Abstract
BACKGROUND
Malignant middle cerebral artery (MCA) infarctions result in cerebral edema that can lead to brain herniation and death. Standard management includes decompressive hemicraniectomy (DHC) and comprehensive neurocritical care. Some patients may continue to decline despite these measures. Reopening of the scalp incision may allow for additional decompression and provide mortality benefit.
OBSERVATIONS
A 47-year-old man developed malignant right MCA territory infarction following intravenous thrombolysis and unsuccessful mechanical thrombectomy. Despite aggressive hyperosmolar therapy and a large DHC, the patient continued to exhibit clinical decline and radiological progression of cerebral edema. In response, the scalp incision was reopened to facilitate maximal external cerebral herniation, a strategy the authors believe was critical in managing the brain swelling. The patient was maintained on prophylactic antibiotics during this period, given the increased infection risk associated with exposed brain surface and potential CSF leakage. Following the resolution of cerebral swelling, a right partial hemispherectomy was performed to excise the infarcted hemisphere. Notably, the patient achieved significant functional recovery following the intervention and an extended period of rehabilitation.
LESSONS
This case highlights the complexities encountered in the surgical management of malignant cerebral infarction, particularly when standard decompressive measures fail.
Keywords: decompressive hemicraniectomy, open scalp incision, hemispherectomy, malignant MCA infarction, ischemic brain edema
ABBREVIATIONS: ATP = adenosine triphosphate, DHC = decompressive hemicraniectomy, ICP = intracranial pressure, MCA = middle cerebral artery
In cases of malignant middle cerebral artery (MCA) infarctions in which patients continue to deteriorate despite decompressive hemicraniectomy (DHC) and aggressive neurocritical care, further surgical interventions may be required. The potential benefits of reducing intracranial pressure (ICP) must be carefully weighed against the inherent risks of such procedures. In this case, reopening the scalp incision followed by partial hemispherectomy provided essential additional decompression and helped manage refractory intracranial hypertension and brain swelling.
Illustrative Case
A 47-year-old man developed malignant right MCA territory infarction following intravenous thrombolysis and unsuccessful mechanical thrombectomy for right M1 thrombosis (thrombolysis in cerebral infarction score 1). Neurological examination 24 hours postintervention revealed a National Institutes of Health Stroke Scale score of 22 for decreased alertness, dense left hemiparesis, left hemianesthesia, and severe dysarthria. CT imaging of the head performed 24 hours after thrombolysis showed an evolving large right MCA infarction, extensive cytotoxic edema causing prominent brain shift, obliteration of the right lateral ventricle, and left lateral ventricular entrapment (Fig. 1A–D). Hyperosmolar therapy was initiated, and permissive hypernatremia was maintained with sodium levels of 150–155 mmol/L. On poststroke day 2, due to a deteriorating neurological examination, the patient underwent a right DHC. A large fronto-temporoparietal bone flap measuring 12.5 cm in its greatest anterior-posterior diameter was achieved with further craniectomy to the floor of the temporal fossa (Fig 1I and J). No internal decompression was performed at this time, as the initial hemicraniectomy was adequate for cerebral swelling. Neurological examination after initial DHC improved the following day; the patient was opening his eyes spontaneously and following commands on the right side of his body, which was unaffected by stroke.
FIG. 1.
A–D: Poststroke day 1. Noncontrast axial CT images of the head showing large right hemispheric hypodensity consistent with evolving infarction, extensive cytotoxic edema, right-to-left midline shift of 9 mm, right lateral ventricle compression, and left lateral ventricular entrapment. E–H: Poststroke day 4. Noncontrast axial CT images of the head showing right DHC, evolving large right hemispheric infarction, extensive cytotoxic edema, transcalvarial herniation, developing leftward midline shift measuring 10 mm, and new right uncal herniation. I and J: Three-dimensional reconstruction of original right DHC.
CT imaging on poststroke day 4 showed extensive vasogenic edema, a 10-mm leftward midline shift, and new right uncal herniation, despite prior decompression (Fig. 1E– H). This correlated with a decrease in alertness observed during the neurological examination. Further bedside decompression was performed with opening of the scalp incision to allow the brain maximal swelling, leading to immediate extracalvarial brain herniation. Urgent bedside decompression was performed in order to avoid delays associated with preparing the operating room. Since the patient did not require prolonged anesthesia, immediate follow-up with neurological examination was possible to assess the effects of the surgical maneuver. Reopening of the scalp incision led to a prompt improvement in mentation. CT imaging on poststroke day 5 after decompression revealed transcalvarial herniation as a result of the severe cerebral edema (Fig. 2). On subsequent days, loose cranial dressing was maintained over exposed brain and open scalp and the patient was maintained on prophylactic broad-spectrum antibiotics. A tracheostomy and percutaneous gastrostomy tube was placed on poststroke day 7. On poststroke day 8, the brain had reached the point of maximal cerebral swelling. Neurological examination had improved to the point that the patient was more alert and again following commands on the right side of his body, which was unaffected by the stroke. The patient underwent a planned right partial hemispherectomy for excision of the infarcted hemisphere and secondary closure of the scalp incision. The scalp flap was reflected back at the site of the previous open incision. A cauterizer was used to resect the infarcted right frontal, temporal, parietal, and occipital lobes. Duraplasty was achieved with DuraGen (Integra LifeSciences), and a Jackson-Pratt drain was left in place, while the previous scalp edges were debrided, cleaned, and approximated, followed by skin closure. Aspirin 81 mg and subcutaneous heparin were initiated on poststroke day 10.
FIG. 2.
Poststroke day 5. Noncontrast axial CT images of the head showing hemorrhagic conversion, prominent cytotoxic edema, and transcalvarial herniation.
On poststroke day 15, CT imaging of the head showed resolving evolving right hemispheric infarction and a hypodense right subdural/extradural fluid collection over the middle cranial fossa (measuring 1.6 cm) (Fig. 3). Forty milliliters of fluid underneath the scalp was aspirated at bedside. The neurological examination was noted for improving alertness with the ability to communicate via yes/no and thumbs up/down, preserved full strength in the right hemibody, left hemiplegia, and the ability to stand with two-person assist. Mechanical ventilation was discontinued with the patient tolerating humidified air via the tracheostomy.
FIG. 3.
Poststroke day 15. Noncontrast axial CT images of the head posthemispherectomy, showing right hemispheric hemorrhage, within resection cavity, rightward midline shift of 7 mm with transcalvarial herniation, and right middle cranial fossa subdural/extradural fluid collection.
On poststroke day 21, the patient was discharged to acute rehabilitation on aspirin 81 mg once daily and subcutaneous heparin for venous thromboembolism prophylaxis. On poststroke day 30, the patient developed right upper lobe segmental artery pulmonary embolism and left common femoral vein thrombosis for which he was initiated on heparin drip and subsequently transitioned to apixaban. CT imaging of the head on poststroke day 37 showed decreasing right hemispheric edema, resolution of transcalvarial herniation, and reduction in size of right subdural fluid collection (Fig. 4).
FIG. 4.
Poststroke day 37. Noncontrast axial CT images of the head showing decreasing right hemispheric edema, resolution of transcalvarial herniation, and decreased right subdural fluid collection.
As of rehabilitation discharge assessment on poststroke day 83, the patient was alert with soft, dysphonic speech, fully oriented, ambulating 10 steps with maximum assistance and use of a hemiwalker, and tolerating a regular diet with full liquids.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
Large hemispheric strokes pose a significant mortality risk due to development of malignant cytotoxic cerebral edema and subsequent brain herniation. Cytotoxic edema develops from loss of ion transport dependent on adenosine triphosphate (ATP), and begins within minutes to hours after the inciting event. Blood-brain barrier breakdown from ischemia results in vasogenic edema, which peaks 24–48 hours after the event.1 Herniation is ultimately the leading cause of death in large hemispheric infarctions.2 DHC along with comprehensive neurocritical care management is the mainstay in relieving the effects of elevated ICP;3,4 however, some patients may continue to decompensate despite this. The size of the DHC is important, and at least 12 cm is recommended in most studies, which we were able to achieve in our patient. For patients who continued to deteriorate, open scalp incision with delayed hemispherectomy or necrosectomy has not been explored in large studies. Following standard DHC, the maximal allowable brain swelling depends on the size of the DHC and is additionally limited by compliance and stretchability of overlying scalp. Reopening of scalp incision proved to be a lifesaving measure leading to effective brainstem decompression, immediate improvement in neurological examination, and resolution of uncal herniation in our case. The focus of neurocritical care management for a patient with an open scalp incision is to preserve normal physiological conditions of the unaffected healthy brain tissue by maintaining normothermia, normoxia, cerebral perfusion, and electrolytic homeostasis.
The subsequent development of malignant cerebral edema following a large hemispheric infarct is a principal contributor to decline and mortality and takes form in three pathophysiological phases, separated in time: cytotoxic edema, ionic edema, and vasogenic edema.1 Shortly after an infarction, cytotoxic edema occurs due to destruction of the Na+/K+ ATP-ase from lack of glucose and oxygen.5 This results in the creation of an ion osmotic gradient across the cell membrane, which leads to influx of Na+, Cl−, and H2O.5 A key feature of cytotoxic edema is an intact blood-brain barrier, and thus cytotoxic edema typically evolves over minutes to hours and begins subsiding within the first 24 hours after initial infarct.1 This phase is immediately succeeded by ionic edema, which is characterized by endothelial dysfunction resulting in water transport across the luminal membrane via channels and transporters.5 This ionic phase is typically short-lived, occurring about 6 hours prior to the development of vasogenic edema.4 Vasogenic edema is characterized by destruction of the blood-brain barrier, leading to permeability pores to be formed in which water and proteins enter the interstitium.5 This phase typically peaks about 24–48 hours after initial infarct.4 In the case of our patient, the initial cytotoxic edema that occurred after infarction necessitated DHC. However, the progression of vasogenic edema resulted in further mass effect and thus neurological decline. Neurocritical care management of cerebral edema follows a stepwise approach to decrease ICP with the use of pharmacological measures, sometimes leading up to necessitating measures such a hypocapnia, heavy sedation, neuromuscular blocking agents, or pentobarbital coma with therapeutic hypothermia.6 These measures, although necessary, do not come without their own harmful side effects. In the case of our patient, the surgical intervention and immediate improvement in neurological status precluded the need for prolonged conventional medical management, thereby avoiding the associated deleterious side effects commonly seen in the treatment of elevated ICP.
Hemispherectomy of the infarcted tissue was performed to allow for closure of the incision. Hemispherectomy or internal decompression, through the removal of infarcted tissue, is usually not performed and has not been explored in stroke patients who continue to deteriorate. Ebise et al. described use of external and internal decompression involving temporal lobectomy in a traumatic brain injury patient with refractory cerebral edema despite decompression.7 Additional internal decompression with hemispherectomy may be a lifesaving surgery leading to effective brainstem decompression and resolution of uncal herniation in our case. To our knowledge, no similar case of open dura DHC with hemispherectomy has been reported in such circumstances. Remarkably, despite the dramatic and potentially catastrophic nature of the exposed brain, the patient was able to follow commands while the brain remained exposed to the external environment and covered with a loose dressing—an outcome that is highly unusual in such cases (Fig. 5).
FIG. 5.
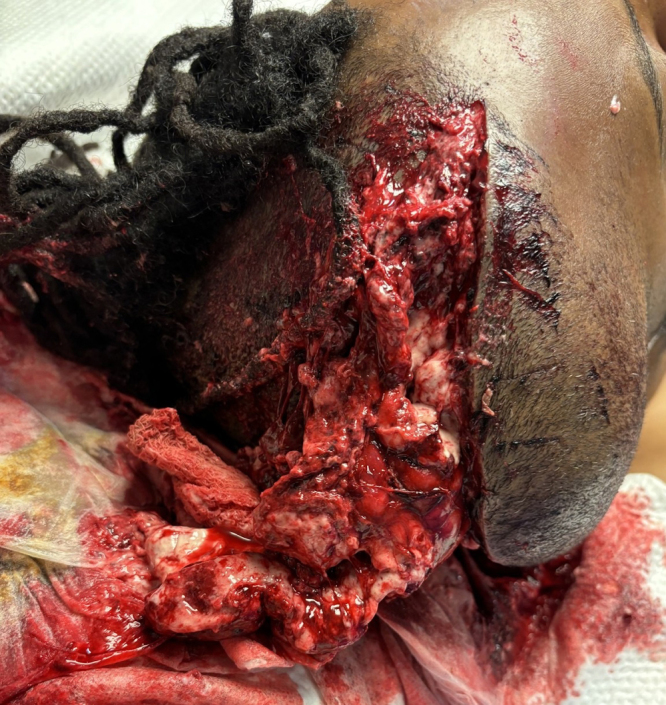
Exposed necrotic brain tissue herniating through open scalp incision.
Potential complications of reopening a scalp incision following hemicraniectomy include wound healing disorders, infection, scalp wound dehiscence, and worsening paradoxical herniation. Our case highlights good functional, neuropsychological, and cognitive outcomes following aggressive surgical management. Neuropsychological assessment on poststroke day 41 showed that the patient was conversational, with an orientation log scale score of 28/30 and cognitive log scale score of 25/30. He was ambulatory to 10 steps with maximum assistance and use of a hemiwalker. He had intact motor functioning of the right side and was tolerating a regular diet with full liquids. The bone flap was replaced on poststroke day 110.
Lessons
Reopening of the scalp incision followed by delayed hemispherectomy, while unconventional, is a lifesaving surgical procedure in patients who continue to deteriorate after external DHC and maximal medical management. Despite the initial grave prognosis, such interventions provide mortality benefit, potentially leading to significant functional gains.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Sherma, To, Muzammil. Acquisition of data: Sherma, To, Muzammil. Analysis and interpretation of data: Olivares, Sherma. Drafting the article: Olivares, Muzammil. Critically revising the article: Olivares, To, Muzammil. Reviewed submitted version of manuscript: Olivares, To, Muzammil. Approved the final version of the manuscript on behalf of all authors: Olivares. Statistical analysis: Olivares. Administrative/technical/material support: Olivares, Sherma. Study supervision: Sherma, To.
Correspondence
Katherine L. Olivares: Wayne State University School of Medicine/Detroit Medical Center, Detroit, MI. kl.olivares@wayne.edu.
References
- 1.Liebeskind DS Jüttler E Shapovalov Y Yegin A Landen J Jauch EC.. Cerebral edema associated with large hemispheric infarction. Stroke. 2019;50(9):2619-2625. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W Schwab S Horn M Spranger M De Georgia M von Kummer R.. “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309-315. [DOI] [PubMed] [Google Scholar]
- 3.Jüttler E, Schwab S, Schmiedek P.Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke. 2007;38(9):2518-2525. [DOI] [PubMed] [Google Scholar]
- 4.Schwab S, Steiner T, Aschoff A.Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29(9):1888-1893. [DOI] [PubMed] [Google Scholar]
- 5.Gu Y, Zhou C, Piao Z.Cerebral edema after ischemic stroke: pathophysiology and underlying mechanisms. Front Neurosci. 2022;16:988283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May CC Smetana KS.. Treatment of elevated intracranial pressure.American College of Pharmacology; 2022. Accessed August 22, 2025. https://www.accp.com/docs/bookstore/ccsap/cc2022b1_sample.pdf [Google Scholar]
- 7.Ebise H, Kubota Y, Ohbuchi H.Aggressive internal and external decompression as a life-saving surgery in a deeply comatose patient with fixed dilated pupils after severe traumatic brain injury: a case report. Surg Neurol Int. 2020;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]






