Abstract
Paramutation is an example of a non-Mendelian-directed allelic interaction that results in the epigenetic alteration of one allele. We describe a paramutation-like interaction between two alleles, bal and cpr1–1 (constitutive expressor of PR genes 1), which map to a complex R-like gene cluster on Arabidopsis chromosome 4. Both alleles cause dwarfing and constitutive defense responses, similar to another dwarf variant, ssi1 (suppressor of SA-insensitivity 1). Previous work has demonstrated that the bal and ssi1 phenotypes are caused by overexpression of an R-like gene from the cluster, which activates an salicylic acid-dependent defense pathway. Here, we show that the cpr1–1 variant does not alter gene expression from the R-like gene cluster. The bal and cpr1–1 alleles did not complement each other in F1 hybrids, but F2 populations that segregated bal and cpr1–1 alleles contained plants with normal morphology at a frequency of 20%. By using molecularly marked bal and cpr1–1 lines, we found that the majority of the normal phenotypes were correlated with inheritance of an altered cpr1–1 allele. Our observation that cpr1–1 is a metastable allele suggests that cpr1–1 is an epigenetic allele. The cpr1–1 allele is the third candidate epigenetic allele originating from this R-like gene cluster, making the region a possible hotspot of epigenetic variation.
In diploids, alleles of a single locus generally operate as independent units that do not affect each other's function. However, there are cases where one allele can transiently or permanently alter the other allele. Transvection is an allelic interaction that involves cross-talk of cis-acting transcriptional regulatory sequences between alleles, leading to pairing-dependent changes in gene expression (1, 2). Transvection effects are transient, and alleles are not altered by their interaction. Conversely, paramutation describes an interaction between two alleles that causes directed, heritable alterations in gene expression without changes in DNA sequence (i.e., epigenetic modifications; refs. 3–5).
Paramutation is best understood in maize, where three loci with alleles sensitive to paramutation have been studied extensively (5, 6). All three loci affect pigmentation of either the kernel or vegetative tissue. Two of the loci, booster1 (b1) (7) and purple plant (pl1) (8), contain single genes, whereas the third locus, red1 (r1) (9–11), has complex haplotypes consisting of paralogous gene segments with interspersed transposon fragments. Paramutagenic alleles at these loci epigenetically alter paired paramutable alleles, and the newly created paramutant alleles can be transmitted in their altered epigenetic state through meiosis. Mechanisms of paramutation are not well understood but seem to involve epigenetic alterations in gene expression imposed by altered chromatin structure and/or differential cytosine methylation (11–14).
One target of epigenetic regulation in the Arabidopsis genome is a complex haplotype on the bottom arm of chromosome 4 (15, 16). In the Columbia strain, the haplotype consists of a cluster of 10 paralogous pathogen Resistance genes or gene fragments belonging to the nucleotide-binding site, leucine rich repeat (NB-LRR) class. The Resistance gene cluster is interspersed with two LTR-containing retrotransposon elements. One of the genes in this cluster (At4g16860) was recently identified as the functional RPP4 locus (17). We have identified an epigenetic variant, bal, that overexpresses another gene from the cluster (At4g16890), resulting in constitutive pathogen response and altered plant morphology (twisted leaves, dwarfing, and reduced fecundity; ref. 18). The semidominant dwarf bal variant was generated in an inbred population of Columbia ddm1 plants (decrease in DNA methylation 1) and is stable in the absence of the ddm1 mutation (19).
The bal variant phenotypically resembles another dwarf called cpr1 (constitutive expressor of PR pathogenesis related proteins 1; refs. 18 and 20). Here, we show that the cpr1–1 mutation is very tightly linked to the chromosome 4 pathogen Resistance gene cluster. Further, we report a paramutation-like interaction between the bal and cpr1–1 alleles that leads to the preferential destabilization of the cpr1–1 allele. Our results suggest that cpr1–1 is an epigenetically modified allele that becomes destabilized in bal/cpr1–1 hybrids.
Materials and Methods
Arabidopsis Genetics.
Arabidopsis plants were grown under long day conditions (16 h day/8 h night, 70% humidity) or short day conditions (8 h day/16 h night, >90% humidity) to differentiate the bal and cpr1–1 variants. All crosses were done by using bal/bal and cpr1–1/cpr1–1, Columbia wild-type, and Landsberg erecta strains with standard methods. The cpr1–1 mutant was provided by X. Dong (Duke University, Durham, NC). cpr1–1 was mapped by using a CAPS marker polymorphic between Columbia and Landsberg strains: forward primer, 5′-GATCAACCAAACTTACAAGAC-3′; reverse primer, 5′-GAGTCAGCAGTGATCTCATC-3′, with BstX1 as the polymorphic restriction enzyme. The bal allele marked with Landsberg came from our previous study and contains Landsberg DNA on the centromeric side of the bal locus that encompasses the SC5 marker (see www.arabidopsis.org).
Nucleic Acid Isolation and Analysis.
Genomic DNA samples were purified with Qiagen reagents and protocols or by the Urea Lysis miniprep protocol (21). Southern blot analyses were performed as described by Jeddeloh et al. (22). Total RNA samples were isolated by using Bio-Rad AquaPure reagents and protocols. Northern blot and reverse transcription (RT)-PCR restriction fragment length polymorphism (RFLP) analyses were performed as described in Stokes et al. (18). In this study, we used the hybridization probes PR2 (23) and R-like (ColF; ref. 15). DNA sequencing was performed by using linear double-stranded templates generated by genomic amplification and Big-Dye Terminator Cycle Sequencing (Perkin–Elmer) protocols/reagents.
Results
Similarities Between the bal and cpr1–1 Variants.
Homozygotes containing the ddm1-induced semidominant bal allele share several characteristics with plants homozygous for the EMS-generated recessive cpr1–1 allele (see Table 1). bal and cpr1–1 variants share morphological similarities such as twisted leaves, reduced fecundity, and dwarfed stature. Interestingly, the morphological phenotypes of the two variants were distinguished by growth in short-day conditions. bal homozygotes grown under short-day conditions showed small, expanded leaves and no chlorosis, whereas cpr1–1 plants grown in parallel exhibited rolled leaves that became chlorotic.
Table 1.
Comparison between bal and cpr1-1 variants
| Characteristic | bal | cpr1-1 | Reference(s) |
|---|---|---|---|
| Strain background | Columbia | Columbia | (19, 20) |
| Generation of variant | ddm1-2 | EMS | (19, 20) |
| Phenotype in long day | Dwarf with twisted leaves | Dwarf with twisted leaves | (19, 20) |
| Phenotype in short day | Small, normal leaves | Small, rolled leaves | This study |
| Genetic behavior relative to Columbia wt allele | Semidominant | Recessive | (18–20) |
| Constitutive PR expression/enhanced defense response | Yes | Yes | (18, 20) |
| Morphological phenotype dependent on EDS1 | Yes | Yes | (18, 24) |
The primary defect in bal and cpr1–1 is constitutive pathogen signal transduction. Both variants constitutively express PR genes that act as downstream effector molecules in plant defense responses (18, 23). In addition, both variants support reduced growth of a bacterial pathogen, Pseudomonas syringae DC3000 (18, 20). Further, both bal and cpr1–1 variant morphologies are suppressed in eds1–1- (enhanced disease susceptibility 1) null genetic backgrounds (24), indicating that the altered signal transduction in both variants affects the TIR-NB-LRR Resistance gene pathway rather than pathways controlled by LZ-NB-LRR class Resistance genes.
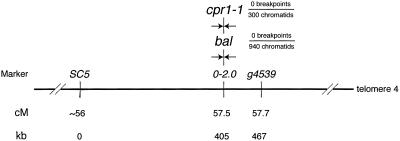
A previous report indicates that the cpr1–1 recessive allele maps to the bottom arm of chromosome 4, closely linked to the BAL locus (25). We refined the position of CPR1 by using a PCR-based molecular marker (0–2.0) tightly linked to the BAL locus (Fig. 1). The 0–2.0 CAPS marker is situated 7 kb telomeric of the Resistance gene cluster that encompasses the BAL locus. We observed no crossover events in 300 chromatids between 0–0.20 and cpr1–1 in a segregating F2 mapping population (Col cpr1–1 × Ler CPR1). In comparison, no crossover events were found between bal and the 0–2.0 marker in 940 chromatids in an F2 Col bal × Ler BAL mapping population (18). These results demonstrate that BAL and CPR1 are tightly linked.
Figure 1.
bal and cpr1–1 map to the same genetic position on chromosome 4.
Genetic Interaction Between bal and cpr1–1 Alleles in F1 Hybrids.
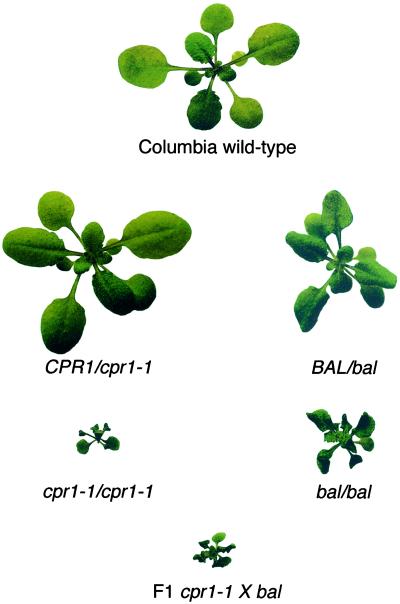
Because the bal and cpr1–1 alleles confer similar phenotypes and are closely linked, we examined the genetic interaction between the two alleles to determine whether they affect the same locus. We performed reciprocal crosses between bal and cpr1–1 homozygotes (both in strain Columbia) and examined the phenotype of the resulting F1 hybrids. As illustrated in Fig. 2, the only phenotypes observed in F1 hybrids were dwarfs (n = 20; 10 from each reciprocal cross). Control crosses confirmed that the cpr1–1 allele is fully recessive for the morphological phenotypes (Fig. 2 and Table 2). The BAL/bal F1 heterozygotes exhibited mild dwarfing and leaf twisting phenotype (Fig. 2), indicating that the bal allele is semidominant, which is consistent with F2 segregation ratios (Table 2). The semidominant nature of the bal allele complicated the genetic interpretation of a straightforward complementation test, but the severe dwarfing in the F1 bal/cpr1–1 hybrids relative to the F1 BAL/bal plants suggests that the two mutations affect the same NB-LRR Resistance gene haplotype.
Figure 2.
Genetic interaction of bal and cpr1–1 in F1 hybrids. The morphology of a representative Columbia wild-type individual is compared with homozygous bal and cpr1–1 individuals. CPR1/cpr1–1 individuals have a wild-type phenotype, whereas BAL/bal plants exhibit mild dwarfing and leaf twisting. In contrast, cpr1–1 × bal F1 hybrids are dwarfed. The plants shown were grown in an 18 h light/6 h dark cycle under 70% humidity in an environmental growth chamber. All plants are 22 days old.
Table 2.
Semidominant segregation of bal variant and recessive segregation of cpr1-1 variant
| Crosses | F2 Phenotypes
|
n = | ||
|---|---|---|---|---|
| Wild type | Intermediate | Dwarf | ||
| WT × bal | 94 | 196 | 109 | 399 |
| WT × cpr1-1 | 369 | 132 | 492 | |
| cpr1-1 × WT | 73 | 22 | 95 | |
R-Like Gene Transcripts Are Not Overexpressed in the cpr1–1 Variant.
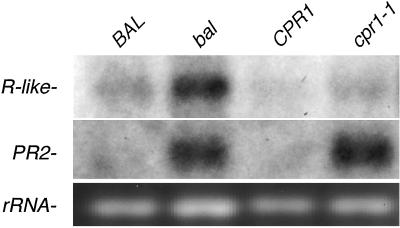
Next, we determined if the cpr1–1 allele leads to the over-expression of a transcript from the NB-LRR Resistance gene cluster, as was previously shown for the bal variant (18). We performed Northern blot analysis by using a hybridization probe that recognizes the first exon of all intact R-like genes in the cluster. We consistently detected little to no increase in gene expression in the R-like gene cluster in cpr1–1 variants (Fig. 3). As a positive control for the Northern blot, we probed the same blot with the pathogenic marker gene PR2, which was constitutively expressed in both bal and cpr1–1 backgrounds in the absence of pathogen attack. Thus, unlike the bal allele, the cpr1–1 allele does not cause a quantitative change in expression from the R-like gene cluster. Moreover, RT-PCR RFLP analysis demonstrated that the At4g16890 gene is the predominant source of the transcripts from the R-like gene cluster in Columbia wild-type, bal and cpr1–1 variants (data not shown). This result indicates that the cpr1–1 allele does not cause a qualitative change in expression from the R-like gene cluster.
Figure 3.
Northern blot shows that an R-like message is not overexpressed in the cpr1–1 background.
Recovery of Plants with Normal Morphology in F2 Populations Arising from F1 bal/cpr1–1 Hybrids.
Because of the extremely tight linkage of bal and cpr1–1 and the severe dwarfing of the bal/cpr1–1 hybrids, we expected to find only dwarf plants in F2 families generated by self-pollination of bal/cpr1–1 F1 hybrids. Unexpectedly, we recovered phenotypically normal plants in these F2 populations at a frequency of approximately ≈20% (Fig. 4 and Table 3). The unexpected phenotypes ranged from plants that were indistinguishable from wild type in terms of size and leaf shape to plants that resembled BAL/bal heterozygotes. The direction of the cross that generated the F1 hybrids did not affect the recovery of phenotypically normal plants in the F2 generation (see below).
Figure 4.
Recovery of phenotypically normal plants in the bal × cpr1–1 F2 generation.
Table 3.
Instability of the dwarfed phenotype in F2 populations from crosses between bal and cpr1-1 homozygotes
| F2 phenotypes | Expected phenotypes in F2 population segregating bal and cpr1-1, % | Observed phenotypes in F2 population segregating bal and cpr1-1, % | Number of plants observed (total = 356) |
|---|---|---|---|
| Wild type | 22 | 78 | |
| Intermediate | 11 | 40 | |
| Dwarf | 100 | 67 | 238 |
Instability Is Predominantly Associated with the cpr1–1 Allele.
Our previous results demonstrated that the bal allele is metastable, leading us to test whether the bal allele is destabilized by passage through bal/cpr1–1 hybrids. Both bal and cpr1–1 were isolated in the Columbia strain, making it necessary to mark one of the alleles to determine which allele was associated with the destabilized phenotype in the F2 generation. We generated a bal/bal genetic background with a polymorphic Landsberg marker, SC5, tightly linked to the Columbia bal allele. Our mapping results (see Fig. 1) indicated that introduction of the portions of the Landsberg genome into the Columbia background do not affect the phenotypic expression of either bal or cpr1–1. The SC5 marker is located 1.5 cM centromere-proximal from the R-like haplotype where bal and cpr1–1 map; therefore, we expect the SC5 genotype to accurately reflect the genotype at the R-like locus except in rare recombinants. We used this line, referred to as balLa, to molecularly distinguish bal and cpr1–1 alleles. Crosses between the balLa and cpr1–1 backgrounds did not show complementation in the F1 generation and exhibited the same instability in F2 populations as crosses using unmarked bal variant lines. We genotyped a total of 461 plants in F2 segregating populations that resulted from reciprocal crosses (Table 4). The polymorphic SC5 marker segregated in the expected codominant Mendelian fashion (see Table 4). However, the distribution of SC5 genotypes relative to the phenotypes was dramatically skewed. In plants with normal morphology, the Columbia allele at the SC5 marker linked to the cpr1–1 allele was overrepresented (167 Columbia alleles vs. 49 Landsberg alleles). The association of the normal phenotype and the Columbia SC5 allele was not affected by the direction of the cross (Table 4). Among the F2 individuals with normal morphology, we recovered 67 Columbia homozygotes compared with only 8 Landsberg F2 homozygotes. These results suggested that instability of the cpr1–1 allele was predominantly responsible for F2 individuals with normal morphology. However, the recovery of phenotypically normal F2 plants homozygous for the Landsberg SC5 allele indicates that the semidominant bal allele also was destabilized in the bal/cpr1–1 hybrids.
Table 4.
Segregation ratios of the SC5 marker that is used to molecularly distinguish the bal and cpr1-1 alleles
| Genotype Phenotype | Col/Col | Col/La | La/La | Total |
|---|---|---|---|---|
| balLa × cpr1-1Col F2 generation* | ||||
| Wild type | 36 | 16 | 6 | 58 (25%) |
| Intermediate | 9 | 12 | 1 | 22 (9%) |
| Dwarf | 25 | 75 | 55 | 155 (66%) |
| Total | 70 | 103 | 62 | 235 |
| cpr1-1Col × balLa F2 generation† | ||||
| Wild type | 31 | 17 | 2 | 50 (22%) |
| Intermediate | 5 | 11 | 2 | 18 (8%) |
| Dwarf | 30 | 82 | 46 | 158 (70%) |
| Total | 66 | 110 | 50 | 226 |
χ2 statistic for 1:2:1 segregation of Col vs. La = 4.12; P > 0.05.
χ2 statistic for 1:2:1 segregation of Col vs. La = 2.43; P > 0.05.
Discussion
We report here an interaction between two Arabidopsis constitutive pathogen response alleles, bal and cpr1–1, that map to the R-like gene cluster on the lower arm of chromosome 4. We have shown previously that the bal allele leads to the overexpression of one of the genes in the R-like gene cluster and is a metastable epigenetic allele (18). Here, we demonstrate that the cpr1–1 mutation is closely linked to the R-like gene cluster and fails to complement the bal allele in F1 bal/cpr1–1 hybrids. cpr1–1 does not cause a quantitative or qualitative gene expression change within this cluster. The bal and cpr1–1 alleles are stable and segregate in the expected Mendelian manner in F2 generations when crossed to wild-type Columbia or Landsberg strains. Surprisingly, approximately 20% of the F2 individuals resulting from self-pollination of F1 bal/cpr1–1 hybrids have a normal morphology. The results cannot be explained by an unlinked extragenic suppressor mutation introduced into the hybrid from the bal or cpr1–1 parent. Rather, the phenotypic and genotypic data shown in Table 4 from F2 populations suggest that both the bal and cpr1–1 alleles are altered by their interaction in the F1 hybrids.
Several models are consistent with our observations. The bal epigenetic allele reverts to a wild-type BAL allele in response to DNA-damaging agents. We propose that a similar stochastic alteration of bal to BAL occurs in response to pairing with the cpr1–1 allele. In turn, we propose that the cpr1–1 allele also is epigenetically destabilized by this pairing interaction and is altered at a higher frequency to a modified allele that conditions normal morphology. We did not see morphologically chimeric F1 plants, suggesting that the bal and cpr1–1 alleles do not change in the vegetative tissues in F1 hybrids, although the systemic nature of the pathogen defense signaling that underlies the dwarf phenotype may obscure such sectoring (26). Further, the segregation data shown in Table 4 are not consistent with the alteration of cpr1–1 in late somatic sectors in F1 plants, because of the under-representation of phenotypically intermediate F2 plants. Destabilization of the recessive cpr1–1 allele ( = > CPR1) would lead to the formation of CPR1Col/balLa self-pollinating F1 flowers and the recovery after Mendelian segregation of twice as many phenotypically intermediate CPR1Col/balLa F2 individuals relative to phenotypically normal CPR1Col/CPR1Col F2 plants. On the other hand, if the semidominant bal allele is destabilized ( = > BAL), creating F1 cpr1Col/BALLa F1 flowers, the most frequent genotypic class among phenotypically normal F2 individuals would be Col/La heterozygotes, which we did not observe. Similar arguments apply to models of allele destabilization in the gametophytes. These considerations suggest that the destabilization of the alleles may occur primarily in the vegetative tissues of F2 plants. In this model, the small number of phenotypically intermediate plants relative to nondwarf individuals indicates that the recessive cpr1–1 allele is destabilized at a higher frequency than the semidominant bal allele.
The induced instability of the cpr1–1 and bal alleles resembles paramutation. Another example of paramutation-like gene silencing among members of a repeated gene family in Arabidopsis has been reported. In this example, the heavily methylated, inverted PAI1-PAI4 repeat leads to the silencing of an unlinked copy of PAI2 (27, 28). It is not known whether newly silencing PAI2 alleles or the altered cpr1–1 and bal alleles described here have gained the ability to silence naïve alleles, as is the case for many paramutable alleles in maize.
We explored various molecular mechanisms to explain our observations of directed change in allele function within the R-like gene cluster. Because transposable elements are often a source of epigenetic variation in gene expression (3), we examined the structure of the two retrotransposable elements embedded within the R-like gene cluster associated with the bal or cpr1–1 allele. We detected no movement or structural changes of either retrotransposon within the R-like gene cluster. In addition, we found no evidence that new transposons have inserted into the region. These results argue against a role for transposition in bal or cpr1–1 allele instability; however, we cannot rule out the possibility that transposons may effect expression of neighboring genes in the absence of structural changes (29–33).
We also searched for other molecular changes in the R-like gene cluster correlated with the cpr1–1 mutation. Our Southern analysis did not detect genomic rearrangements in the gene cluster in the cpr1–1 variant. We also tested the cpr1–1 background for DNA methylation polymorphisms relative to wild-type Columbia or bal plants by Southern analysis by using a battery of seven different cytosine methylation-sensitive restriction enzymes. We previously detected cytosine methylation polymorphisms in both the Columbia bal variant and the related dwarf ssi1 (suppressor of SA insensitivity 1) by using hybridization probes that recognize the chromosome 4 R-like gene cluster (data not shown). However, in the cpr1–1 background, no DNA methylation polymorphisms were detected at either retrotransposon nor within 1 kb upstream of the At4g16890 gene. Finally, we determined the nucleotide sequence of the At4g16890 gene, which is the most abundantly expressed R-like gene in the cluster in Columbia backgrounds, including wild type, bal, and cpr1–1. We found no nucleotide sequence differences in the coding and 5′ upstream regions (≈7 kb total) in the cpr1–1 background.
Our results argue that cpr1–1 is a metastable epigenetic allele mapping to the chromosome 4 R-like gene cluster. Two other candidate epigenetic alleles, bal and ssi1, have been associated with this gene cluster (18). The first variant, bal, was isolated from a ddm1 hypomethylation mutant background, which has generated other epigenetic alleles in the AG, FWA, PAI2, and SUPERMAN loci (22, 34–36). Both the cpr1–1 and ssi1 variants were originally identified in EMS-mutagenized populations (20, 25), consistent with the observation that the DNA-damaging agent can epigenetically modify certain loci (34, 36–38). Our results show a directed change in the cpr1–1 allele occurs in bal/cpr1–1 hybrids. Our study also demonstrates the potential role of epigenetic regulation to generate heritable variation within the Arabidopsis genome.
Acknowledgments
We thank Xinnian Dong for providing cpr1–1 material and Mike Dyer for greenhouse management. This work was supported by National Science Foundation Grants MCB9604972 and MCB9985348 (to E.J.R.). T.L.S. was supported in part by a predoctoral fellowship from the Monsanto Company.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pirrotta V. Biochim Biophys Acta. 1999;1424:M1–M8. doi: 10.1016/s0304-419x(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 2.Wu C T, Morris J R. Curr Opin Genet Dev. 1999;9:237–246. doi: 10.1016/S0959-437X(99)80035-5. [DOI] [PubMed] [Google Scholar]
- 3.Martienssen R. Curr Biol. 1996;6:810–813. doi: 10.1016/s0960-9822(02)00601-2. [DOI] [PubMed] [Google Scholar]
- 4.Hollick J B, Dorweiler J E, Chandler V L. Trends Genet. 1997;13:302–308. doi: 10.1016/s0168-9525(97)01184-0. [DOI] [PubMed] [Google Scholar]
- 5.Chandler V L, Eggleston W B, Dorweiler J E. Plant Mol Biol. 2000;43:121–145. doi: 10.1023/a:1006499808317. [DOI] [PubMed] [Google Scholar]
- 6.Brink R A. Annu Rev Genet. 1973;7:129–152. doi: 10.1146/annurev.ge.07.120173.001021. [DOI] [PubMed] [Google Scholar]
- 7.Patterson G I, Thorpe C J, Chandler V L. Genetics. 1993;135:881–894. doi: 10.1093/genetics/135.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollick J B, Patterson G I, Coe E H, Jr, Cone K C, Chandler V L. Genetics. 1995;141:709–719. doi: 10.1093/genetics/141.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kermicle J L, Eggleston W B, Alleman M. Genetics. 1995;141:361–372. doi: 10.1093/genetics/141.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panavas T, Weir J, Walker E L. Genetics. 1999;153:979–991. doi: 10.1093/genetics/153.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker E L, Panavas T. Genetics. 2001;159:1201–1215. doi: 10.1093/genetics/159.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker E L. Genetics. 1998;148:1973–1981. doi: 10.1093/genetics/148.4.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoekenga O A, Muszynski M G, Cone K C. Genetics. 2000;155:1889–1902. doi: 10.1093/genetics/155.4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollick J B, Patterson G I, Asmundsson I M, Chandler V L. Genetics. 2000;154:1827–1838. doi: 10.1093/genetics/154.4.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker J E, Coleman M J, Szabo V, Frost L N, Schmidt R, van der Biezen E A, Moores T, Dean C, Daniels M J, Jones J D. Plant Cell. 1997;9:879–894. doi: 10.1105/tpc.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel L, Moores T L, van Der Biezen E A, Parniske M, Daniels M J, Parker J E, Jones J D. Plant Cell. 1999;11:2099–2112. [PMC free article] [PubMed] [Google Scholar]
- 17.van der Biezen E A, Freddie C T, Kahn K, Parker J E, Jones J D. Plant J. 2002;29:439–451. doi: 10.1046/j.0960-7412.2001.01229.x. [DOI] [PubMed] [Google Scholar]
- 18.Stokes T L, Kunkel B N, Richards E J. Genes Dev. 2002;16:171–182. doi: 10.1101/gad.952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakutani T, Jeddeloh J A, Flowers S K, Munakata K, Richards E J. Proc Natl Acad Sci USA. 1996;93:12406–12411. doi: 10.1073/pnas.93.22.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowling S A, Guo A, Cao H, Gordon A S, Klessig D F, Dong X. Plant Cell. 1994;6:1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocciolone S M, Cone K C. Genetics. 1993;135:575–588. doi: 10.1093/genetics/135.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeddeloh J A, Bender J, Richards E J. Genes Dev. 1998;12:1714–1725. doi: 10.1101/gad.12.11.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X, Mindrinos M, Davis K R, Ausubel F M. Plant Cell. 1991;3:61–72. doi: 10.1105/tpc.3.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke J D, Aarts N, Feys B J, Dong X, Parker J E. Plant J. 2001;26:409–420. doi: 10.1046/j.1365-313x.2001.2641041.x. [DOI] [PubMed] [Google Scholar]
- 25.Shah J, Kachroo P, Klessig D F. Plant Cell. 1999;11:191–206. doi: 10.1105/tpc.11.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell J M, Dangl J L. Trends Biochem Sci. 2000;25:79–82. doi: 10.1016/s0968-0004(99)01532-7. [DOI] [PubMed] [Google Scholar]
- 27.Bender J, Fink G R. Cell. 1995;83:725–734. doi: 10.1016/0092-8674(95)90185-x. [DOI] [PubMed] [Google Scholar]
- 28.Luff B, Pawlowski L, Bender J. Mol Cell. 1999;3:505–511. doi: 10.1016/s1097-2765(00)80478-5. [DOI] [PubMed] [Google Scholar]
- 29.Argeson A C, Nelson K K, Siracusa L D. Genetics. 1996;142:557–567. doi: 10.1093/genetics/142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan H D, Sutherland H G, Martin D I, Whitelaw E. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 31.Barkan A, Martienssen R A. Proc Natl Acad Sci USA. 1991;88:3502–3506. doi: 10.1073/pnas.88.8.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh C P, Chaillet J R, Bestor T H. Nat Genet. 1998;20:116–117. doi: 10.1038/2413. [DOI] [PubMed] [Google Scholar]
- 33.Michaud E J, van Vugt M J, Bultman S J, Sweet H O, Davisson M T, Woychik R P. Genes Dev. 1994;8:1463–1472. doi: 10.1101/gad.8.12.1463. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen S E, Meyerowitz E M. Science. 1997;277:1100–1103. doi: 10.1126/science.277.5329.1100. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen S E, Sakai H, Finnegan E J, Cao X, Meyerowitz E M. Curr Biol. 2000;10:179–186. doi: 10.1016/s0960-9822(00)00324-9. [DOI] [PubMed] [Google Scholar]
- 36.Soppe W J, Jacobsen S E, Alonso-Blanco C, Jackson J P, Kakutani T, Koornneef M, Peeters A J. Mol Cell. 2000;6:791–802. doi: 10.1016/s1097-2765(05)00090-0. [DOI] [PubMed] [Google Scholar]
- 37.Jones P A, Taylor S M, Wilson V L. Recent Res Cancer Res. 1983;84:202–211. doi: 10.1007/978-3-642-81947-6_15. [DOI] [PubMed] [Google Scholar]
- 38.Wilson V L, Jones P A. Cell. 1983;32:239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]