Abstract
Functional organization of human cerebral hemispheres is asymmetrically specialized, most typically along a verbal/nonverbal axis. In this event-related functional MRI study, we report another example of the asymmetrical specialization. Set-shifting paradigms derived from the Wisconsin card sorting test were used, where subjects update one behavior to another on the basis of environmental feedback. The cognitive requirements constituting the paradigms were decomposed into two components according to temporal stages of task events. Double dissociation of the component brain activity was found in the three bilateral pairs of regions in the lateral frontal cortex, the right regions being activated during exposure to negative feedback and the corresponding left regions being activated during updating of behavior, to suggest that both hemispheres contribute to cognitive set shifting but in different ways. The asymmetrical hemispheric specialization within the same paradigms further implies an interhemispheric interaction of these task components that achieve a common goal.
Flexible adaptation to changing environments is one of the central functions of the prefrontal cortex (1–3). The function called cognitive set shifting is prerequisite to this goal, and is most often instantiated in the Wisconsin card sorting test (WCST) (4), an effective detector of frontal lobe dysfunction (5–11). In this task paradigm, subjects intermittently update one behavioral pattern maintained for a prolonged period to another on the basis of feedback from changing environments. The performance of the set-shifting paradigm is known to be characteristically impaired by lesions in the lateral frontal cortex of both humans (5–10) and monkeys (12, 13). Consistent with neuropsychological studies, a number of neuroimaging studies have demonstrated prominent activation in the lateral frontal cortex during the set-shifting paradigm (14–21).
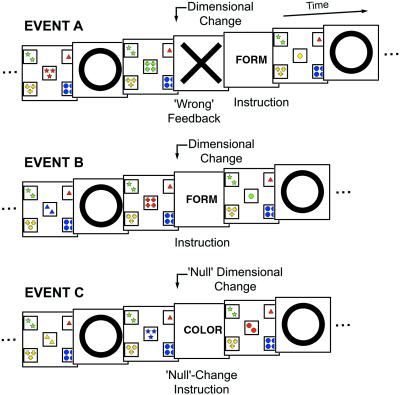
Because of the multipartite nature of cognitive components required for set shifting, however, it remains underdetermined which brain region is implicated in each of the components. In addition, such ambiguity may also have resulted in the controversy as to which hemisphere, right (8) or left (6, 10), of the frontal cortex contributes more critically to cognitive set shifting. As the first step toward direct resolution of this issue, in the present study, the multiple cognitive requirements at the time of set shifting were decomposed into two components according to temporal stages of two task events, that is, exposure to negative feedback and updating of cognitive set. Three task variants (Fig. 1) were used in this study, in which contents of these task events were systematically modified to reveal brain activity derived from each of the two task components. We found double dissociation of brain activity elicited by the two components in the lateral frontal cortex, and the dissociation was observed, unexpectedly, across hemispheres in bilateral homologous regions. Three right lateral frontal regions were activated during exposure to negative feedback, and the corresponding three left frontal regions were activated during updating of cognitive set, suggesting that both hemispheres contribute to cognitive set shifting in different ways. The asymmetrical hemispheric specialization is shared with that in previous neuropsychological (6, 22) and functional neuroimaging (23–26) studies concerning verbal/nonverbal materials, and that related to different stages of memory encoding and retrieval tasks (27). Moreover, a previously undescribed aspect of the double dissociation in the present study would be that the dissociation was observed within the sequential task components, implying that these two regions interact online across hemispheres to adapt to changing environments.
Figure 1.
Three variants of the WCST used in this study. Presented stimuli (card, feedback, and instruction) before and after dimensional changes are shown in temporal order. In this figure the original dimension is “color,” and the dimension is changed into “form” (Top and Middle) or remains the same (Bottom). The transient events that take place at the time of dimensional changes were modified in three ways to examine cognitive components involved in the set-shifting paradigms.
Materials and Methods
Subjects and Functional MRI (fMRI) Procedures.
Informed consent was obtained from 16 healthy right-handed subjects (10 males; 6 females, age, 19–35 years). They were scanned by using experimental procedures approved by the institutional review board of the University of Tokyo School of Medicine. Experiments were conducted by using a 1.5 T fMRI system. Scout images were first collected to align the field of view centered on the subjects' brain. Then T2-weighted spin-echo images were obtained for anatomical reference [repetition time (TR) = 5.5 s; echo time (TE) = 30 ms; 75 slices; slice thickness = 2 mm; in-plane resolution = 2 × 2 mm2). For functional imaging, gradient echo echo-planar sequences were used (TR = 4 s, TE = 50 ms, flip angle = 90°). Each functional run consisted of 68 whole-brain acquisitions (28 slices, in plane resolution 4 mm, 4-mm thickness, no skip between slices, interleaved slice acquisition). The first four functional images in each run were excluded from analysis to take into account the equilibrium of longitudinal magnetization.
Behavioral Procedures.
Visual stimuli were presented to subjects by projecting the stimuli onto a screen. Subjects viewed the screen through prism glasses. A magnet-compatible button press based on a fiber-optic switch was used to record subjects' performance.
The tasks used in this study were derived from the original WCST (5) computerized in our previous studies (17, 28). In each WCST trial, a five-card stimulus was presented until subjects responded to one of four card stimuli at the corner of a screen by matching the attribute of a central card on the basis of the dimension of color, form, or number. A four-channel button was pressed by using the right thumbs for the choice of one of the four card stimuli. A feedback stimulus (right: O; wrong: X) was then presented. After six or more successive correct trials, the currently relevant dimension was changed to one of the others without warning. In the original WCST condition (17), subjects identified subsequent dimensions by trial and error based on the feedback stimuli.
The events that take place at the time of dimensional changes were varied in three ways in the present study (events A, B, and C). In this first modification, at the time of dimensional changes, the negative feedback stimulus was presented, and then a subsequent dimension was signaled by visual presentation of the word “color,” “form,” or “number” (event A) (28). This modification was simplified by omitting the negative feedback stimulus (event B). Finally this modification was further simplified by presenting a “null” change instruction (event C) in a manner otherwise equivalent to the event B. This modification eliminated the execution of cognitive set shifting, but control for perceptual and oddball effects was preserved. As a result, the contrast A minus B extracted brain regions whose activity was modulated dependent on presentation of the negative feedback, whereas the contrast B minus C extracted brain regions whose activity was modulated dependent on updating of cognitive set (29, 30). These three modifications and the original WCST condition were intermixed within runs. The task used a self-paced design, and the instruction and feedback stimuli were presented for 0.5 s, with each stimulus separated by a blank image for 0.25 s.
Data Analysis.
Data were analyzed by using SPM99 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were first realigned, and slice timing was corrected, normalized to the default template with interpolation to a 2 × 2 × 2 mm space, and spatially smoothed (full width, half maximum = 6 mm). Correction for odd/even slice intensity differences was not used because the present study used a relatively long repetition time (4 s). Then event timing was coded into a general linear model (31–34). Four types of transient events during dimensional changes (or “null” changes) in the three modified conditions and the original condition, together with error trials in these conditions, were coded by using the default canonical function in SPM99, time-locked to the onset of the negative feedback stimulus, or time-locked to the onset of the instruction stimulus when the negative feedback stimulus was not presented (Fig. 1). Note that this event-related fMRI design subtracts out the sustained activity related to the card-sorting trials. Images of parameter estimates for signal response magnitudes in these event types were compared between events A and B and between events B and C by using a random effect model. Significant activation was detected above the threshold level of P < 0.05 (corrected, t > 6.6) obtained by masking with the t map for the original WCST condition (P < 0.001, uncorrected).
The difference images were further flipped along midline to test hemispheric asymmetry. The resultant parameter images for each subject were entered into a second-level analysis by using a two-way ANOVA (“A minus B vs. B minus C” × “flipped vs. nonflipped”). Significant interaction effects (23) (P < 0.001, uncorrected, masked with a t map image that kept voxels above P < 0.001 by either contrast “A minus B” or “B minus C”) were used to detect double dissociation patterns of the two subtractions across hemispheres. Correction for whole-brain multiple comparisons was not used because the detected interaction effects were derived from the activation already identified for each contrast. The estimated value of temporal and dispersion derivatives for contrasts A minus B and B minus C in a random effect model were not significant in the three bilateral foci detected (P > 0.05, Bonferroni corrected), indicating that the canonical function fitted well to the signal time courses in these foci.
Results
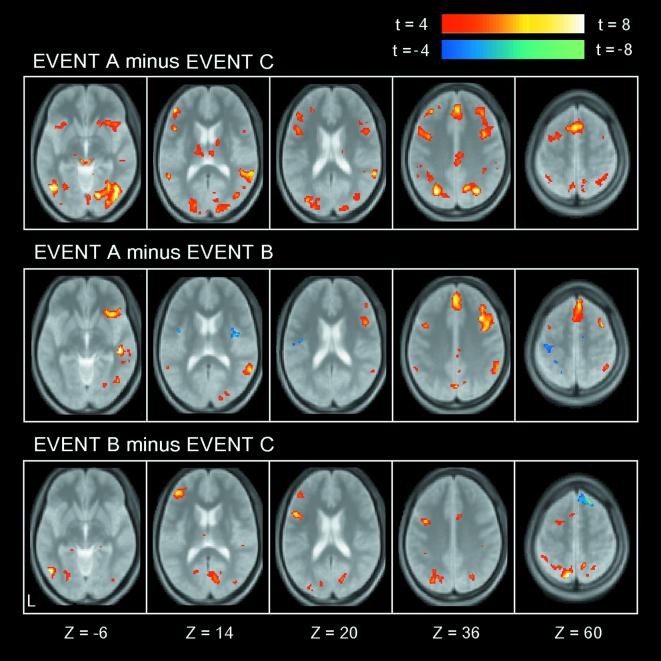
Subjects made correct responses in 99.8 ± 0.1% (mean ± SD) of trials in the three task variants (Fig. 1), excluding inevitable error trials immediately after dimensional changes. The image data set from a pool of sixteen subjects was analyzed by a general linear model implemented in SPM99, and was applied to a random effect model. As shown in Fig. 2 Top, the contrast A minus C revealed prominent bilateral frontal activation. This activation was decomposed into A minus B and B minus C. First, the contrast A minus B revealed a mostly right-dominant activation pattern (Fig. 2 Middle and Table 1). Prominent activations included multiple lateral frontal regions, the medial frontal cortex, anterior insula, precuneus, and the temporo-parietal junction in the right hemisphere. On the other hand, the contrast B minus C revealed a mostly left-lateralized activation including prominent activations in multiple lateral frontal regions and superior parietal lobule in the left hemisphere (Fig. 2 Bottom and Table 1).
Figure 2.
Statistical activation maps for signal increase and decrease in contrasts “A minus C” (Top), “A minus B” (Middle), and “B minus C” (Bottom). The contrast A minus C was decomposed into A minus B and B minus C. The color scale in the maps reflects statistical significance, by using the threshold of t > 4.07, P < 0.001 (uncorrected) for a display purpose. Activation maps are displayed as transverse sections and are overlaid on top of the anatomic image averaged across subjects. The transverse section level is indicated by the Z coordinates of Talairach space at the bottom.
Table 1.
Brain regions showing signal increase in contrasts “A minus B” and “B minus C”
| Contrast | Coordinates
|
Significance level
|
BA | |||
|---|---|---|---|---|---|---|
| X | Y | Z | t value | P value | ||
| A minus B | ||||||
| Lateral frontal cortex | 36 | 18 | 36 | 8.3 | 0.005 | 9 |
| 38 | 10 | 58 | 8.3 | 0.005 | 6 | |
| 38 | 2 | 40 | 7.7 | 0.05 | 6/44 | |
| 46 | 10 | 24 | 7.2 | 0.05 | 45/44 | |
| 36 | 24 | −6 | 7.2 | 0.05 | 47/12* | |
| −40 | 8 | 38 | 6.7 | 0.05 | 9 | |
| Medial frontal cortex | 0 | 36 | 40 | 10.3 | 0.001 | 8 |
| 6 | 18 | 50 | 7.6 | 0.05 | 8/6 | |
| 4 | 30 | 58 | 7.5 | 0.05 | 6/8 | |
| 0 | 48 | 34 | 7.1 | 0.05 | 9/8 | |
| Others | 48 | −24 | −6 | 9.9 | 0.001 | 21 |
| 4 | −74 | 40 | 8.2 | 0.01 | 7 | |
| 60 | −52 | 12 | 7.9 | 0.01 | 22/39 | |
| 60 | −22 | −14 | 7.2 | 0.05 | 20/21 | |
| 12 | −32 | 46 | 7.0 | 0.05 | 7/31 | |
| −56 | −48 | 42 | 6.7 | 0.05 | 40 | |
| B minus C | ||||||
| Lateral frontal cortex | −48 | 12 | 20 | 6.8 | 0.05 | 45/44 |
| −40 | 42 | 14 | 6.7 | 0.05 | 46 | |
| −38 | 2 | 36 | 6.6 | 0.05 | 6/44 | |
| Others | −48 | −64 | −10 | 10.2 | 0.001 | 37/19 |
| −10 | −70 | 60 | 8.2 | 0.01 | 7 | |
| 14 | −76 | 18 | 7.0 | 0.05 | 18 | |
| 12 | −58 | 62 | 6.8 | 0.05 | 7 | |
| −52 | −36 | 42 | 6.7 | 0.05 | 40/7 | |
| −28 | −78 | 34 | 6.6 | 0.05 | 19/39 | |
Coordinates are listed in the Talairach space. BA is the Brodmann area near the coordinates and is approximate. This table lists only main activations above the threshold level of P < 0.05 (corrected, t > 6.6) obtained by masking with the t-map for the original WCST condition (P < 0.001, uncorrected) (see Behavioral Procedures for the original WCST condition). When plural significant peaks occurred within 12 mm of one another, the most significant peak location was kept.
, Area definition by Petrides and Pandya (35).
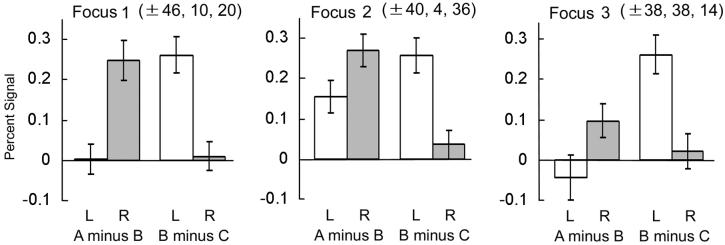
From these findings, the lateral frontal cortex thus has an apparent tendency of double dissociation between the right frontal regions activated in contrast A minus B and the left frontal regions activated in contrast B minus C. To quantify this dissociation pattern, parameter estimates of the difference magnitude from each subject were entered into a voxel-wise two-way ANOVA with laterality (right vs. left) and contrast (A minus B vs. B minus C) as the main effects, and the interaction effect (23) was examined. This analysis revealed three bilateral pairs of frontal areas that showed a significant interaction effect. The Talairach coordinates (36), statistical significance, spatial extent of suprathreshold voxels, and approximate Brodmann area of the three foci are listed in Table 2. The signal magnitude pattern of each contrast and laterality in these foci are further represented in Fig. 3. In the first focus presented in Table 2 (±46, 10, 20), the interaction effect was highly significant, showing a clear “cross” pattern of double dissociation as defined by the interaction of the two-way ANOVA. Importantly, the interaction effect of this focus is consistent with the presence of activation peaks from each contrast at (46, 10, 24) and (−48, 12, 20) (Table 1). This focus conforms to the one reported in our previous studies of set shifting located in the posterior part of the inferior frontal sulcus near Brodmann area 45/44 (see table 2 in ref. 37), functionally homologous to monkey area 45 (38, 39). In the second focus, located at the junction of precentral and inferior frontal sulci (±40, 4, 36), in addition to the double dissociation component, the contrast A minus B in the left hemisphere was also present (t test, P < 0.005) but without any significant main effects. The interaction effect of this focus is also consistent with the presence of activation peaks from each contrast at (38, 2, 40) and (−38, 2, 36) (Table 1). In the third focus (±38, 38, 14), in addition to the double dissociation component, the main effect of the “A minus B vs. B minus C” was also significant (P < 0.05). The left activation peak in the contrast B minus C (−40, 42, 14) (Table 1) fitted well to the focus, but in the right hemisphere the contrast A minus B only revealed an activation peak (40, 36, 12) below P < 0.001 (t = 3.4, P < 0.005). These three bilateral pairs of peak coordinates from contrasts A minus B and B minus C, that is (46, 10, 24) and (−48, 12, 20), (38, 2, 40) and (−38, 2, 36), and (40, 36, 12) and (−40, 42, 14), were separated by less than 5 mm from the peaks of the interaction effects (neglecting the laterality of X coordinates), which is comparable to smoothing filter size (full width, half maximum = 6 mm). It should be noted that no region was detected that showed significant interaction effects in the opposite direction, i.e., right hemisphere activated in contrast B minus C and left hemisphere activated in contrast A minus B.
Table 2.
Lateral frontal regions showing an interaction effect in the two-way ANOVA
| Focus | Coordinates
|
Significance level
|
No. voxels | BA | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | F value | P value | |||
| 1 | ±46 | 10 | 20 | 34.8 | 1 × 10−6 | 122 | 45/44 |
| 2 | ±40 | 4 | 36 | 17.5 | 1 × 10−4 | 13 | 6/44 |
| 3 | ±38 | 38 | 14 | 16.1 | 0.001 | 27 | 46 |
Coordinates are listed in the Talairach space. BA is the Brodmann area near the coordinates and is approximate. The number of voxels was counted based on the threshold level of P < 0.001 (uncorrected). The significant foci were based on the activation detected after correction of multiple comparisons (see Fig. 2 and Table 1).
Figure 3.
Percent signal for contrasts “A minus B” and “B minus C” in the three foci 1, 2, and 3 in the lateral frontal cortex that showed significant interaction effects in the two-way ANOVA. The ordinates indicate percent signal, and the abscissas indicate laterality and contrast. The coordinates are taken from Table 2 and are labeled above each histogram. End stopped lines in the graph bars show SE of means across subjects.
Discussion
The cognitive requirements constituting the set shifting paradigms were decomposed into two components according to the temporal stages of two task events, exposure to negative feedback (contrast A minus B) and updating of cognitive set (contrast B minus C). These contrasts revealed multiple activated regions including prominent activation in the lateral frontal cortex as listed in Table 1. One notable finding regarding the activation pattern was the asymmetrical distribution of the task components in the lateral frontal cortex, i.e., the double dissociation of brain activity modulated by exposure to negative feedback in right lateral frontal regions and that modulated by updating of cognitive set in the corresponding left lateral frontal regions.
One caveat regarding the task design used in this study is the use of serial subtraction on the assumption of pure insertion, where event C serves as baseline for event B, which in turn serves as baseline for event A. However, the data presented in Figs. 2 and 3 collectively indicate that the assumption holds. As shown in Fig. 2, each activation in the foci 1, 2, and 3 was mostly attributable either to contrast A minus B or to B minus C, supporting that the additive model of these contrasts works. Thus, the detection of the double dissociation pattern itself would be an indicator of the additivity in the foci 1, 2, and 3. Outside the foci, if the pure insertion does not hold, violation patterns of the assumption should include those like (i) significant positive signals in contrast A minus B and significant negative signals in contrast B minus C, or (ii) significant negative signals in contrast A minus B and significant positive signals in contrast B minus C in regions where no significant positive or negative signals were observed in contrast A minus C (30). Such a region did not exist except for the right superior frontal regions at the Z level of around 60 mm (Fig. 2 Middle and Bottom), although the activation pattern might be derived from multiple comparisons through whole brain exploration. These results suggest that the additive model works in most regions including the foci 1, 2, and 3 highlighted in this study.
In a recent study by Monchi et al. (21), activity related to individual task events in the WCST was decomposed by using event-related fMRI. The activity related to receiving negative feedback was found bilaterally in the lateral frontal cortex in their study, whereas mainly right-lateralized activation was observed in the present study. Although the right frontal activation during negative feedback is highlighted in the present study, left activation was also present (see Fig. 2 Middle). For example, the left frontal activation in focus 2 (see Fig. 3) was significant (t test, P < 0.005). On the other hand, the two studies differed in many ways, including task parameters. The left frontal activation observed by Monchi et al. might have been accelerated by the longer interval between the presentation of negative feedback and the presentation of a new test card used in their study to dissociate signals from individual task events. The signal isolation strategies used in the two studies were also different. In their study, each type of trial event was coded separately, whereas in the present study, activity of interest was detected relative to a baseline activity related to card-sorting trials sustained throughout runs.
Cognitive set shifting that effectively detects frontal lobe pathology is most often instantiated in the WCST. Salient characteristics of frontal lobe dysfunction can be found in careful observation of the behavior of patients with frontal-lobe lesions while performing the task. As reported in one of the initial neuropsychological studies of the WCST (5), despite his/her knowing that the next card sorting response was wrong, a frontal patient was still unable to change the card-sorting dimension and perseverated on that inappropriate dimension. Several previous articles (6, 10, 40, 41) have attributed the executive aspect of frontal lobe function to the lateral frontal cortex, consistent with the left frontal activation observed in this study during the updating of cognitive set. This left hemisphere dominance in the updating of cognitive set is also shared with prior neuroimaging studies of Stroop test (42) and inhibition of proactive interference (43, 44). On the other hand, a right hemisphere dominance for frontal activation associated with response inhibition, another type of executive function, has repeatedly been reported (45–51). Indeed, right and left frontal activation has also been reported in working memory paradigms that would require executive functions (52, 53). The right lateral frontal activation during exposure to negative feedback in the present study might reflect, as one possibility, the inference made when a subject concluded that the error resulted from a dimensional change rather than from his/her own mistake. It is also possible that the right frontal activation is associated with processing of the negative feedback stimulus in the context of, for instance, reward systems (54, 55). However, it seems unlikely that perceptual processing of a symbolic cue activated the right frontal cortex, because the present study detected transient activity by subtracting out the sustained activity related to card-sorting trials that include perceptual processing of positive feedback stimuli. On the other hand, the right frontal activation might be understood in terms of the right hemisphere activation and interhemispheric interaction rather than as specific regional role. For instance, one might speculate that the right frontal regions might be activated, implying a preparation for later set shifting implemented in the left hemisphere (37).
Another aspect of the asymmetrical functional specialization presented in this study is the fact that it was observed within a sequence of cognitive operations that constitute the same behavioral paradigm, unlike the functional asymmetry observed for verbal vs. nonverbal material specialization or for the different mnemonic stages of encoding and retrieval. This finding may imply that the left and right regions interact online (56, 57), although this study does not provide any evidence at the temporal level. It could be further argued that such cognitive processing is transmitted to the other hemisphere through callosal fibers. Prior neuropsychological studies in split-brain patients and in monkeys have demonstrated that the anterior part of the corpus callosum is capable of transmitting to the other hemisphere higher-order semantic information but not perceptual properties of stimulus input (58, 59), which might share underlying neural mechanisms for the cognitive interaction suggested in the present study. Although open to interpretation as to what is benefited by this hemispheric asymmetry across the same bilateral frontal regions, the present result suggests the interhemispheric interaction of cognitive processes implemented in each hemisphere by which they function for the efficient control of behavior.
Acknowledgments
This work was supported by a Grant-in-Aid for Specially Promoted Research (07102006) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- WCST
Wisconsin card sorting test
- fMRI
functional MRI
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stuss D T, Benson D F. The Frontal Lobes. New York: Raven; 1986. [Google Scholar]
- 2.Fuster J M. The Prefrontal Cortex. New York: Raven; 1997. [Google Scholar]
- 3.Roberts A C, Robbins T W, Weiskrantz L, editors. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford: Oxford Univ. Press; 1998. [Google Scholar]
- 4.Grant D A, Berg E A. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- 5.Milner B. Arch Neurol. 1963;9:90–100. [Google Scholar]
- 6.Milner B. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 7.Nelson H E. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 8.Robinson A L, Heaton R K, Lehman R A W, Stilson D W. J Consult Clin Psychol. 1980;48:605–614. doi: 10.1037//0022-006x.48.5.605. [DOI] [PubMed] [Google Scholar]
- 9.Owen A M, Roberts A C, Hodges J R, Summers B A, Polkey C E, Robbins T W. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- 10.Rogers R D, Sahakian B J, Hodges J R, Polkey C E, Kennard C, Robbins T W. Brain. 1998;121:815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 11.Dehaene S, Changeux J-P. Cereb Cortex. 1991;1:62–79. doi: 10.1093/cercor/1.1.62. [DOI] [PubMed] [Google Scholar]
- 12.Passingham R E. Neuropsychologia. 1972;10:41–46. doi: 10.1016/0028-3932(72)90041-3. [DOI] [PubMed] [Google Scholar]
- 13.Dias R, Robbins T W, Roberts A C. Nature (London) 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 14.Berman K F, Ostrem J L, Randolph C, Gold J, Goldberg T E, Coppola R, Carson R E, Herscovitch P, Weinberger D R. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 15.Nagahama Y, Fukuyama H, Yamaguchi H, Matuszaki S, Konishi J, Shibasaki H, Kimura J. Brain. 1996;119:1667–1675. doi: 10.1093/brain/119.5.1667. [DOI] [PubMed] [Google Scholar]
- 16.Barcelo F, Sanz M, Molina V, Rubia F J. Neuropsychologia. 1997;35:399–408. doi: 10.1016/s0028-3932(96)00096-6. [DOI] [PubMed] [Google Scholar]
- 17.Konishi S, Nakajima K, Uchida I, Kameyama S, Nakahara K, Sekihara K, Miyashita Y. Nat Neurosci. 1996;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 18.Dove A, Pollmann S, Schubert T, Wiggins C J, von Cramon D Y. Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 19.Rogers R D, Andrews T C, Grasby P M, Brooks D J, Robbins T W. J Cogn Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald A W, III, Cohen J D, Stenger V A, Carter C S. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 21.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazzaniga M S. Neuron. 1995;14:217–228. doi: 10.1016/0896-6273(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 23.Kelley W M, Miezin F M, McDermott K B, Buckner R L, Raichle M E, Cohen N J, Ollinger J M, Akbudak E, Conturo T E, Snyder A Z, Petersen S E. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 24.Wagner A D, Poldrack R A, Eldridge L L, Desmond J E, Glover G H, Gabrieli J D E. NeuroReport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- 25.Buckner R L, Kelley W M, Petersen S E. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 26.McDermott K B, Buckner R L, Petersen S E, Kelley W M, Sanders A L. J Cogn Neurosci. 1999;11:631–640. doi: 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- 27.Tulving E, Kapur S, Craik F I, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y. Cereb Cortex. 1999;9:745–753. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- 29.Friston K J, Price C J, Fletcher P, Moore C, Frackowiak R S, Dolan R J. NeuroImage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- 30.Petersen S E, van Mier H, Fiez J A, Raichle M E. Proc Natl Acad Sci USA. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friston K J, Jezzard P, Turner R. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- 32.Worsley K J, Friston K J. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 33.Miezin F M, Maccotta L, Ollinger J M, Petersen S E, Buckner R L. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 34.Donaldson D I, Petersen S E, Ollinger J M, Buckner R L. NeuroImage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- 35.Petrides M, Pandya D N. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- 36.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 37.Konishi S, Donaldson D I, Buckner R L. NeuroImage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- 38.Rushworth M F S, Nixon P D, Eacott M J, Passingham R E. J Neurosci. 1997;17:4829–4838. doi: 10.1523/JNEUROSCI.17-12-04829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakahara K, Hayashi T, Konishi S, Miyashita Y. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 40.Perret E. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 41.Thompson-Schill S L, Swick D, Farah M J, D'Esposito M, Kan I P, Knight R T. Proc Natl Acad Sci USA. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor S F, Kornblum S, Lauber E J, Minoshima S, Koeppe R A. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- 43.Jonides J, Smith E E, Marshuetz C, Koeppe R A, Reuter-Lorenz P A. Proc Natl Acad Sci USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Esposito M, Postle B R, Jonides J, Smith E E. Proc Natl Acad Sci USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Brain Res. 1996;728:79–89. [PubMed] [Google Scholar]
- 46.Garavan H, Ross T J, Stein E A. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama S, Miyashita Y. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 48.Hazeltine E, Poldrack R, Gabrieli J D E. J Cogn Neurosci. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- 49.de Zubicaray G I, Andrew C, Zelaya F O, Williams S C R, Dumanoir C. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- 50.Braver T S, Barch D M, Gray J R, Molfese D L, Snyder A. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 51.Bunge S A, Dudukovic N M, Thomason M E, Vaidya C J, Gabrieli J D E. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ungerleider L G, Courtney S M, Haxby J V. Proc Natl Acad Sci USA. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith E E, Jonides J. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 54.Elliott R, Friston K J, Dolan R J. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Doherty J, Kringelbach M L, Rolls E T, Hornak J, Andrews C. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 56.Gazzaniga M S. Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 57.Houde O, Zago L, Mellet E, Moutier S, Pineau A, Mazoyer B, Tzourio-Mazoyer N. J Cogn Neurosci. 2000;12:721–728. doi: 10.1162/089892900562525. [DOI] [PubMed] [Google Scholar]
- 58.Sidtis J J, Volpe B T, Holtzman J D, Wilson D H, Gazzaniga M S. Science. 1981;212:344–346. doi: 10.1126/science.6782673. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa I, Fukushima T, Ihara T, Miyashita Y. Science. 1998;281:814–818. doi: 10.1126/science.281.5378.814. [DOI] [PubMed] [Google Scholar]