Abstract
Prokaryotic repressor–operator systems provide exemplars for the sequence-specific interactions between DNA and protein. The crucial atomic contacts of the two macromolecules are attained in a compact, geometrically defined structure of the DNA–protein complex. The pitch of the DNA interface seems an especially sensitive part of this architecture because changes in its length introduce new spacing and rotational relations in one step. We discovered a natural system that may serve as a model for investigating this problem: the repressor of the 16-3 phage of Rhizobium meliloti (helix-turn-helix class protein) possesses inherent ability to accommodate to various DNA twistings. It binds the cognate operators, which are 5′-ACAA-4 bp-TTGT-3′ (OL) and 5′-ACAA-6 bp-TTGT-3′ (OR) and thus differ 2 bp in length, and consequently the two half-sites will be rotated with respect to each other by 72° in the idealized B-DNA (64° by dinucleotide steps calculations). Furthermore, a synthetic intermediate (DNA sequence) 5′-ACAA-5 bp-TTGT-3′ (O5) also binds specifically the repressor. The natural operators and bound repressors can form higher order DNA–protein complexes and perform efficient repression, whereas the synthetic operator-repressor complex cannot do either. The natural operators are bent when complexed with the repressor, whereas the O5 operator does not show bending in electrophoretic mobility assay. Possible structures of the complexes are discussed.
Keywords: DNA–protein interactions‖transcription regulation‖repressor–operator binding‖Rhizobium phage
In many intracellular functions, one of them being transcription regulation, the specific binding of protein to DNA ensures the fidelity of control. In a specific protein–DNA complex, the target DNA surface juxtaposes the cognate peptide sequences, and both molecules should fold properly to form a biologically active structure. In major of prokaryotic repressor–operator systems, the unit regulatory complex (i.e., one DNA site plus the bound protein) is rotationally symmetrical, consisting of a homodimer of the protein and two invertedly repeated “half” binding sites of the DNA (1–4). The DNA counterpart at the protein–DNA interface of the complex looks especially vulnerable to mutations, because, beyond base changes that directly influence the protein–DNA contacts, insertions and deletions destroy the original geometry by introducing new rotational relations and spacings. There are only a few examples, either proven (5, 6) or suggestive (7, 8,) where repressors recognized operators of two different lengths. In this paper, we report on a natural case when a repressor protein is able to bind to three (and regulate on two) geometric variants of the operator DNA and form stable and specific complexes with them. The repressor is the C protein of the 16-3 temperate phage of Rhizobium meliloti strain 41 (R. meliloti 41), the DNA counterparts are the cognate operators. The main regulatory circle of 16-3 consists of a c repressor gene and two operator-promoter regions at its rightward and leftward flanks, both containing two palindromic DNA sequences, called operator units (OR1 and OR2, OL1 and OL2 in Fig. 1A; refs. 9–12). Although Rhizobium phage 16-3 does not show DNA homology to lambdoid phages, its repressor still shares 55% identity in the helix-turn-helix motif with the repressor of the Escherichia coli phage 434; the OR operator units of 16-3 are identical in the conserved positions of the 434 consensus operator 5′-ACAA-6 bp-TTGT-3′; and the known, naturally occurring operator mutation of 16-3 is also identical to one of the canonical 434 operator mutations. The 16-3 repressor and the OR operator also could recognize weakly the corresponding 434 elements (11). Whereas the location of the OR2 operator has been confirmed by a point mutation (regained from virulent stocks), no pinpointing genetic evidence has been achieved for the OL operator: the virulent mutants and the relevant backcross derivatives (avirC in ref. 10) carried overlapping deletions on the left side of gene c. These mutants and subsequent clonings, coupled with functional assays in E. coli, reduced the suspected area to a 150-bp segment (10, 11). In this paper, we report on the identification of the operators that exhibited unexpected structural differences. Complex formation of these operators with 16-3 repressor was analyzed, and the results are discussed in the light of the adaptive capability of the protein.
Figure 1.
Components of the central regulatory circuits of phage 16-3 (A). OL1 and OL2 leftward, OR1 and OR2 rightward operators (formerly O and Or in ref. 7); PL and PR, cognate promoters; PC, promoter for repressor gene. Nucleotide sequence of the region is available in GenBank under accession no. AJ131679. O
and Or in ref. 7); PL and PR, cognate promoters; PC, promoter for repressor gene. Nucleotide sequence of the region is available in GenBank under accession no. AJ131679. O -1, Mutation contributes to immunity insensitivity in virulent mutants. Bar indicates deletion. * and ♦ point mutations led to operator malfunction (see C). (B) Schematic diagram of the in vivo system for monitoring repression in R. meliloti 41. Promoter activities measured via lacZ activities in R. meliloti 41; repressor provided from integrated 16-3 prophage in lysogenic derivatives. (C) Effect of mutations on the repression of promoters PL and PR. +, wild-type elements; −, no control element; ▵, deletion; * and ♦, point mutations as indicated in A (for base changes, see Fig. 2A). a, Definition of R value (see Materials and Methods). b, The intervening 1,182 bp DNA for separating OL2 OL1 and OR1 OR2 PR regions in pEP223 is derived from wheat gliadin sequences, for separating OR2 and OR2PR units (by 198 bp) in pEP224, derived from M13 sequences.
-1, Mutation contributes to immunity insensitivity in virulent mutants. Bar indicates deletion. * and ♦ point mutations led to operator malfunction (see C). (B) Schematic diagram of the in vivo system for monitoring repression in R. meliloti 41. Promoter activities measured via lacZ activities in R. meliloti 41; repressor provided from integrated 16-3 prophage in lysogenic derivatives. (C) Effect of mutations on the repression of promoters PL and PR. +, wild-type elements; −, no control element; ▵, deletion; * and ♦, point mutations as indicated in A (for base changes, see Fig. 2A). a, Definition of R value (see Materials and Methods). b, The intervening 1,182 bp DNA for separating OL2 OL1 and OR1 OR2 PR regions in pEP223 is derived from wheat gliadin sequences, for separating OR2 and OR2PR units (by 198 bp) in pEP224, derived from M13 sequences.
Materials and Methods
Constructing Plasmids Carrying Operator Alleles.
pEP plasmids (for assays in R. meliloti 41) were constructed as follows: (i) pEP82: πvx polylinker (13) was inserted in a slightly modified pRK290 (14), then a promoter lacking the E. coli lacZ gene was spliced at the ClaI site; (ii) operator-promoter units, amplified by PCR [for OLPL on a 145-bp fragment leftwards from chromosome site P57 and for ORPR on a 92-bp fragment from site H60 (12), leftwards, from bp 431 to 523], were cloned in pEP82 BglII-PstI backbone (OL), called pEP106, and in pBluescript KS II at the EcoRV site (OR), then transferred to pEP82 as a 105-bp HindIII-PstI fragment [the conventional methods were used according to Sambrook et al. (15)], resulting in plasmid pEP86. Mutations [induced by site-directed technique (16)] homologous to spontaneous operator mutation O -1 (11) were introduced in one or both of the consensus boxes in the OL region, pEP130 and pEP109, respectively. In pEP144, the promoter-distal half of OR1 was deleted from pEP86; in pEP148, a G to A transition was introduced in OR2. In plasmid pEP223, the 16-3 phage sequences (≈1 kbp, including gene c) separating OL and OR operator regions in the phage were replaced by 1,182-bp plant DNA derived from wheat gliadin sequences. Plasmid pEP224 was derived from pEP144 by inserting M13 polylinker sequences and the second OR2 operator (the center to center distance of the two OR2s is 198 bp). Plasmid maps and sequences are provided by the authors on request.
-1 (11) were introduced in one or both of the consensus boxes in the OL region, pEP130 and pEP109, respectively. In pEP144, the promoter-distal half of OR1 was deleted from pEP86; in pEP148, a G to A transition was introduced in OR2. In plasmid pEP223, the 16-3 phage sequences (≈1 kbp, including gene c) separating OL and OR operator regions in the phage were replaced by 1,182-bp plant DNA derived from wheat gliadin sequences. Plasmid pEP224 was derived from pEP144 by inserting M13 polylinker sequences and the second OR2 operator (the center to center distance of the two OR2s is 198 bp). Plasmid maps and sequences are provided by the authors on request.
pPV plasmids (for assays in E. coli) are derivatives of pMLB1109 (17) carrying synthetic promoter-operator units (cloned via EcoRI/BamHI ends). The 16-3 operators were placed in between the −35 and −10 boxes (both selected according to ref. 18). The structures of these promoter-operator units are as follows: 5′-(EcoRI site)-(TTGACT, for −35 box)-(CT or TCT, for space filling, Table 1)-(16-3 operators, Table 1)-(A or AC, for space filling, Table 1)-(TATGAT, for −10 box)-(TACTCAGAT)-(BamHI site)-3′.
Table 1.
Activity of 16-3 operators in E. coli using “one copy” setups
| Plasmid inserted in chromosome | Operator | DNA sequence between −10 and −35 boxes | β-Gal activities
|
R* | |
|---|---|---|---|---|---|
| No repressor | Repressor provided | ||||
| pPV4 | OL2 | TCTACAATTGATTGTAC | 235 | 37 | 0.84 |
| pPV5 | O5 | TCTACAATTGAGTTGTA | 3,293 | 1,735 | 0.47 |
| pPV6 | OR2 | CTACAATTGTAGTTGTA | 3,577 | 496 | 0.86 |
| pPV6M | O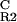
|
CTACGATTGTAGTTGTA | 74 | 67 | 0.095 |
| pPV4M | O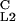
|
TCTACGATTGATTGTAC | 3,256 | 2,699 | 0.17 |
For definition of R value, see Materials and Methods.
Measuring Repression, R Values.
In R. meliloti 41 setups, the pEP plasmids were introduced in E. coli HB101 and then transferred parallel to R. meliloti 41 and R. meliloti 41(16-3); then Rhizobium transconjugants were selected on glucose Tris·succinate minimal medium (GTS) tetracycline (Tc) plates (19). The lacZ activity of the corresponding nonlysogenic (R. meliloti 41) and lysogenic (R. meliloti 41(16-3) strains was measured in the same experiment to minimize the fluctuations of R (repression) values. The starter cultures were grown for 22 h in yeast tripton broth (YTB) (9) containing Tc (15 μg/ml) at 30°C with vigorous aeration and then diluted (OD600 = 0.05 in 2 ml YTB-Tc), grown further for 5 h at 30°C, chilled on ice, and assayed for β-galactosidase (β-gal) activity according to Miller (20). Each experiment was repeated three times with three parallel cultures. Parallels varied around the average within a range ±<2%, R values within ±<5%. The degree of repression (R value) was calculated as: R = 1 − [β-gal in R. meliloti 41(16-3) per β-gal in R. meliloti 41].
In E. coli “one copy” setups, E. coli promoter of medium strength and the 16-3 operators (OL2, OR2, O5, and mutants) were combined (see pPV plasmids in the first section) and introduced in E. coli DH5α (21). The λ RS45 procedure (22) was then applied to complete the reporter gene as well as to transfer this reporter unit to the bacterium chromosome by prophage insertion. Each of the reporter strains was then transformed parallel with plasmid pPM232 (source for 16-3 repressor) and with pSEM91 (ref. 23; for control). pPM232 is a derivative of pSEM91, carrying the c repressor gene of 16-3 spliced into the XbaI-EcoRV sites. The c+ allele was used, obtained from wild-type 16-3 by PCR amplification. Repression (R value) was calculated, as described for the R. meliloti 41 setups, from the β-gal activities of the pSEM91 and pPM232 carrying couples. Each experiment was repeated in five parallels. SDs for β-gal measurements varied ±<4%, for R values ±<3%. The degree of repression (R value) was calculated as follows: R = 1 − [β-gal in E. coli with pPM232 per β-gal in E. coli with pSEM91].
Band Shift.
Experimental setup for band shift (24) assays was as follows. Sequences of OL2 and OR2 regions (see triads 1–7 below the gel photos in Fig. 2 and that in Fig. 3A) were synthesized in vitro and cloned in pBluescript KS II. O5 sequence (Fig. 3A) was designed (then synthesized) by omitting the middle thymine from OR2 (see triad 6 in Fig. 2B). DNA fragments (66–74 bp long) were isolated after ApaI-BamHI digestions and end-labeled with [α-32P]dATP by Klenow fragment of E. coli DNA polymerase I in fill-in reaction. Wild-type 16-3 repressor (encoded by the c+ allele) was overexpressed from T7 promoter in E. coli BL21(DE3), extracted by freeze-thaw cycles followed by 35% ammonium sulfate precipitation, purified on hydroxylapatite column, and stored in 50% glycerol at −20°C. The repressor content of the protein samples was about 80% and kept binding activity for months. (Details on constructing plasmids, repressor-producing strains, and protein purifications are provided on request. The repressor preparation was checked by protein sequencing from its N-terminal end and from the Arg-88 position. DNA sequence: GenBank accession no. AJ131679). Each binding reaction contained 50 ng poly(dI:dC) (as competitor DNA), 100 pg α-32P operator DNA, and repressor as described above (see Fig. 2 for quantities), in binding buffer (10 mM Tris, pH 7.5/40 mM KCl/1 mM EDTA/2 mM CaCl2/2 mM MgCl2/500 mM glucose/10% glycerol) for 10 min at 0°C. PAGE was as follows: 5% polyacrylamide (49:1), TBE buffer, and voltage 8 V/cm.
Figure 2.
Effect of operator mutations on repressing the cognate promoter activities (A) and determining the boundary of the binding sites of 16-3 repressor in band shift experiments (B; ref. 24). Vertical lines in A correspond to the relative strength of repressor binding normalized for wild type: relR = R value for mutant per R value for wild type (see Fig. 1C for details). O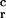 -1 is a natural operator mutation (10, 11). (B) Sequences 1–7 below the gel photos refer to the sequence variations (within 66- to 74-bp-long ApaI/BamHI fragments; see Band Shift in Materials and Methods) tested from the operator regions. Lanes a, b, and c refer to the increasing quantities of 16-3 repressor protein added in the reaction mixture: a, no repressor; b, 100 ng; c, 300 ng. The free (F) and repressor bound (B) DNAs are indicated (multiple bands may correspond to sandwich structures, indicative for cooperative bindings between DNA bound repressors).
-1 is a natural operator mutation (10, 11). (B) Sequences 1–7 below the gel photos refer to the sequence variations (within 66- to 74-bp-long ApaI/BamHI fragments; see Band Shift in Materials and Methods) tested from the operator regions. Lanes a, b, and c refer to the increasing quantities of 16-3 repressor protein added in the reaction mixture: a, no repressor; b, 100 ng; c, 300 ng. The free (F) and repressor bound (B) DNAs are indicated (multiple bands may correspond to sandwich structures, indicative for cooperative bindings between DNA bound repressors).
Figure 3.
(A) Band shift demonstration for 16-3 repressor binds artificial operator sequence of 5 bp spacing (i.e., O5; 5′-ACAAttgagTTGT-3′, i.e., the central T of OR2 is missing). Experimental circumstances and setup were identical to that of Fig. 2B. F, free; B, repressor bound DNA. Note that the higher order complex (sandwich structure) is absent. (B) Repressor–operator binding isoterms for OL2, OR2, and O5. KD values derived from the curves are as follows: 2.4 × 10−7 M for OL2, 1.95 × 10−7 M for OR2, and 4.5 × 10−7 M for O5.
Determination of Binding Constants (KD).
Band shift experiments were used to characterize the binding of repressor protein on different operator sequences. The final DNA concentration was 1.1 × 10−10 M in each reaction. Repressor concentrations were between 4 × 10−8 M and 2 × 10−6 M. KD values, defined as the repressor concentration required to bind 50% of the operator DNA, were determined from the binding curves.
DNA Bending.
The circular permutation method (25) was applied by using plasmid pBend2 (26). The following sequences were inserted with SalI/XbaI ends at the cognate sites of pBend2: (pTN91) for OL2 (sequence with bold letters in the underlined section), SalI ClaI/Bsp106 HindIII gattACAAttgaTTGTaatc EcoRI PstI SmaI BamHI SpeI XbaI; (pTN51) for OR2 (sequence with bold letters in the underlined section), SalI ClaI/Bsp106 HindIII caACAAttgtagTTGTc EcoRI PstI SmaI BamHI SpeI XbaI; (pTN121) for O5 (sequence with bold letters in the underlined section), SalI ClaI/Bsp106 HindIII ACAAttgagTTGTaattc PstI SmaI BamHI SpeI XbaI. The operator carrying fragments were regained by MluI, XhoI, and NruI digestions. Fragment lengths are as follows: 204 bp for OL2, 201 bp for OR2, and 196 bp for O5. All other circumstances were identical to those described for band shift assays. Operators are in bold. Bases are in normal letters. Flanking the operators are remnants of cloning procedures.
Results
Operator Units and Cooperativity Identified in the Natural Host R. meliloti.
For the quantitative characterization of the repression by the 16-3 C protein, a reporter system was developed in Rhizobium (see Materials and Methods for details). The putative operator-promoter regions were fused to the E. coli lacZ (reporter) gene, and the fusion was cloned in a broad host range conjugative plasmid in E. coli (14). To test the operator-promoter functions, these reporter plasmids were transferred simultaneously to R. meliloti 41 and to its lysogenic derivatives, which carried wild-type 16-3 prophage and hence provided the repressor (Fig. 1B). A significant (2.5- to 5-fold) repression of β-gal activity is recorded in the lysogenic strains (rows of pEP86, pEP106 in Fig. 1C). The measured values can be said to derive from the incoming lacZ reporter system because, omitting the plasmids, β-gal activity was very low (pEP82 vs. other pEPs in Fig. 1C). Dramatic increase of this repression is observed when the topology of the regulatory unit of the reporter gene imitated the complexity of the natural, “in phage” situation, i.e., when wild-type OR and OL operator regions, separated by 1.2 kbp, were both present (pEP223). Similarly, high repression is recorded when two OR2 unit operators, separated by 200 bp, were present (pEP223 and pEP224 in Fig. 1C). In the DNA sequence to the left of the c gene (<6 kbp), only one sequence was found that resembled the rightward controlling element. Furthermore, this region was within the shortest DNA fragment that donated leftward operator-promoter activity (Fig. 1A). Two 5′-ACAA + TTGT-3′ boxes overlapping the −35 sequence of the leftward promoter, both with 4-bp spacings, 2 bp less than in the right counterpart, were the most striking features seen. To prove that the above palindromic structures were functional operators, in vivo and in vitro approaches were taken: (i) site-directed mutagenesis at both the OL and OR regions followed by in vivo assays in R. meliloti 41 (Figs. 1C and 2A); (ii) band shift assays with both operator DNAs and purified 16-3 repressor (Fig. 2B); and (iii) in vivo assays in “one copy” reporter system in E. coli (Table 1).
Effect of Site-Directed Mutations.
Mutations at both operators in the 5′-ACAA-3′/5′-TTGT-3′ boxes rendered the lacZ reporter gene expression constitutive to various degrees in both R. meliloti 41 and E. coli (Fig. 1C, pEP86 vs. pEP101, pEP144, pEP148, and pEP106 vs. pEP103, pEP108, pEP109, pEP130; Table 1, pPV6 vs. pPV6M, pPV4 vs. pPV4M). We introduced an A to G transition at the third position of the putative leftward recognition boxes, because it had been well established that this mutation in OR2 (named O -1 in ref. 11) resulted in operator malfunction. This mutation contributes to the development of immunity insensitive (virulent) phenotype in 16-3 (as well as in 434); in its presence, the rightward promoter activity was not sensitive to the cloned repressor gene; the O
-1 in ref. 11) resulted in operator malfunction. This mutation contributes to the development of immunity insensitive (virulent) phenotype in 16-3 (as well as in 434); in its presence, the rightward promoter activity was not sensitive to the cloned repressor gene; the O -1 operator was not able to bind the DNA recognizing headpiece of the 16-3 repressor (10–12). It is worthy of note that, in a quantitative sense, as shown in this work, the reference O
-1 operator was not able to bind the DNA recognizing headpiece of the 16-3 repressor (10–12). It is worthy of note that, in a quantitative sense, as shown in this work, the reference O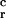 -1 mutation leads to the weakest phenotypic shift. Analogous changes in the OLs as well as other mutations in the ORs harm the corresponding operator functions more severely (Fig. 1C, pEP101 vs. pEP103, pEP108, pEP130, pEP148). On the other hand, mutations in the 5′ or 3′ flanking positions, next to the 5′-ACAA + TTGT-3′ edges behaved neutral at the OR2 binding site, and at OL2 only milder effects were recorded (Fig. 2A). We believe that the latter effects attest to the complexity (i.e., the relation of the operator vs. promoter boxes) of the (in vivo) repression process (27).
-1 mutation leads to the weakest phenotypic shift. Analogous changes in the OLs as well as other mutations in the ORs harm the corresponding operator functions more severely (Fig. 1C, pEP101 vs. pEP103, pEP108, pEP130, pEP148). On the other hand, mutations in the 5′ or 3′ flanking positions, next to the 5′-ACAA + TTGT-3′ edges behaved neutral at the OR2 binding site, and at OL2 only milder effects were recorded (Fig. 2A). We believe that the latter effects attest to the complexity (i.e., the relation of the operator vs. promoter boxes) of the (in vivo) repression process (27).
Identification of the Binding Site Boundaries.
DNA fragments 66–74 bp long and carrying the putative binding sites were combined with purified 16-3 C protein in band shift assays. These experiments unambiguously demonstrated that the presence of the 5′-ACAA-4/6 bp-TTGT-3′ sequences are sufficient and essential to bind the 16-3 repressor efficiently. Truncating at the two edges by removing the A:T and T:A base pairs demolished the specific binding, whereas the length of the central spacer, be it 4, 5, or 6 bp, did not influence that (Figs. 2 and 3). At higher repressor concentrations, extra bands appeared in the case of OL and OR, but not O5. We shall return to this phenomenon in the Discussion.
Deviant Behavior of the “Intermediate Operator” O5 in Band Shift and Bending Assays.
Both the mutation analyses and the band shift assay (Fig. 1C, Fig. 2, and Table 1) defined OLs and ORs as “shorter and longer” operators with identical palindromic flanks separated by 4 and 6 bp, respectively. A simple possibility could be that the flexibility of the 16-3 repressor protein is enough to adjust for the steric difference of the operators. This view motivated the investigation of repressor binding to an operator in the “intermediate state” between OL2 and OR2. The intermediate operator O5 was designed to be as close to both natural operators as possible, i.e., O5 minus 1 bp = OL2, O5 plus 1 bp = OR2. The band shift assays with the 16-3 repressor clearly showed that O5 is competent in binding to the repressor (Fig. 3A). To characterize the binding between the repressor and the different operators, binding isoterms were obtained. The KD values for OL2, OR2, and O5 were determined: 2.4 × 10−7 M, 1.95 × 10−7 M, and 4.5 × 10−7 M, respectively. However, three striking differences were seen: (i) repressor binding on O5 operator is significantly weaker than on OL2 and OR2; (ii) O5, unlike OL2 and OR2, did not make complexes of higher order (Fig. 3A vs. Fig. 2 B); and (iii) O5, unlike OL2 and OR2, migrated as straight, not bent DNA (in the repressor/operator complex). The bending angle calculated from the pBend2 permutation data proved to be 34 ± 3° for both OL2 and OR2 (Fig. 4; calculation, according to ref. 26).
Figure 4.
DNA bending (pBend2) assays with operator sequences OL2, O5, and OR2, carried out on DNAs of 204 bp, 196 bp, and 201 bp, respectively (see Materials and Methods for details). Repressor quantity applied: 250 ng for each reaction. Experimental circumstances were identical to that of Fig. 2B. (The assay for O5 was done in a separate gel, then centered between OL2 and OR2, aligned at the shifted bands).
O5 Operator Function in Repression.
For these studies, we built a “one copy” reporter system in E. coli in which the 16-3 operator sequences were inserted in between the −10 and −35 boxes of an E. coli promoter sequence. The reporter unit was integrated in the E. coli chromosome (at the att λ attB site—therefore, the copy number of the reporter unit mimics the prophage state). The 16-3 repressor was provided from plasmid pPM232 (see Materials and Methods for details). As seen in Table 1, O5 operator is much less efficient in the control function than the “natural” sequences, OL2 and OR2. This result is consistent with the binding efficiencies measured in vitro (Fig. 3B). Mutations corresponding to the O -1 allele when introduced in the “natural” operators demolished their activity. Despite the difference in complexity of the E. coli and R. meliloti 41 reporter setups (the former is considerably less complex), the operator functions paralleled well.
-1 allele when introduced in the “natural” operators demolished their activity. Despite the difference in complexity of the E. coli and R. meliloti 41 reporter setups (the former is considerably less complex), the operator functions paralleled well.
Discussion
We identified four operator loci of bacteriophage 16-3 that bind the 16-3 C (repressor) protein, and determined the extension of the two strongest sites, OL2 and OR2. We discovered that the two sites are identical in the inversely repeated “recognition” sequences but differ in the length of the intervening section, the OR2 binding site being 2 bp longer than OL2 (Fig. 2). Among the natural, well-studied examples, the E. coli cAMP receptor protein (CRP) system is known for a similar topology for DNA–protein sequence-specific interactions. However the relative orientations of the DNA binding peptide motifs (helix-turn-helices) of the proteins are opposite in the two systems, and the geometries of the binding sites are also different. In the CRP case, the cognate DNA binding sites have 6- and 8-bp central spacers bracketed by the recognition sequences 5′-TGTGA + TCACA-3′ (5), whereas in 16-3 the 5′-ACAA + TTGT-3′ sequences are separated by 4 and 6 bp. The question is how the contacting residues of the protein and DNA bases can be held in correct positions despite the spacing and twisting differences. In the CRP–DNA complexes, as is proven experimentally, a conformation shift from a B- to A-form DNA over one helical-turn covering the longer spacer smoothes the inequalities (28). The 16-3 repressor–operator cases may represent a more complex situation. Let's assume first that, in the 16-3 repressor–operator complexes, the DNA adapts to and is bent by the protein, as is in the CRP–DNA complexes (3, 4). In this case, a conformation shift from B- to A-form DNA over the 5′-AA-6 bp-TT-3′ section of OR would be needed according to Adhya's suggestion (28). However, in the light of recent calculations, this conformational shift is hindered in the TT and AT dinucleotide steps, which the ORs are rich in (29). On the other hand, if the overall geometry of the 16-3 repressor–operator complexes follows that of the related 434 repressor–operator complex, in which the DNA is bent only slightly (30), conformational change of the repressor is a more likely solution. Protein flexibility in DNA binding was demonstrated for yeast GCN4 (leucine zipper-basic region class) and Mat alpha 2 (homeodomain class) regulatory (31–33) proteins. However, this solution is unlikely to be adaptable for the more compact helix-turn-helix class proteins, where the DNA binding domains, as the well-studied examples show, contain interfaces for dimerization that align with rather rigid and constant geometries. This observation has not been challenged even by the single chain 434 repressors, in which two DNA binding headpieces are joined to each other in direct (head-to-tail) orientation by long and flexible peptide linkers (34, 35). Although we have no direct experimental evidences for the structural basis for the flexibility of the 16-3 repressor, an indication for its conformational adaptivity is shown in Fig. 3: the C protein binds specifically not only to operators with natural spacings, but also to an intermediate operator O5, 5′-ACAA-5 bp-TTGT-3′.
The repressor flexibility is also apparent when “band shift assays” in Figs. 2 and 3 are compared. The repressor makes only one kind of complex with the “intermediate” operator, whereas with the natural operators higher order complexes are also formed. These higher order complexes are most probably DNA–protein “sandwich” structures (36). Their appearances at higher protein concentration are in good agreement with the in vivo observations (Fig. 1C, pEP223 and 224), which show that distant (200-bp, 1200-bp) operator regions of 16-3 can cooperate. We believe that conformations of repressors that bind the natural operators are apt for cooperative interactions, whereas binding the intermediate operator interferes with this ability.
We do not think that the operators deviate from the standard B-DNA. The naked DNAs that carry the operators (regained from pBend2) migrate uniformly (Fig. 4, and other observations, not shown). Twisting angles for OL2 and OR2 are nearly symmetrical in relation to O5 as computed by the dinucleotide steps calculations (37). Between the outer edges (i.e., ACAA/TTGT) the overall twisting for O5 is 409.6°, and 30.4° less for OL2 and 33.4° more for OR2. The same relations were obtained for the inner spacings (200.6° at O5, 30.6° less and 33.4° more at OL2 and OR2, respectively).
The behavior of the O5 operator in DNA bending assay (Fig. 4) raises a complex structural problem. One explanation (model) could be that O5, like OL2 and OR2, is also bent when complexed with the 16-3 repressor. Bends, as generally accepted for helix-turn-helix repressor/operator complexes, are assembled from two symmetrically positioned kinks. Bent DNA (formally) is in plane when the two kinks are integral or half integral helical turns apart. In the latter case, if the two kinks are identical, the dihedral angle defined by them is 180°: they counterbalance each other. If in O5 the two kinks are 5 or 15 bp apart (i.e., half integral turn/s), at the inner or at the outer edges of the 5′-ACAA-3′ boxes, the DNA fragment carrying O5 would hardly differ from a straight (rod) shape, consequently escaping from detection in the bending assay. If O5 bends are in one plane, OL2 and OR2 bends cannot be so, and therefore would be detected in bending assays (Fig. 4). A further consequence for this shape of the bent OL2 and OR2 is that they may relate to each other more or less as (quasi) mirror images. This geometry creates further enigmas, as illustrated in Fig. 5, concerning the structure of the bound dimer (Fig. 5A; see orientations of the monomers relative to each other; they rotate at the monomer/monomer interface).
Figure 5.
(A) Putative model for structures of 16-3 operator–repressor complexes “rotationally flexible protein homodimers.” Note that OL2 (i.e., O5 − 1 bp) and OR2 (i.e., O5 + 1 bp) DNAs show (quasi) mirror symmetry (see more details in the text). Protein monomers (egg-like shapes) join differently in the two dimers bound to OL2 and OR2. (B) “Geometric homeostasis” model for OL2 and OR2, which is not compatible with the behavior of O5.
An alternative to the above “rotationally flexible protein homodimers” model, operator conformations could differ that maintain the specific intermolecular contacts (geometric homeostasis model; Fig. 5B). Assuming that the bending directions are identical for both OL2 and OR2 (unlike the situation in the first model), in order for them to achieve the same “pitch,” three pathways can be envisioned: (i) OL2 is converted to “OR2-pitch” by stretching; (ii) the opposite, i.e., compressing OR2 to “OL2- pitch”; and (iii) making an intermediate from both by parallel stretching OL2 and compressing OR2 into the same pitch. The experimental data, however, are inconsistent with these scenarios (Table l and Figs. 3 and 4). Unlike the first model (Fig. 5A), which is compatible with all our observations with OL2, O5, and OR2, none of the versions of the second model (Fig. 5B) can explain the behavior of the O5 operator. In the second model, O5 should behave like the other two operators, because, at sequence level, O5 is the closest relative of both OL2 and OR2, and more close to them than they are to each other. But O5 behaves like the mutant alleles of those and does not show the characteristics of OL2 and OR2 in band shift and in pBend assays (Fig. lC, Fig. 2B vs. Fig. 3 A and B, Table l). O5 apparently also cannot be converted to function as efficiently as the wild-type OL2 and OR2 operators.
A better understanding of the steric relations outlined for the 16-3 operators would require three dimensional studies. It is worth mentioning that, in the 434 repressor–operator complex, the operator DNA is slightly kinked at the inner edges of the 5′-ACAA-3′ “recognition” boxes (38). In the 16-3 repressor–operator complexes, kinks at the same sites of the operators would be compatible with the model shown in Fig. 5A.
Acknowledgments
We thank Mark Garner and Sankar Adhya for valuable discussions; Tibor Sík and Éva Kárpáti for critically reading the manuscript; Kornélia Szóráth Gál, Andrea Nagy, Magdolna Tóth Péli, and Csilla Sánta Török for excellent technical assistance; and József Antal for artwork. This work was supported by grants from the Hungarian National Scientific Research Fund (OTKA) (T 016092, T 023695, T 032205, and T 032255), from the Ministry of Culture (MKM Fund FKFP 0868/97), from the Hungarian Academy of Sciences (MTA/AKT-F 1999-2001), and from the Ph.D. program for Genetics led by L.O. at the Eötvös Loránd Science University in Budapest and at the Agricultural University in Gödöllõ.
Abbreviations
- β-gal
β-galactosidase
- CRP
cAMP receptor protein
References
- 1.Aggarwal A K, Rodgers D W, Drottar M, Ptashne M, Harrison S C. Science. 1988;242:899–907. doi: 10.1126/science.3187531. [DOI] [PubMed] [Google Scholar]
- 2.Jordan S R, Pabo C O. Science. 1988;242:893–899. doi: 10.1126/science.3187530. [DOI] [PubMed] [Google Scholar]
- 3.Schultz S C, Shields G C, Steitz T A. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 4.Parkinson G, Wilson C, Gunasekera A, Ebright Y W, Ebright R E, Berman H M. J Mol Biol. 1996;260:395–408. doi: 10.1006/jmbi.1996.0409. [DOI] [PubMed] [Google Scholar]
- 5.Barber A M, Zhurkin V B, Adhya S. Gene. 1993;130:1–8. doi: 10.1016/0378-1119(93)90339-5. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen H, Valentin-Hansen P. EMBO J. 1997;16:2108–2118. doi: 10.1093/emboj/16.8.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller J, Oehler S, Müller-Hill B. J Mol Biol. 1996;257:21–29. doi: 10.1006/jmbi.1996.0143. [DOI] [PubMed] [Google Scholar]
- 8.Dodd I B, Egan J B. J Biol Chem. 1996;271:11532–11540. doi: 10.1074/jbc.271.19.11532. [DOI] [PubMed] [Google Scholar]
- 9.Orosz L, Rostás K, Hotchkiss R D. Genetics. 1980;94:249–263. doi: 10.1093/genetics/94.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallmann G, Olasz F, Orosz L. Mol Gen Genet. 1980;178:443–446. [Google Scholar]
- 11.Dallmann G, Papp P, Orosz L. Nature (London) 1987;330:398–401. [Google Scholar]
- 12.Dallmann G, Marincs F, Papp P, Gaszner M, Orosz L. Mol Gen Genet. 1991;227:106–112. doi: 10.1007/BF00260714. [DOI] [PubMed] [Google Scholar]
- 13.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. p. 7. [Google Scholar]
- 14.Ditta G, Stanfield S, Corbin D, Helinski D R. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 17.Papp P P, Chattoraj D K, Schneider T D. J Mol Biol. 1993;233:219–230. doi: 10.1006/jmbi.1993.1501. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg M, Court D. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- 19.Kiss G B, Vincze É, Kálman Z, Forrai T, Kondorosi A. J Gen Microbiol. 1979;113:105–118. [Google Scholar]
- 20.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. p. 352. [Google Scholar]
- 21.Hanahan D. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 22.Simons R W, Houman F, Kleckner N. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 23.Semsey Sz, Papp I, Buzas Z, Patthy A, Orosz L, Papp P P. J Bacteriol. 1999;181:4185–4192. doi: 10.1128/jb.181.14.4185-4192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garner M M, Revzin A. Nucleic Acids Res. 1981;9:3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H M, Crothers D M. Nature (London) 1984;308:509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Zwieb C, Wu C, Adhya S. Gene. 1989;85:15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- 27.Johnson A D. Cell. 1995;81:655–658. doi: 10.1016/0092-8674(95)90524-3. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov V I, Minchenkova L E, Chernov B K, McPhie P, Ryu S, Garges S, Barber A M, Zhurkin V B, Adhya S. J Mol Biol. 1995;245:228–240. doi: 10.1006/jmbi.1994.0019. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Yagi N, Finch J T. FEBS Lett. 1996;379:148–152. doi: 10.1016/0014-5793(95)01506-x. [DOI] [PubMed] [Google Scholar]
- 30.Koudelka G B. Nucleic Acids Res. 1991;19:4115–4119. doi: 10.1093/nar/19.15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sellers J W, Vincent A C, Struhl K. Mol Cell Biol. 1990;10:5077–5086. doi: 10.1128/mcb.10.10.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pu W T, Struhl K. Proc Natl Acad Sci USA. 1991;88:6901–6905. doi: 10.1073/pnas.88.16.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer R T, Smith D L, Johnson A D. Genes Dev. 1988;2:807–816. doi: 10.1101/gad.2.7.807. [DOI] [PubMed] [Google Scholar]
- 34.Simoncsits A, Chen J, Percipalle P, Wang S, Törö I, Pongor S. J Mol Biol. 1997;267:118–131. doi: 10.1006/jmbi.1996.0832. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Pongor S, Simoncsits A. Nucleic Acids Res. 1997;25:2047–2054. doi: 10.1093/nar/25.11.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krämer H, Niemöller M, Amouyal M, Revet B, von Wilcken-Bergmann B, Müller-Hill B. EMBO J. 1987;6:1481–1491. doi: 10.1002/j.1460-2075.1987.tb02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabsch W, Sander C, Trifonov E N. Nucleic Acids Res. 1982;10:1097–1104. doi: 10.1093/nar/10.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson J E, Ptashne M, Harrison S C. Nature (London) 1987;326:846–852. doi: 10.1038/326846a0. [DOI] [PubMed] [Google Scholar]







