Full text
PDF



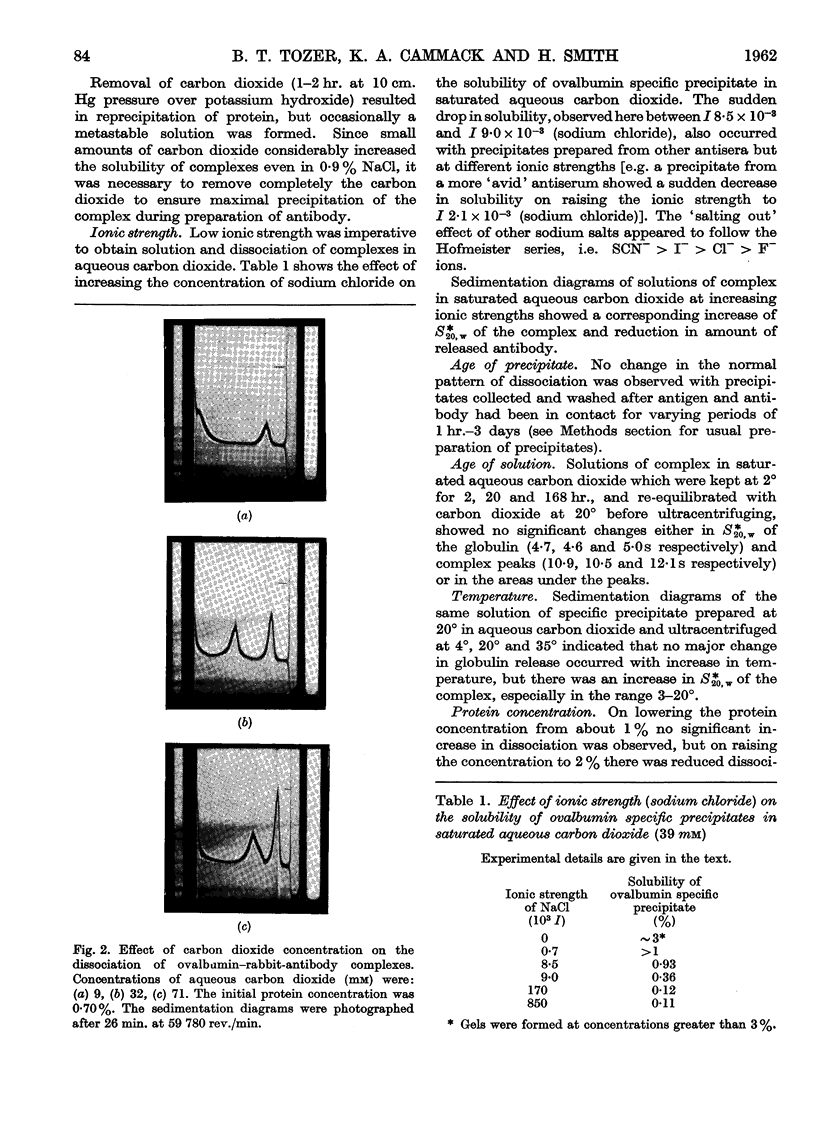

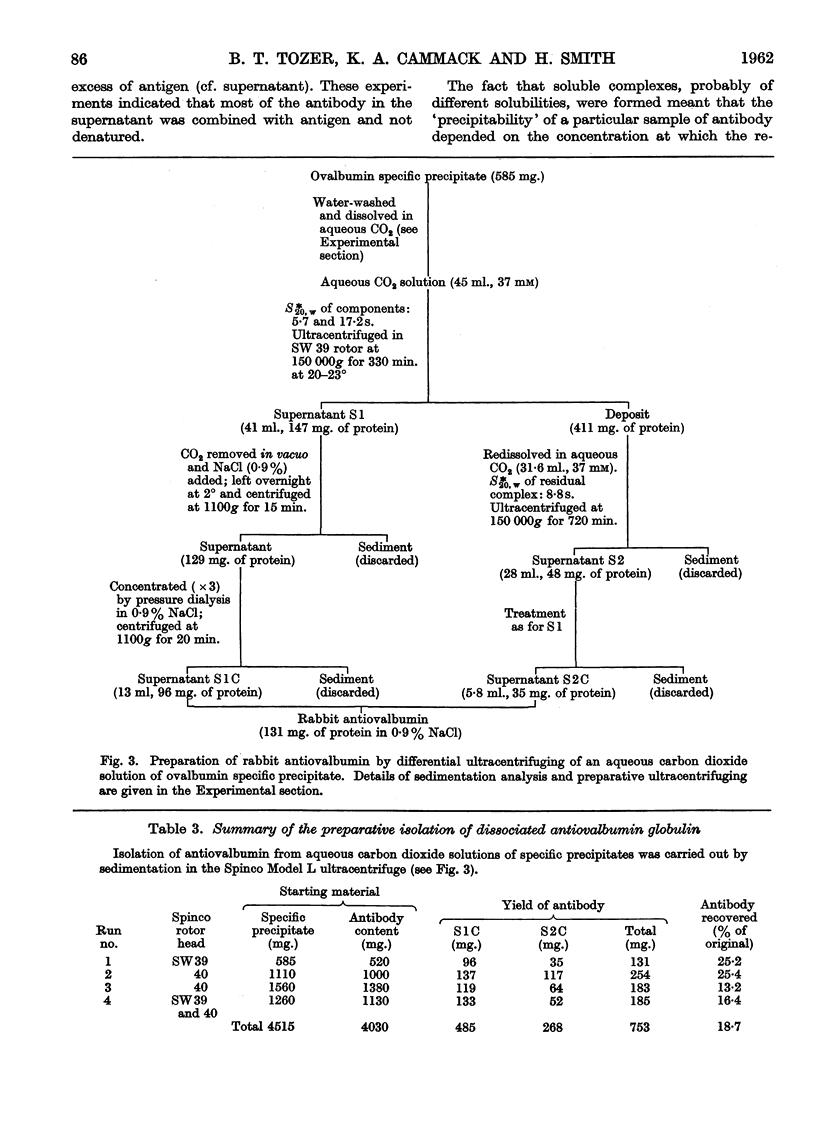
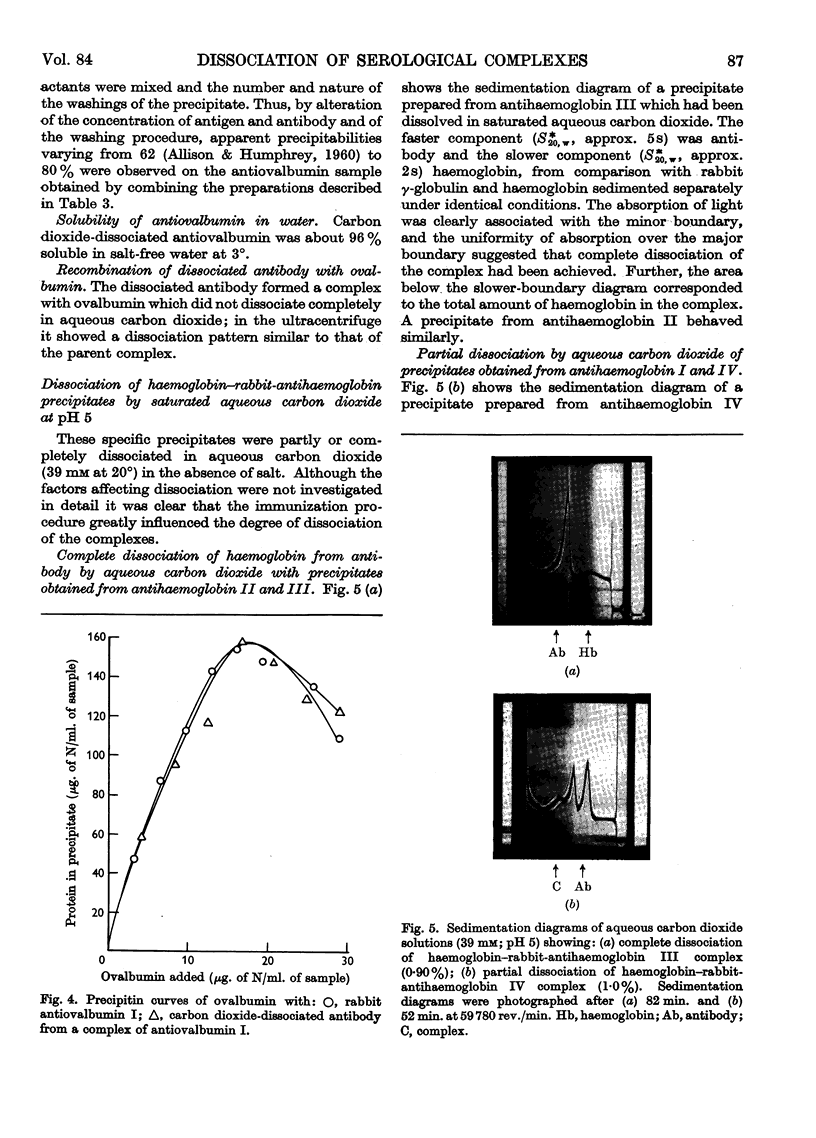






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., JOHNSON P. Macroglobulins. I. Studies on the isolation and physical properties of pathological macroglobulins. Biochem J. 1961 Dec;81:658–669. doi: 10.1042/bj0810658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., HUMPHREY J. H. A theoretical and experimental analysis of double diffusion precipitin reactions in gels, and its application to characterization of antigens. Immunology. 1960 Jan;3:95–106. [PMC free article] [PubMed] [Google Scholar]
- CAMMACK K. A. Molecular weight of rabbit gamma-globulin. Nature. 1962 May 26;194:745–747. doi: 10.1038/194745a0. [DOI] [PubMed] [Google Scholar]
- CINADER B. Antibodies against enzymes. Annu Rev Microbiol. 1957;11:371–390. doi: 10.1146/annurev.mi.11.100157.002103. [DOI] [PubMed] [Google Scholar]
- CREETH J. M. The use of the Gouy diffusiometer with dilute protein solutions; an assessment of the accuracy of the method. Biochem J. 1952 Apr;51(1):10–17. doi: 10.1042/bj0510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. A study on the effects of sodium salicylate and some structurally related compounds on antigen-antibody reaction in vitro. J Immunol. 1953 Feb;70(2):141–146. [PubMed] [Google Scholar]
- GILBERT G. A., JENKINS R. C. Boundary problems in the sedimentation and electrophoresis of complex systems in rapid reversible equilibrium. Nature. 1956 May 5;177(4514):853–854. doi: 10.1038/177853a0. [DOI] [PubMed] [Google Scholar]
- GRANT R. A. The inhibition by dioxan of antigen-antibody precipitation. I. Precipitation reactions with protein antigens. Br J Exp Pathol. 1959 Dec;40:551–558. [PMC free article] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- HUMMELER K., KETLER A. Dissociation of poliomyelitis virus from neutralizing antibody. Virology. 1958 Aug;6(1):297–299. doi: 10.1016/0042-6822(58)90082-5. [DOI] [PubMed] [Google Scholar]
- KATZ J. J. Anhydrous trifluoroacetic acid as a solvent for proteins. Nature. 1954 Sep 11;174(4428):509–509. doi: 10.1038/174509a0. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT W. J., BOYER P. D. Interaction of protein antigens and antibodies. I. Inhibition studies with the egg albumin-antiegg albumin system. J Immunol. 1952 Sep;69(3):247–255. [PubMed] [Google Scholar]
- MARRACK J. R., HOCH H., JOHNS R. G. S. The valency of antibodies. Br J Exp Pathol. 1951 Jun;32(3):212–230. [PMC free article] [PubMed] [Google Scholar]
- MARRACK J. R. The relation of the rates of flocculation and amounts of precipitate in precipitin reactions to the concentration of hydrogen ion and of neutral salts. Immunology. 1958 Jul;1(3):251–267. [PMC free article] [PubMed] [Google Scholar]
- MITZ M. A. The solubility of proteins in the presence of carbon dioxide. Biochim Biophys Acta. 1957 Aug;25(2):426–426. doi: 10.1016/0006-3002(57)90493-6. [DOI] [PubMed] [Google Scholar]
- POPE C. G. Observations on the diphtheria toxin-antitoxin reaction. Br J Exp Pathol. 1957 Apr;38(2):207–216. [PMC free article] [PubMed] [Google Scholar]
- REES E. D., SINGER S. J. A preliminary study of the properties of proteins in some nonaqueous solvents. Arch Biochem Biophys. 1956 Jul;63(1):144–159. doi: 10.1016/0003-9861(56)90018-2. [DOI] [PubMed] [Google Scholar]
- TOZER B. T., CAMMACK K. A., SMITH H. Dissociation of serological complexes of ovalbumin and haemoglobin using aqueous carbon dioxide. Nature. 1958 Sep 6;182(4636):668–669. doi: 10.1038/182668a0. [DOI] [PubMed] [Google Scholar]
- TURNBULL J. H. Antigen-antibody interaction at specific binding sites: a mechanism involving iminazole. Experientia. 1959 Aug 15;15:304–305. doi: 10.1007/BF02158536. [DOI] [PubMed] [Google Scholar]






