Abstract
Tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6) is an adapter protein that links signals from members of the TNFR superfamily and Toll/IL-1 receptor family to activation of transcription factors NFκB and AP-1. Analysis of TRAF6-deficient mice revealed that TRAF6 is essential for normal bone formation and establishment of immune and inflammatory systems. Here we report that TRAF6 deficiency results in defective development of epidermal appendixes, including guard hair follicles, sweat glands, sebaceous glands of back skin, and modified sebaceous glands such as meibomian glands, anal glands, and preputial glands. Except the sebaceous gland impairment, these abnormal phenotypes are identical to those observed in Tabby (Ta), downless (dl), and crinkled (cr) mice, which are models of hypohidrotic (anhidrotic) ectodermal dysplasia in human. β-catenin and mucosal addressin cell adhesion molecule-1, an early marker of developing guard-hair follicles is absent in the skin of TRAF6-deficient embryos. Thus, TRAF6 is essential for development of epidermal appendixes. TRAF6 does not associate with the cytoplasmic tail of the dl protein (DL)/ectodysplasin receptor (EDAR) receptor, which, when mutated, results in hypohidrotic (anhidrotic) ectodermal dysplasia. However, TRAF6 associates with X-linked ectodysplasin-A2 receptor (XEDAR) and TNFR super family expressed on the mouse embryo (TROY/toxicity and JNK inducer (TAJ), which are EDAR-related members of the TNFR superfamily that are expressed at high level in epidermal appendixes. Furthermore, TRAF6 is essential for the XEDAR-mediated NFκB activation. Our results suggest that TRAF6 may transduce signals emanating from XEDAR or TROY/TAJ that are associated with development of epidermal appendixes.
Clinical hypohidrotic (anhidrotic) ectodermal dysplasia (HED) is a congenital disorder of ectodermal differentiation in which the individuals have no sweat glands; they also have sparse scalp hair and abnormal teeth (1). The molecular defects that cause HED have been uncovered recently. Kere et al. (2) reported that a transmembrane protein, ectodysplasin (EDA), is disrupted in some HED patients. A mouse model of HED, Tabby (Ta), was found to be caused by a mutation in the murine EDA gene (3, 4). Structural analysis of the Ta protein revealed that EDA/Ta is a member of the tumor necrosis factor (TNF) family (5). Therefore, the ectodysplasin receptor (EDAR) was hypothesized to be a member of the TNFR superfamily. As expected, the novel TNF receptor (TNFR) family gene was mapped to the downless (dl) locus (6, 7). dl is also a mouse model of HED. Mutations of this newly identified receptor [the dl protein (DL)/EDAR] were found to be the cause of some clinical HED patients (7). These studies indicated that EDA/EDAR (Ta/DL) signal transduction plays important roles in the formation of hair follicles and skin eccrine glands. Recently, two other EDAR-related members of the TNFR superfamily, X-linked ectodysplasin-A2 receptor (XEDAR; ref. 8) and TROY/TAJ (9, 10) were reported. They also are thought to be involved in ectodermal differentiation because they are expressed in skin, especially in hair follicles. Signals from these three receptors were shown to activate transcription factor NFκB (8–11), and the specific missense mutations in NFκB essential modifier (NEMO)/IκB kinase γ(IKKγ), which is essential for NFκB activation (12), result in HED and immunodeficiency (13, 14). Thus, HED could result from impaired NFκB signaling, possibly triggered by members of the TNFR superfamily expressed in skin and hair follicles.
The TNFR-associated factor 6 (TRAF6) is a cytoplasmic adapter protein that links signals from members of the TNFR superfamily and the Toll/IL-1 receptor family (15) to activation of transcription factors, such as NFκB through IKK activation and AP-1 through activation of mitogen-activated protein kinase including Jun N-terminal kinase, p38, and extracellular signal-regulated kinase. We and others reported (16–18) that TRAF6-deficient (TRAF6−/−) mice are defective in osteoclast formation because of defective signaling from receptor activator of NFκB upon binding of osteoclast differentiation factor/receptor activator of NFκB ligand (also known as OPGL and TRANCE). Therefore, TRAF6−/− mice exhibit severe osteopetrosis. Furthermore, TRAF6−/− mice are defective in normal B cell differentiation, lymph node organogenesis, IL-1 signaling, lipopolysaccharide signaling, and neural tube closure (16, 17, 19). Thus, TRAF6 plays critical roles in normal bone formation and immune and inflammatory systems in vivo. However, involvement of TRAF6 in ectodermal development has not been reported.
In the present study, we found that TRAF6−/− mice display HED, which may be caused by defective signals from XEDAR or TROY/TAJ.
Materials and Methods
Mice, Cell Culture, Abs, and Plasmids.
The generation of TRAF6−/− mice has been described previously (16). 293T cells were cultured in DMEM supplemented with 10% (vol/vol) FBS. mAbs against mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (MECA367, BD PharMingen), the FLAG epitope (Sigma), and human CD40 (B-B20, Serotec), and rabbit polyclonal Abs against β-catenin (H-102) and TRAF6 (H-274, Santa Cruz Biotechnology) were purchased. Anti-human CD40 mAb (G28–5) was purified from culture medium of American Type Culture Collection HB-9110. For generation of vectors expressing glutathione S-transferase (GST) fused with the cytoplasmic tail of mouse DL (amino acid 212–448), mouse TROY (amino acid 194–416), or human XEDAR (amino acid 158–297), each cDNA fragment encoding the corresponding peptide was ligated into pME18S (20) with the DNA fragment encoding GST. An expression vector for FLAG-tagged TRAF6 has been described (21). For generation of a retrovirus vector expressing a chimeric receptor, pMX-CD40/XEDAR, in which the extracellular domain of human CD40 (amino acid 1–188) was fused to the cytoplasmic tail of XEDAR (amino acid 135–297), the cDNA fragments encoding the corresponding peptide were ligated into pMXpuro (22).
Histology and Immunohistochemistry.
For flat-mount tissue preparations, neonatal mouse skin was incubated in fixing solution [4% (wt/vol) paraformaldehyde in PBS] for 2 h and then flattened on a slide glass under dissecting microscope and mounted with Aquatex (Merck) and a coverslip. Excised fetuses were subjected to whole-mount immunostaining performed by using anti-MAdCAM-1 mAb (2 μg/ml) and horseradish peroxidase-conjugated anti-rat Ig Ab (1 μg/ml) as described (23). The enzymatic reaction was allowed to proceed until the desired color intensity was reached. For section staining, embryos [from embryonic day (E)15.5 to E18.5] and skin and hand specimens from 5-day-old mice were fixed in 2% (wt/vol) paraformaldehyde in PBS for 2–12 h, washed for 30 min, and embedded in OCT compound. Sections (5 μm) were dried up by microwave and were incubated in 2% (wt/vol) paraformaldehyde for 20 min followed by two time washing in PBS. To block endogenous peroxidase activity, sections were incubated in 0.1% NaN3 in PBS for 20 min, and then H2O2 was added to 0.1% final concentration and incubated for 5 min. After washing three times in PBS, nonspecific binding of Abs was blocked in PBSMT [3% (wt/vol) skim milk/0.1% Triton X-100 in PBS] for overnight at 4°C. Anti-β-catenin or anti-TRAF6 Ab was incubated on slides for 12 h at 4°C and detected by using horseradish peroxidase conjugated anti-rabbit Ig Abs with 3-h incubation at room temperature. After washing five times, sections were soaked in PBST containing 300 μg/ml diaminobenzidine (Dojin, Kumamoto, Japan) for 20 min, and then H2O2 was added to 0.01% final concentration.
In Vivo GST Pull-Down Assay.
293T cells were cotransfected with expression vectors for FLAG-tagged TRAF6 and GST-tagged cytoplasmic tail of DL, TNFR superfamily expressed on the mouse embryo (TROY), or XEDAR. After transfection (36 h), cells were harvested and lysed with TNE buffer (50 mM Tris·HCl, pH 8.0/1% NP-40/1 mM EDTA/150 mM NaCl/0.5 mM dithiothreitol) (18) followed by centrifugation. The supernatant was incubated with glutathione-Sepharose beads (Amersham Pharmacia) for 1 h at 4°C. After the beads were washed, GST fusion protein complexes were separated on an SDS/10% polyacrylamide gels. An aliquot of the lysate before being subjected to GST pull-down assay was separated on an SDS/10% polyacrylamide gels for analysis of the expression level of each TRAF protein and GST fusion protein. FLAG-tagged TRAF6 proteins and GST-fusion proteins were detected by Western blotting with anti-FLAG Ab M2 and anti-GST Ab, respectively.
Flow Cytometric Analysis and Electrophoretic Mobility Shift Assays.
Wild-type and TRAF6−/− mouse embryonic fibroblasts were infected with virus generated by introducing pMX-CD40/XEDAR into packaging cell line BOSC23 (22). Puromycin-resistant cell pools were immunostained with biotinylated anti-human CD40 mAb (B-B20) and streptavidin-RED670 and were subjected to flow cytometric analysis for checking expression of the chimeric receptor. Nuclear extracts were prepared from cells either untreated or stimulated with anti-CD40 mAb (G28–5, 5 μg/ml) for 20 min. Equal amounts of extract (5 μg of protein) were incubated with 32P-labeled double-stranded oligonucleotide containing a κB site from the mouse κ-light chain enhancer. The DNA-protein complex was analyzed on a 4% polyacrylamide gel.
Results
Phenotype of TRAF6−/− and Mutant Mice with Disorders Similar to HED.
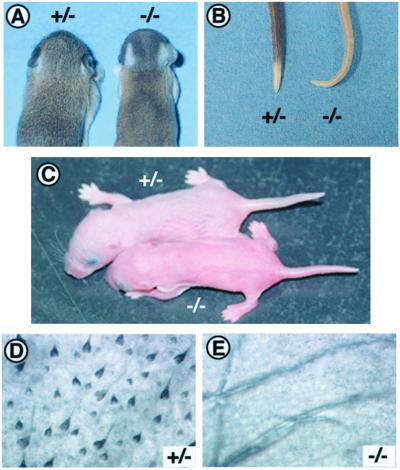
Studying TRAF6−/− mice, we noticed that they have focal alopecia behind their ears (Fig. 1A) and alopecia of the tail (Fig. 1B). They also have a distinctive kink near the tip of their tail (Fig. 1B). Identical phenotypes are reported for Ta, dl, and crinkled (cr), which have disorders analogous to HED (24). Interestingly, the skin color of TRAF6−/− is lighter than that of their wild-type or heterozygous littermates in the first few days after birth (Fig. 1C), but the skin color of TRAF6−/− mice become gradually pigmented afterward (16). Although it was reported that TRAF6−/− mice suffer from various developmental defects including severe osteopetrosis, impaired responses to several cytokines, and abnormal central nervous system development (16, 17, 19), none of these characteristics appear to be associated with the abnormal phenotypes described here. These observations prompted us to perform pathologic studies of skin tissue from TRAF6−/− mice. We first made flat-mount sections of back skin from 1-day-old TRAF6−/− and heterozygous mice. Unexpectedly, there were no pigmented hair follicles in the dorsal skin of TRAF6−/− mice (Fig. 1E); pigmented follicles were detected in heterozygous or wild-type mice (Fig. 1D). However, the numbers of developing small hair follicles without melanocytes (Fig. 1 D and E) was comparable between TRAF6−/− and heterozygous mice. This result indicates that development of only large hair follicles or melanocytes is affected in TRAF6−/− mice. The distribution and numbers of melanocytes in skin of TRAF6−/− neonates as detected by anti-c-kit mAb (25) were comparable to those of the wild-type littermates (data not shown), suggesting that the melanocyte development is normal in TRAF6−/− mice. Thus, we next analyzed hair follicle development in TRAF6−/− mice.
Figure 1.
Abnormal phenotypes in skin and hair of TRAF6−/− mice. (A and B) Abnormal external appearances common to TRAF6−/− mice and mutant mice with HED. (C) Appearance of TRAF6−/− mice (lower) and heterozygous (upper) mice at day 1. TRAF6−/− mice have more translucent skin than their littermates and appear similar to albino neonates. (D and E) Flat-mount sections of back skin of TRAF6−/− mice and their heterozygous littermates, respectively, at day 1. Note that small unpigmented hair follicles (clear small spots) are present even in the back skin of TRAF6−/− mice.
Abnormal Hair Follicle Formation in TRAF6−/− Mice.
We used a dissecting microscope to analyze the surface appearance of embryos harvested from heterozygous parents. Until E13, there were no gross differences in the appearance of homozygous and heterozygous or wild-type mice, with the exception of occasional exencephaly and failure of neural tube closure (ref. 19 and A.N., unpublished data). From E14 to E17, we found a clear difference between TRAF6−/− mice and their littermates. TRAF6−/− mice had no hair follicle buds, which are distributed over the entire bodies of wild-type and heterozygous embryos (Fig. 2 A and B). Expression of β-catenin, which is involved in hair follicle development (26), was observed in hair follicle buds of heterozygous E15.5 embryos but not in those of TRAF6−/− embryos (Fig. 2 C and D). After E18, hair follicles were detected on the belly of TRAF6−/− mice but were absent in the dorsal region (data not shown).
Figure 2.
Lack of hair follicle buds in skin of TRAF6−/− mouse embryos at E15.5. (A and B) Sagittal sections of embryos at E15.5. Sections were stained with hematoxylin and eosin. (C and D) Expression of β-catenin in hair follicle at E15.5. (E and F) Expression of MAdCAM-1 in hair follicle buds of embryos at E15.5 visualized by whole-mount immunostaining. TRAF6−/− mice (B, D, and F) and heterozygous littermates (A, C, and E) .
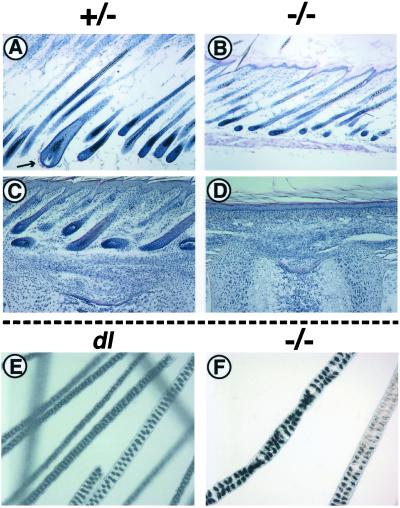
The coat of the mouse consists of two kinds of hairs: the underhairs (zigzag) and the overhairs, which include guard, awl, and auchen hairs. These types are classified based on the time of formation of hair follicle buds and the structure of hair shafts (24, 27, 28). The initiation of guard hair follicles begins from E14 to E15. The awl hair follicles start to form from E16 to E17. The formation of auchen and zigzag hair follicles begins from E19 to birth. Numbers of rows of air cells and those of constrictions in a hair shaft are varied among the types of pelage. Guard hairs have two rows of air cells. Awl and auchen hairs have two or more rows of air cells whereas zigzag hairs have a single row. Guard and awl hairs are straight without constrictions. Auchen hairs have a single constriction, and zigzag hairs usually have three flat constrictions. In TRAF6−/− mice, formation of hair follicle buds begins from E17 to E18 (data not shown). Because MAdCAM-1 is the earliest marker expressed in developing guard hair follicles in mouse embryos (E.N., unpublished data), we examined its expression in TRAF6−/− E15.5 embryos and their littermates. Although vascular expression of MAdCAM-1 (29) was comparable between TRAF6−/− mice and their littermates, MAdCAM-1 was not expressed in the skin of TRAF6−/− mouse embryos (Fig. 2F), whereas its expression in skin was clearly observed in their heterozygous littermates (Fig. 2E). This skin-specific MAdCAM-1 expression defect also was found in Ta and dl mice (E.N., unpublished data), which have defects in guard hair follicle formation (24). In juvenile wild-type mice, hair follicles of various sizes developed and became pigmented (Fig. 3A). Although many hair follicles also developed in TRAF6−/− mice, there were no guard hair follicles (Fig. 3B), which are present in heterozygous mouse skin (Fig. 3A, arrow). Total absence of guard hair follicles was apparent in the tail skin of juvenile TRAF6−/− mice (Figs. 1B and 3D), where the hair follicles comprise only guard hairs in their heterozygous littermates (Fig. 3C). In fine structure of hair shafts, TRAF6−/− mice have a single type of pelage hairs, which has two or three rows of air cells and many constrictions (Fig. 3F). The pelage hairs of dl mice also have two or three rows of air cells but they have no constriction (Fig. 3E). Based on the time of hair follicle formation and the structure of hair shafts described above, hair type of TRAF6−/− mice is not guard hairs but may be abnormal awl hairs.
Figure 3.
Lack of guard hair follicles in juvenile TRAF6−/− mice. (A and B) Sections of back skin of mice at day 11. Guard hair follicles, which are larger than other follicles, are visible in back skin of their heterozygous littermates (A, arrow). (C and D) Sections of tail skin at day 8. (E and F) Fine structure of pelage hairs of dl mice and that of TRAF6−/− mice. TRAF6−/− mice (B, D, and F), heterozygous littermates (A and C), and dl mice (E).
Lack of Sweat Glands and Impaired Formation of Sebaceous Glands in TRAF6−/− Mice.
Humans with HED exhibit a predisposition to hyperthermia because of the absence of sweat glands (1). Furthermore, Ta, dl, and cr mice lack sweat glands (24). Thus, we examined development of skin appendixes in TRAF6−/− mice. There were no sweat glands in the palmar skin of 8-day-old TRAF6−/− mice (Fig. 4B), whereas both sweat glands and sweat ducts were developed in their heterozygous littermates (Fig. 4A). In addition, formation of sebaceous glands, clearly observed in back skin of the heterozygous 11-day-old mice (Fig. 4C), was severely impaired in TRAF6−/− littermates (Fig. 4D). Impairment of the sebaceous gland was not observed in Ta, dl, and cr mice (24) but sometimes found in the HED patients (30). However, as observed in the dl mice, the modified sebaceous glands such as meibomian glands (Fig. 4 E and F), anal glands (Fig. 4 G and H), and preputial glands (Fig. 4 I and J) are severely impaired in TRAF6−/− mice.
Figure 4.
Developmental defects in skin eccrine glands in TRAF6−/− mice. (A and B) Sections of palmar skin at day 8. Arrows and arrowheads in A indicate sweat ducts and sweat glands, respectively. (C and D) Horizontal sections of back skin at day 11. Arrowheads indicate sebaceous glands. (E and F) Sections of an eyelid. Arrows in E indicate meibomian glands. (G and H) Sections of an anal. Arrow in H indicates a significantly reduced anal gland of the TRAF6−/− mouse. (I and J) Photographs of abdomen of a male mouse. Arrows in I indicate preputial glands. TRAF6−/− mice (B, D, F, H, and J) and heterozygous littermates (A, C, E, G, and I).
To analyze expression of the TRAF6 protein in skin tissue, immunohistochemistry was performed. TRAF6 was weakly expressed in hair follicle buds of E15.5 embryos and highly expressed in hair follicles of E16.5 and E18.5 embryos (Fig. 5 A–C). These results support the notion that TRAF6 is expressed in guard hair follicles because TRAF6-positive hair follicles migrate deeply into the dermal tissue by E16.5. Expression of TRAF6 was observed in sweat glands (Fig. 5D), sebaceous glands, and hair follicles (Fig. 5E) of 5-day-old mice. Signals were not detected when experiments were performed without first Ab (data not shown).
Figure 5.
In utero and postnatal expression of TRAF6 in epidermal appendixes. Saggital sections of embryos at E15.5 (A), E16.5 (B), and E18.5 (C). Arrows in A–C indicate hair follicles. (D and E) Sections of palmer skin at day 5. Arrows in D indicate sweat glands. An arrowhead and arrows in E indicate a sebaceous gland and hair follicles, respectively.
Mouse models of HED and human HED patients usually have dental defects, such as absent or abnormally shaped teeth (1, 24). However, one obvious abnormal phenotype observed in TRAF6−/− mice is failure of tooth eruption, which is characteristic of osteopetrosis (16, 17). Thus, it is unclear whether the tooth development defect in TRAF6−/− mice is similar to that in other mouse models of HED. Taken together, these histological findings indicate that TRAF6−/− mice have HED.
TRAF6 Binds to the Cytoplasmic Tail of XEDAR and TROY but Not to That of DL/EDAR.
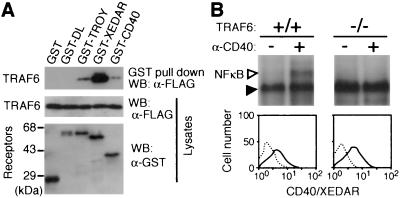
Three members of the TNFR superfamily, DL/EDAR, TROY, and XEDAR are expressed in skin and hair follicles (6, 8, 9) and are thus thought to be involved in hair follicle formation. In fact, impairment of DL/EDAR-mediated signaling results in HED (7). However, some phenotypes of TRAF6−/− mice are different from those of dl and Ta mice. Therefore, it is interesting to identify roles of TRAF6 in signaling from these receptors. Binding of TRAF6 to the cytoplasmic tails of DL/EDAR, TROY, and XEDAR was analyzed (Fig. 6A). TRAF6 bound strongly to the cytoplasmic tail of XEDAR, weakly to that of TROY, and not at all to that of DL. We next expressed chimeric receptors in which the cytoplasmic tail of XEDAR was fused to the extracellular domain of CD40 in wild-type and TRAF6−/− mouse embryonic fibroblasts. When the chimeric receptor was stimulated with anti-CD40 agonistic Ab, NFκB was activated only in the presence of TRAF6 (Fig. 6B), indicating that TRAF6 is an essential signal transducer of XEDAR. These results suggest that HED in TRAF6−/− mice may not be because of defective signaling from DL and that the TRAF6-mediated signal from TROY or XEDAR may be essential for the normal development of ectoderm-derived tissues.
Figure 6.
Roles of TRAF6 in signalings from various members of the TNFR superfamily expressed in hair follicles. (A) Binding of TRAF6 with various members of the TNFR family known to be expressed in hair follicles. An expression plasmid encoding FLAG-tagged TRAF6 was cotransfected into 293T cells with expression plasmids for GST-tagged cytoplasmic tails of various receptors. GST pull-down assays and Western blotting analysis of cell lysates were performed as described in Materials and Methods. (B) Requirement of TRAF6 in the XEDAR signaling. Flow cytometric analysis of expression of the CD40/XEDAR chimeric receptor in wild-type (Left) and TRAF6−/− (Right) mouse embryonic fibroblasts (Lower). Cells were stained with biotinylated anti-CD40 mAb (B-B20) and streptavidin-RED670 (solid lines) or without the Ab (dotted lines). Nuclear extracts prepared from cells untreated (−) or stimulated with anti-CD40 mAb (G28–5) were subjected to electrophoretic mobility shift assays as described in Materials and Methods (Upper). An open triangle indicates NFκB-DNA complexes and a solid triangle indicates nonspecific bands.
Discussion
We report here that the following epidermal phenotypes of TRAF6−/− mice are identical to those of Ta, dl, or cr mice (24) and related to HED in human (1). TRAF6−/− mice exhibit focal alopecia behind the ears, alopecia in the tail, a distinctive kink near the tip of the tail (Fig. 1 A and B), absence of guard hair follicles (Figs. 2 and 3 B and D), lack of sweat glands (Fig. 4B), and severe impairment of the modified sebaceous glands such as meibomian glands (Fig. 4 E and F), anal glands (Fig. 4 G and H), and preputial glands (Fig. 4 I and J). These findings indicate that TRAF6 is essential for transmitting signals that lead to development of epidermal appendixes.
TRAF6 mediates signals from members of the TNFR superfamily, including CD40, receptor activator of NFκB, and p75 nerve growth factor receptor, and those from members of the Toll/IL-1 receptor family (15). These signals activate at least two distinctive groups of transcription factors: AP-1 is activated by mitogen-activated protein kinase and NFκB is activated by IKK. These kinases are activated by TRAF6 via adapter molecules or other kinases such as transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) (31) or evolutionarily conserved signaling intermediate in Toll pathways (ECSIT) (32) in a signal-dependent manner. Several groups reported recently (13, 14) that hypomorphic mutations in the IKBKG gene in men result in HED with immunodeficiency. IKBKG encodes NEMO/IKKγ, the regulatory subunit of the IKK complex, which is essential for NFκB activation (12). Furthermore, mice that ubiquitously express a dominant-active mutant of IκBα, an inhibitor of NFκB, exhibit phenotypes identical to Ta, dl, and cr mice phenotypes (33). Taken together, these findings indicate that full activation of NFκB is essential for normal development of epithelial appendixes. In fact, abundant activation of NFκB in hair follicles and sweat glands was observed in mice carrying an NFκB responsive β-galactosidase reporter transgene (34). Therefore, we first thought that the EDA/EDAR signal-induced NFκB activation through TRAF6 and the IKK complex could be the only pathway that is critical for ectodermal development. However, in vivo binding assays revealed that TRAF6 binds to XEDAR and TROY but not to DL. Previous studies suggest that TRAF6 may be involved in transducing signals from XEDAR and TROY (8, 9). Furthermore, we confirmed that XEDAR cannot activate NFκB in TRAF6−/− embryonic fibroblasts (Fig. 6B). Thus, although TRAF6−/− mice have an epidermal phenotype similar to Ta, dl, and cr mice, the causative defects could be distinct. In fact, severe impairment of the sebaceous gland formation, which is observed in TRAF6−/− mice (Fig. 4D), does not occur in Ta and dl mice. Furthermore, pelage hairs of TRAF6−/− mice have many constrictions whereas those of dl mice do not (Fig. 3 E and F). These results suggest that TRAF6 may not be involved in the EDA/EDAR signaling. However, involvement of TRAF6 in hair follicle formation also is supported by the abundant expression of TRAF6 in hair follicles of embryos (Fig. 5 A–C). Therefore, it is possible that XEDAR- or TROY-induced activation of NFκB via TRAF6 is essential for normal development of epithelial appendixes including the sebaceous glands. In this case, the three receptors may cooperate to form hair follicle buds. Because both EDAR and XEDAR recognize the EDA gene products as their ligand (8), cooperation of the signals from these two receptors could be essential for development of the guard hair follicle buds. Furthermore, we cannot rule out the possibility that EDAR may indirectly bind to TRAF6 via a recently identified adapter molecule, EDARADD, a product of cr gene (35). However, identification of the precise mechanisms must await detailed analyses of XEDAR- and TROY-deficient mice.
At what point in hair follicle development does TRAF6 play its critical role? β-catenin plays important roles in the development of various organs and is expressed specifically in hair follicle buds (26). In these mice with skin-specific inactivation of β-catenin gene, hair follicle development was disrupted, but early hair follicle bud formation and EDAR expression were observed, suggesting that EDAR must participate in very early step in hair follicle bud formation. Total absence of guard hair follicle buds and MAdCAM-1 expression in the skin surface was observed in both TRAF6−/− mice and mice with defects in EDA/EDAR signal (E.N., unpublished data). Because MAdCAM-1 is one of the earliest markers of the hair follicle development and also functions in their development (E.N., unpublished data), induction of MAdCAM-1 expression by TRAF6-mediated signals or EDA/EDAR signals must be an essential early event in development of hair follicle buds. Importantly, the promoter region of the MAdCAM-1 gene contains an NFκB-binding site, and transcription from the promoter is enhanced by TNFα or lymphotoxin stimulation (36, 37). Therefore, in the formation of guard hair follicle buds, one of the critical target genes of NFκB activated by a TRAF6-mediated signal may be MAdCAM-1. Furthermore, an increased rate of apoptosis in many hair follicles was reported in mice expressing dominant-active IκBα (33), indicating that NFκB also acts as a survival factor during hair follicle formation. The total absence of sweat glands in TRAF6−/− mice also suggests that TRAF6-mediated signals may be essential for the initial stage of sweat gland development. We have already established a culture system for MAdCAM-1+ epithelial cells derived from skin of E13–E15 mouse embryos. Further investigation by using such primary cells is needed to understand the role of TRAF6 in the formation of skin appendixes.
Acknowledgments
This work was supported by a grant-in-aid for Scientific Research on Priority Areas and the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government, a grant for AIDS Research from the Japan Health Science Foundation, and a grant from the Takeda Science Foundation.
Abbreviations
- TNFR
tumor necrosis factor receptor
- TRAF
TNFR-associated factor
- HED
hypohidrotic ectodermal dysplasia
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- EDAR
ectodysplasin receptor
- GST
glutathione S-transferase
- En
embryonic day n
- IKK
IκB kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pinheiro M, Freire-Maia N. Am J Med Genet. 1994;53:153–162. doi: 10.1002/ajmg.1320530207. [DOI] [PubMed] [Google Scholar]
- 2.Kere J, Srivastava A K, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, et al. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava A K, Pispa J, Hartung A J, Du Y, Ezer S, Jenks T, Shimada T, Pekkanen M, Mikkola M L, Ko M S, et al. Proc Natl Acad Sci USA. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson B M, Brockdorff N, Formstone E, Ngyuen T, Kronmiller J E, Zonana J. Hum Mol Genet. 1997;6:1589–1594. doi: 10.1093/hmg/6.9.1589. [DOI] [PubMed] [Google Scholar]
- 5.Mikkola M L, Pispa J, Pekkanen M, Paulin L, Nieminen P, Kere J, Thesleff I. Mech Dev. 1999;88:133–146. doi: 10.1016/s0925-4773(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 6.Headon D J, Overbeek P A. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- 7.Monreal A W, Ferguson B M, Headon D J, Street S L, Overbeek P A, Zonana J. Nat Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- 8.Yan M, Wang L C, Hymowitz S G, Schilbach S, Lee J, Goddard A, de Vos A M, Gao W Q, Dixit V M. Science. 2000;290:523–527. doi: 10.1126/science.290.5491.523. [DOI] [PubMed] [Google Scholar]
- 9.Kojima T, Morikawa Y, Copeland N G, Gilbert D J, Jenkins N A, Senba E, Kitamura T. J Biol Chem. 2000;275:20742–20747. doi: 10.1074/jbc.M002691200. [DOI] [PubMed] [Google Scholar]
- 10.Eby M T, Jasmin A, Kumar A, Sharma K, Chaudhary P M. J Biol Chem. 2000;275:15336–15342. doi: 10.1074/jbc.275.20.15336. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Eby M T, Sinha S, Jasmin A, Chaudhary P M. J Biol Chem. 2001;276:2668–2677. doi: 10.1074/jbc.M008356200. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 13.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, et al. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Ma C A, Liu S, Brown M, Cohen J, Strober W. Nat Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 15.Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T. Exp Cell Res. 2000;254:14–24. doi: 10.1006/excr.1999.4733. [DOI] [PubMed] [Google Scholar]
- 16.Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, et al. Genes Cells. 1999;4:353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 17.Lomaga M A, Yeh W C, Sarosi I, Duncan G S, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomaga M A, Henderson J T, Elia A J, Robertson J, Noyce R S, Yeh W C, Mak T W. J Neurosci. 2000;20:7384–7393. doi: 10.1523/JNEUROSCI.20-19-07384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiio Y, Yamamoto T, Yamaguchi N. Proc Natl Acad Sci USA. 1992;89:5206–5210. doi: 10.1073/pnas.89.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, et al. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura T. Int J Hematol. 1998;67:351–359. doi: 10.1016/s0925-5710(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida H, Kunisada T, Kusakabe M, Nishikawa S, Nishikawa S I. Development (Cambridge, UK) 1996;122:1207–1214. doi: 10.1242/dev.122.4.1207. [DOI] [PubMed] [Google Scholar]
- 24.Sundberg J P. In: Handbook of Mouse Mutations with Skin and Hair Abnormalities. Maibach H I, editor. Boca Raton, FL: CRC; 1994. [Google Scholar]
- 25.Kunisada T, Lu S Z, Yoshida H, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams D A, Wang X, Longley B J. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 27.Mann S J. Anat Rec. 1962;144:135–141. [Google Scholar]
- 28.Vielkind U, Hardy M H. Acta Anat. 1996;157:183–194. doi: 10.1159/000147880. [DOI] [PubMed] [Google Scholar]
- 29.Hashi H, Yoshida H, Honda K, Fraser S, Kubo H, Awane M, Takabayashi A, Nakano H, Yamaoka Y, Nishikawa S. J Immunol. 2001;166:3702–3709. doi: 10.4049/jimmunol.166.6.3702. [DOI] [PubMed] [Google Scholar]
- 30.Al-Jassim A H, Swift A C. J Laryngol Otol. 1996;110:379–382. doi: 10.1017/s0022215100133687. [DOI] [PubMed] [Google Scholar]
- 31.Ninomiya T J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. Nature (London) 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 32.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway C A, Ghosh S. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Ullrich R, Aebischer T, Hulsken J, Birchmeier W, Klemm U, Scheidereit C. Development (Cambridge, UK) 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. Development (Cambridge, UK) 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- 35.Headon D J, Emmal S A, Ferguson B M, Tucker A S, Justice M J, Sharpe P T, Zonana J, Overbeek P A. Nature (London) 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi M, Baichwal V R. Proc Natl Acad Sci USA. 1995;92:3561–3565. doi: 10.1073/pnas.92.8.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ettinger R, Mebius R, Browning J L, Michie S A, van Tuijl S, Kraal G, van Ewijk W, McDevitt H O. Int Immunol. 1998;10:727–741. doi: 10.1093/intimm/10.6.727. [DOI] [PubMed] [Google Scholar]