Abstract
Cannabis is the most frequently used illicit drug during pregnancy, with use steadily increasing in the United States as legalization and decriminalization expand to more states. Many pregnant individuals use cannabis to reduce adverse symptoms of pregnancy, considering it to be less harmful than other pharmaceuticals or alcohol. The primary psychoactive component of cannabis, delta-9-tetrahydrocannabinol (THC), is a partial agonist of the candidate receptors of the endocannabinoid (eCB) system cannabinoid receptor 1 (CB1R) and 2 (CB2R). However, whether it perturbs neural development of the fetus is poorly understood. Previously we have shown that androgen mediated eCB tone in the developing amygdala promotes microglial phagocytosis of newborn astrocytes which has enduring consequences on the neural circuits regulating sex differences in social behavior. Microglia are the resident immune cells of the brain and express both CB1R and CB2R, making them likely targets of modulation by THC. It is also plausible that exposure to THC at differing gestational timepoints can result in distinct outcomes, as is the case with alcohol exposure. To model human cannabis use during either late or early pregnancy, we exposed rodents to THC either directly during the early postnatal period via intraperitoneal (IP) injection or in utero during the prenatal period via dam subcutaneous (SC) injection respectively. Here we show that postnatal THC exposure results in sex specific changes in microglial phagocytosis during development as well as social behavior during the juvenile period. Interestingly prenatal exposure to THC resulted in inverse changes to phagocytosis and social behavior. These findings highlight the differential effects of THC exposure across gestation.
Subject terms: Neuroimmunology, Glial development
Introduction
With decreasing perception of danger and increasing legalization of cannabis, self-reported use among pregnant populations is steadily increasing [1–3]. Pregnant individuals report using cannabis for many reasons, including to reduce adverse symptoms of pregnancy. Among pregnant individuals that use cannabis to treat at least 1 adverse symptom, most used it to treat nausea and vomiting, with the other most common reason being to reduce anxiety or for general pain [4, 5]. This wide-ranging use of cannabis leads to individuals self-reporting using at varying timepoints across their pregnancy, although on average use is reported to be higher during the first trimester than the later trimesters [1]. Because of the dose and time dependent outcomes associated with prenatal alcohol exposure [6–8], it is possible that the primary psychoactive component of cannabis, delta-9-tetrahydrocannabinol (THC), also elicits specific effects based on the timing of exposure.
THC is a partial agonist of both receptors of the endocannabinoid (eCB) system, cannabinoid receptor 1 (CB1R) and 2 (CB2R) [9]. The eCB system of the developing brain comes online early and signaling via the endogenous ligands 2-arachidonylglycerol (2-AG) and anandamide (AEA) play a critical role in a plethora of developmental processes including the formation of proper brain circuitry and subsequent behavior [10–12]. Previous work from our lab has shown that eCB tone varies by sex in the developing amygdala and promotes microglial phagocytosis of newborn astrocytes which ultimately contributes to a sexually differentiated play circuitry [13, 14]. Here, we sought to model human THC exposure during either the third or second trimesters and determine the impact on microglia of the developing amygdala and subsequent social behavior. In rodents, the early postnatal period, specifically the day of birth through the first week of life, is considered roughly analogous to the third trimester of human pregnancy, while gestational days 15–21 in the rat are analogous to the second trimester [15].
During these developmental windows, microglia, the brain’s resident immune cell, are integral to appropriate brain maturation and development. They are involved in many processes including synapse formation and pruning [16–18], and importantly performing phagocytosis, the act of engulfing and digesting various structures, cells, or debris in both healthy and disease states [19, 20]. These resident macrophages perform phagocytosis to not only clear cellular debris after injury [21], but also regulate a number of processes across healthy brain development including maintaining a balance of neural precursors in the developing cortex [22], maintaining homeostasis of the baseline neurogenic cascade in the hippocampus [23], and regulating the number of newborn astrocytes in the developing amygdala [13]. Microglia possess CB1R and CB2R, and thus are targets for modulation by THC exposure [24].
Here we test the hypothesis that THC exposure during two distinct developmental periods differentially alters social behavior as well as microglial activity in the developing amygdala. Behaviorally, postnatal THC exposure increases social play in males and females, while prenatal THC exposure decreased play in males only. Interestingly, both treatment paradigms impaired social recognition in both sexes. The results from a behavioral battery during development and the juvenile period revealed no effects of either THC exposure paradigm on weight gain, reflex development, or nonsocial behaviors such as open field, novel object recognition, and elevated plus maze. At the cellular level, we find that postnatal THC exposure increases the number of phagocytic microglia in the developing amygdala in females, while prenatal THC exposure decreases the number of phagocytic microglia in both sexes. Taken together, these findings reveal that the timing of THC exposure produces specific changes to social behaviors and differential effects on microglia of the developing amygdala.
Materials and methods
Below is a condensed version of the Materials and Methods, detailed Materials and Methods can be found in Supplementary Materials.
Animal studies and treatments
Adult Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were maintained on a 12:12 h reverse light/dark cycle with ad libitum food and water. Rats were mated in our facility, and pregnant females were designated as gestational day 0 (GD0) on the date of sperm positivity. Pregnant females were allowed to deliver naturally with the day of birth being designated as postnatal day 0 (P0). On P0, pups were sexed and culled to no more than 14 pups per dam. Male and female pups were used in these studies, and treatment groups and sexes were balanced across litters. For prenatal THC exposure studies, all pups were cross fostered to untreated dams with equal numbers of males and females, and equal numbers of treated and control pups per dam. All animal procedures were performed in accordance with the Animal Care and Use Committee’s regulations at the University of Maryland School of Medicine.
Bromodeoxyuridine (BrdU) (50 mg/kg; Sigma-Aldrich Cat#B5002) was dissolved in saline and delivered intraperitoneally in a volume of 0.1 mL per pup per day.
THC was acquired from the NIDA Drug Supply Program pre-dissolved in ethanol and prepared as a 1:1:18 solution of THC:Tween-80:saline (or ethanol:Tween-80:saline for vehicle control). For postnatal THC exposure pups were given an intraperitoneal (IP) injection of THC (5 mg/kg) or vehicle either once daily for 7 consecutive days from P0 to 6 for behavioral studies or once daily for 4 consecutive days from P0 to 3 for histological studies. For prenatal THC exposure pregnant dams were given a subcutaneous (SC) injection of THC (5 mg/kg) or vehicle once daily for 7 consecutive days from GD15 to 21. These periods were chosen to roughly model an exposure to cannabis during the third trimester and second trimester in humans respectively [15, 25]. Appropriate dosing of THC was determined by prior work [26].
Immunohistochemistry, image acquisition, and cell counting
Rats were deeply anesthetized with Fatal Plus (Vortech Pharmaceuticals) and transcardially perfused with phosphate-buffered saline (PBS; 0.1M, pH 7.4) followed by 4% paraformaldehyde (PFA; 4% in PBS, pH 6.8). Brains were removed and postfixed for 24 h in 4% PFA at 4 °C, then kept in 30% sucrose at 4 °C until fully submerged. Coronal sections were cut at a thickness of 45 μm on a cryostat (Leica CM2050S) and directly mounted onto silane-coated slides. Immunohistochemistry was performed to stain for Iba1 and BrdU using either fluorescent or 3,3′-diaminobenzidine (DAB) based staining protocols. Stereological cell counts were performed using StereoInvestigator (MBF Bioscience) on a computer interfaced with a Nikon Eclipse E600 microscope and MBF Bioscience CX9000 camera. Confocal fluorescence images were acquired using the Nikon CSU-W1 or A1 microscope equipped with 405, 488, 561, and 647 lasers, using a 20x (0.75 NA) and Nikon Elements software. Widefield fluorescence and brightfield images were captured on a Keyence BZ-X700 microscope using a 4x (0.2 NA), 20x (0.75 NA), 100x oil (1.40 NA) objective and BZ-X Viewer software. Density of microglia was determined by using Imaris.
Developmental battery
The developmental battery was performed as previously described [27]. Behavior testing took place during the dark phase of the rat’s light/dark cycle under red light illumination. Weights were taken from P0 to 90, dam latency to retrieve pups was performed on P5, surface righting from P5 to 10, developmental locomotion from P5 to 15, negative geotaxis from P5 to 15, and wire hang from P10 to 15.
Juvenile behavioral battery
The behavioral battery was performed as previously described [27]. All behavior testing took place during the dark phase of the rat’s light/dark cycle under red light illumination. Rats were weaned on P21 and housed in same-sex, same-treatment sibling pairs. Open field was performed on P26, novel object recognition on P27, social recognition on P29, social play on P30, and elevated plus maze on P32.
Quantification and statistical analysis
All values are shown as the mean ± SEM. Statistical analyses were performed using GraphPad Prism version 10.4.1 for Windows, GraphPad Software, www.graphpad.com. Data in the figures represent individual offspring values and means. Statistical details of experiments can be found in figure legends (tests used, exact n, p value). Quantification and scoring of behavior was done by researchers blinded to treatment and sex.
Results
Postnatal THC exposure increases social play and decreases social recognition without impacting development or other nonsocial behaviors
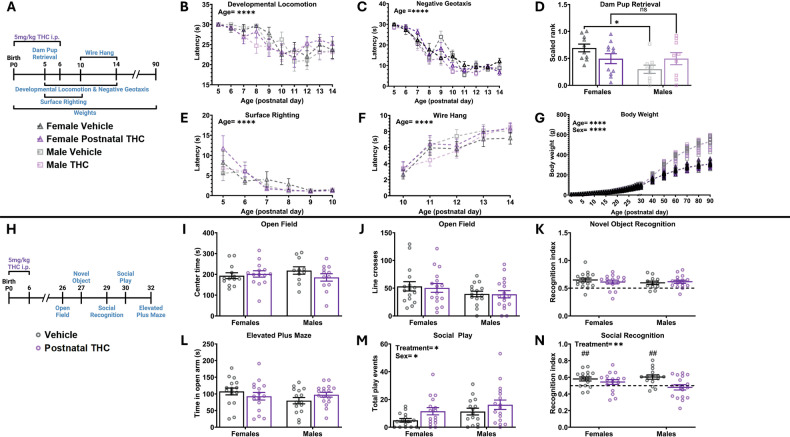
We began by investigating the effects of postnatal THC exposure on typical developmental milestones and behavior. We treated rat pups with THC (5 mg/kg) from P0 to 6 and then assessed behavior using a series of tests (Fig. 1A). Overall, postnatal exposure to THC did not alter developmental locomotion, negative geotaxis reflex, surface righting reflex, wire hang ability, or body weight gain (Fig. 1B, C, E–G). Dams have a natural propensity to retrieve male pups before females [28], however after postnatal THC exposure there was no difference in dam latency to retrieve either sex, thereby abrogating this tendency (Fig. 1D). We then performed a series of behavioral tests during the juvenile period on these same rats (Fig. 1H) and found no effect on open field center time or line crosses, novel object recognition, or open arm time in the elevated plus maze (Fig. 1I–L).
Fig. 1. Postnatal THC exposure increases social play and decreases social recognition without impacting development or other nonsocial behaviors.
Bars represent the mean ± SEM, open circles represent individual data points for each rat. A Schematic showing treatment paradigm, timeline, and legend for (B–G). B Quantification of the latency to locomote. Repeated measures three-way ANOVA revealed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed decrease in latency to locomote as rats aged (F (6.176, 382.9) = 12.12; P < 0.0001) and no effect of treatment on latency. n = 17–18 rats per sex per treatment. ****P < 0.0001. C Quantification of the latency to respond to gravitational cues. Repeated measures three-way ANOVA showed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed decrease in latency to respond to gravitational cues as rats aged (F (4.896, 181.2) = 75.32; P < 0.0001) and no effect of treatment on latency. n = 17–18 rats per sex per treatment. ****P < 0.0001. D Quantification of dam latency to retrieve pups. Repeated measures two-way ANOVA showed sex x treatment interaction (F (1, 35) = 4.882; P = 0.0338). Bonferroni post hoc comparison between groups. n = 9–11 rats per sex per treatment. *P < 0.05. E Quantification of the latency to surface right. Repeated measures three-way ANOVA showed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed decrease in latency to surface right as pups aged (F (2.027, 74.99) = 17.60; P < 0.0001) and no effect of treatment on latency. n = 17–18 rats per sex per treatment. ****P < 0.0001. F Quantification of the latency to fall from a hanging wire. Repeated measures three-way ANOVA showed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed increased latency to fall from a hanging wire as pups aged (F (3.544, 219.7) = 31.29; P < 0.0001) and no effect of treatment on latency. n = 17–18 rats per sex per treatment. ****P < 0.0001. G Quantification of body weight, individual rat data points shown with mean line displayed due to overlapping averages. Mixed-effects analysis showed a main effect of sex (F (1, 60) = 246.9; P < 0.0001) so analysis was repeated as two-way repeated measures ANOVAs to assess the effect of treatment separately in male and female rats. Mixed-effects analysis of females showed an increase in weight across age (F (1.782, 52.47) = 2676; P < 0.0001) with no effect of treatment on weight. Mixed-effects analysis of males showed an increase in weight across age (F (1.442, 41.82) = 3142; P < 0.0001) with no effect of treatment on weight. n = 9–12 rats per sex per treatment. ****P < 0.0001. H Schematic showing treatment paradigm, timeline, and legend for (I–N). I Quantification of open field center time. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 11–14 rats per sex per treatment. J Quantification of open field line crosses. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 11–14 rats per sex per treatment. K Quantification of recognition index during novel object recognition. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 12–16 rats per sex per treatment. Horizontal dashed line indicates recognition index of 0.5. L Quantification of the effect of postnatal THC on time spent in open arm of elevated plus maze. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 15–17 rats per sex per treatment. M Quantification of the total number of play events. Two-way ANOVA showed main effect of sex with males playing more than females (F (1, 59) = 4.424; P = 0.0397) and a main effect of treatment with THC exposure increasing play (F (1, 59) = 4.807; P = 0.0323). n = 15–17 rats per sex per treatment. *P < 0.05. N Quantification of the recognition index score. Two-way ANOVA showed main effect of treatment with THC exposure decreasing recognition index (F (1, 60) = 8.024; P = 0.0063). **P < 0.01. One sample t-test against a hypothetical value of 0.5 of vehicle treated females (t = 3.312, P = 0.0047), THC treated males (t = 1.485, P = 0.1584), vehicle treated males (t = 3.944, P = 0.0015), and THC treated males (t = 0.6330, P = 0.5357). ##P < 0.01. n = 15–17 rats per sex per treatment. Horizontal dashed line indicates recognition index of 0.5.
Interestingly, while postnatal exposure to THC appeared to have no effect on development as well as many nonsocial juvenile behaviors, we found that it significantly altered juvenile social behaviors. Postnatal THC exposure increased the total number of play behaviors in the social play test (Fig. 1M) and decreased the recognition index in the social recognition test (Fig. 1N) in both female and male juvenile rats. Importantly, the recognition indices of THC-treated females and males were not significantly different than a hypothetical value of 0.5 (i.e. value that indicates equal time spent with the familiar and novel stimulus rats; one sample t-test), indicating that THC-treated rats had impaired social memory.
Postnatal THC exposure alters microglial phagocytosis and newborn cell number in the developing amygdala
To assess whether the observed changes in juvenile social behaviors were correlated with altered microglial dynamics in the developing amygdala, we treated pups with both THC and BrdU, to mark newborn cells, from P0-3 (Fig. 2A). This time course and brain region were chosen based on our prior observation that eCB signaling directs microglia-mediated phagocytosis of newborn cells across the first 4-days of life in rats, which is responsible for establishing the sex difference in juvenile play behavior [13]. We analyzed the developing amygdala, determining its boundary using the optic tract (OT) (Fig. 2B) at P4 and visualized microglia via immunohistochemistry using an antibody for ionized calcium binding adaptor molecule 1 (Iba1) (Fig. 2D). Postnatal THC exposure did not alter the total number of microglia (Fig. 2E), but increased the total number of phagocytic microglia, determined by the presence of a phagocytic cup (Fig. 2C), in THC-treated females (Fig. 2F). This was reflected by an increase in the percentage of phagocytic microglia in THC-treated females, with no effect in THC-treated males (Fig. 1G). We then quantified the number of newborn cells and found a corresponding decrease in BrdU+ cell number only in THC-treated females (Fig. 1H). This being consistent with the notion that postnatal THC exposure increased phagocytosis in females, which lead to more newborn cells being phagocytosed, and a subsequent decrease in total newborn cell number.
Fig. 2. Postnatal exposure to THC alters microglial phagocytosis and newborn cell number in the developing amygdala.
Bars represent the mean ± SEM, open circles represent individual data points for each rat. A Schematic showing treatment paradigm, timeline, and legend for (E–H). B 4x hematoxylin and Iba1 stained coronal section of the P4 brain with dashed black line that indicates the boundaries of the developing amygdala used for analysis. Optic tract (OT) and amygdala denoted as landmarks for regions of analysis. Scale bar represents 1000 um. C 100x hematoxylin and Iba1 stained microglia denoting morphology of phagocytic cup (red arrow) used for quantification. Scale bar represents 15 um. D Representative 100x images of hematoxylin and Iba1 labeling from P4 developing amygdala. Black dashed line denotes boundary between amygdala and OT as landmark. Red arrowheads indicate phagocytic microglia. Scale bars represents 40 µm. E Quantification of the total number of all microglia. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 7–8 rats per sex per treatment. F Quantification of the number of phagocytic microglia. Two-way ANOVA showed sex x treatment interaction (F (1, 25) = 17.42; P = 0.0003). Bonferroni post hoc comparison between groups. n = 7–8 rats per sex per treatment. ****P < 0.0001. G Quantification of the percentage of microglia that are phagocytic. Two-way ANOVA showed sex x treatment interaction (F (1, 25) = 19.37; P = 0.0002). Bonferroni post hoc comparison between groups. n = 7–8 rats per sex per treatment. ****P < 0.0001. H Quantification of the total number of BrdU+ cells in the developing amygdala. Two-way ANOVA showed sex x treatment interaction (F (1, 20) = 11.30; P = 0.0031). Bonferroni post hoc comparison between groups. n = 7–8 rats per sex per treatment. ***P < 0.001.
Prenatal exposure to THC alters social play and decreases social recognition without impacting development or other nonsocial behaviors
After observing altered social behavior and microglial dynamics following postnatal THC exposure, we sought to determine if these effects were specific to a defined developmental window. To test this, we exposed pups to THC in utero by treating pregnant dams with THC (5 mg/kg) from GD15-21, then cross fostered pups at birth to an untreated lactating dam. We then assessed the development of the offspring using the same developmental behavior battery as before (Fig. 3A). Similar to our findings above, prenatal THC exposure did not affect developmental locomotion, negative geotaxis reflex, dam latency to retrieve pups, surface righting reflex, wire hang ability, or body weight gain (Fig. 3B–G). We again assessed juvenile behaviors in these same rats (Fig. 3H) and found no changes in open field center time or line crosses, novel object recognition index, or open arm time in elevated plus maze (Fig. 3I–L).
Fig. 3. Prenatal exposure to THC alters social play and decreases social recognition without impacting development or other nonsocial behaviors.
A Schematic showing treatment paradigm, timeline, and legend for (B–G). B Quantification of the latency to locomote. Repeated measures three-way ANOVA revealed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed decrease in latency to locomote as rats aged (F (9, 621) = 8.404; P < 0.0001) and no effect of treatment on latency. n = 15–17 rats per sex per treatment. ****P < 0.0001. C Quantification of the latency to respond to gravitational cues. Repeated measures three-way ANOVA revealed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed decrease in latency to respond to gravitational cues as rats aged (F (3.925, 270.8) = 78.50; P < 0.0001) and no effect of treatment on latency. n = 9–11 rats per sex per treatment. ****P < 0.0001. D Quantification of the dam latency to retrieve pups. Two-way ANOVA shoed no interaction or main effects of sex or treatment. n = 11–12 rats per sex per treatment. E Quantification of the latency to surface right. Repeated measures three-way ANOVA revealed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed decrease in latency to surface right as rats aged F (2.354, 162.4) = 32.38; P < 0.0001) and no effect of treatment on latency. n = 9–11 rats per sex per treatment. ****P < 0.0001. F Quantification of the latency to fall from a hanging wire. Repeated measures three-way ANOVA revealed no effect of sex, data was then collapsed. Repeated measures two-way ANOVA showed an increase in latency to fall from a hanging wire as rats aged (F (3.581, 247.1) = 14.51; P < 0.0001) and no effect of treatment on latency. n = 15–17 rats per sex per treatment. ****P < 0.0001. G Quantification of body weight, individual rat data points shown with mean line displayed due to overlapping averages. Repeated measures three-way ANOVA showed a main effect of sex (F (1, 38) = 129.6; P < 0.0001) so analysis was repeated as two-way repeated measures ANOVAs to assess the effect of treatment separately in male and female rats. Two-way ANOVA of females showed an increase in weight across age (F (1.515, 28.78) = 1759; P < 0.0001) with no effect of treatment on weight. Two-way ANOVA of males showed an increase in weight across age (F (1.427, 27.11) = 3185; P < 0.0001) with no effect of treatment on weight. n = 11–17 rats per sex per treatment. ****P < 0.0001. H Schematic showing treatment paradigm, timeline, and legend for (I–N). I Quantification of open field center time. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 17–18 rats per sex per treatment. J Quantification of open field line crosses. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 17–18 rats per sex per treatment. K Quantification of recognition index during novel object recognition. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 15–18 rats per sex per treatment. Horizontal dashed line indicates recognition index of 0.5. L Quantification of the total number of play events. Two-way ANOVA showed sex x treatment interaction (F (1, 66) = 8.422; P = 0.0050). Bonferroni post hoc comparison between groups. N = 17–18 rats per sex per treatment. *P < 0.05, ***P < 0.001. M Quantification of recognition index score. Two-way ANOVA showed main effect of treatment with THC exposure decreasing recognition index (F (1, 67) = 8.421; P = 0.0050). **P < 0.01. One sample t-test against a hypothetical value of 0.5 of vehicle treated females (t = 3.173, p = 0.0059), THC treated females (t = 1.178, p = 0.2549), vehicle treated males (t = 5.159, p < 0.0001), and THC treated males (t = 0.7001, p = 0.4933). ##P < 0.01 ####P < 0.0001. n = 15–17 rats per sex per treatment. Horizontal dashed line indicates recognition index of 0.5. N Quantification of time spent in open arm of elevated plus maze. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 17–18 rats per sex per treatment.
In line with our prediction that the timing of THC exposure would change its impact on behavior, we found that prenatal THC exposure decreased the total number of play behaviors in males, while females were unaffected. (Fig. 3M). Prenatal THC exposure also decreased the social recognition index in both sexes (Fig. 3N). For both females and males, the recognition index in THC-exposed groups was not statistically different than a hypothetical value of 0.5 while the vehicle treated rat’s index scores were, once again indicating an impairment in social memory.
Prenatal exposure to THC decreases microglial phagocytosis and newborn cell number in the developing amygdala
Because prenatal exposure to THC had distinct effects on social behavior when compared to postnatal exposure to THC, we next sought to determine if microglia were also differentially altered in the developing amygdala. To test this, we again exposed pups to THC in utero by treating pregnant dams from GD15 to 21, cross fostered offspring at birth, and subsequently treated pups with BrdU from P0 to 3 to label newborn cells (Fig. 4A). We quantified microglia (Fig. 4B) and found that prenatal exposure to THC surprisingly decreased the total number of microglia in both sexes (Fig. 4C) and decreased the number of phagocytic microglia (Fig. 4D). However, the decrease in total and phagocytic population of microglia was proportional, meaning that the percentage of phagocytic microglia was unchanged (Fig. 4E). Interestingly, the decrease in phagocytosis did not correspond to an increase in the number of newborn cells in the developing amygdala; rather, we found that prenatal THC exposure also reduced BrdU+ cell number in both sexes (Fig. 4F).
Fig. 4. Prenatal exposure to THC decreases microglial phagocytosis and newborn cell number in the developing amygdala.
A Schematic showing treatment paradigm, timeline, and legend for (C–F). B Representative 100x images of methyl green and Iba1 labeling from P4 developing amygdala. Black dashed line denotes boundary between amygdala and OT as landmark. Red arrowheads indicate phagocytic microglia. Scale bars represent 40 µm. C Quantification of the total number of all microglia. Two-way ANOVA showed main effect of treatment with THC exposure decreasing the number of microglia (F (1, 31) = 33.76; P < 0.0001). n = 8–10 rats per sex per treatment. ****P < 0.0001. D Quantification of the number of phagocytic microglia. Two-way ANOVA showed main effect of treatment with THC exposure decreasing the number of phagocytic microglia (F (1, 31) = 57.16; P < 0.0001) n = 8–10 rats per sex per treatment. ****P < 0.0001. E Quantification of the percentage of microglia that are phagocytic. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 8–10 rats per sex per treatment. F Quantification of the total number of BrdU+ cells. Two-way ANOVA showed main effect of treatment with prenatal THC decreasing the total number of BrdU+ cells (F (1, 31) = 10.65; P = 0.0027). n = 8–10 rats per sex per treatment. **P < 0.01.
Prenatal exposure to THC diminishes microglial population transiently during development
To further investigate the decrease in microglia number at P4 following prenatal THC exposure, we quantified the microglia proliferation within the developing amygdala. We treated pregnant dams with THC from GD15-21 to expose pups in utero, then treated pups with BrdU on P0 to label newborn cells, followed by euthanasia 3 h post injection (Fig. 5A). We then quantified the number of microglia, as well as proliferating microglia (BrdU +) in the developing amygdala (Fig. 5B). Paradoxically, we found no differences in microglia number at P0 (Fig. 5C) and found an increase in BrdU+ microglia (Fig. 5D). We interpret these findings to suggest that the decrease in microglia seen at P4 occurs after the cessation of THC exposure between P0 and P4, rather than being an acute effect of prenatal THC exposure.
Fig. 5. Prenatal exposure to THC alters diminishes microglial population transiently during development.
Bars represent the mean ± SEM. Open circles represent individual data points for each rat. A Schematic showing treatment paradigm and timeline for (C, D). B Representative 20x image of Iba1, DAPI, and BrdU labeling from P0 developing amygdala. Separate images with BrdU and Iba1 channels individually for clearer viewing. White dashed line denotes boundary between amygdala and OT as landmark. White arrowheads indicate proliferating microglia (double positive for Iba1 and BrdU). Scale bars represent 100 µm. C Quantification of the density of microglia. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 6–11 rats per sex per treatment. D Quantification of the density of Brdu+ Microglia. Two-way ANOVA showed main effect of treatment with THC exposure increasing the density of BrdU+ microglia (F (1, 27) = 7.889; P = 0.0091) n = 6–11 rats per sex per treatment. **P < 0.01. E Schematic showing treatment paradigm and timeline for (F). F Quantification of density of microglia in adulthood. Two-way ANOVA showed no interaction or main effects of sex or treatment. n = 8–9 rats per sex per treatment. G Representative 20x image of Iba1 and DAPI from P60+ amygdala. Dashed line denotes medial amygdala. Scale bar represents 1000 µm. H Representative 20x images of Iba1 from P60+ amygdala. Vehicle and THC treated rats to show microglial populations. White dashed line denotes boundary between amygdala and OT as landmark. Scale bars represent 50 µm.
Finally, to determine whether the changes in microglia number after prenatal THC exposure endured into adulthood, we treated pregnant dams with THC from GD15 to 21 and subsequently allowed the offspring to grow to adulthood (P60+) (Fig. 5E). We then quantified microglia within the medial amygdala (Fig. 5G, H) and found no differences in the number of microglia in either sex (Fig. 5F), indicating that the decrement in microglia number is temporary during development.
Discussion
The primary psychoactive component of cannabis, THC, is a partial agonist of the eCB system’s cannabinoid receptors [9]. Cannabis use among pregnant populations is relatively understudied and the need for models of developmental exposure is evident when considering that the amount and timing of exposure to substances such as alcohol during pregnancy can result in vastly different outcomes [6–8]. It is possible that differences reported in cognitive and behavioral outcomes of children exposed to THC [29] could be a consequence of the developmental period during which exposure occurred. Here using a rodent model we find that the timing of THC exposure resulted in some common and some distinct changes to juvenile social behavior while leaving many developmental milestones and behaviors unchanged. Specifically with regards to social play, the changes in play behavior were dependent upon the timing of THC exposure and were accompanied by distinct effects on microglial dynamics within the developing amygdala.
We chose 5 mg/kg IP injection for the postnatal paradigm to deliver consistent levels of THC based on previous work using this method of injection [30–32] as this results in translationally relevant concentrations of THC in the brain (~72 ng/mL) [32–34]. Similarly, when treating pregnant dams we used a 5 mg/kg SC injection as this also results in translationally relevant concentrations of THC in maternal blood and fetal brain (~150 ng/mL) [26]. Both of these concentrations fall within the range of THC concentration seen in human blood following cannabis use (60–200 ng/mL) [35, 36]. Subcutaneous injection was used for pregnant dams to avoid possible misinjection and damaging of fetuses or the uterus. One potential shortcoming of these routes of administration when compared to an inhalation model is that they do result in elevated levels of the metabolite of THC, 11-hydroxy-tetrahydrocannabinol (11-OH-THC), in the brain of pups/fetuses (~98 ng/mL and ~125 ng/mL, respectively) [26, 32]. Importantly, our present study highlights time dependent alterations caused by THC exposure and thus establishes a framework for future investigations to investigate THC dose dependent alterations, as not only does metabolism of THC differ between sexes [37], but exposure to other drugs during development such as alcohol have dose dependent effects [6–8].
THC exposure during the prenatal and postnatal periods in rats does not affect the acquisition of normal developmental milestones and non-social behaviors
We found that neither exposure paradigm (prenatal or postnatal) altered the acquisition of major developmental milestones, and did not affect juvenile locomotor, object memory, or anxiety-like behaviors (Supplementary Table 1). While in humans prenatal THC exposure has been associated with increased anxiety and anhedonia in adolescence [30], other groups working on rodent models have reported no effect on anxiogenic behavior [31–33]. Additionally, studies of prenatal THC exposure in rodents often report mixed effects on birth weight [34, 38], locomotion [34, 39], and ultrasonic vocalizations (USVs) [40, 41], with a meta-analysis suggesting low weighted effect size of THC exposure on behavior and locomotor activity [42]. Here we find only postnatal THC exposure disrupted dam-pup interactions: dams retrieved control male pups faster than females and postnatal THC exposure equalized latencies, delaying male pup retrieval. This change may stem from alterations in ultrasonic vocalizations (USVs), as male pups vocalize more frequently than females which promotes quicker retrieval by the dam [43]. Other reports show that prenatal THC exposure increases pup USVs [44], however, prenatal THC exposure did not alter pup retrieval suggesting that pup-dam communication is unaffected in our paradigm. Overall, these findings indicate THC exposure during these distinct time periods does not dramatically alter development or impact non-social behaviors by the juvenile age.
Juvenile social behavior is specifically altered based on timing of THC exposure
Social play is one of the earliest pro-social behaviors, is highly conserved, and is most robustly displayed during the juvenile period [45–47]. In nearly every species that plays, males engage in more frequent and vigorous rough-and-tumble play than females [48]. The amygdala controls sex differences seen in social play [14], and we have previously shown this difference in rats to be mediated by eCB signaling in the developing amygdala [13]. Given that THC is a partial agonist of eCB receptors, we hypothesized THC exposure might alter the expression of juvenile play behavior. We found that the effects of THC on later-life play behavior were specific to the time of exposure: prenatal THC exposure reduced play behavior in males but did not affect females, while postnatal THC exposure increased play behavior in both sexes. Interestingly, injection of 3 mg/kg or inhalation of cannabis from G6-20 has no effect on social behavior, but does alter pre pulse inhibition, locomotion, and object memory [49]. In this study exposure to THC began earlier than our prenatal paradigm, highlighting that the timing of THC exposure during development may produce differential outcomes.
Both THC exposure paradigms resulted in impaired social memory in both sexes, highlighting the behavioral specificity of the effects of THC exposure. These alterations in social behaviors are consistent with similar work showing cannabinoid exposure altering social behaviors [31, 40, 44, 50]. Given that we found no effect of either THC exposure paradigm on locomotor or anxiety-like behaviors, it seems likely that the observed changes in play are due to specific changes in the development of social behavior circuitry [51, 52]. Furthermore, we found no changes in novel object recognition memory following either THC exposure paradigm, suggesting that specifically social memory, not memory overall, was impaired. This points to both a time dependent and region dependent effect of THC exposure, which could be explained by the timing of neurogenesis and development of various brain structures during early development [15, 53]. Exposure to THC beginning at earlier time points (G5-20) shows male specific deficits in stress-induced pre-pulse inhibition (PPI) and altered ventral tegmental area glucocorticoid receptor expression [54], once again highlighting the importance of timing on what brain structures are affected.
A further caveat to the current findings is the still limited understanding of the nature of sex differences in social play which is scored based on the frequency of pre-determined “elements” such as pouncing, pinning and boxing. The advent of machine learning tools to use unbiased metrics to observe the patterns and syllables of play may reveal heretofore unknown complexity in play elements [55, 56], and that shifts in the nature of play rather than just the amount could be occurring due to THC exposure. This warrants further characterization to how the quality of play could also be changed following THC exposure at varying timepoints in both sexes.
Microglial dynamics in the developing amygdala are specifically altered based on timing of THC exposure
Microglia are critical for neurodevelopment, through production of neurotrophic factors and cytokine secretion, synapse pruning, and, relevant to our study, phagocytosis [57–59]. We found that postnatal THC exposure increased microglial phagocytosis in females but not males and was accompanied by a reduction in BrdU+ cells, consistent with our previous findings demonstrating engulfment of astrocyte progenitors in the developing amygdala. Increased phagocytic activity, and therefore fewer astrocyte progenitors in females, may explain the observed increase in social play, as a lower astrocyte density within the medial amygdala is permissive for higher levels of play [13]. In males, however, postnatal THC exposure increased social play without detectable changes in phagocytosis, suggesting THC exposure may alter the development of social behavior circuitry through additional modulation independent of microglial dynamics. These effects could be related to findings showing that cannabinoid exposure can delay the GABA switch [43], ablate eCB mediated long-term depression in the prefrontal cortex (PFC) as well as nucleus accumbens [31, 50], alter dopaminergic activity [60], and dysregulate striatal epigenetic expression [61].
How eCBs directly influence phagocytic state is unknown, but postnatal THC exposure could be inducing this state directly or it could be indirectly modulating the eCB system by changing the levels of circulating eCBs or their synthetic and degrative enzymes. The eCB ligand 2-AG has been implicated to be a chemoattractant “find me” signal for microglia [62] and thus by modulating its synthetic or degrative enzymes, THC exposure could be indirectly increasing this “find me” signal. Activation of CB1R during adolescence has been shown to mediate microglial apoptosis [63], while activation of CB2R has been shown to not only switch microglia to their more anti-inflammatory phenotype [64], but also switch microglia to their more activated phagocytic phenotype [65]. These highly variable outcomes emphasize the importance of investigating the specific microglial profiles following THC exposure at specific timepoints to determine how alterations in their function may impact brain development.
Prenatal THC exposure reduced the microglia population and microglial phagocytosis in both sexes during the postnatal period. Paradoxically, the decrease in phagocytosis did not increase, but rather decreased newborn cell number in both sexes following prenatal THC exposure. Microglia secrete important trophic factors that promote newborn cell proliferation and survival, namely tumor necrosis factor alpha (TNFα) [66], and THC exposure may alter the expression of these factors during development. THC has been shown to be potently anti-inflammatory in vitro by decreasing macrophage inflammatory protein-1α and β [67], as well as reducing the levels of tumor necrosis factor-α (TNF-α) and interleukin 6 and 8 [68]. However, the full mechanism of THC action during development remains understudied. Prenatal THC exposure from G6-22, alters maternal blood cytokine profiles with supplementation with omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) reversing changes in cytokine expression [69]. However, supplementation with EPA reduces inflammatory cytokines such as TNF-α in maternal blood but neither DHA nor EPA supplementation affect placenta cytokine concentrations [70]. Whether it be direct or indirect, it appears that THC exposure most likely influences cytokine profile in the developing brain.
After cessation of prenatal THC treatment, the microglia population rebounds rapidly via microglia proliferation postnatally. This suggests that microglia during prenatal development may be uniquely sensitive to the effects of THC, and the functional consequences of in utero exposure may be dramatically different than postnatal exposure in rodents. Prenatal THC exposure could be altering the distinct transcriptomic temporal stages of microglial developmental [71]. Similarly, given THC’s effect on chemotaxis [72, 73], the normal colonization and migration patterns of microglia may be interrupted during development.
Effects of THC are likely not limited to the developing amygdala
The current findings paired with our previous work [13] demonstrate the eCB system’s role in sculpting social behavior during brain development. The amygdala is an individual node in the larger social behavior network (SBN) that governs social behavior [52]. Another critical node in social behavior circuitry is the PFC [74]. THC exposure via lactation from THC injected dams from P1 to 10 alters synaptic plasticity and cell excitability in the PFC [75] suggesting THC exposure’s effects are not limited to the amygdala but most likely are altering multiple nodes of the SBN or global circuit dynamics. In our study THC exposure also blunted social recognition, possibly due to alterations in the bidirectional connection between the PFC and amygdala [76]. Additionally, prenatal exposure to THC from G7 to 22 resulted in dysregulated endocannabinoid signaling, an imbalance of excitatory and inhibitory neurons in the PFC, and reduced social recognition [77]. The timing of THC exposure in this study begins earlier than our prenatal paradigm, highlighting that there are likely many critical windows of sensitivity to modulation by THC that differ between brain regions. In conclusion, our findings demonstrate the importance of modeling different periods of gestational cannabis exposure by showing that the timing of THC exposure has differential effects on social behavior as well as microglial dynamics during development. Interestingly, our findings show that social behaviors are uniquely sensitive to disruption by THC exposure, whereas major developmental milestones and behaviors associated with cognition and emotional reactivity were unaffected. Play behavior is critical for the development of later-life social behaviors [78–80]; thus, the behavioral changes due to THC exposure in juvenile rats may have lasting impacts on behavior during adulthood. As society decreases stigma surrounding cannabis, and increases access, it will be increasingly important to understand the enduring but latent effects of use during pregnancy.
Supplementary information
Supplemental Methods and Supplemental Table 1
Author contributions
Conceptualization, JVR, AEM, MMM; Methodology, JVR, AEM, MMM; Formal Analysis, ALP, JVR; Investigation, ALP, AEM, KNS, KRM, JVR; Writing- Original Draft, ALP, JVR, MMM; Writing- Review and Editing, ALP, JVR, MMM; Supervision, JVR, MMM; Funding Acquisition, MMM.
Funding
This work was supported by R01 5R01DA039062-08 to MMM. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Data availability
The datasets generated during and or/analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-025-02173-5.
References
- 1.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322:167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark K, Gryczynski J, Axenfeld E, Schwartz RP, Terplan M. Pregnant women’s current and intended cannabis use in relation to their views toward legalization and knowledge of potential harm. J Addict Med. 2017;11:211–6. [DOI] [PubMed] [Google Scholar]
- 3.Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996-1998. Obstet Gynecol. 2003;101:374–9. [DOI] [PubMed] [Google Scholar]
- 4.Wicken C, Walia A, Solhjoo S, Mark K. Prevalence of cannabis use disorder among pregnant people who test positive for cannabis at time of delivery. AJOG Glob Rep. 2022;2:100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westfall RE, Janssen PA, Lucas P, Capler R. Survey of medicinal cannabis use among childbearing women: patterns of its use in pregnancy and retroactive self-assessment of its efficacy against ‘morning sickness’. Complement Ther Clin Pr. 2006;12:27–33. [DOI] [PubMed] [Google Scholar]
- 6.Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23:726–34. [DOI] [PubMed] [Google Scholar]
- 7.Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. [DOI] [PubMed] [Google Scholar]
- 8.Pierce DR, West JR. Alcohol-induced microencephaly during the third trimester equivalent: relationship to dose and blood alcohol concentration. Alcohol. 1986;3:185–91. [DOI] [PubMed] [Google Scholar]
- 9.Chayasirisobhon S. Mechanisms of action and pharmacokinetics of cannabis. Perm J. 2020;25:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maccarrone M, Guzmán M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharm Sci. 2007;28:83–92. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Ruiz JJ, Berrendero F, Hernández ML, Romero J, Ramos JA. Role of endocannabinoids in brain development. Life Sci. 1999;65:725–36. [DOI] [PubMed] [Google Scholar]
- 13.VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, et al. Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron. 2019;102:435–49.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci USA. 2010;107:20535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeiss CJ. Comparative milestones in rodent and human postnatal central nervous system development. Toxicol Pathol. 2021;49:1368–73. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–9. [DOI] [PubMed] [Google Scholar]
- 18.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33:2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GC. Cell death by phagocytosis. Nat Rev Immunol. 2024;24:91–102. [DOI] [PubMed] [Google Scholar]
- 20.Paolicelli RC, Sierra A, Stevens B, Tremblay ME, Aguzzi A, Ajami B, et al. Microglia states and nomenclature: a field at its crossroads. Neuron. 2022;110:3458–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galloway DA, Phillips A, Owen D, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baglot SL, VanRyzin JW, Marquardt AE, Aukema RJ, Petrie GN, Hume C, et al. Maternal-fetal transmission of delta-9-tetrahydrocannabinol (THC) and its metabolites following inhalation and injection exposure during pregnancy in rats. J Neurosci Res. 2022;100:713–30. [DOI] [PubMed] [Google Scholar]
- 27.VanRyzin, JW, Yu SJ, Perez-Pouchoulen M, McCarthy MM. Temporary depletion of microglia during the early postnatal period induces lasting sex-dependent and sex-independent effects on behavior in rats. eNeuro. 2016;3:ENEURO.0297-16.2016. [DOI] [PMC free article] [PubMed]
- 28.Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013;33:3276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, BF, Salmons KA, Hoyt AT, Swenson KS, Bates EA, Sauder KA, et al. Associations between prenatal and postnatal exposure to cannabis with cognition and behavior at age 5 years: the healthy start study. Int J Environ Res Public Health, 2023;20:4880. [DOI] [PMC free article] [PubMed]
- 30.Leech SL, Larkby CA, Day R, Day NL. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry. 2006;45:223–30. [DOI] [PubMed] [Google Scholar]
- 31.Bara, A, Manduca A, Bernabeu A, Borsoi M, Serviado M, Lassalle O, et al. Sex-dependent effects of in utero cannabinoid exposure on cortical function. Elife. 2018;7:e36234. [DOI] [PMC free article] [PubMed]
- 32.Manduca A, Servadio M, Melancia F, Schiavi S, Manzoni OJ, Trezza V. Sex-specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212-2 depend on mGlu5 receptor signalling. Br J Pharm. 2020;177:449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frau R, Miczán V, Traccis F, Aroni S, Pongor CI, Saba P, et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nat Neurosci. 2019;22:1975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borgen LA, Marvin Davis W, Pace HB. Effects of prenatal Δ9-tetrahydrocannabinol on the development of rat offspring. Pharmacol Biochem Behav. 1973;1:203–6. [Google Scholar]
- 35.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharm Ther. 2007;82:572–8. [DOI] [PubMed] [Google Scholar]
- 36.Fuentes-Verdugo E, Pellon R, Miguens M. Repeated Delta-9-Tetrahydrocannabinol administration dose dependently increases stablished schedule-induced drinking. Psychopharmacology. 2024;241:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot S, Grace LM, et al. Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci Rep. 2021;11:23990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abel EL, Dintcheff BA, Day N. Effects of marihuana on pregnant rats and their offspring. Psychopharmacology. 1980;71:71–4. [DOI] [PubMed] [Google Scholar]
- 39.Fried PA. Short and long-term effects of pre-natal cannabis inhalation upon rat offspring. Psychopharmacology. 1976;50:285–91. [DOI] [PubMed] [Google Scholar]
- 40.Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratù MR, et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology. 2008;198:529–37. [DOI] [PubMed] [Google Scholar]
- 41.Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex. 2005;15:2013–20. [DOI] [PubMed] [Google Scholar]
- 42.Ramírez S, Miguez G, Quezada-Scholz VE, Pardo L, Alfaro F, Varas FI, et al. Behavioral effects on the offspring of rodent mothers exposed to Tetrahydrocannabinol (THC): a meta-analysis. Front Psychol. 2022;13:934600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheyer AF, Borsoi M, Wager-Miller J, Pelissier-Alicot AL, Murphy MN, Mackie K, et al. Cannabinoid exposure via lactation in rats disrupts perinatal programming of the gamma-aminobutyric acid trajectory and select early-life behaviors. Biol Psychiatry. 2020;87:666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weimar HV, Wright HR, Warrick CR, Brown AM, Lugo JM, Freels TG, et al. Long-term effects of maternal cannabis vapor exposure on emotional reactivity, social behavior, and behavioral flexibility in offspring. Neuropharmacology. 2020;179:108288. [DOI] [PubMed] [Google Scholar]
- 45.Palagi E, Antonacci D, Cordoni G. Fine-tuning of social play in juvenile lowland gorillas (gorilla gorilla gorilla). Dev Psychobiol. 2007;49:433–45. [DOI] [PubMed] [Google Scholar]
- 46.Argue KJ, McCarthy MM. Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biol Sex Differ. 2015;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanRyzin JW, Marquardt AE, McCarthy MM. Developmental origins of sex differences in the neural circuitry of play. Int J Play. 2020;9:58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–20. [DOI] [PubMed] [Google Scholar]
- 49.Black T, Barnard IL, Baccetto SL, Greba Q, Orvold SN, Austin-Scott F, et al. Differential effects of gestational Cannabis smoke and phytocannabinoid injections on male and female rat offspring behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2025;136:111241. [DOI] [PubMed] [Google Scholar]
- 50.Scheyer AF, Borsoi M, Pelissier-Alicot AL, Manzoni O. Maternal exposure to the cannabinoid agonist WIN 55,12,2 during lactation induces lasting behavioral and synaptic alterations in the rat adult offspring of both sexes. eNeuro. 2020;7:ENEURO.0144–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J Neurosci. 2017;37:4103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen VS, Morrison JP, Southwell MF, Foley JF, Bolon B, Elmore SA. Histology atlas of the developing prenatal and postnatal mouse central nervous system, with emphasis on prenatal days E7.5 to E18.5. Toxicol Pathol. 2017;45:705–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valeria S, Francesco T, Sonia A, Laura VP, Luca C, Marcello S, et al. Sex-specific maladaptive responses to acute stress upon in utero THC exposure are mediated by dopamine. Pharm Res. 2024;210:107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinreb C, Pearl JE, Lin S, Osman M, Zhang L, Annapragada S, et al. Keypoint-MoSeq: parsing behavior by linking point tracking to pose dynamics. Nat Methods. 2024;21:1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klibaite U, Li T, Aldarondo D, Akoad JF, Ölveczky BP, Dunn TW. Mapping the landscape of social behavior. bioRxiv. 2024. https://www.biorxiv.org/content/10.1101/2024.09.27.615451v1. [DOI] [PMC free article] [PubMed]
- 57.Perez-Pouchoulen M, VanRyzin JW, McCarthy MM. Morphological and phagocytic profile of microglia in the developing rat cerebellum. eNeuro. 2015;2:ENEURO.0036-15.2015. [DOI] [PMC free article] [PubMed]
- 58.Mordelt A, de Witte LD. Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: hype or hope? Curr Opin Neurobiol. 2023;79:102674. [DOI] [PubMed] [Google Scholar]
- 59.Mastenbroek LJM, Kooistra SM, Eggen B, Prins JR. The role of microglia in early neurodevelopment and the effects of maternal immune activation. Semin Immunopathol. 2024;46:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez de Fonseca F, Cebeira M, Hernández ML, Ramos JA, Fernández-Ruiz JJ. Changes in brain dopaminergic indices induced by perinatal exposure to cannabinoids in rats. Brain Res Dev Brain Res. 1990;51:237–40. [DOI] [PubMed] [Google Scholar]
- 61.Ellis RJ, Bara A, Vargas CA, Frick AL, Loh E, Landry J, et al. Prenatal delta(9)-tetrahydrocannabinol exposure in males leads to motivational disturbances related to striatal epigenetic dysregulation. Biol Psychiatry. 2022;92:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasegawa Y, Kim J, Ursini G, Jouroukhin Y, Zhu X, Miyahara Y, et al. Microglial cannabinoid receptor type 1 mediates social memory deficits in mice produced by adolescent THC exposure and 16p11.2 duplication. Nat Commun. 2023;14:6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young AP, Denovan-Wright EM. The dynamic role of microglia and the endocannabinoid system in neuroinflammation. Front Pharm. 2021;12:806417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mecha M, Feliú A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutiérrez S, de Sola RG, et al. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233–45. [DOI] [PubMed] [Google Scholar]
- 66.VanRyzin JW, Marquardt AE, McCarthy MM. Feminization of social play behavior depends on microglia. bioRxiv. 2024. https://www.biorxiv.org/content/10.1101/2024.08.19.608675v1.full.
- 67.Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suryavanshi SV, Zaiachuk M, Pryimak N, Kovalchuk I, Kovalchuk O. Cannabinoids alleviate the LPS-induced cytokine storm via attenuating NLRP3 inflammasome signaling and TYK2-mediated STAT3 signaling pathways in vitro. Cells. 2022;11:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee K, Sarikahya MH, Cousineau SL, Yeung KK, Lucas A, Loudon K, et al. Maternal dietary DHA and EPA supplementation ameliorates adverse cardiac outcomes in THC-exposed rat offspring. Sci Rep. 2025;15:8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mozurkewich EL, Berman DR, Vahratian A, Clinton CM, Romero VC, Chilimigras JL, et al. Effect of prenatal EPA and DHA on maternal and umbilical cord blood cytokines. BMC Pregnancy Childbirth. 2018;18:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matcovitch-Natan O, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. [DOI] [PubMed] [Google Scholar]
- 72.Raborn ES, Marciano-Cabral F, Buckley NE, Martin BR, Cabral GA. The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor. J Neuroimmune Pharm. 2008;3:117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizzo MD, Crawford RB, Bach A, Sermet S, Amalfitano A, Kaminski NE. Delta(9)-tetrahydrocannabinol suppresses monocyte-mediated astrocyte production of monocyte chemoattractant protein 1 and interleukin-6 in a toll-like receptor 7-stimulated human coculture. J Pharm Exp Ther. 2019;371:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sotoudeh N, Namavar MR, Bagheri F, Zarifkar A. The medial prefrontal cortex to the medial amygdala connections may affect the anxiety level in aged rats. Brain Behav. 2022;12:e2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheyer AF, Borsoi M, Pelissier-Alicot AL, Manzoni O. Perinatal THC exposure via lactation induces lasting alterations to social behavior and prefrontal cortex function in rats at adulthood. Neuropsychopharmacology. 2020;45:1826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goetschius LG, Hein TC, Mattson WI, Lopez-Duran N, Dotterer HL, Welsh RC, et al. Amygdala-prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. Neuroimage. 2019;191:278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVuono MV, Nashed MG, Sarikahya MH, Kocsis A, Lee K, Vanin SR, et al. Prenatal tetrahydrocannabinol and cannabidiol exposure produce sex-specific pathophysiological phenotypes in the adolescent prefrontal cortex and hippocampus. Neurobiol Dis. 2024;199:106588. [DOI] [PubMed] [Google Scholar]
- 78.Vanderschuren LJ, Achterberg EJ, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellis SM, Pellis VC, Ham JR, Stark RA. Play fighting and the development of the social brain: the rat’s tale. Neurosci Biobehav Rev. 2023;145:105037. [DOI] [PubMed] [Google Scholar]
- 80.Marquardt AE, VanRyzin JW, Fuquen RW, McCarthy MM. Social play experience in juvenile rats is indispensable for appropriate socio-sexual behavior in adulthood in males but not females. Front Behav Neurosci. 2022;16:1076765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods and Supplemental Table 1
Data Availability Statement
The datasets generated during and or/analyzed during the current study are available from the corresponding author on reasonable request.