Abstract
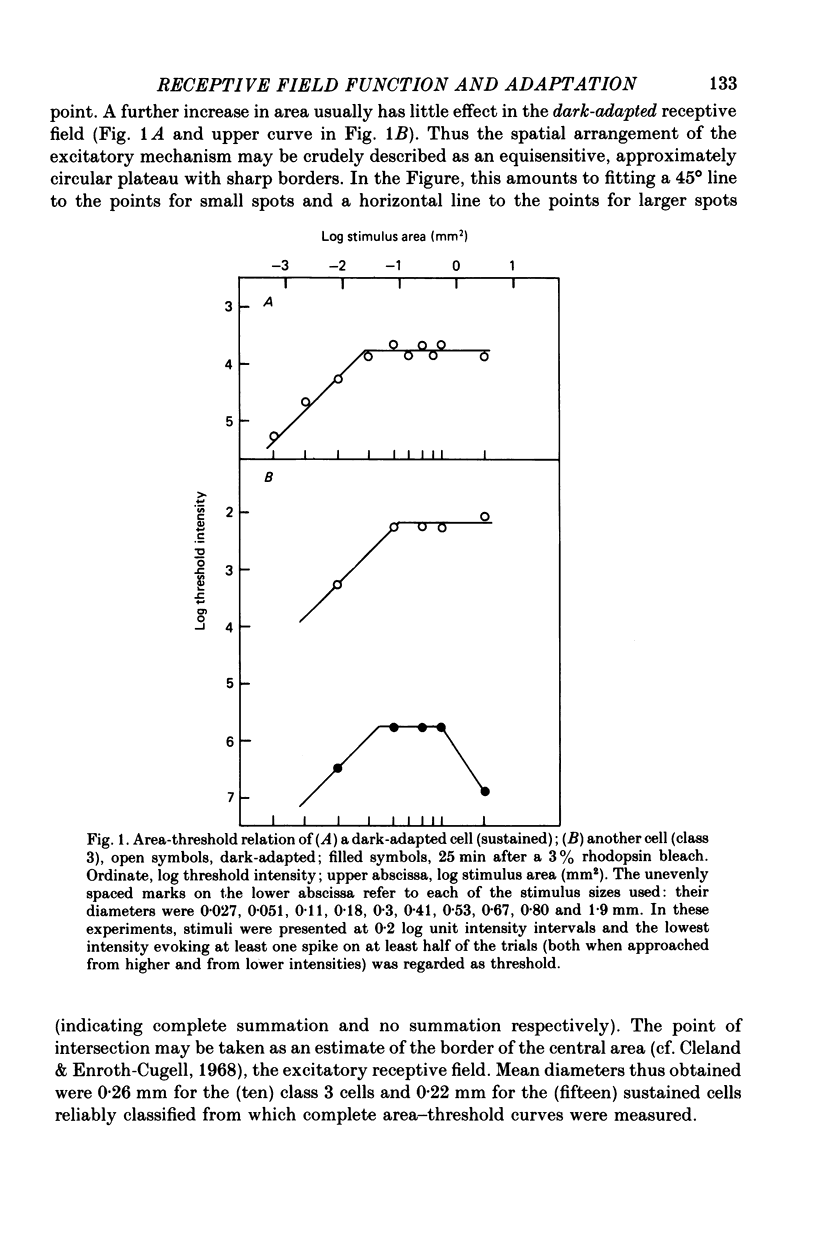
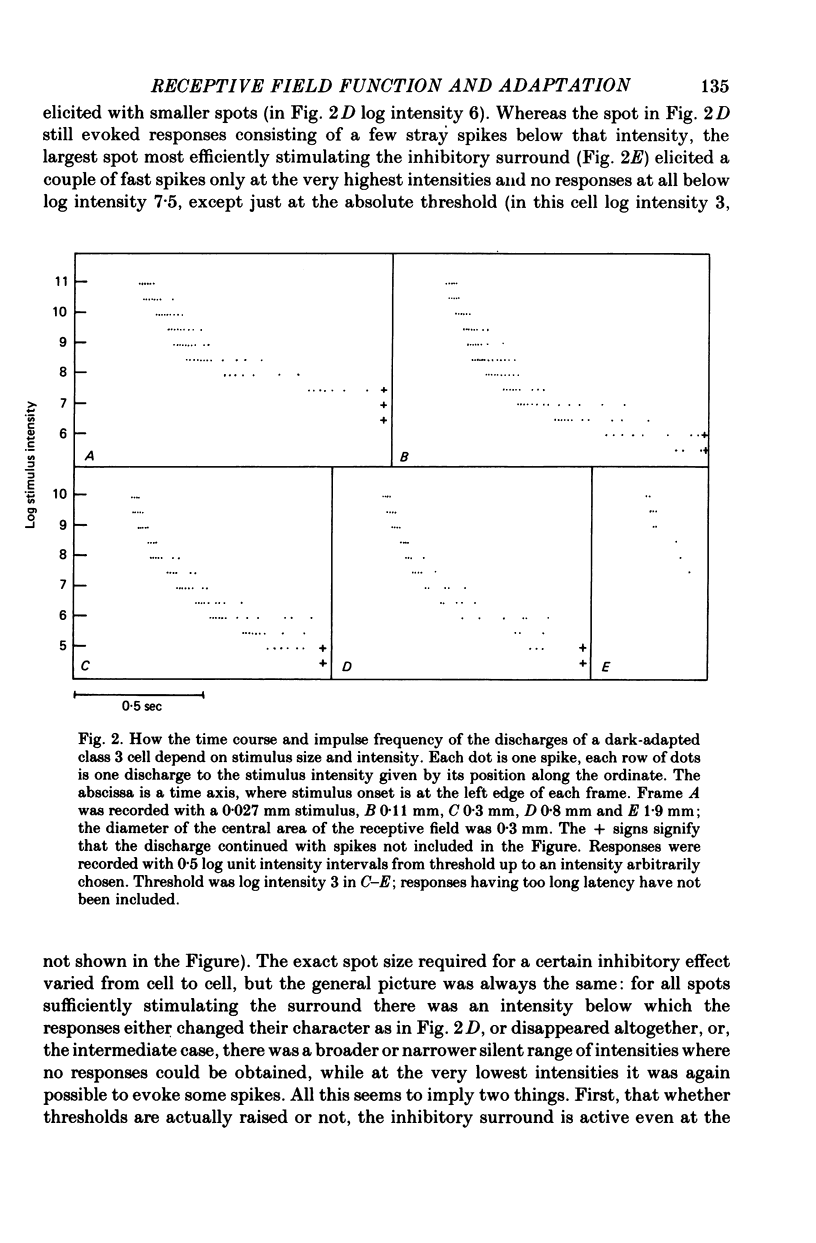
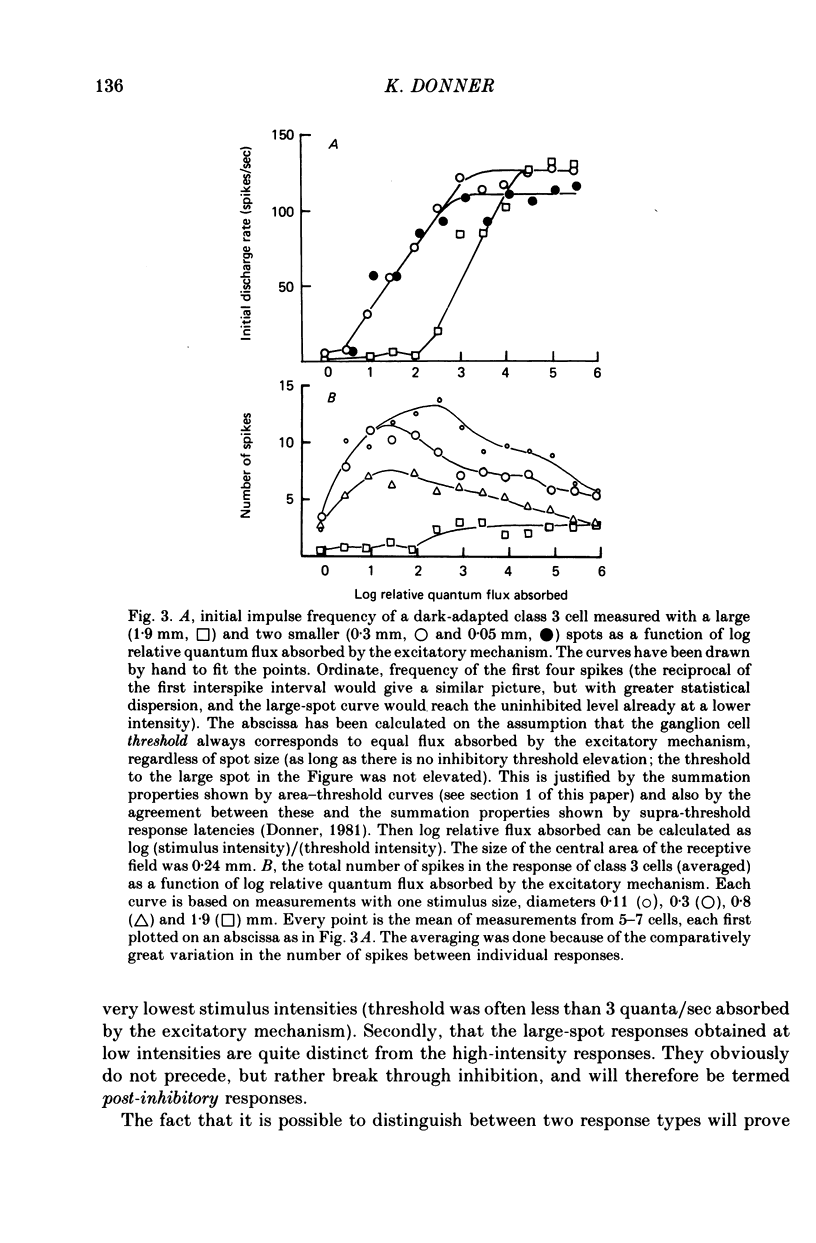
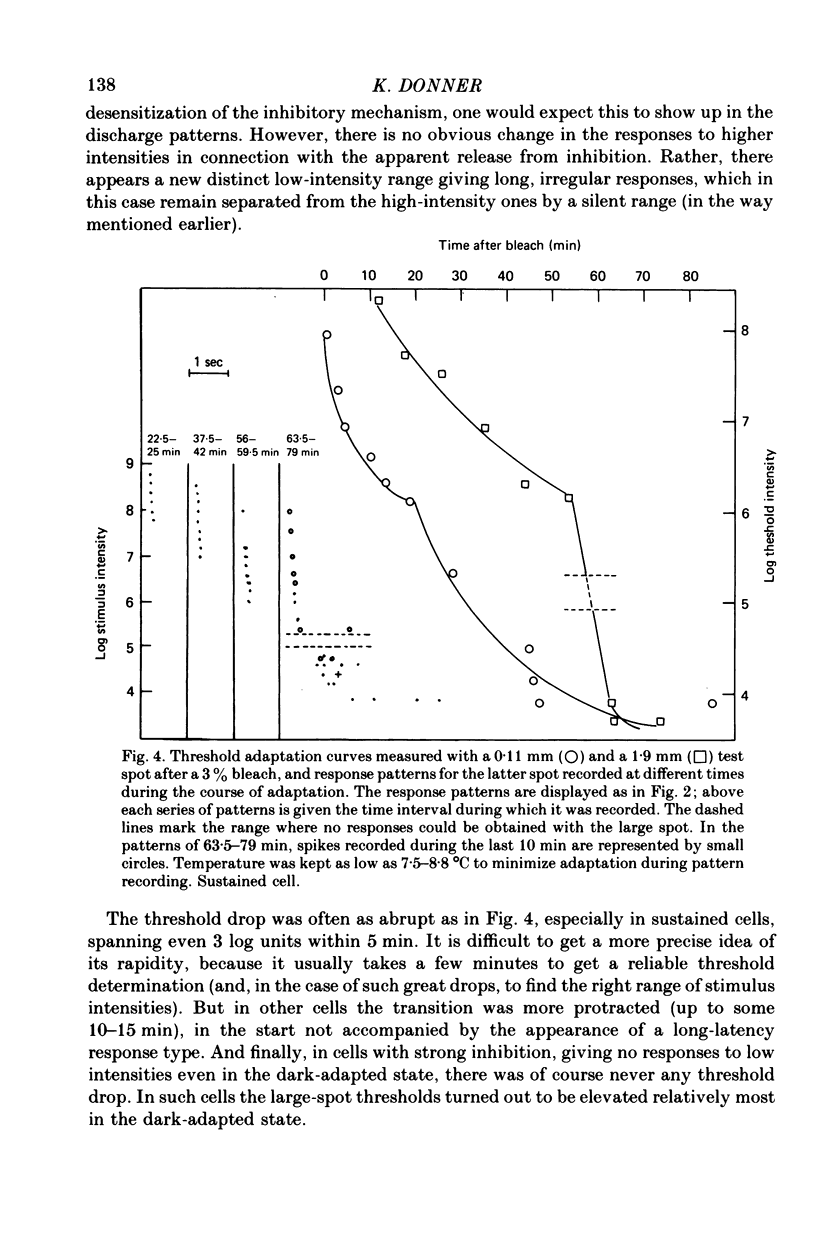
1. The excitatory and inhibitory receptive field mechanisms of retinal ganglion cells were studied by extracellular recording from the eyecup of Rana temporaria in order to elucidate the nature of adaptational changes in the functioning of the receptive field. 2. The responses to large stimuli were always strongly depressed relative to responses evoked by smaller spots. This was true even in the fully dark-adapted state and at the very lowest stimuli intensities. 3. Threshold measurements confirmed earlier findings, usually revealing the surround only in light-adapted states. However, in more than 10% of fully dark-adapted cells thresholds to large stimuli were significantly elevated. 4. The central summation area of the receptive field was found to shrink with light-adaptation. There was a gradual decrease in diameters, amounting to some 20-30%, from the dark-adapted, rod-determined receptive fields to the cone-determined ones. 5. Adaptation by bleaching and adaptation by backgrounds changed the effects of the surround in different ways. After a rhodopsin bleach the transition from a light-adapted to a dark-adapted situation was seen as an abrupt drop of large-stimulus thresholds at some time during adaptation. Steady backgrounds produced no such dramatic changes, but the increment threshold lines were somewhat steeper with test spots stimulated the surround than with smaller spots. 6. Although the discharge patterns generally show the strength of the surround influence, they underwent no qualitative change at the time of the drop of large-stimulus thresholds after a bleach. 7. It is suggested that the drop does not reflect a sudden reorganization of the receptive field, but is the consequence of the different ways the response to large stimuli are formed in different ranges of stimulus intensity (pre-inhibitory at high intensities, post-inhibitory at low intensities), and of gradual changes in signal dynamics.
Full text
PDF





Selected References
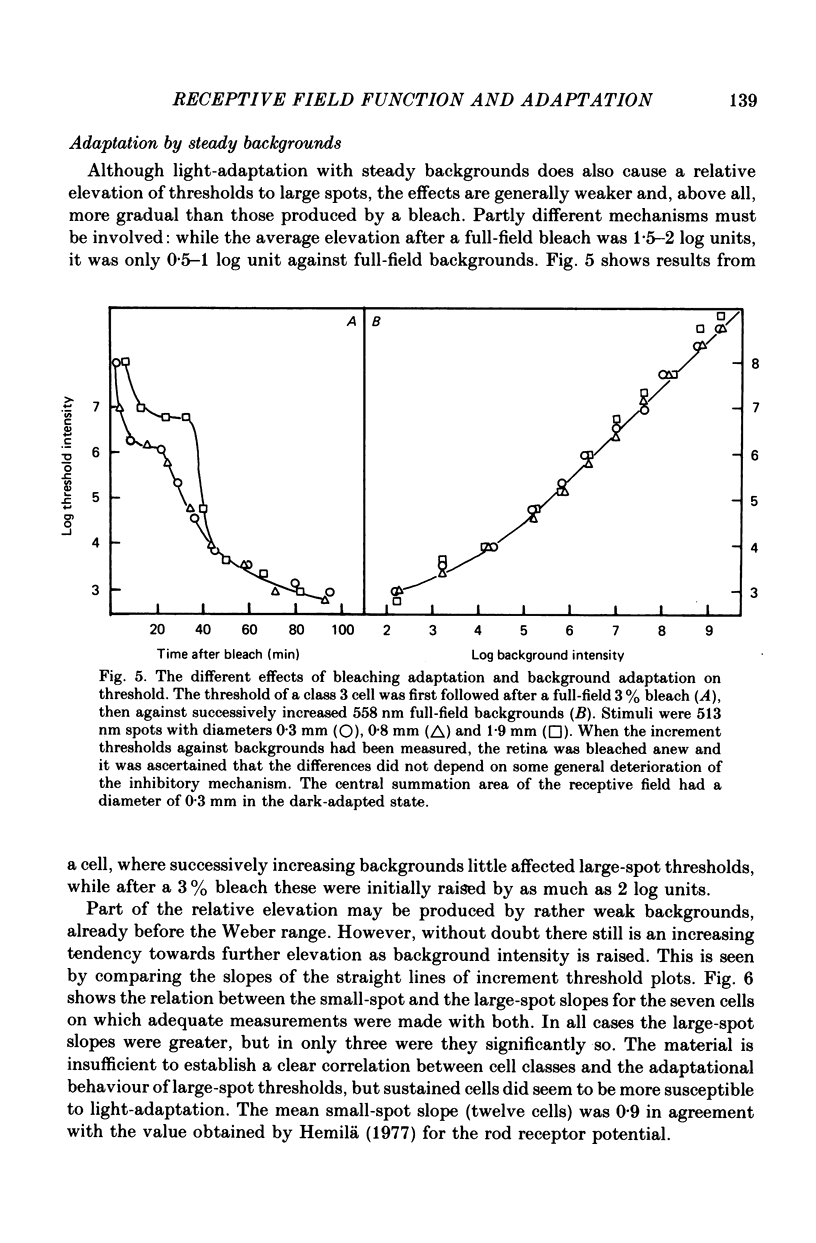
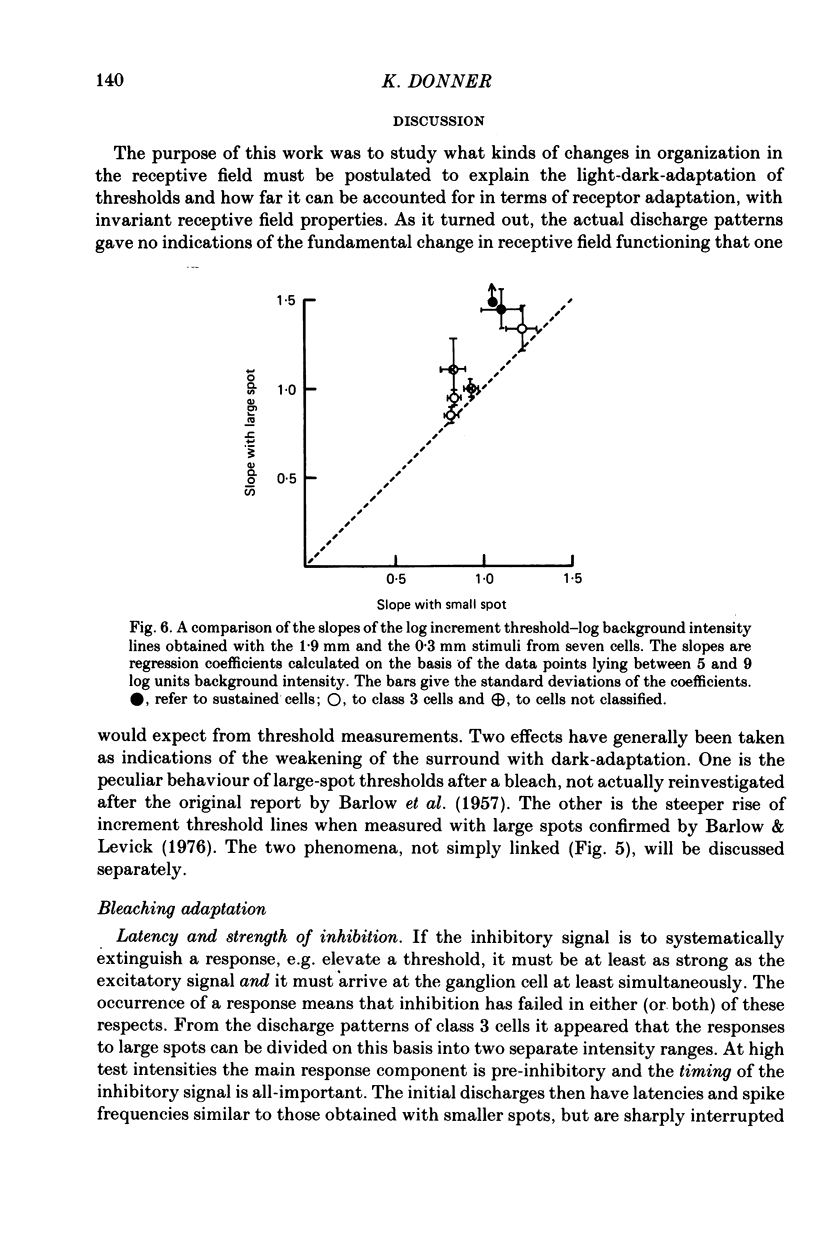
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Threshold setting by the surround of cat retinal ganglion cells. J Physiol. 1976 Aug;259(3):737–757. doi: 10.1113/jphysiol.1976.sp011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström A. C., Hemilä S., Reuter T. Directional selectivity and colour coding in the frog retina. Med Biol. 1978 Apr;56(2):72–83. [PubMed] [Google Scholar]
- Bäckström A. C., Reuter T. Receptive field organization of ganglion cells in the frog retina: contributions from cones, green rods and red rods. J Physiol. 1975 Mar;246(1):79–107. doi: 10.1113/jphysiol.1975.sp010881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. The dark-adaptation of single units in the frog's retina and its relation to the regeneration of rhodopsin. Vision Res. 1965 Dec;5(11):615–632. doi: 10.1016/0042-6989(65)90035-0. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. Visual adaptation of the rhodopsin rods in the frogs retina. J Physiol. 1968 Nov;199(1):59–87. doi: 10.1113/jphysiol.1968.sp008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä S. Background adaptation in the rods of the frog's retina. J Physiol. 1977 Mar;265(3):721–741. doi: 10.1113/jphysiol.1977.sp011740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye P. W., Naka K. I. The dynamics of inhibitory interaction in a frog receptive field: a paradigm of paracontrast. Vision Res. 1971 May;11(5):377–392. doi: 10.1016/0042-6989(71)90081-2. [DOI] [PubMed] [Google Scholar]


