Abstract
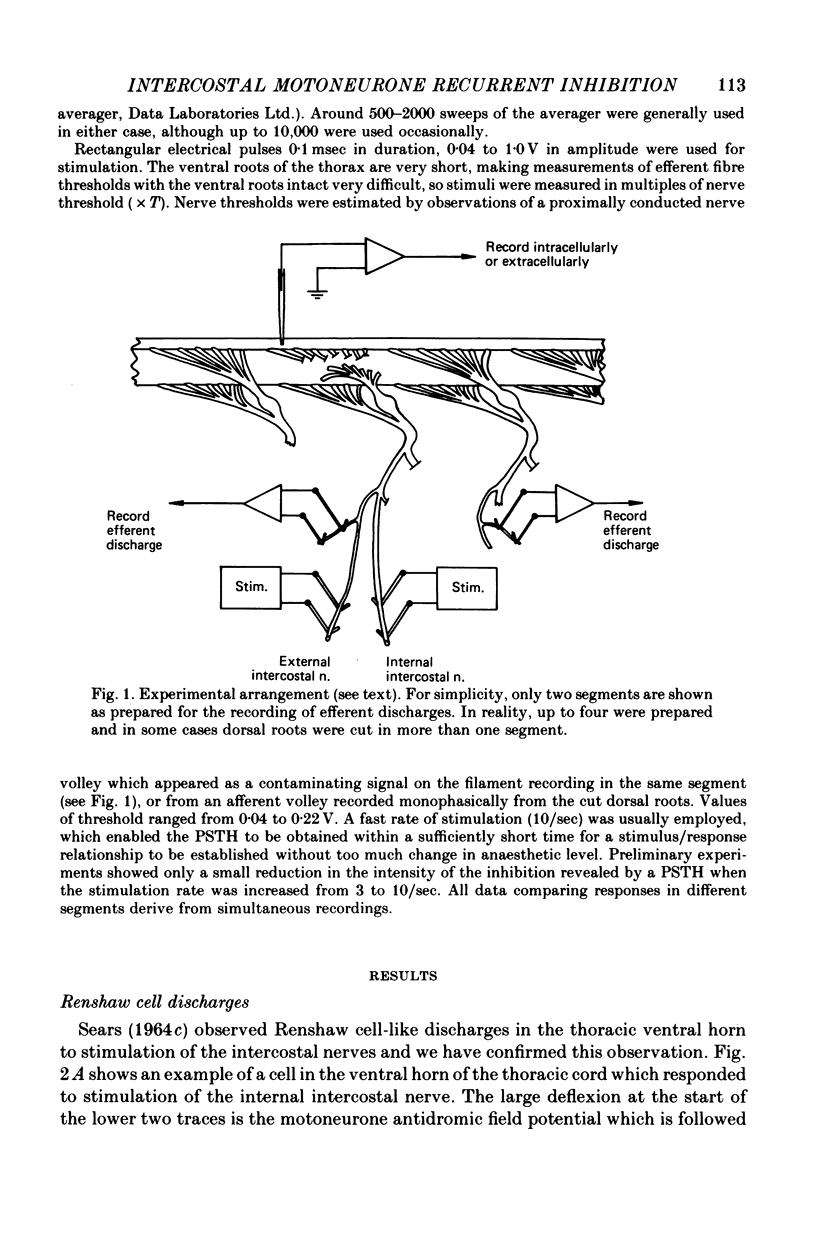
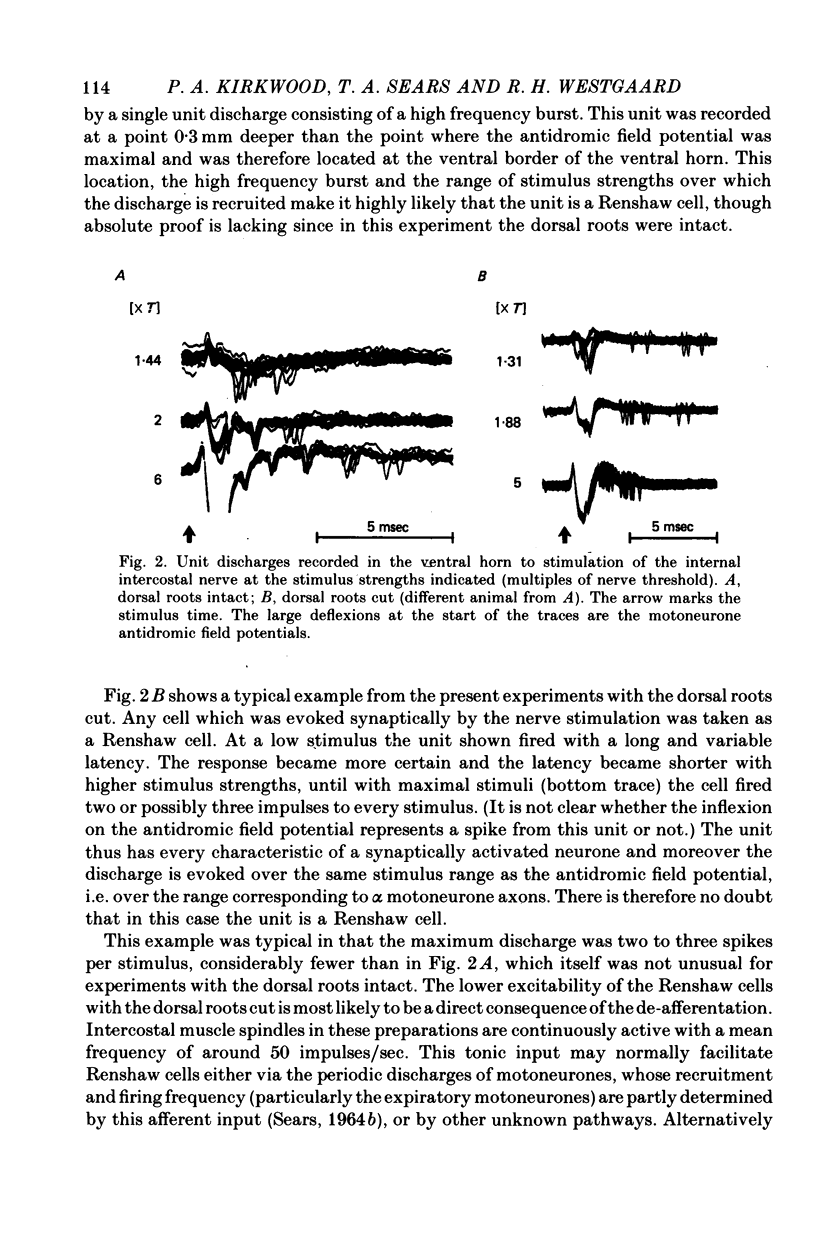
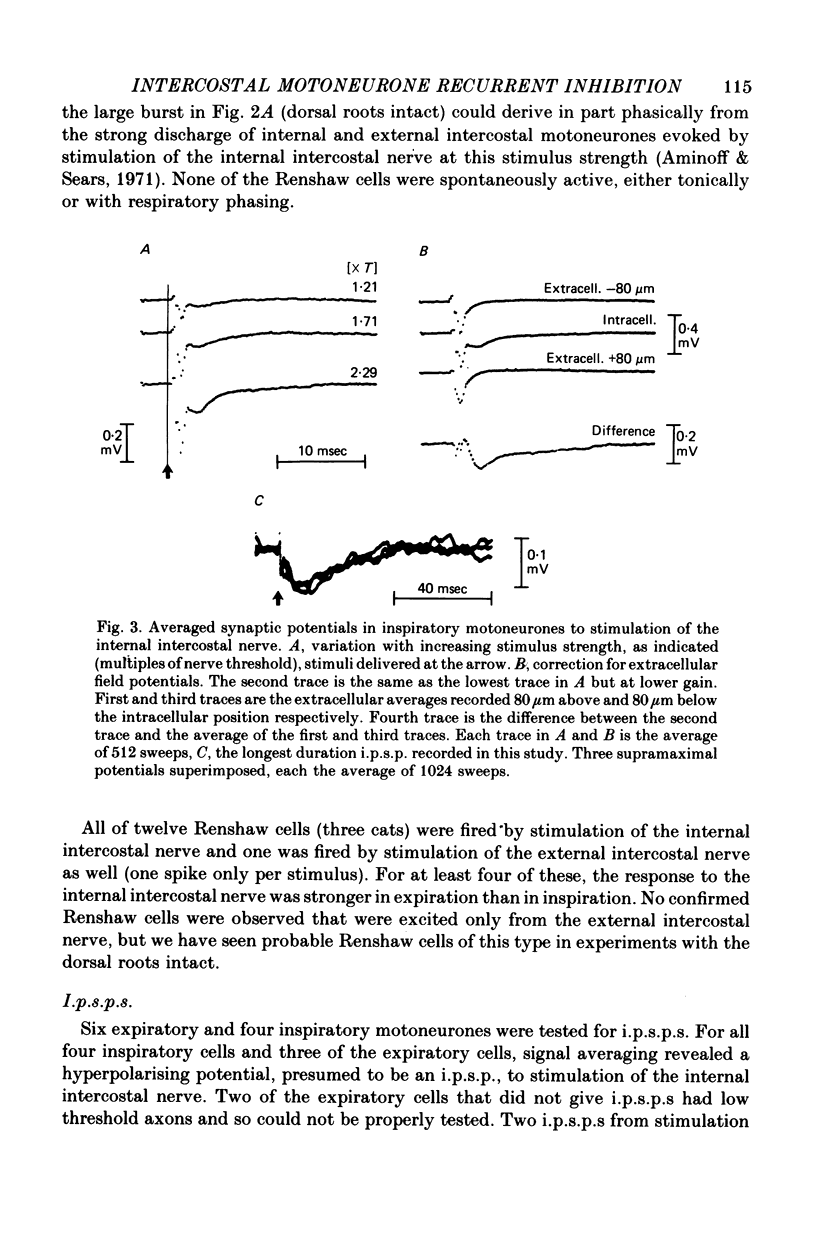
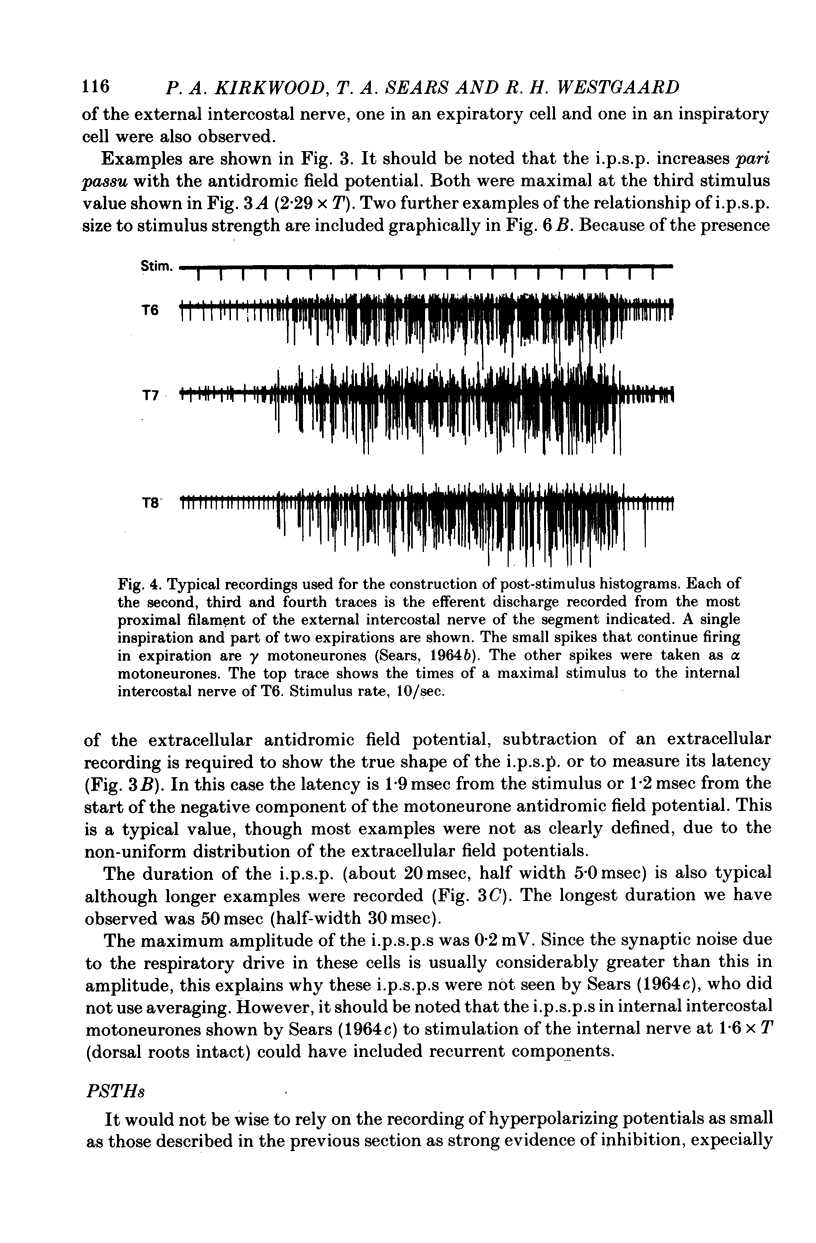
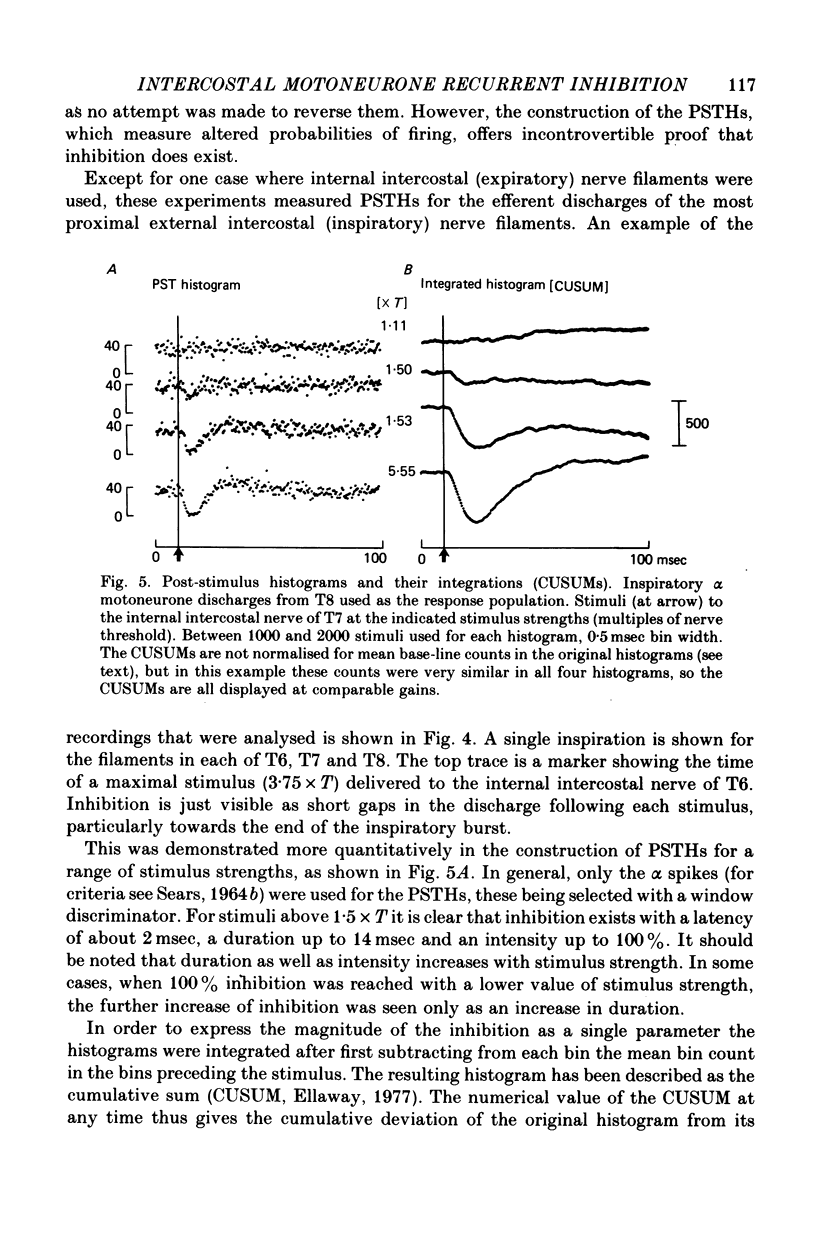
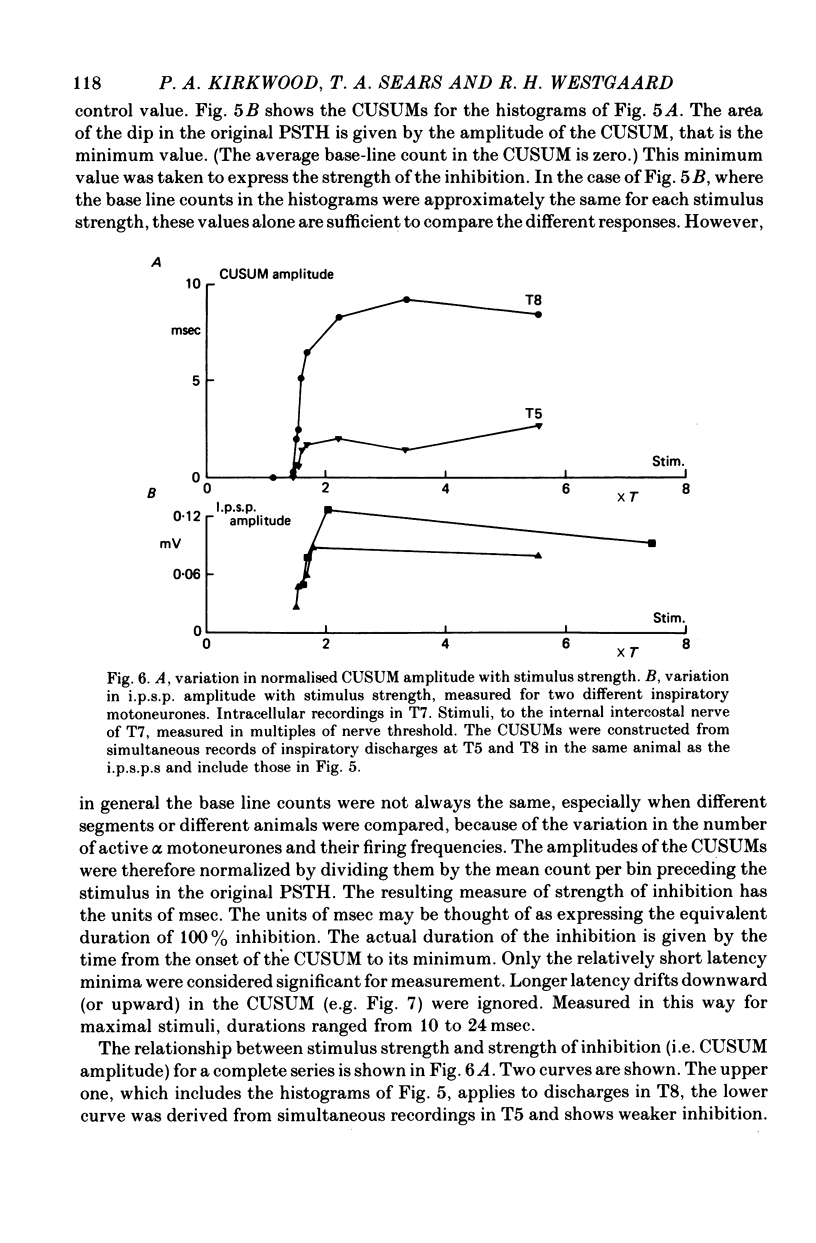
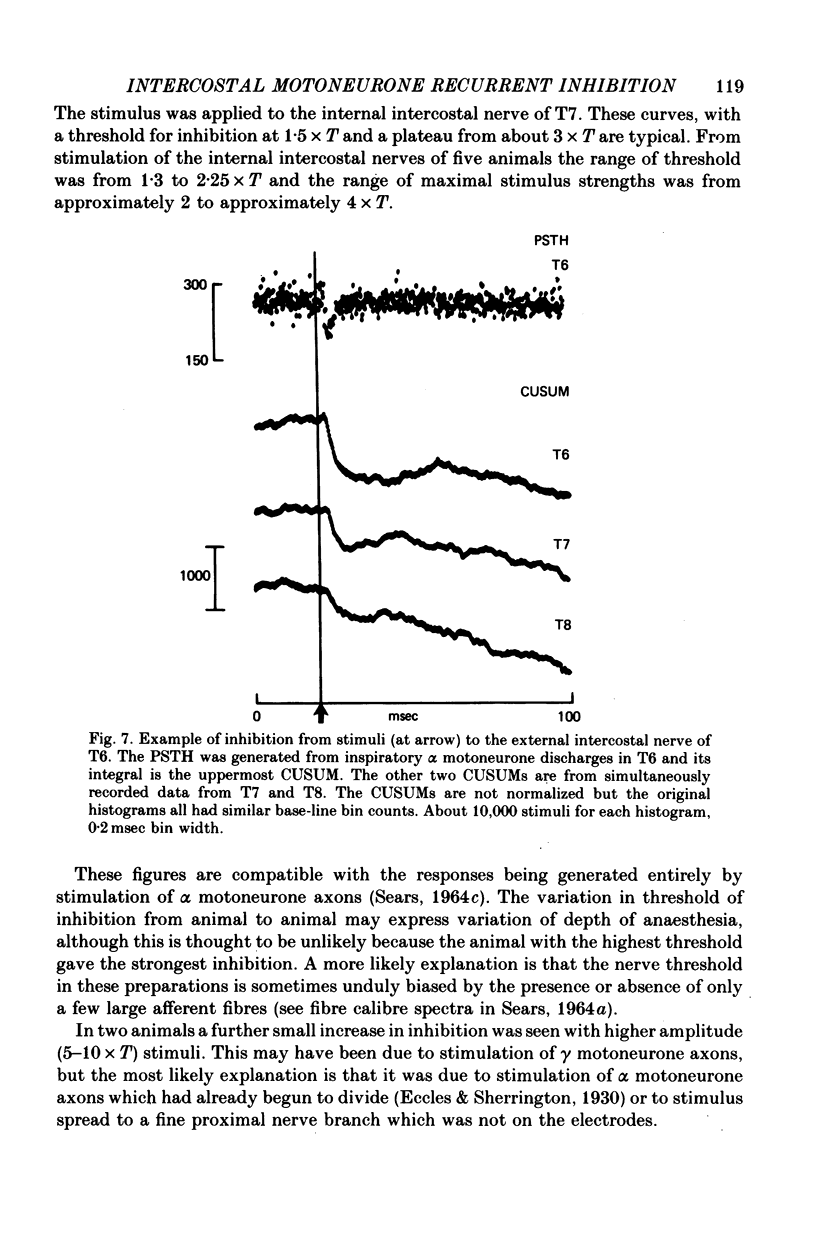
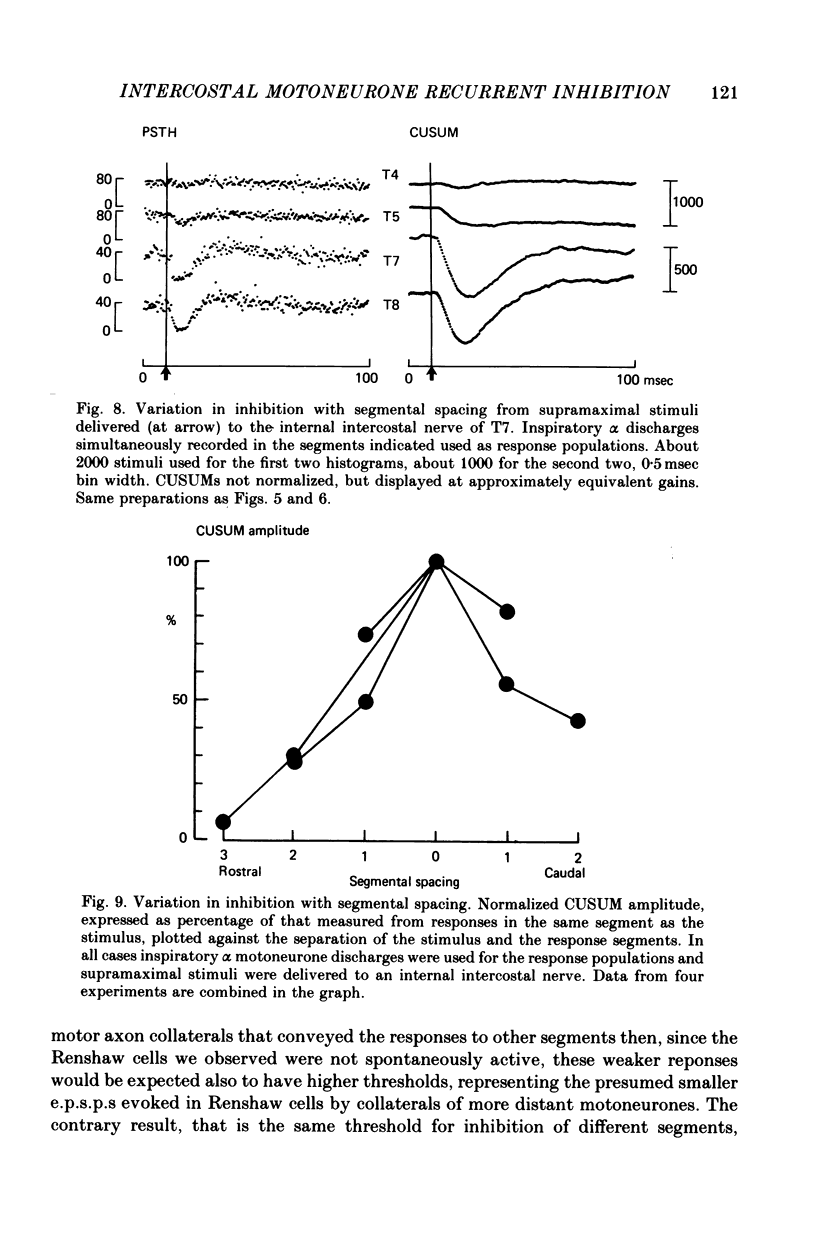
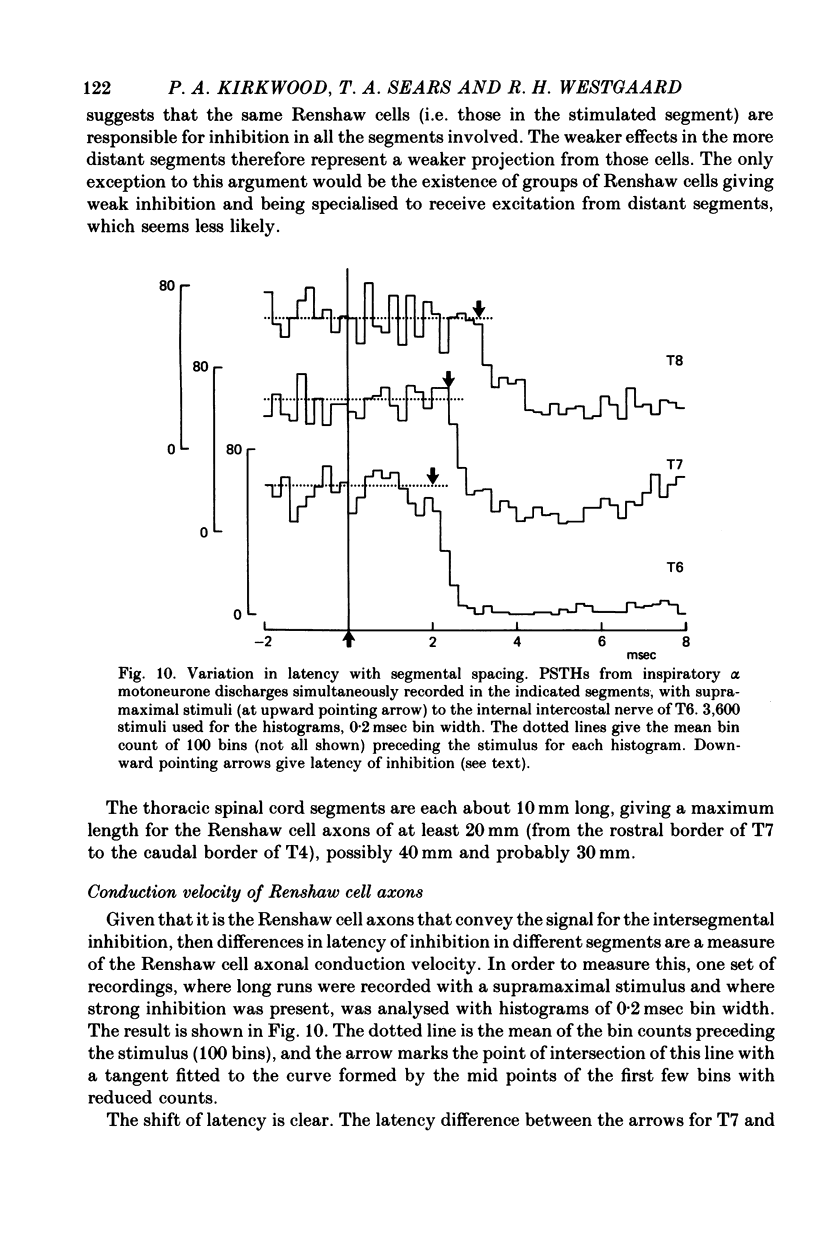
1. The external and internal intercostal nerves of a single intercostal space were stimulated in anaesthetized paralysed cats with dorsal roots cut in the corresponding spinal cord segment. 2. Extracellular recording in the ventral horn revealed single units which fired short high frequency bursts of spikes at short latency to stimulation of either or both of the two nerves at stimulus strengths appropriate to the activation of alpha motor axons. These units were deduced to be Renshaw cells. 3. Small (0.1-0.2 mV) hyperpolarizing potentials of duration up to 50 msec were recorded intracellularly in both inspiratory and expiratory motoneurones of the same segment. Latencies and thresholds were appropriate for disynaptic i.p.s.p.s evoked by collaterals of alpha motor axons. 4. The changes in probability of firing following the stimuli were examined for inspiratory alpha motoneurones by constructing post-stimulus histograms of efferent discharges recorded from filaments of the external intercostal nerve of the segment stimulated and from other segments. 5. A period of reduced probability of firing of up to 24 msec duration, corresponding in all respects to disynaptic inhibition from alpha motor axon collaterals, was seen in the segment stimulated and up to three segments distant, though declining in intensity with distance. Either nerve could evoke such inhibition although that evoked from the internal intercostal nerve was stronger, as were the intensities of the Renshaw cell discharges. 6. We conclude that recurrent inhibition, via Renshaw cells which have axons up to 30 mm in length, is present for intercostal motoneurones. Arguments are adduced to show that although the effects from stimulating any one segmental nerve may be relatively weak, the over-all effect resulting from the widely spread projections of the Renshaw cells concerned is an inhibition comparable intensity with that seen in many hind limb motor nuclei.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., O'Donovan M. J., Somjen G., Stephens J. A., Taylor A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J Physiol. 1978 Jun;279:409–423. doi: 10.1113/jphysiol.1978.sp012353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN L. A. Localization of stretch reflex. J Neurophysiol. 1953 May;16(3):272–285. doi: 10.1152/jn.1953.16.3.272. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat sciatic alpha-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1978 Apr 1;178(3):537–557. doi: 10.1002/cne.901780309. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O., Conradi S. Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977 Aug 19;132(1):1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- Duron B. Postural and ventilatory functions of intercostal muscles. Acta Neurobiol Exp (Wars) 1973;33(1):355–380. [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKLUND G., VON EULER, RUTKOWSKI S. SPONTANEOUS AND REFLEX ACTIVITY OF INTERCOSTAL GAMMA MOTONEURONES. J Physiol. 1964 May;171:139–163. doi: 10.1113/jphysiol.1964.sp007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M. D., Freeman N. C., Proshansky E. Morphology of spinal motoneurones mediating a cutaneous spinal reflex in the cat. J Physiol. 1980 Sep;306:349–363. doi: 10.1113/jphysiol.1980.sp013401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. PROPERTIES OF PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S., Roberts W. Neuronal pathway of the recurrent facilitation of motoneurones. J Physiol. 1971 Oct;218(2):495–514. doi: 10.1113/jphysiol.1971.sp009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979 Dec;297(0):229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Input-output relations in the pathway of recurrent inhibition to motoneurones in the cat. J Physiol. 1979 Dec;297(0):267–287. doi: 10.1113/jphysiol.1979.sp013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Lindström S. Morphological identification of Renshaw cells. Acta Physiol Scand. 1971 Mar;81(3):428–430. doi: 10.1111/j.1748-1716.1971.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Smith D. O. Antidromic activation of Renshaw cells and their axonal projections. Acta Physiol Scand. 1973 Jun;88(2):198–214. doi: 10.1111/j.1748-1716.1973.tb05447.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K. Cross-correlation functions for a neuronal model. Biophys J. 1974 Aug;14(8):567–582. doi: 10.1016/S0006-3495(74)85936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea D. A., Pratt C. A., Jordan L. M. Renshaw cell activity and recurrent effects on motoneurons during fictive locomotion. J Neurophysiol. 1980 Sep;44(3):475–488. doi: 10.1152/jn.1980.44.3.475. [DOI] [PubMed] [Google Scholar]
- Nelson P. G. Interaction between spinal motoneurons of the cat. J Neurophysiol. 1966 Mar;29(2):275–287. doi: 10.1152/jn.1966.29.2.275. [DOI] [PubMed] [Google Scholar]
- Pratt C. A., Jordan L. M. Recurrent inhibition of motoneurons in decerebrate cats during controlled treadmill locomotion. J Neurophysiol. 1980 Sep;44(3):489–500. doi: 10.1152/jn.1980.44.3.489. [DOI] [PubMed] [Google Scholar]
- Rapoport S. Reflex connexions of motoneurones of muscles involved in head movement in the cat. J Physiol. 1979 Apr;289:311–327. doi: 10.1113/jphysiol.1979.sp012739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C. Intersegmental and intrasegmental distribution of mutual inhibition of Renshaw cells. J Neurophysiol. 1971 Jul;34(4):700–707. doi: 10.1152/jn.1971.34.4.700. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. SOME PROPERTIES AND REFLEX CONNEXIONS OF RESPIRATORY MOTONEURONES OF THE CAT'S THORACIC SPINAL CORD. J Physiol. 1964 Dec;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. THE FIBRE CALIBRE SPECTRA OF SENSORY AND MOTOR FIBRES IN THE INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Jul;172:150–161. doi: 10.1113/jphysiol.1964.sp007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Recurrent interactions between motoneurons of known location in the cervical cord of the cat. J Neurophysiol. 1967 Jul;30(4):661–674. doi: 10.1152/jn.1967.30.4.661. [DOI] [PubMed] [Google Scholar]
- Willis W. D. The case for the Renshaw cell. Brain Behav Evol. 1971;4(1):5–52. doi: 10.1159/000125422. [DOI] [PubMed] [Google Scholar]


