during pregnancy and lactation.
The control of differentiation of the mammary gland is important for understanding its biology and may be relevant for understanding the evolution of cancer. However, the study of biological and signaling pathways involved in mammary gland differentiation is made difficult in the animal by the multiple steps of its development. The mammary gland is one of the few organs that undergoes numerous rounds of proliferation and regression throughout adult life. Development of the mammary gland occurs in several different stages, begins in the embryo, progresses after birth, and is completed in the mature mammal. The full development of the gland proceeds in distinct phases: embryonic, puberal, pregnancy, lactation, and involution (1). A rudimentary system of small ducts is present in newborn mice (2). Later, moderate ductal growth occurs until puberty, when a more pronounced development of the ducts leads to the formation of a ductal tree (2). In cycling virgin mice, the parenchyma consists of a highly organized system of ducts with terminal end-buds that represent the major sites of ductal growth (3–5) and give rise to alveolar buds (6). Alveolar buds in turn generate alveoli that eventually may secrete milk proteins during pregnancy and lactation (7). In the puberal phase, the growth pattern is characteristically ductal and very few alveoli are formed (8). Full alveolar development occurs during pregnancy when alveoli increase in number and size and (during the second half of pregnancy) start to form larger and fully differentiated secretory lobules. Terminal differentiation of alveolar epithelial cells is completed at parturition with the production and secretion of milk (2). Finally, the end of weaning suppresses lactation and leads to involution of the lobulo-alveolar compartment, returning the mammary gland to its nonpregnant state. Mammary gland functional differentiation occurs with distinct morphological and molecular changes of the epithelial cells and allows for the production and secretion of milk. The secretory alveolar cells represent therefore the final cellular state of the differentiation process within the mammary gland (9). These differentiation steps taking place during pregnancy and lactation are defined and characterized by the sequential activation of genes encoding the milk proteins WDNM1, β-casein, whey acidic protein (WAP), and α-lactalbumin (5). In particular, early pregnancy stage is characterized by the expression of WDNM and β-casein genes, the expression of which increases during alveolar proliferation in the second half of pregnancy and during lactation. Late pregnancy and lactation are characterized by the additional expression of WAP and α-lactalbumin genes (1, 5).
The study of mammary gland differentiation is additionally complicated by the difficulty of obtaining uncontaminated cellular samples at definite stages of differentiation. These problems can be overcome by using, as a model, epithelial cultures derived from the mammary gland that are capable of in vitro differentiation. For this purpose, we have been using a system formed by two cell lines, LA7 and 106, which are clonal lines derived from the cell line RAMA-25 isolated from mammary adenocarcinoma induced in Sprague–Dawley rats by chemicals (10). The RAMA-25 line is considered to contain stem cells of the mammary gland (10–13). The derived LA7 cell line spontaneously undergoes differentiation in vitro by forming domes, and this differentiation can be strongly promoted by differentiation inducers, such as DMSO. In contrast, the 106 cell line does not undergo this differentiation either spontaneously or under the action of inducers (14–16). The two cell lines also differ in their ability to produce tumors in nude mice, which is reduced for the LA7 cells (I.Z. and R.D., unpublished observations). By differential cloning of genes, using subtractive cDNA library construction and proteomic-based technologies, both performed on DMSO-induced LA7 and 106 cells, we determined that dome formation in LA7 cells is under strict gene regulation (16–18). Among the genes we identified differentially expressed between the two cell lines, we demonstrated to be critical in dome formation: rat8, tropomyosin-5B, maspin, the β subunit of the epithelial sodium channel, and the rat homolog of the human EMP3 gene. Using in situ hybridization and immunofluorescence technology, we determined the in vivo sites of action of the identified genes, and using RNA antisense oligonucleotide technology for blocking specific protein synthesis, we determined that these genes have a functional role in dome formation.
We have assumed that the domes may correspond to the tubular structures present in the mammary gland, which also express rat8 and the epithelial sodium channel β subunit (ref. 16 and I.Z. and R.D., unpublished observations), moreover domes, like the epithelial structures, express α6β1 integrin, E-cadherin, and cytokeratin 8 (16, 17). As mentioned before, the mammary epithelium consists of a branched ductal system that develops mainly during puberty and a lobulo-alveolar compartment that develops during pregnancy. Ducts arbor into smaller ductules that terminate in lobules. Lobules are composed of alveoli, which in turn consist of secretory epithelial cells that undergo functional differentiation and secrete milk proteins at parturition (1). Although we have shown that several of the genes we identified as differentially expressed were essential in dome formation and were expressed in LA7 cells as well as in tubular structures of the lactating gland (ref. 16 and I.Z. and R.D., unpublished observations), we had yet no indication of the type of tubules to which the domes correspond.
In this article, we provide evidence that domes correspond to a distinct stage of development of tubules and alveoli present in the mammary gland of pregnant or lactating mammals, based on these two findings: First, the lactogenic hormones hydrocortisone (HC) and prolactin (PRL) induce dome formation in LA7 cells, accompanied by the expression of the complete repertoire of the genes we previously identified as critical in dome formation; second, DMSO-induced LA7 cells express the milk protein genes WDNM1 and β-casein, as detected by using the cDNA library subtraction approach. WDMN1, originally identified as a gene whose expression is diminished in metastatic rat mammary adenocarcinomas, is a member of a family of protease inhibitors and encodes a secreted protein that may inhibit metastases by inhibiting proteases (19–21). WDNM1 and β-casein genes appear to be specific markers of mammary gland functional differentiation because in vivo the expression of these two genes occurs earlier in pregnancy (5–22), suggesting that their expression in LA7 cells may be indicative of a possible relationship between the biological processes resulting in dome formation in vitro and the processes resulting in the alveoli differentiation of the mammary gland at pregnancy.
We also report on two gene products differentially expressed between LA7 and 106 cells and identified by proteomic analysis. They are annexin I and HSP90-β, which are highly expressed in DMSO-induced LA7 cells. These two proteins appear also to be involved in the secretory differentiation process and in the developmental regulation of the mammary gland (23–26). Interestingly, alveolar epithelial cells of the mammary gland display the highest level of annexin I expression, suggesting a specialized function of annexin I in these cells (23). Both genes are relevant for dome formation, because when their protein synthesis is blocked by RNA antisense oligonucleotides, dome formation is inhibited.
Materials and Methods
Cell Lines and Differentiation Inducers.
The cell lines LA7 and 106, both clonal derivatives from the RAMA-25 line (10) were cultured as described (14). The LA7 cells, after having grown to confluence, were exposed to 1.5% DMSO as an inducer of cell differentiation (15). Differentiation of LA7 cells also was induced by the addition of HC (5 μg/ml) and PRL (2 μg/ml), both purchased from Sigma. Cells were inspected for dome formation, photographed at ×4 magnification, and harvested for RNA isolation as described (16).
mRNA Isolation and Northern Analysis.
Total RNA was isolated by the single-step acid–guanidine isothiocyanate–phenol–chloroform extraction method (27). For Northern blot analysis, total RNA (5–10 μg) from each cell line was fractionated by electrophoresis as described in Sambrook and Russel (28). Probes were prepared by using [α-32P]dCTP and the random priming labeling kit Ready Prime (Amersham Pharmacia). Ribosomal cDNA was used as a loading control.
Reverse Transcription–PCR Assay.
Uninduced and induced LA7 and 106 cells were used for RNA isolation. One microgram of RNA was treated with 1 unit of RNase-free DNase (Invitrogen) for 15 min at room temperature to remove any possible DNA contamination. cDNA strand synthesis and reverse transcription–PCR analysis were performed by using conditions previously described (16). For PCR amplification of the β-casein cDNA, we used a forward primer designed at nucleotides 298–317 (5′-TCCCATTCCACAAAACATCC-3′) that, combined with a reverse primer designed at nucleotides 732–712 (5′-AATGAAGTTGTTGACTGGCAAG-3′) from the β-casein mRNA sequence (accession no. J00711), amplified a PCR fragment of 435 bp. For the epithelial sodium channel β subunit, we used primers and PCR conditions as described (17). Efficiency of each reverse transcription reaction was tested by using a forward primer designed at nucleotides 123–142 (5′-CAGGACTGAAAGACTTGCTC-3′) from the hypoxanthine phosphoribosyltransferase sequence (accession no. AF009656) that, combined with a reverse primer designed at nucleotides 277–257 (5′-TCATAGGAATGGACCTATCAC-3′) from the same sequence, amplified a fragment of expected size by using PCR conditions already described (17). Concurrently, additional samples containing LA7 and 106 mRNAs after DNase treatment and before reverse transcription were subjected to PCR in identical conditions to exclude the presence of genomic DNA.
Proteomic Analysis.
High-resolution two-dimensional gel electrophoresis and matrix-assisted laser desorption ionization–time-of-flight mass spectrometry of DMSO-induced LA7 cells and uninduced 106 cells were carried out as described in detail in Zucchi et al. (18).
Antisense Oligonucleotide Methodology.
For the inhibition study of HSP90-β mRNA expression, three oligonucleotides of 20 bases were synthesized: the antisense oligomer (5′-CTCCACCTCTTCCTCTCCAT-3′), designed as the complementary sequence to the HSP90-β mRNA sequence (accession no. S45932) between nucleotides 97–116, the sense oligomer (5′-ATGGAGAGGAAGAGGTGGAT-3′) from the same region, and the scrambled oligomer (5′-TCTCACCCCTTCCCTCATTC-3′), a scrambled sequence with the same nucleotide composition used for the antisense oligomer. For the inhibition study of annexin I mRNA expression, three oligonucleotides of 20 bases were synthesized: the antisense oligomer (5′-GCCTCATCCACACCTTTA-3′), designed as the complementary sequence to the annexin I mRNA sequence (accession no. M19967), between nucleotides 221 and 237, the sense oligomer (5′-TAAAGGTGTGGATGAGGC-3′) from the same region, and the scrambled oligomer (5′-TGTGGGCTGAAAGGGATA-3′). The experiments were performed as already described (16). Cells were maintained in culture for 60–72 h, inspected for dome formation, photographed at ×4 final magnification, and harvested for RNA extraction.
Results
Functional Differentiation of LA7 by Lactogenic Hormones.
To determine which kind of structures of the mammary gland correspond to domes, we tested the effect of HC and PRL on the LA7 cells because it has been reported that the action of these hormones is essential for the differentiation of mammary epithelium, for the development of the tubulo-alveolar structures during pregnancy, and for the secretion of milk (1, 5, 8, 29–32). Confluent cultures of LA7 cells were treated with either HC (at a concentration of 5 μg/ml) or PRL (at a concentration of 2 μg/ml), after a preinduction of the cells with HC at a concentration of 1 μg/ml (33). HC is one of the major regulators of lactogenesis (34), and it also is reported that a synergistic action of HC and PRL controls the differentiation of the secretory mammary epithelium (1). Approximately 30–72 h after treatment by either inducer, domes appeared in the confluent monolayers. The domes varied considerably in size relative to DMSO-induced cells. Few and small domes occurred spontaneously in postconfluent LA7 cells after 96–110 h; HC-induced domes developed after 27–30 h and appeared numerically very abundant but slightly smaller than those developed by DMSO; domes induced after PRL treatment developed 24–48 h after the HC preinduction and appeared in shape, numbers, and size very similar to those induced by DMSO. The HC- and PRL-induced cultures expressed the epithelial sodium channel β subunit and also the milk proteins WDNM1 and β-casein as well as E-cadherin and gene rat8, as in domes arising after induction with DMSO (data not shown).
Expression of Milk Protein Genes on DMSO Induction.
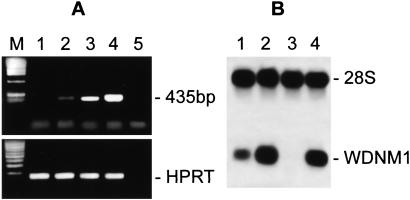
The differentiation steps during pregnancy and lactation are defined by the sequential activation of genes coding for milk proteins WDNM1, β-casein, WAP, and α-lactalbumin (5). In particular, the early pregnancy stage is characterized by the expression of WDNM1 and β-casein, whereas late pregnancy and lactation are characterized by the additional expression of WAP and α-lactalbumin genes (1, 5). In our previous work, using cDNA library subtraction approach, we found that the genes for two milk proteins, β-casein and WDNM1, were differentially expressed in DMSO-induced LA7 cells with respect to 106 cells. We show here that both genes are expressed in both DMSO-induced LA7 and 106 cells, as shown in Fig. 1A (β-casein expression pattern), lanes 2 and 4, and Fig. 1B (WDNM1 expression pattern), lanes 2 and 4. Both genes are moderately expressed in uninduced, postconfluent LA7 cultures (Fig. 1 A, lane 3 and B, lane 1) but are not expressed in uninduced, postconfluent 106 cells (Fig. 1 A, lane 1 and B, lane 3).
Figure 1.
Cells were cultured as described in the text. Total RNA was obtained from postconfluent cultures of uninduced and DMSO-induced 106 and LA7 cells. (A) β-Casein gene expression. Reverse transcription–PCR reactions were performed with β-casein pairs of specific primers by using cDNAs of uninduced and DMSO-induced 106 cells (lanes 1 and 2) and uninduced and DMSO-induced LA7 cells (lanes 3 and 4); reverse transcription–PCR negative control containing no template (lane 5); molecular size marker (lane M). Reverse transcription reaction efficiency test performed with hypoxanthine phosphoribosyltransferase (HPRT)-specific primers (Lower). To control for any DNA contamination, a PCR, using the same primers, was performed on the same samples after DNase treatment, before retro-transcription (data not shown). (B) WDNM1 expression pattern. Northern blots were hybridized with radioactive WDNM1 probe: uninduced LA7 (lane 1), DMSO-induced LA7 (lane 2), uninduced 106 (lane 3), and DMSO-induced 106 (lane 4). A cDNA probe for 28S ribosomal RNA was used as a loading control.
In contrast, with these experimental conditions, no expression was detected for the WAP and α-lactalbumin genes in both LA7 and 106 cells, either in the presence or in the absence of DMSO (data not shown). The induction of domes by lactogenic hormones, together with the presence of the milk proteins WDNM1 and β-casein in the doming cells, but the lack of the expression of WAP and α-lactalbumin genes under these experimental conditions, tend to identify dome-forming cells as components of the lobulo-alveolar structures that develop in the mammary gland at early stage of pregnancy.
Role of Annexin I and HSP90-β in Dome Formation.
In our previous work, we reported on the proteomic analysis of DMSO-induced LA7 and 106 cells by two-dimensional gel electrophoresis (18). Computer-assisted analysis of the results, performed by using the program MELANIE 3, identified >200 differentially expressed proteins between the two lines. Several of the differentially expressed spots were analyzed by mass spectrometry, using matrix-assisted laser desorption ionization–time-of-flight, and the data obtained were compared to Swiss-Prot and Trembl databases to identify matches to known proteins. We have previously discussed the roles of maspin and tropomyosin-5b, two of the differentially expressed proteins identified (18).
Two additional proteins, annexin I and HSP90-β, also showed a high level of expression in DMSO-induced LA7 cells, when compared to 106 cells (Fig. 2). Using the previously described mRNA antisense methodology (16), we determined whether the expression of these genes was essential for dome formation. Inhibition of annexin I or HSP90-β protein synthesis was carried out by the addition of mRNA antisense oligonucleotides to LA7 cultures induced by 1.5% DMSO, to see whether dome formation was inhibited. The results, reproduced in Figs. 3D and 4D, show the complete inhibition of dome formation by either antisense oligonucleotide; in contrast, the corresponding sense (Figs. 3B and 4B) or scrambled oligonucleotides (Fig. 4 B and C) were without effect. The expression of these genes is therefore essential for dome formation.
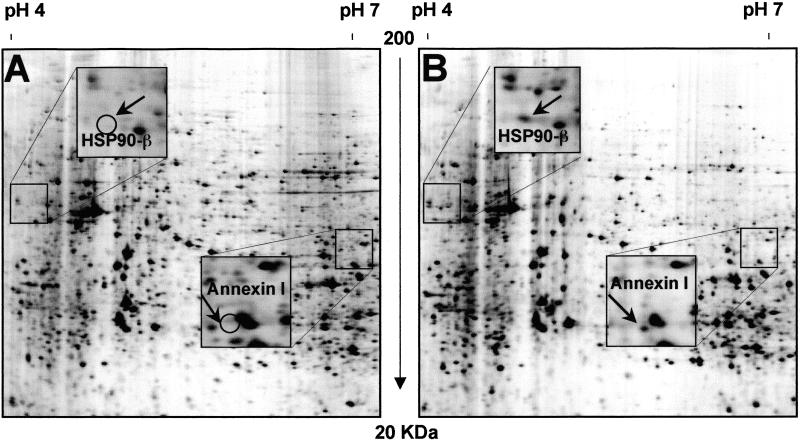
Figure 2.
Silver-stained details of two-dimensional gel electrophoresis polypeptide maps of uninduced 106 cells (A) and DMSO-induced LA7 cells (B). Spots were named after protein identification by mass spectrometry analysis. Squares in the pictures indicate regions magnified containing HSP90-β and annexin 1 spots. Differential expression of HSP90-β and annexin I proteins is indicated with open circles (A).
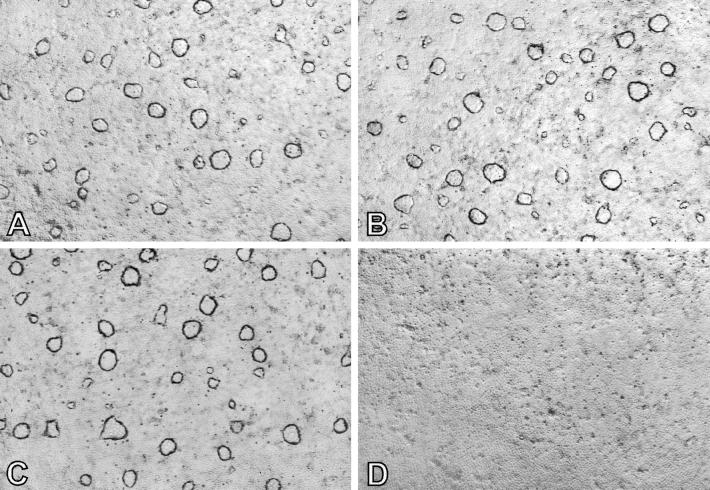
Figure 3.
Antisense anti-annexin I oligonucleotide effect on dome formation in DMSO-induced LA7 cells. (A) Cells not treated with oligonucleotides. (B) Cells treated with sense oligonucleotides. (C) Cells treated with scrambled oligonucleotides. (D) Cells treated with antisense anti-annexin I oligonucleotides. (×40.)
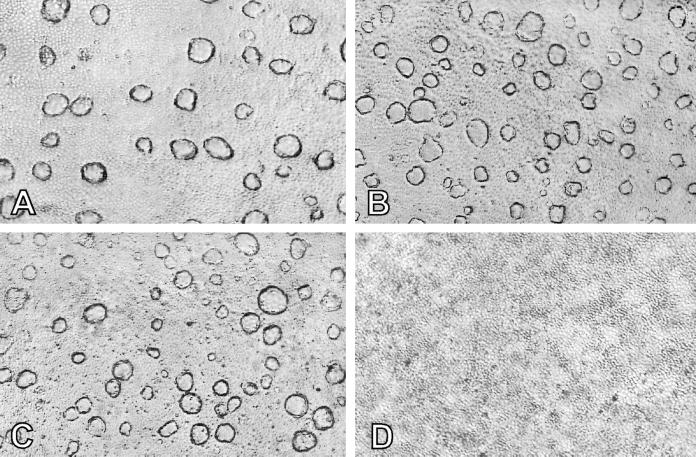
Figure 4.
Antisense anti-HSP90-β oligonucleotide effect on dome formation in DMSO-induced LA7 cells. (A) Cells not treated with oligonucleotides. (B) Cells treated with sense oligonucleotides. (C) Cells treated with scrambled oligonucleotides. (D) Cells treated with antisense anti-HSP90-β oligonucleotides. (×40.)
Discussion
In the past, we have studied dome formation in LA7 cells as an example of in vitro differentiation, and we considered dome formation as a possible model for studying a specific stage in mammary gland development. This idea was supported by the origin of the cell line LA7 as a clonal derivative from the line RAMA-25 (10), which is obtained from a rat mammary adenocarcinoma and contains mammary stem cells (10–13). Here we present data that confirm that a correlation exists between dome formation in vitro and the differentiation processes occurring in the mammary gland at pregnancy. This is based on two new findings: one is that the formation of domes can be induced by the lactogenic hormones HC and PRL, the other is that the formation of domes, induced by either DMSO or lactogenic hormones, in the LA7 cells is accompanied by the production of the milk proteins β-casein and WDNM1, which both are specific markers of mammary gland functional differentiation. In addition we show that the DMSO-induced domes express at a high level the annexin I and HSP90-β proteins, the expression of which also have been shown to be stage-specific during pregnancy and lactation in other in vitro models and in the animal in vivo (25, 26). It has been shown that both of these genes are involved in the secretory and functional differentiation of the mammary gland in vivo (23, 25, 26); now we demonstrate that their expression in LA7 is required for the formation of domes. We therefore can conclude that the formation of domes corresponds to a specific stage in the lobulo-alveolar development of the mammary gland occurring during pregnancy and lactation.
Distinct steps of cellular differentiation take place during the terminal differentiation of the alveolar epithelial cells; these steps are defined by the sequential activation of genes coding for milk proteins (5). The β-casein gene is expressed early during mammary differentiation at pregnancy, closely followed by WDNM1 (5, 22). Both genes are expressed in the LA7 cells after induction with DMSO. The WAP and the α-lactalbumin genes are, in contrast, expressed later in pregnancy, when the terminal differentiation takes place. These two genes were not detected in DMSO-induced LA7 cells, indicating that the developmental stage of DMSO-induced domes in this cell line may correlate to an early stage of lobulo-alveolar development, preceding mammary gland terminal differentiation. Therefore domes in the LA7 cultures correspond to the initial stage of mammary gland development, associated with the formation of tubules and of early alveoli during pregnancy. Consistent with these observations, as reported by Schwarz-Albiez and coworkers (23), the mammary alveolar epithelial cells are the most immunoreactive to annexin I. The LA7 line, therefore, when treated with dome inducers, appears to parallel a distinct stage of the mammary development in vivo.
The expression of β-casein and WDNM1 in the 106 cells may indicate that this line shows an aberrant stage of development, as is possible in a cancer-derived line. In contrast, the LA7 cells has a more physiological behavior. The difference between the two cell lines is supported by the more pronounced tumorigenic growth of 106 cells in nude mice, compared to LA7 cells (I.Z. and R.D., unpublished observations).
The availability of our tissue culture system for studying a stage-specific phase of mammary gland development allows for a simpler method to analyze the expression of individual genes compared to the study carried out in animals because it is directed at a single cell type that differentiates in vitro. Moreover, the roles of the expressed genes can be determined by the simple approach of the antisense RNA technology. The same system also should be suitable for determining the signals that cause the undifferentiated LA7 cells to differentiate in the lobulo-alveolar direction.
Acknowledgments
We acknowledge Prof. M. Neville for her suggestions and Prof. A. Albertini and Dr. R. Reinbold for their constant input into this work. This work was partially funded by grants from Associazione Italiana per la Ricerca sul Cancro (to I.Z.), Progetto Finalizzato Biotecnologie Consiglio Nazionale delle Ricerche (to P.V.), Progetto Strategico Consiglio Nazionale delle Ricerche, Tecnologie di base della postgenomica (to P.V.), and grants from Ministero dell'Università e della Ricerca Scientifica e Tecnologica (to L.B. and P.V.). D.A. is supported by the Mario e Valeria Rindi Postdoctoral Fellowship from Fondazione Italiana per la Ricerca sul Cancro. This is manuscript no. 62 of the Genoma 2000/Istituto Tecnologie Biomediche Avanzate Project funded by Cariplo.
Abbreviations
- HC
hydrocortisone
- PRL
prolactin
- WAP
whey acidic protein
References
- 1.Hennighausen L, Robinson G W. Dev Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 2.Hennighausen L, Robinson G W, Wagner K U, Liu W. J Biol Chem. 1997;272:7567–7569. doi: 10.1074/jbc.272.12.7567. [DOI] [PubMed] [Google Scholar]
- 3.Dulbecco R, Unger M, Armstrong B, Bowman M, Syka P. Proc Natl Acad Sci USA. 1983;80:1033–1037. doi: 10.1073/pnas.80.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ormerod E J, Rudland P S. Am J Anat. 1984;170:631–652. doi: 10.1002/aja.1001700408. [DOI] [PubMed] [Google Scholar]
- 5.Robinson G W, McKnight R A, Smith G H, Hennighausen L. Development (Cambridge, UK) 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- 6.Russo I H, Russo J. J Natl Cancer Inst. 1978;6:1439–1449. [PubMed] [Google Scholar]
- 7.Russo J, Tay L K, Russo I H. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 8.Topper Y J, Freeman C S. Physiol Rev. 1980;60:1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- 9.Hennighausen L, Robinson G W. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- 10.Bennett D C, Peachey L A, Durbin H, Rudland P S. Cell. 1978;15:283–298. doi: 10.1016/0092-8674(78)90104-6. [DOI] [PubMed] [Google Scholar]
- 11.Warburton M J, Head L P, Ferns S A, Rudland P S. Eur J Biochem. 1983;133:707–715. doi: 10.1111/j.1432-1033.1983.tb07520.x. [DOI] [PubMed] [Google Scholar]
- 12.Paterson F C, Warburton M J, Rudland P S. Dev Biol. 1985;107:301–313. doi: 10.1016/0012-1606(85)90313-6. [DOI] [PubMed] [Google Scholar]
- 13.Rudland P S. J Cell Physiol. 1992;153:157–168. doi: 10.1002/jcp.1041530120. [DOI] [PubMed] [Google Scholar]
- 14.Dulbecco R, Bologna M, Unger M. Proc Natl Acad Sci USA. 1979;76:1256–1260. doi: 10.1073/pnas.76.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulbecco R, Okada S. Proc R Soc London Ser B. 1980;208:399–408. doi: 10.1098/rspb.1980.0058. [DOI] [PubMed] [Google Scholar]
- 16.Zucchi I, Montagna C, Susani L, Vezzoni P, Dulbecco R. Proc Natl Acad Sci USA. 1998;95:1079–1084. doi: 10.1073/pnas.95.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucchi I, Montagna C, Susani L, Montesano R, Affer M, Zanotti S, Redolfi E, Vezzoni P, Dulbecco R. Proc Natl Acad Sci USA. 1999;96:13766–13770. doi: 10.1073/pnas.96.24.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zucchi I, Bini L, Valaperta R, Ginestra A, Albani D, Susani L, Sanchez J C, Liberatori S, Magi B, Raggiaschi R, et al. Proc Natl Acad Sci USA. 2001;98:5608–5613. doi: 10.1073/pnas.091101898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dear T N, Ramshaw I A, Kefford R F. Cancer Res. 1988;48:5203–5209. [PubMed] [Google Scholar]
- 20.Dear T N, Kefford R F. Biochem Biophys Res Commun. 1988;176:247–254. doi: 10.1016/0006-291x(91)90916-u. [DOI] [PubMed] [Google Scholar]
- 21.Steeg P S. Invasion Metastasis. 1989;9:351–359. [PubMed] [Google Scholar]
- 22.Morrison B W, Leder P. Oncogene. 1994;9:3417–3426. [PubMed] [Google Scholar]
- 23.Schwartz-Albiez R, Koretz K, Moller P, Wirl G. Differentiation. 1993;52:229–237. doi: 10.1111/j.1432-0436.1993.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 24.Solito E, de Coupade C, Parente L, Flower R J, Russo-Marie F. Cell Growth Differ. 1998;9:327–336. [PubMed] [Google Scholar]
- 25.Catelli M G, Ramachandran C, Gauthier Y, Legagneux V, Quelard C, Baulieu E E, Shyamala G. Biochem J. 1989;258:895–901. doi: 10.1042/bj2580895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe A, Miyamoto T, Katoh N, Takahashi Y. J Dairy Sci. 1997;80:2372–2379. doi: 10.3168/jds.S0022-0302(97)76188-5. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Russel D W. Molecular Cloning: A Laboratory Manual. 3rd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 2001. [Google Scholar]
- 29.Neville M C. Ann NY Acad Sci. 1990;586:1–11. doi: 10.1111/j.1749-6632.1990.tb17783.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt-Ney M, Happ B, Hofer P, Hynes N E, Groner B. Mol Endocrinol. 1992;6:1988–1997. doi: 10.1210/mend.6.12.1491685. [DOI] [PubMed] [Google Scholar]
- 31.Brisken C, Kaur S, Chavarria T E, Binart N, Sutherland R L, Weinberg R A, Kelly P A, Ormandy C J. Dev Biol. 1999;210:96–106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- 32.McManaman J L, Hanson L, Neville M C, Wright R M. Arch Biochem Biophys. 2000;373:318–327. doi: 10.1006/abbi.1999.1573. [DOI] [PubMed] [Google Scholar]
- 33.Mizoguchi Y, Yamaguchi H, Aoki F, Enami J, Sakai S. Mol Cell Endocrinol. 1997;132:177–183. doi: 10.1016/s0303-7207(97)00134-2. [DOI] [PubMed] [Google Scholar]
- 34.Cassy S, Charlier M, Belair L, Guillomot M, Laud K, Djiane J. Domest Anim Endocrinol. 2000;18:41–55. doi: 10.1016/s0739-7240(99)00062-4. [DOI] [PubMed] [Google Scholar]