Abstract
The diagnostic classification of differentiated thyroid cancer has been a longstanding topic of debate among pathologists, largely due to high interobserver variability. This complexity has increased with the expansion of tumor types and subtypes. However, molecular studies have revealed a simpler and less controversial approach, categorizing these lesions into RAS‐like and BRAF p.V600E‐like neoplasms. In this review, the authors propose a classification that is based on, but does not require, the confirmation of molecular alterations. This approach aligns with and helps inform the pattern‐based assessment of tumor growth and cytologic atypia that is already widely used in clinical practice for preoperative patient stratification and tumor diagnosis, and promises a simpler conceptual understanding. © 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: thyroid carcinoma, classification, molecular, morphology, immunohistochemistry, Papillary thyroid carcinoma, follicular thyroid carcinoma, invasion, nuclear atypia
Scope of the problem
Thyroid nodules are common and can be detected in up to 60% of the population, depending on the diagnostic method employed. Their prevalence is highest in females, older individuals, those in iodine‐deficient regions, and those exposed to ionizing radiation [1, 2, 3, 4]. Cytopathology and surgical pathology play a central role in diagnosing these lesions and represent the most frequent specimens in endocrine pathology. However, to date uniform diagnosis of follicular patterned thyroid nodules remains one of the most highly controversial topics in pathology, with significant inter‐ and intraobserver variation [5, 6, 7, 8, 9, 10, 11, 12]. While the field has been considered complex, advances in the molecular profiling of thyroid lesions provide opportunities for simplification and clarity that should not be dismissed.
This discussion will focus on the diagnostic classification of well‐differentiated follicular cell‐derived thyroid neoplasms, which represents an area in need of major reform. Neuroendocrine tumors of thyroid C‐cells, as well as rare tumors of other or uncertain histogenesis (such as those resembling salivary gland neoplasms or derived from thymic and ultimobranchial body remnants), will not be covered [13]. The discussion of high‐grade differentiated thyroid carcinoma, poorly differentiated thyroid carcinoma, and anaplastic thyroid carcinoma is also beyond the scope of this review.
Historical perspectives: understanding how we got here
In the 1950s, thyroid pathology was relatively straightforward [14]. Tumors of follicular epithelial cells were classified as either follicular or papillary based on their architecture; some tumors were classified as mixed papillary and follicular when they displayed both growth patterns. Lesions that were invasive were classified as carcinomas (papillary thyroid carcinoma [PTC], follicular thyroid carcinoma [FTC], mixed carcinoma), while noninvasive lesions were referred to as adenomas (papillary adenoma, follicular adenoma). Less differentiated lesions were recognized as anaplastic thyroid carcinomas.
Over time, clinical experience led to major changes to this simplistic approach [15, 16]. The recognition of tumors with papillary architecture but without capsular invasion – yet still leading to metastatic disease – led to the belief that all papillary thyroid neoplasms should be considered malignant [17, 18]. The term ‘papillary adenoma’ became tainted and all tumors with papillary architecture were subsequently classified as malignant. Once the cytologic features of PTCs were appreciated, they became a key criterion for the diagnosis; these features included enlarged and elongated nuclei with peripheral margination of chromatin and clearing of nucleoplasm, giving them an empty appearance that resembled the eyes of the cartoon character Little Orphan Annie [19]. This concept of ‘the nuclear features of papillary thyroid carcinoma’ further evolved as this type of nuclear atypia was identified in follicular thyroid lesions, including some that had been classified as FTCs and others as follicular adenomas, and the concept of a follicular variant of papillary thyroid carcinoma (FVPTC) was proposed by Lindsay in 1960 [20] then popularized by Chen and Rosai in 1977 [21]. Metastasis was not always accompanied by identifiable invasion. Cases were reported in which encapsulated FVPTC led to bone metastasis despite the absence of detectable invasion [22]. Additionally, ~27% of noninvasive encapsulated classic PTCs were found to develop lymph node metastases [23]. These findings challenged the traditional paradigm that overt invasion is a requisite criterion for metastasis and further highlighted the diagnostic challenges faced by pathologists in accurately determining the status of capsular and vascular invasion, emphasizing the inherent difficulties in establishing definitive diagnostic criteria [12, 24, 25].
As nuclear morphology was recognized to be a key feature of lesions with metastatic potential, a controversy arose between pathologists who relied on invasion only and those who relied on cytologic features, particularly nuclear features. The threshold for nuclear atypia required to classify a lesion as malignant was undoubtedly the primary contributor to much inter‐ and intraobserver variability [5, 6, 7, 8, 9, 10, 11, 12]. Some experts saw nuclear atypia in all malignant lesions, resulting in the demise of follicular thyroid carcinoma, which had been defined based only on invasion and for which nuclear atypia had become an exclusion criterion [26].
Molecular advancements: what insights have we gained?
With the advent of molecular technologies, the molecular basis of thyroid cancer began to emerge. The identification of RET fusions in PTCs [27, 28, 29, 30, 31, 32, 33] was followed by the identification of other gene fusions involving NTRK and ALK. In 2003 the BRAF mutation resulting in the BRAF p.V600E variant protein was reported [34, 35, 36], and it quickly became evident that this was the most common driver event in PTCs. As data accumulated, BRAF codon 600 mutations and the various fusions were discovered to be mutually‐exclusive drivers; the fusions appeared to be more frequent in pediatric thyroid carcinomas [37] and in the setting of ionizing radiation exposure [38, 39, 40, 41, 42, 43, 44, 45, 46], which was already a known cause of PTCs [44, 47, 48].
A distinct signaling pathway was identified in tumors associated with clinical or subclinical hyperthyroidism. Activation of the TSH receptor or its Gsα partner, encoded by GNAS, became the hallmark of these ‘hot nodules,’ which clinically behave in a benign fashion in most cases [49, 50, 51]. These mutations lead to the constitutive activation of adenylyl cyclase, increasing intracellular cAMP and stimulating both function and proliferation of thyroid follicular cells. Some of these lesions also harbor alterations in EZH1, usually occurring as a second event in combination with TSHR or GNAS mutation [52], or rarely, in association with pathogenic DICER1 variants [53]. These tumors may have a papillary architecture but are not characterized by nuclear atypia and morphologists had to concede that there was indeed a benign papillary neoplasm, based on these features. The 5th edition of the WHO finally recognized these as ‘follicular adenomas with papillary architecture’ [13].
The study of FTCs identified the presence of RAS mutations as a common event [54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64] with occasional other alterations such as the PAX8::PPARG fusion [65, 66]; THADA and FGFR2 fusions and mutations in EIF1AX were also identified in follicular‐patterned neoplasms [67]. Indeed, it also became evident that the same molecular alterations found in FTCs were also present in FVPTCs [68]. These alterations can also be identified in more aggressive high‐grade and poorly differentiated neoplasms, consistent with the concept of progression of thyroid carcinomas [69].
The most important insight gained from molecular studies of differentiated thyroid carcinoma is the concept of ‘RAS‐like’ and ‘BRAF p.V600E‐like’ cancers that was proposed by the TCGA [68]. RAS‐like tumors (including those with RAS mutations but also those with PAX8::PPARG, THADA, and FGFR fusions, mutations in EIF1AX, and mutations in BRAF that differ from p.V600E) exhibit characteristic architectural features traditionally associated with FTCs. They have colloid‐containing follicles and they tend to grow as expansile tumors with well‐defined borders, often with capsules. They lack the infiltrative, sclerosing growth pattern of most BRAF p.V600E‐like PTCs (which include the infiltrative tumor that has predominant follicular growth known as ‘infiltrative follicular variant papillary carcinoma’). In the absence of identified invasion of a capsule or local boundary (as opposed to the infiltrative growth of BRAF p.V600E‐like tumors), RAS‐like follicular‐patterned thyroid neoplasms generally have minimal metastatic potential. Even in the presence of minimal capsular invasion only, their behavior is indolent, warranting clinical observation alone after resection. Unlike classic PTCs (prototypic of BRAF p.V600E‐like disease), they tend not to exhibit lymphatic invasion or involvement of regional lymph nodes. When they become more aggressive, they invade blood vessels and can give rise to systemic disease.
As the many similarities between FTC and invasive encapsulated FVPTC have become more apparent, their distinction now hinges solely on the identification – or interpretation – of nuclear atypia. The previously published literature, in this setting, is not helpful, as the definition of ‘follicular variant of papillary thyroid carcinoma’ has varied. This term has been used to diagnose both infiltrative follicular BRAF p.V600E‐like and encapsulated follicular RAS‐like lesions (both invasive and noninvasive), and the diagnosis has depended on one's diagnostic threshold for what constitutes the ‘nuclear atypia sufficient for papillary thyroid carcinoma.’ The historical diagnosis of FVPTC, therefore, has been highly variable and has encompassed a wide range of biologically and genetically diverse follicular cell‐derived thyroid neoplasms. When interpretations about infiltrative growth are also factored in, what in one country or by one pathologist is regarded as malignant may be regarded as benign by another. At present, the reliance on a specific threshold of nuclear atypia in RAS‐like neoplasms (when, in truth, subtle/papillary‐like nuclear atypia exists along a histologic spectrum both within the category as a whole and even in individual tumors) to subdivide them into either FTC or FVPTC unnecessarily complicates diagnostic consistency and contributes to variability in interpretation across pathologists and studies [70].
The inherent subjectivity, challenge, and even futility of subdividing RAS‐like neoplasms into two distinct diagnostic entities becomes even more apparent when contrasted with the objective clarity provided by molecular profiling. This underscores the necessity for integrated approaches that combine molecular insights with morphological assessments to improve and simplify disease classification, as opposed to relying solely on individual interpretation of histopathological criteria.
Definitional ambiguities
One of the fundamental problems in thyroid pathology is the lack of clear definitions. The simplest one is one of the most troublesome: ‘invasion.’ Although the diagnosis of malignancy has been based on the presence of invasion for over 60 years, the term itself still lacks a clear definition and consensus.
The diagnosis of malignancy in many tissues depends on the identification of invasive features. Indeed, the word ‘cancer’ comes from the ancient Greek word καρκίνος that means ‘crab’; the ancient Greeks identified malignant tumors that looked like crabs because of their irregular, infiltrative growth patterns. Invasion in many epithelial structures is not difficult to define; where there are basement membranes, it is readily apparent that tumor cells infiltrating through the basement membrane constitutes evidence of malignancy. However, in the thyroid, the concept of invasion through a basement membrane of a follicle has not been considered evidence of malignancy, with the possible exception of C‐cell proliferations, which are not the subject of this work.
Some thyroid tumors, including the classic forms of PTC, infiltrate through the gland in a clearly crab‐like fashion; this is characteristic of BRAF p.V600E‐like tumors (even those that are follicular‐predominant, i.e. infiltrative follicular subtype) [68]. In contrast, RAS‐like tumors are usually surrounded by compressed thyroid tissue that may, in some cases, form a fibrous capsule. However, in rare instances the tumor may lack a capsule altogether [71]. The distinction of benign from malignant nodules in this scenario often depends on the identification of invasion into or through the tumor capsule or, if there is no capsule, into the surrounding thyroid.
While it is intuitive that pathologists use the criteria of invasion to reach a diagnosis of malignancy, the definition of invasion in various scenarios is not clear or consistent, resulting in uncertainty and discrepancies in diagnoses. This uncertainty and resultant discrepancy in diagnosis mirrors the problems in adjudicating nuclear atypia. In the following three sections, this article will review what is known and, more important, what is unknown, about the criteria for invasion and nuclear atypia that should be used for the diagnosis of thyroid carcinomas.
Capsular invasion: what qualifies as invasion and what if the capsule is absent?
The term ‘capsular invasion’ is widely used in publications that include research papers and textbooks, but there is no clear definition of this term. The term ‘capsule’ generally refers to the capsule surrounding the tumor. Some authors have used it to describe tumors that invade beyond the thyroid, but this has been clarified as a misuse of the terminology, since the thyroid gland does not have an anatomic capsule [72]. In modern publications, it is now usually clear that the discussion of tumor invasion beyond the thyroid gland is ‘extra‐thyroidal extension.’ While this too, has been a subject of controversy, the staging of thyroid carcinomas has been redefined based on clarity concerning this subject [73].
More critical, however, is the definition of invasion of a tumor capsule, as this has become the criterion of malignancy for follicular‐patterned neoplasms, regardless of one's threshold for nuclear atypia. While many articles simply assume that pathologists can clearly define capsular invasion, this is not necessarily the case. When invasion is equivocal, the term “tumor of uncertain malignant potential (UMP)" has been used in some practices.
The identification of capsular invasion is not difficult if invasion through the full thickness of a capsule can be readily identified (Figures 1 and 2). However, there is no consensus on whether tumor cells infiltrating INTO, but not through a capsule, represent invasion. Is invasion identified when the infiltration extends into the inner third of the capsule, must it go halfway through, or should the term be restricted only to invasion that penetrates through the full thickness of the capsule? The answer to this dilemma can often be obtained by examining multiple sections taken at different levels through the region of interest [74]. In some cases, the invasion will become unequivocal (Figure 2), but in others, it disappears. The challenge in the latter situation is that the invasion may have been present in sections in the opposite direction, but that will never be known. In fact, the lack of invasive growth can never be proven, even if one submits the entire intersection of the tumor and nontumorous parenchyma for microscopic examination [24, 75, 76]. The problem is compounded by the fact that almost no patient comes to surgery without a previous biopsy of the lesion, and that procedure can create artifact with fibrosis that can trap tumors and cause irregularity of the capsule, mimicking capsular invasion [77] (supplementary material, Figure S1). The clue to this phenomenon is the identification of a biopsy site in close proximity to the focus of apparent invasion. In reality, additionally, the aggressiveness of diagnosing capsular invasion may also be driven by the identification of RAS‐like cytologic atypia in follicular patterned thyroid tumors.
Figure 1.
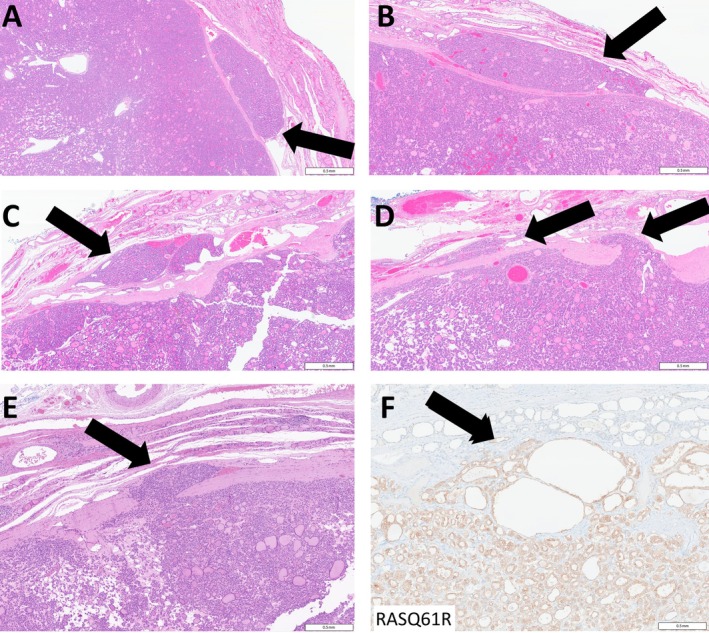
Unequivocal capsular invasion by thyroid neoplasms. (A–C) Capsular invasion is readily identified when there are nests of tumor cells beyond the capsule of a thyroid neoplasm (arrows) or (D, E) when there are tumor cells invading through the capsule (arrows). (F) Immunohistochemistry for RASQ61R stains the tumor cells and identifies tumor cells infiltrating through the capsule (arrow). Scale bars, 0.5 mm.
Figure 2.
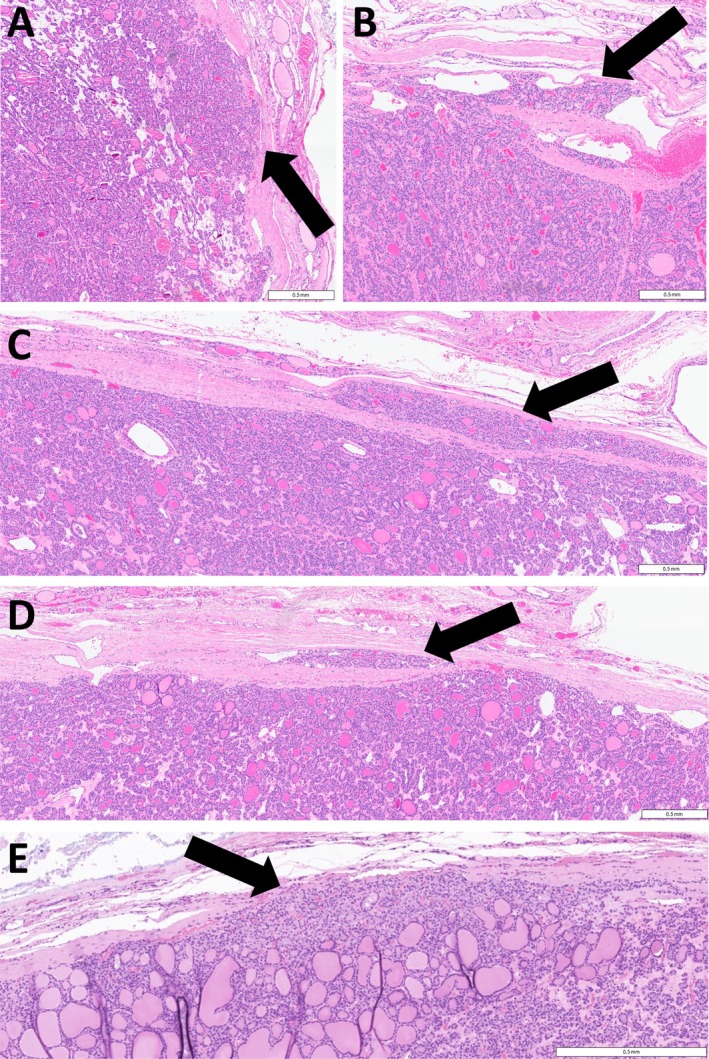
Capsular invasion by thyroid neoplasms. Capsular invasion is not always classified as such when (A) there are tumor cells pushing into the capsule (arrow) or (B) trapped within the fibrous tissue surrounding a thyroid neoplasm (arrow). (C, D) When invasion is questionable, it can be proven by examining sections cutting through the lesion (arrows). (E) In this RAS‐mutant thyroid follicular neoplasm, tumor cells are seen percolating through the capsule (arrow) but not through its full thickness. While some pathologists consider this evidence of invasive behavior, others would not classify this as capsular invasion. Scale bars, 0.5 mm.
Identifying invasion becomes even more challenging in the absence of a well‐defined fibrous capsule. Traditionally, invasion in such cases is defined as tumor cells extending beyond the lesion's outline into the surrounding thyroid tissue (Figure 3). However, some instances of this can be considered simply an irregular border, often indented by a vascular channel.
Figure 3.

Local invasion by thyroid neoplasms. (A) Local invasion by an unencapsulated lesion is usually recognized as nests of tumor cells beyond the edge of the thyroid neoplasm, infiltrating into and around adjacent nontumorous follicles (arrows); this invasive behavior is not currently defined, as it does not represent ‘capsular invasion.’ Scale bar, 0.5 mm. (B) In some instances, tumor cells with marked cytologic atypia forming microfollicles (arrows) can be seen infiltrating around cytologically and architecturally benign components of a follicular neoplasm (*), suggesting that this represents malignancy arising in a follicular adenoma; does this invasive behavior qualify as ‘capsular invasion’? Scale bar, 0.25 mm.
Thyroid tumors can undergo degeneration, such as fibrosis, hemorrhage, and cystic change. These changes can distort the tumor's shape, resulting in an irregular appearance that complicates the assessment of whether there is true capsular invasion. In these situations, it becomes increasingly difficult to distinguish between a reactive irregular border and genuine invasive growth. Again, there is often bias in the process of searching for invasion, since benign lesions with clearly benign nuclear morphology do not raise concern for this being true invasion.
The ability to truly classify a lesion as ‘noninvasive’ remains controversial, since one can never unequivocally prove the lack of invasion in any circumscribed follicular neoplasm, as the entire periphery of the lesion will never be examined microscopically, based on the limitations of our practice [24, 75]. Despite the challenges in identification of capsular invasion, RAS‐like follicular‐patterned neoplasms that are – (1) noninvasive, (2) with equivocal capsular invasion, or (3) with capsular invasion ONLY (minimally‐invasive) and no high‐grade features or angioinvasion – are ALL treated similarly, with clinical observation alone without completion thyroidectomy or radioactive iodine ablation, in light of the low recurrence risk [78]. Therefore, a diagnosis of minimally invasive FTC, noninvasive follicular tumor with papillary‐like nuclei (NIFTP) [79], and minimally invasive encapsulated FVPTC, will likely result in a similar clinical treatment paradigm.
Vascular invasion: what are the relevant criteria?
Perhaps an even more controversial method of invasion is vascular invasion, also called angioinvasion, which represents invasion into blood vessels (not lymphatic vessels) at the periphery of the tumor. In some studies, the number 4 was regarded as biologically significant, as patients with 4 or more foci of vascular invasion in a follicular‐patterned tumor were placed in a high‐recurrence‐risk group and were treated with completion thyroidectomy and radioactive iodine therapy [80, 81]. However, some data suggest that, when using rigid criteria for diagnosis of angioinvasion (tumor cells invading through a vessel wall / present within a vascular lumen with associated fibrin thrombus) (Figure 4), any amount of true invasion is biologically relevant [82]. Challenges in the diagnosis of angioinvasion (supplementary material, Figure S2), which include ‘undermining’ of endothelium by tongues of tumor, close association of tumor nests with capsular vessels in richly‐vascular tumors, and detachment/fragmentation of tumor cells with sloughing into vascular lumina without morphologic evidence of endothelial injury/activation, may explain the lack of clinical relevance of vascular invasion in some studies [83]. The diagnosis of both vascular invasion and capsular invasion is made increasingly difficult with extensive manual manipulation of the thyroid gland (including fresh sectioning), causing architectural distortions that may mimic invasion.
Figure 4.

Vascular invasion by thyroid neoplasms. (A) Vascular invasion is characterized by tumor cells within blood vessels associated with fibrin thrombus (*). When equivocal, (B) vascular channels can be confirmed by staining for CD34 that decorates endothelium, and (C) thrombus can be identified by staining for CD61 that stains platelets/fibrinogen. Scale bars, 0.25 mm.
The controversy of nuclear atypia
Prior to the recognition of BRAF p.V600E‐like and RAS‐like neoplasms, those who believed in the concept of FVPTC recognized that nuclear atypia, even when subtle, was a feature of tumors with metastatic potential. This claim, qualified by the differing thresholds for nuclear atypia worldwide, formed the crux of debates surrounding reproducibility in thyroid diagnostics. The ‘hawks’ would identify nuclear irregularity and classify the lesion as FVPTC, while others with a higher threshold for nuclear atypia would call the lesion FTC; if they were not convinced that they saw capsular invasion, they would call the lesion a benign follicular adenoma. This interobserver (and even geography‐dependent) variability was highlighted in a recent study showing high rates of diagnosis of nuclear atypia in North America and the lowest rates in Asia/Oceania [70]. Additionally, studies using deep learning have suggested that the distinction between follicular adenoma and FTC can be based on nuclear features [84], raising the possibility that invasion may not be required for the diagnosis of FTC.
The concept of nuclear atypia remains one of the most contentious issues in thyroid pathology. Nuclear atypia is readily identified in classic papillary thyroid carcinomas (BRAF p.V600E‐like malignancies) where the nuclei are extremely large and often elongated with thick, dark nuclear membranes and nucleoplasm that is almost completely clear; they have irregular nuclear contours and invaginations that form grooves and intranuclear cytoplasmic pseudoinclusions [85] (Figure 5A,B). These nuclear changes are also often associated with cytoplasmic characteristics of BRAF p.V600E‐like tumors (dense, eosinophilic cytoplasm) that are more frequently emphasized by cytopathologists than surgical pathologists. However, nuclear atypia is not unique to BRAF p.V600E‐like tumors as RAS‐like lesions also usually display subtle nuclear atypia, typically less well developed than in BRAF p.V600E‐like conventional PTC [86] (Figure 5C,D); they may even have ‘pseudo‐pseudoinclusions,’ but these do not contain cytoplasm [86]. Additionally, nuclear changes can also occur in areas of chronic inflammation, fibrosis, injury, or follicular‐cell activation, underscoring nuclear changes as a historical gray zone of thyroid pathology [87]. The distinction between RAS‐like and BRAF p.V600E‐like nuclear atypia can be difficult in some borderline cases and, as is the case with invasion, it must be interpreted in the context of invasive behavior and architecture; in some cases, immunohistochemistry may be required to accurately classify the lesion.
Figure 5.
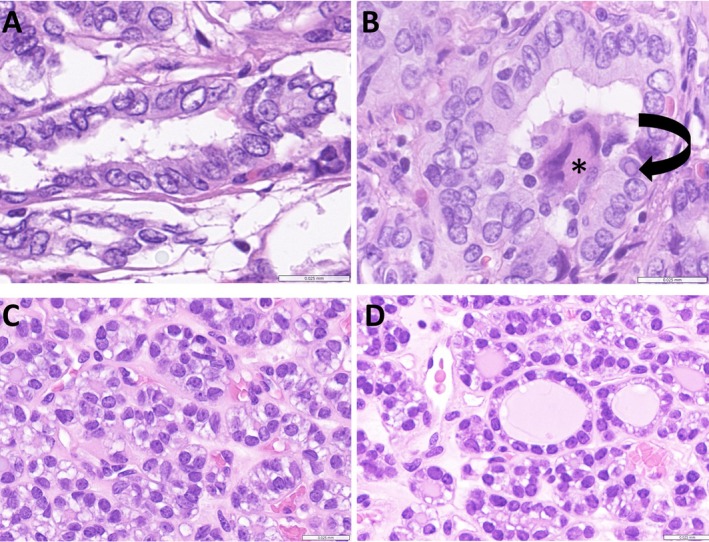
Nuclear atypia in thyroid neoplasms. (A, B) The nuclear atypia of BRAF p.V600E‐like papillary carcinoma is florid. The tumor cells have enlarged, overlapping nuclei with irregular borders, peripheral margination of chromatin, and clearing of nucleoplasm. The irregular nuclear contours can result in cytoplasmic invaginations known as pseudoinclusions (B, arrow); note also the multinucleated giant cell in the lumen (*). (C, D) The nuclei of RAS‐like thyroid carcinomas are enlarged and irregular in shape, frequently overlapping and may have prominent nucleoli, but they rarely have peripheral margination of chromatin or cytoplasmic pseudoinclusions. Scale bars, 0.025 mm.
There clearly remains a need to evaluate nuclear atypia in order to make a diagnostically relevant distinction between the classic nuclear features of BRAF p.V600E‐like PTCs and the more subtle nuclear features of RAS‐like follicular neoplasms. However, there appears to be no rational need, within the family of RAS‐like follicular neoplasms, to distinguish the nuclear features in tumors previously classified as FVPTC by some experts (including some of the authors of this article) from those classified as FTC by others. Likewise, there appears to be no need to persist with the historical definition of FTC, which narrowly defines it as ‘a thyroid malignancy with follicular architecture that does not have the nuclear atypia of papillary thyroid carcinoma’ as that excludes a substantial portion of the spectrum of RAS‐like nuclear atypia for no clinically apparent reason. Molecular genetics and clinical outcome data have shown the paradigm for follicular thyroid neoplasms with subtle papillary‐like nuclear atypia, including nuclear enlargement, nuclear elongation, and irregular contours (which pathologists interpret unreliably): these carcinomas, like classic FTC, are RAS‐like and exhibit behavior similar to classic FTC [88]. Both are indolent when minimally invasive but more aggressive when angioinvasive or widely invasive. The 5th edition WHO Classification of Endocrine and Neuroendocrine Tumours addressed this, in part, by clearly removing invasive encapsulated FVPTC from the category of PTC and placing it next to FTC [13], but failed to garner consensus on merging invasive encapsulated FVPTC with FTC, despite the fact that the two lesions are similar in epidemiology, clinical behavior, gross morphology, molecular pathology, and outcomes.
Impact on cytologic diagnosis of fine‐needle aspiration (FNA) biopsies
The use of fine‐needle aspiration (FNA) for the preoperative management of patients with thyroid nodules is now considered the standard of care. Cytopathologists have identified the florid nuclear features of PTC as unequivocal evidence of malignancy. At the other end of the spectrum, they define benign lesions based on the lack of any nuclear atypia, as well as background features, such as thin colloid, and the absence of architectural atypia, such as microfollicular patterns. They have also defined less florid nuclear atypia, the type that is seen in RAS‐like malignancies, as atypia of uncertain significance (AUS) [89]. There is justification for this hesitation in making a clear‐cut diagnosis of malignancy, because this type of atypia is also seen in reactive lesions, such as thyroiditis, and because of the lack of consensus, as previously discussed, on the definition and diagnosis of invasion on subsequent histology.
Clinical implications
The implications of the data that we have summarized have a clear impact on the management of patients with thyroid carcinomas. First, there is an unequivocal role for molecular testing of thyroid cytology samples that are classified as ‘AUS’ (Bethesda III) and ‘follicular neoplasm’ (Bethesda IV) [89]. For example, the identification of a RAS‐like clonal alteration generally implies a low‐risk follicular neoplasm that requires surgical resection, in light of possible progression [69], whereas the presence of an additional high‐risk alteration (e.g. TERT‐promoter or TP53 mutations, or adverse epigenetic alterations) may direct total thyroidectomy in a single procedure in light of possible aggressive behavior. Conversely, the management of PTCs relies less on molecular testing, as the diagnosis can usually be made through routine cytologic examination. The decision for total thyroidectomy in these cases is more dependent on factors such as tumor size, local disease extent, or the identification of metastases prior to surgery, often identified by preoperative radiologic studies [90].
The molecular data suggest that RAS‐like thyroid neoplasms clinically exhibit a spectrum, ranging from, in most cases, indolent minimally invasive malignancies (often lacking additional secondary molecular alterations), to, less commonly, more aggressive cancers that invade locally and may invade blood vessels to give rise to distant metastases in lungs, liver, brain, and bone. The former can be treated conservatively with directed surgical resection (lobectomy or hemithyroidectomy), but usually do not require total thyroidectomy and certainly do not warrant more aggressive management with radioactive iodine therapy. In contrast, the latter requires complete removal of all normal thyroid tissue to allow high‐dose radioactive iodine therapy to target metastatic malignancy. Fortunately, most RAS‐like thyroid neoplasms tend to be relatively well‐differentiated malignancies that retain normal function and regulation of the sodium iodide symporter, except in those cases that progress to high‐grade differentiated thyroid carcinomas, poorly differentiated thyroid carcinomas, or anaplastic thyroid carcinomas.
The classic PTCs that are BRAF p.V600E‐like, including the majority of the kinase fusion cancers, are by definition less differentiated, since they express thyroid‐specific genes at a much lower level [68]. They tend to be infiltrative in growth pattern ab initio; the rare cases that are truly encapsulated may have a better prognosis [91, 92]. The size of the tumor plays an important role, and these cancers have well‐defined TNM staging criteria that guide the therapeutic approach [73, 93].
A practical roadmap moving forward
Broadly speaking, pathology plays a critical role in determining appropriate patient management, but the difficulty is that pathology remains an interpretive discipline, highly influenced by experience, practice environment, and training. Because of the importance and responsibility of pathology to patient care, changes in classification can be met with resistance. However, in this setting, the clinical, molecular, and outcome data all suggest that patient care can be improved by simplifying the classification of differentiated thyroid carcinomas [94].
The next iteration of this classification (Figure 6) should abandon the complex terminology of invasive encapsulated FVPTC, which essentially represents the variation in interpretation of nuclear atypia seen in RAS‐like neoplasms, and, in parallel fashion, FTC should no longer be defined as unequivocally lacking nuclear features of PTC. This simplified classification of follicular cell‐derived differentiated thyroid carcinoma more accurately reflects the uniformity in their architectural pattern of tumor growth and in their underlying molecular basis of disease.
Figure 6.

Suggested revised terminology and diagnostic approach for thyroid follicular‐patterned neoplasms, (A) and diagnostic approach for thyroid follicular‐patterned neoplasms (B). The revised classification does away with follicular variant papillary carcinoma; instead, the infiltrative subtype of that entity, which is BRAF‐like, is classified as classic papillary carcinoma with predominant follicular growth (*). The encapsulated subtype is now folded in with other RAS‐like thyroid carcinomas as follicular carcinoma. Note the subtypes of papillary carcinoma have graded aggressiveness and include the diffuse sclerosing and solid variants that may be fusion carcinomas (#). Fusions involving RET, NTRK, and ALK are implicated in some of these subtypes; however, they may give rise to tumors that have morphologic heterogeneity and cannot be easily classified as BRAF‐like or RAS‐like. In addition, some studies have shown that a subset of columnar cell papillary thyroid carcinomas are RAS‐like [95, 96]. (B) This algorithm indicates the approach to classifying a follicular‐patterned lesion in the revised classification based on the presence or absence of nuclear atypia and invasion. TFND, thyroid follicular nodular disease; FTA, follicular thyroid adenoma; FT‐UMP, follicular tumor of uncertain malignant potential; NIFTP, noninvasive follicular tumor with papillary‐like nuclei; WDT‐UMP, well‐differentiated tumor of uncertain malignant potential.
While an ideal scenario would involve comprehensive molecular classification, its implementation remains limited by cost and accessibility. Nevertheless, such an approach could resolve some of the diagnostic challenges posed by borderline tumors, particularly fusion carcinomas that display molecular heterogeneity – some aligning with BRAF‐like, and others with RAS‐like signatures. In settings where molecular testing would help with risk stratification but is not available or practical, we are fortunate to have biomarkers that can provide rapid answers. For example, immunohistochemistry can localize the BRAF p.V600E mutated protein [97, 98], and RAS Q61R antibodies are becoming more widely used [99]. Additionally, ALK and TRK expression in follicular epithelial cells, which typically do not express these proteins at significant levels, can be detected via immunohistochemistry, as can RET, provided appropriate reagents are used [98]. However, there are well‐known limitations to these ancillary techniques based on biological considerations related to the correlation between protein expression of intact versus fusion proteins, and molecular testing remains the gold standard for accurate classification and risk stratification.
Incorporating molecular terminology into the classification not only enhances diagnostic precision but also reduces interobserver variability, aligns pathology with targeted therapeutic strategies, and facilitates clearer communication among pathologists, clinicians, and researchers. This approach allows for a more standardized framework that can adapt to evolving molecular insights while maintaining clinical relevance.
It is essential to modernize our terminology, to more accurately reflect the biological and clinical coherence of RAS‐like thyroid lesions. The phrase ‘nuclear features of papillary thyroid carcinoma’ has become so ambiguous and inconsistently applied that its meaning is often obscured without detailed context, or familiarity with the diagnosing pathologist's perspective. We must reframe our diagnostic language through the lens of both historical experience and the advancements offered by molecular profiling to inform our current practice.
Author contributions statement
All authors contributed to the conception and design of the study, article preparation, approval of the final article and consent for publication. Figure preparation was performed by SLA, OM and CCJ.
Supporting information
Figure S1. Pseudoinvasion of the capsule in a thyroid neoplasm
Figure S2. Pseudoinvasion of vessels in a thyroid neoplasm
No conflicts of interest were declared.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Ezzat S, Sarti DA, Cain DR, et al. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med 1994; 154: 1838–1840. [DOI] [PubMed] [Google Scholar]
- 2. Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009; 39: 699–706. [DOI] [PubMed] [Google Scholar]
- 3. Trerotoli P, Ciampolillo A, Marinelli G, et al. Prevalence of thyroid nodules in an occupationally radiation exposed group: a cross sectional study in an area with mild iodine deficiency. BMC Public Health 2005; 5: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burguera B, Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinol Metab Clin North Am 2000; 29: 187–203. [DOI] [PubMed] [Google Scholar]
- 5. Saxen E, Franssila K, Bjarnason O, et al. Observer variation in histologic classification of thyroid cancer. Acta Pathol Microbiol Scand A 1978; 86A: 483–486. [DOI] [PubMed] [Google Scholar]
- 6. Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 2002; 26: 1508–1514. [DOI] [PubMed] [Google Scholar]
- 7. Franc B, de la SP, Lange F, et al. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol 2003; 34: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 8. Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol 2004; 28: 1336–1340. [DOI] [PubMed] [Google Scholar]
- 9. Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 2008; 130: 736–744. [DOI] [PubMed] [Google Scholar]
- 10. Du E, Wenig BM, Su HK, et al. Inter‐observer variation in the pathologic identification of extranodal extension in nodal metastasis from papillary thyroid carcinoma. Thyroid 2016; 26: 816–819. [DOI] [PubMed] [Google Scholar]
- 11. Hernandez‐Prera JC, Machado RA, Asa SL, et al. Pathologic reporting of tall‐cell variant of papillary thyroid cancer: have we reached a consensus? Thyroid 2017; 27: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 12. Zhu Y, Li Y, Jung CK, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms‐an observer variation study. Endocr Pathol 2020; 31: 132–140. [DOI] [PubMed] [Google Scholar]
- 13. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol 2022; 33: 27–63. [DOI] [PubMed] [Google Scholar]
- 14. Warren S, Meissner WA. Tumors of the Thyroid Gland. Atlas of Tumor Pathology, Series 1, Fascicle 14. Armed Forces Institute of Pathology: Washington, DC, 1953. [Google Scholar]
- 15. Asa SL. The current histologic classification of thyroid cancer. Endocrinol Metab Clin North Am 2019; 48: 1–22. [DOI] [PubMed] [Google Scholar]
- 16. Cipriani NA. The metamorphosis of papillary thyroid carcinoma. Histopathology 2022; 81: 168–170. [DOI] [PubMed] [Google Scholar]
- 17. Meissner WA, Adler A. Papillary carcinoma of the thyroid. A study of the pathology of two hundred twenty‐six cases. Arch Pathol 1958; 66: 518–525. [PubMed] [Google Scholar]
- 18. Silverberg SG, Vidone RA. Adenoma and carcinoma of the thyroid. Cancer 1966; 19: 1053–1062. [DOI] [PubMed] [Google Scholar]
- 19. Hapke MR, Dehner LP. The optically clear nucleus. A reliable sign of papillary carcinoma of the thyroid? Am J Surg Pathol 1979; 3: 31–38. [DOI] [PubMed] [Google Scholar]
- 20. Lindsay S. Carcinoma of the Thyroid Gland. A Clinical and Pathological Study of 293 Patients at the University of California Hospital. Charles C. Thomas: Springfield, 1960. [Google Scholar]
- 21. Chen KTK, Rosai J. Follicular variant of thyroid papillary carcinoma: a clinicopathologic study of six cases. Am J Surg Pathol 1977; 1: 123–130. [DOI] [PubMed] [Google Scholar]
- 22. Baloch ZW, LiVolsi VA. Encapsulated follicular variant of papillary thyroid carcinoma with bone metastases. Mod Pathol 2000; 13: 861–865. [DOI] [PubMed] [Google Scholar]
- 23. Rivera M, Tuttle RM, Patel S, et al. Encapsulated papillary thyroid carcinoma: a clinico‐pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern). Thyroid 2009; 19: 119–127. [DOI] [PubMed] [Google Scholar]
- 24. Stojanov IJ, Mete O, Asa SL. Obstacles to tumor capsule assessment in noninvasive follicular thyroid neoplasm with papillary‐like nuclear features (NIFTP). Endocr Pathol 2023; 34: 484–486. [DOI] [PubMed] [Google Scholar]
- 25. Mete O, Asa SL. Thyroid tumor capsular invasion: the bottom line or much ado about nothing? Endocr Pathol 2020; 31: 141–142. [DOI] [PubMed] [Google Scholar]
- 26. LiVolsi VA, Asa SL. The demise of follicular carcinoma of the thyroid gland. Thyroid 1994; 4: 233–235. [DOI] [PubMed] [Google Scholar]
- 27. Fusco A, Grieco M, Santoro M, et al. A new oncogene in human thyroid papillary carcinomas and their lymph‐nodal metastases. Nature 1987; 328: 170–172. [DOI] [PubMed] [Google Scholar]
- 28. Bongarzone I, Pierotti MA, Monzini N, et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene 1989; 4: 1457–1462. [PubMed] [Google Scholar]
- 29. Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the ret proto‐oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990; 60: 557–563. [DOI] [PubMed] [Google Scholar]
- 30. Santoro M, Carlomagno F, Hay ID, et al. Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest 1992; 89: 1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lanzi C, Borrello MG, Bongarzone I, et al. Identification of the product of two oncogenic rearranged forms of the RET proto‐oncogene in papillary thyroid carcinomas. Oncogene 1992; 7: 2189–2194. [PubMed] [Google Scholar]
- 32. Santoro M, Dathan NA, Berlingieri MT, et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RET proto‐oncogene in a human thyroid papillary carcinoma. Oncogene 1994; 9: 509–516. [PubMed] [Google Scholar]
- 33. Viglietto G, Chiappetta G, Martinez‐Tello FJ, et al. RET/PTC oncogene activation is an early event in thyroid carcinogenesis. Oncogene 1995; 11: 1207–1210. [PubMed] [Google Scholar]
- 34. Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003; 95: 625–627. [DOI] [PubMed] [Google Scholar]
- 35. Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene 2003; 22: 6455–6457. [DOI] [PubMed] [Google Scholar]
- 36. Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003; 88: 5399–5404. [DOI] [PubMed] [Google Scholar]
- 37. Stosic A, Fuligni F, Anderson ND, et al. Diverse oncogenic fusions and distinct gene expression patterns define the genomic landscape of pediatric papillary thyroid carcinoma. Cancer Res 2021; 81: 5625–5637. [DOI] [PubMed] [Google Scholar]
- 38. Ito T, Seyama T, Iwamoto KS, et al. In vitro irradiation is able to cause RET oncogene rearrangement. Cancer Res 1993; 53: 2940–2943. [PubMed] [Google Scholar]
- 39. Nishisho I, Rowland JM, Bove KE, et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation‐induced and sporadic thyroid papillary carcinoma in children. Cancer Res 1997; 57: 1690–1694. [PubMed] [Google Scholar]
- 40. Nikiforov YE, Rowland JM, Bove KE, et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation‐induced and sporadic thyroid papillary carcinomas in children. Cancer Res 1997; 57: 1690–1694. [PubMed] [Google Scholar]
- 41. Nikiforov Y, Koshoffer A, Nikiforova M, et al. Chromosomal breakpoint positions sugeest a direct role for radiation in inducing illegitimate recombination between the ELE1 and RET genes in radiation‐induced thyroid carcinomas. Oncogene 1999; 18: 6330–6334. [DOI] [PubMed] [Google Scholar]
- 42. Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation‐induced RET and NTRK1 rearrangements in 191 post‐chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res 2000; 6: 1093–1103. [PubMed] [Google Scholar]
- 43. Nikiforova MN, Ciampi R, Salvatore G, et al. Low prevalence of BRAF mutations in radiation‐induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett 2004; 209: 1–6. [DOI] [PubMed] [Google Scholar]
- 44. Williams D. Radiation carcinogenesis: lessons from Chernobyl. Oncogene 2008; 27: S9–S18. [DOI] [PubMed] [Google Scholar]
- 45. Hamatani K, Mukai M, Takahashi K, et al. Rearranged anaplastic lymphoma kinase (ALK) gene in adult‐onset papillary thyroid cancer amongst atomic bomb survivors. Thyroid 2012; 22: 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leeman‐Neill RJ, Kelly LM, Liu P, et al. ETV6‐NTRK3 is a common chromosomal rearrangement in radiation‐associated thyroid cancer. Cancer 2014; 120: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sampson RJ, Key CR, Buncher CR, et al. Thyroid carcinoma in Hiroshima and Nagasaki. I. Prevalence of thyroid carcinoma at autopsy. JAMA 1969; 209: 65–70. [PubMed] [Google Scholar]
- 48. Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster: Pathomorphologic study of 84 cases (1991‐1992) from the Republic of Belarus. Cancer 1994; 74: 748–766. [DOI] [PubMed] [Google Scholar]
- 49. Parma J, Duprez L, van Sande J. Somatic mutations in the thyrotropin recptor gene cause hyperfunctioning thyroid adenomas. Nature 1993; 365: 649–651. [DOI] [PubMed] [Google Scholar]
- 50. van Sande J, Parma J, Tonacchera M, et al. Genetic basis of endocrine disease. Somatic and germline mutations of the TSH receptor gene in thyroid disease. J Clin Endocrinol Metab 1995; 80: 2577–2585. [DOI] [PubMed] [Google Scholar]
- 51. Parma J, Duprez L, Van SandemH, et al. Diversity and prevalence of somatic mutations in the thyrotropin receptor and Gs alpha genes as a cause of toxic thryoid adenomas. J Clin Endocrinol Metab 1997; 82: 2695–2701. [DOI] [PubMed] [Google Scholar]
- 52. Calebiro D, Grassi ES, Eszlinger M, et al. Recurrent EZH1 mutations are a second hit in autonomous thyroid adenomas. J Clin Invest 2016; 126: 3383–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wasserman JD, Sabbaghian N, Fahiminiya S, et al. DICER1 mutations are frequent in adolescent‐onset papillary thyroid carcinoma. J Clin Endocrinol Metab 2018; 103: 2009–2015. [DOI] [PubMed] [Google Scholar]
- 54. Suarez HG, du Villard JA, Caillou B, et al. Detection of activated ras oncogenes in human thyroid carcinomas. Oncogene 1988; 2: 403–406. [PubMed] [Google Scholar]
- 55. Lemoine NR, Mayall ES, Wyllie FS, et al. Activated ras oncogenes in human thyroid cancers. Cancer Res 1988; 48: 4459–4463. [PubMed] [Google Scholar]
- 56. Lemoine NR, Mayall ES, Wyllie FS, et al. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989; 4: 159–164. [PubMed] [Google Scholar]
- 57. Wright PA, Lemoine NR, Mayall ES, et al. Papillary and follicular thyroid carcinomas show a different pattern of ras oncogene mutation. Br J Cancer 1989; 60: 576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Namba H, Gutman RA, Matsuo K, et al. H‐ras protooncogene mutations in human thyroid neoplasms. J Clin Endocrinol Metab 1990; 71: 223–229. [DOI] [PubMed] [Google Scholar]
- 59. Suarez HG, du Villard JA, Severino M, et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene 1990; 5: 565–570. [PubMed] [Google Scholar]
- 60. Namba H, Rubin SA, Fagin FA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol 1990; 4: 1474–1479. [DOI] [PubMed] [Google Scholar]
- 61. Shi Y, Zou M, Schmidt H, et al. High rates of ras codon 61 mutation in thyroid tumors in an iodide‐deficient area. Cancer Res 1991; 51: 2690–2693. [PubMed] [Google Scholar]
- 62. Karga H, Lee J‐K, Vickery AL Jr, et al. Ras oncogene mutations in benign and malignant thyroid neoplasms. J Clin Endocrinol Metab 1991; 73: 832–836. [DOI] [PubMed] [Google Scholar]
- 63. Schark C, Fulton N, Yashiro T, et al. The value of measurement of RAS oncogenes and nuclear DNA analysis in the diagnosis of Hürthle cell tumors of the thyroid. World J Surg 1992; 16: 745–752. [DOI] [PubMed] [Google Scholar]
- 64. Goretzki PE, Lyons J, Stacy‐Phipps S, et al. Mutational activation of RAS and GSP oncogenes in differentiated thyroid cancer and their biological implications. World J Surg 1992; 16: 576–582. [DOI] [PubMed] [Google Scholar]
- 65. Kroll TG, Sarraf P, Pecciarini L, et al. PAX8‐PPARgamma1 fusion oncogene in human thyroid carcinoma. Science 2000; 289: 1357–1360. [DOI] [PubMed] [Google Scholar]
- 66. Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8‐PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 2003; 88: 2318–2326. [DOI] [PubMed] [Google Scholar]
- 67. Karunamurthy A, Panebianco F, Hsiao S, et al. Prevalence and phenotypic characteristics of EIF1AX mutations in thyroid nodules. Endocr Relat Cancer 2016; 23: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. The Cancer Genome Atlas Research Network . Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159: 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular‐cell neoplasia. Nat Rev Cancer 2006; 6: 292–306. [DOI] [PubMed] [Google Scholar]
- 70. Williams MD, Liu Z, Rossi ED, et al. Seven years of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features (NIFTP): rate of acceptance and variation of diagnostic approaches across different continents. J Clin Endocrinol Metab 2024; 110: 166–175. [DOI] [PubMed] [Google Scholar]
- 71. Kahn NF, Perzin KH. Follicular carcinoma of the thyroid: an evaluation of the histologic criteria used for diagnosis. Pathol Annu 1983; 1: 221–253. [PubMed] [Google Scholar]
- 72. Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol 2010; 17: 386–391. [DOI] [PubMed] [Google Scholar]
- 73. Brierley JD, Gospodarowicz MK, Wittekind C.TNM Classification of Malignant Tumours (8th edn). Wiley Blackwell: Oxford, 2017. [Google Scholar]
- 74. Asa SL, de Jesus AC, Kerr D, et al. Thyroid. In Endocrine Pathology, Mete O, Asa SL (eds). Cambridge University Press: Cambridge, 2016; 398–572. [Google Scholar]
- 75. Glomski K, Nose V, Faquin WC, et al. Metastatic follicular thyroid carcinoma and the primary thyroid gross examination: institutional review of cases from 1990 to 2015. Endocr Pathol 2017; 28: 177–185. [DOI] [PubMed] [Google Scholar]
- 76. Yamashina M. Follicular neoplasms of the thyroid. Total circumferential evaluation of the fibrous capsule. Am J Surg Pathol 1992; 16: 392–400. [DOI] [PubMed] [Google Scholar]
- 77. LiVolsi VA, Merino MJ. Worrisome histologic alterations following fine needle aspiration of the thyroid. Pathol Annu 1994; 29: 99–120. [PubMed] [Google Scholar]
- 78. van Heerden JA, Hay ID, Goellner JR, et al. Follicular thyroid carcinoma with capsular invasion alone: a nonthreatening malignancy. Surgery 1992; 112: 1130–1138. [PubMed] [Google Scholar]
- 79. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016; 2: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Collini P, Sampietro G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non‐Hurthle cell follicular carcinoma of the thyroid gland: a clinicopathological study of 18 consecutive cases from a single institution with a 11‐year median follow‐up. Histopathology 2004; 44: 35–39. [DOI] [PubMed] [Google Scholar]
- 81. Ghossein RA, Hiltzik DH, Carlson DL, et al. Prognostic factors of recurrence in encapsulated Hurthle cell carcinoma of the thyroid gland: a clinicopathologic study of 50 cases. Cancer 2006; 106: 1669–1676. [DOI] [PubMed] [Google Scholar]
- 82. Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol 2011; 24: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 83. Wreesmann VB, Nixon IJ, Rivera M, et al. Prognostic value of vascular invasion in well‐differentiated papillary thyroid carcinoma. Thyroid 2015; 25: 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nojima S, Kadoi T, Suzuki A, et al. Deep learning‐based differential diagnosis of follicular thyroid tumors using histopathological images. Mod Pathol 2023; 36: 100296. [DOI] [PubMed] [Google Scholar]
- 85. Turchini J, Sioson L, Clarkson A, et al. The presence of typical ‘BRAFV600E‐like’ atypia in papillary thyroid carcinoma is highly specific for the presence of the BRAFV600E mutation. Endocr Pathol 2023; 34: 112–118. [DOI] [PubMed] [Google Scholar]
- 86. Ip YT, Dias Filho MA, Chan JK. Nuclear inclusions and pseudoinclusions: friends or foes of the surgical pathologist? Int J Surg Pathol 2010; 18: 465–481. [DOI] [PubMed] [Google Scholar]
- 87. Kim C, Agarwal S, Bychkov A, et al. Differentiating BRAF V600E‐ and RAS‐like alterations in encapsulated follicular patterned tumors through histologic features: a validation study. Virchows Arch 2024; 484: 645–656. [DOI] [PubMed] [Google Scholar]
- 88. Hernandez‐Prera JC, Wenig BM. RAS‐mutant follicular thyroid tumors: a continuous challenge for pathologists. Endocr Pathol 2024; 35: 167–184. [DOI] [PubMed] [Google Scholar]
- 89. Ali SZ, Baloch Z, Cochand‐Priollet B, et al. The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid 2023; 33: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 90. Haugen BR, Alexander EK, Bible KC, et al. American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2015; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Akbulut D, Kuz ED, Kursun N, et al. Capsular invasion matters also in ‘papillary patterned’ tumors: a study on 121 cases of encapsulated conventional variant of papillary thyroid carcinoma. Endocr Pathol 2021; 32: 357–367. [DOI] [PubMed] [Google Scholar]
- 92. Giani C, Torregrossa L, Ramone T, et al. Whole tumor capsule is prognostic of very good outcome in the classical variant of papillary thyroid cancer. J Clin Endocrinol Metab 2021; 106: e4072‐e4083. [DOI] [PubMed] [Google Scholar]
- 93. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual (8th edn). Springer, 2017. [Google Scholar]
- 94. Asa SL, Giordano TJ, LiVolsi VA. Implications of the TCGA genomic characterization of papillary thyroid carcinoma for thyroid pathology: does follicular variant papillary thyroid carcinoma exist? Thyroid 2015; 25: 1–2. [DOI] [PubMed] [Google Scholar]
- 95. Janovitz T, Williamson DFK, Wong KS, et al. Genomic profile of columnar cell variant of papillary thyroid carcinoma. Histopathology 2021; 79: 491–498. [DOI] [PubMed] [Google Scholar]
- 96. Higgins KE, Sadow PM, Johnson DN, et al. Columnar cell thyroid carcinoma: a heterogeneous entity demonstrating overlap between papillary thyroid carcinoma and follicular neoplasms. Head Neck Pathol 2024; 18: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Singarayer R, Mete O, Perrier L, et al. A systematic review and meta‐analysis of the diagnostic performance of BRAF V600E immunohistochemistry in thyroid histopathology. Endocr Pathol 2019; 30: 201–218. [DOI] [PubMed] [Google Scholar]
- 98. Baloch Z, Mete O, Asa SL. Immunohistochemical biomarkers in thyroid pathology. Endocr Pathol 2018; 29: 91–112. [DOI] [PubMed] [Google Scholar]
- 99. Alzumaili BA, Fisch AS, Faquin WC, et al. Detection of RAS p.Q61R by immunohistochemistry in practice: a clinicopathologic study of 217 thyroid nodules with molecular correlates. Endocr Pathol 2024; 35: 219–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pseudoinvasion of the capsule in a thyroid neoplasm
Figure S2. Pseudoinvasion of vessels in a thyroid neoplasm
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


