Abstract
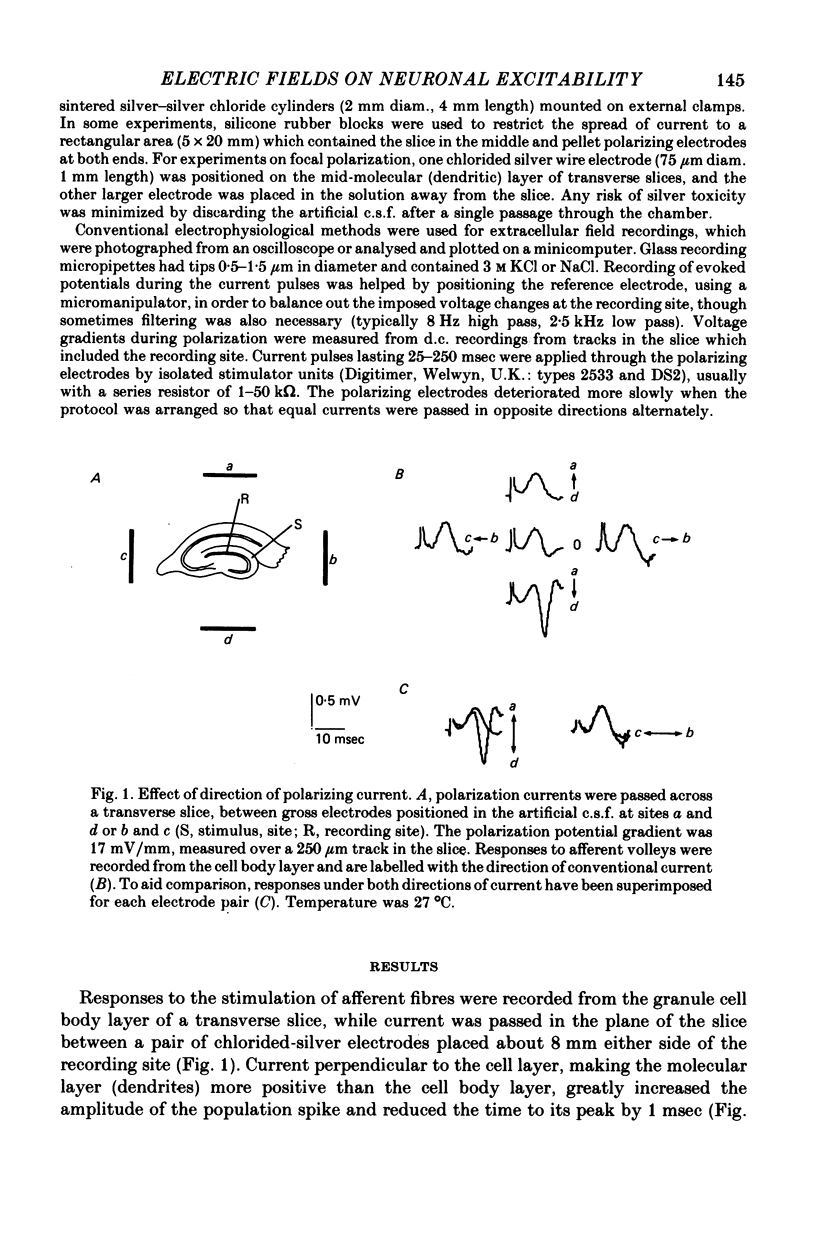
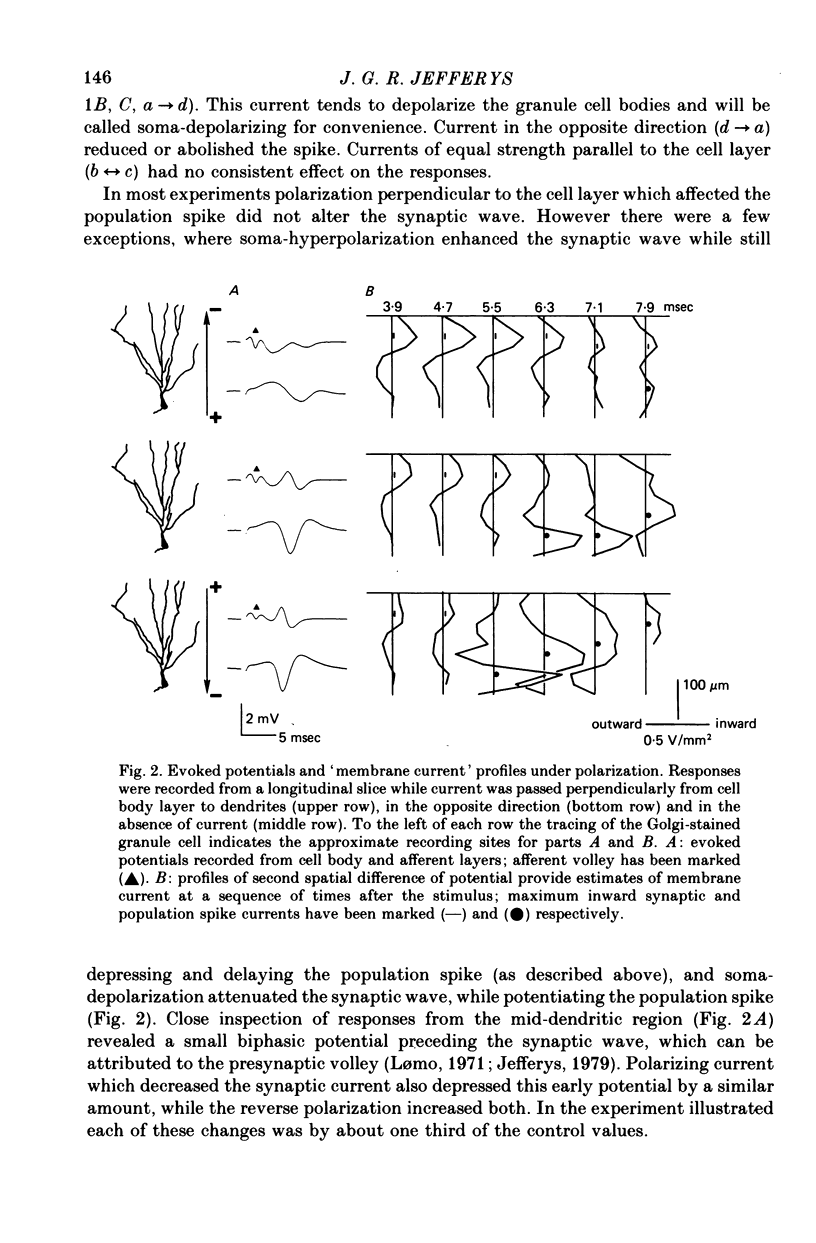
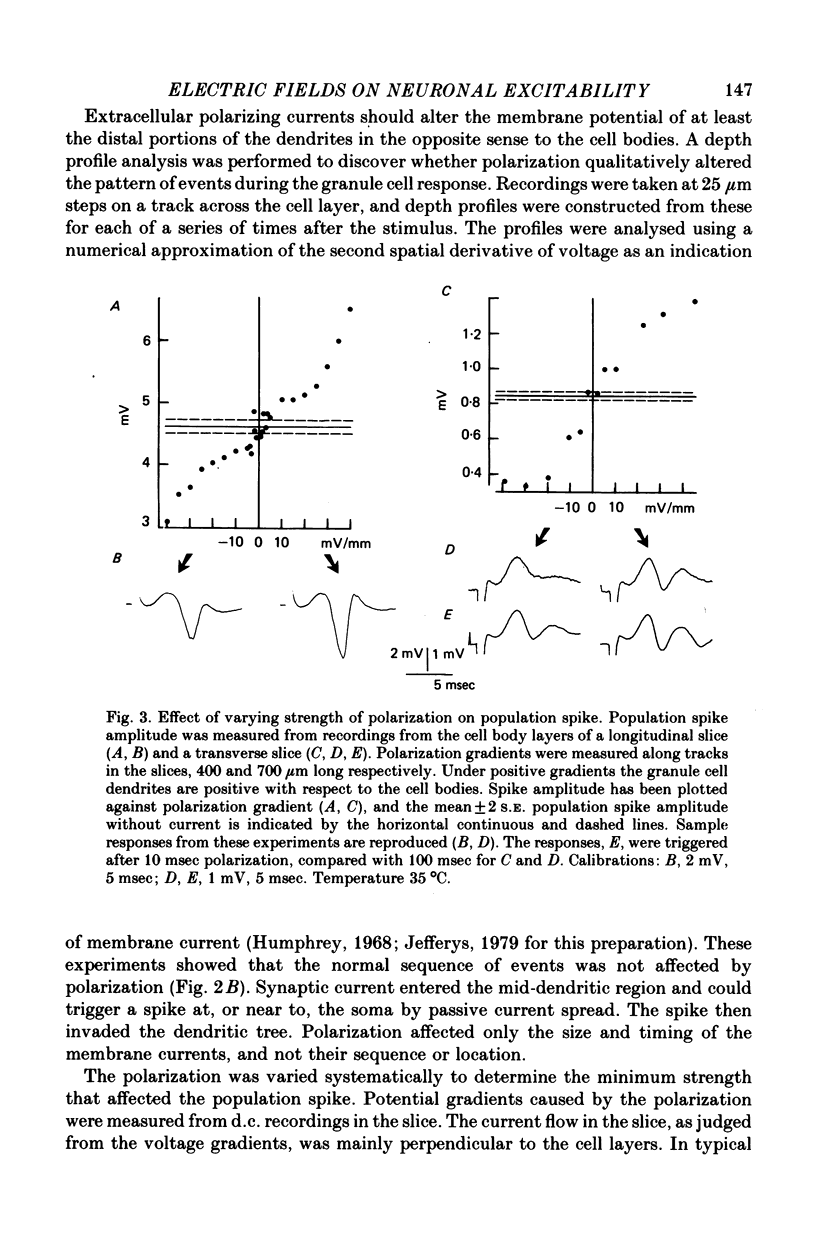
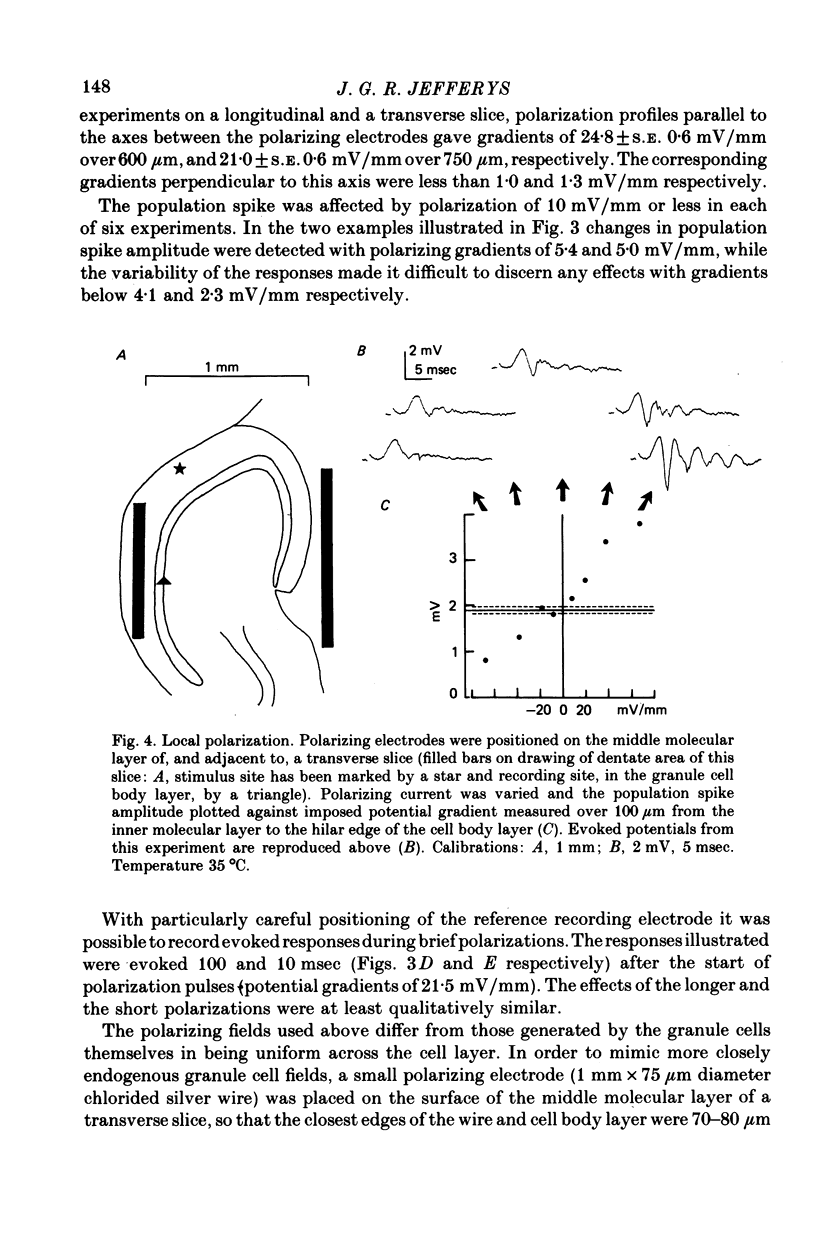
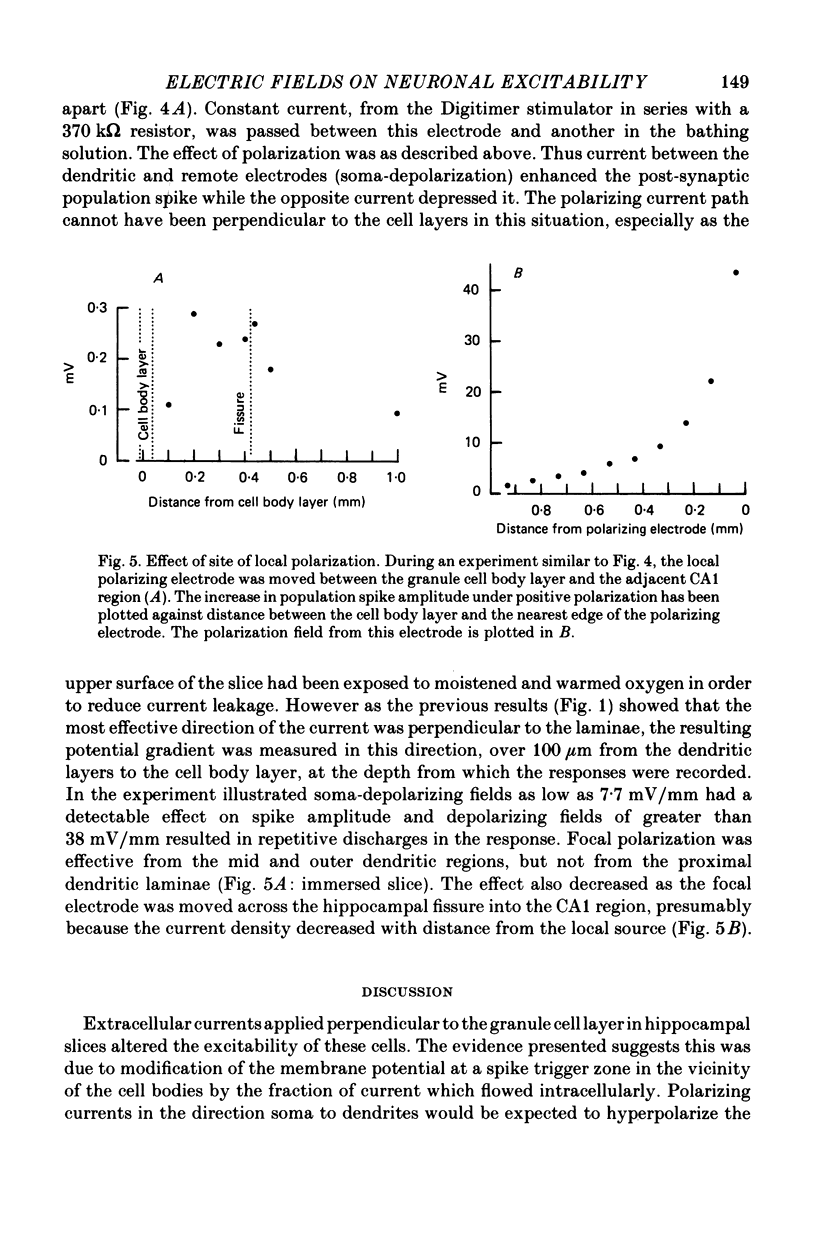
1. Monosynaptic evoked potentials were recorded from the granule cell layer of slices of guinea-pig hippocampus maintained in vitro. Current pulses of 25-250 msec duration were passed across the slices, between gross electrodes in the bathing liquid. 2. Polarizing current modified the excitability of the granule cells as judged by changes in their population discharge during postsynaptic responses. All durations of polarization had at least qualitatively similar effects. Conventional current from dendrites to cell bodies increased excitability (and vice versa). This is consistent with altered membrane potential of a spike trigger zone, at or close to the granule cell bodies, imposed by the fraction of polarizing current which flows intracellularly. 3. In some experiments polarization also affected the presynaptic volley and (hence?) the synaptic potential. When this occurred it was in the wrong sense to explain the concomitant changes in population spike. 4. Focal polarization, where currents were applied across the cell body layer between a small electrode on the mid or outer dendritic regions and a remote gross electrode, altered granule cell excitability in the same direction as in (2). Thus conventional current injected at the dendritic electrode increased excitability. 5. The smallest effect polarizing currents caused extracellular voltage gradients of 5-10 mV/mm, which is less than occurs in this tissue during synchronous activation of the neurons or during seizure activity. Therefore such field potentials could increased the synchrony of discharge of the granule cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BINDMAN L. J., LIPPOLD O. C., REDFEARN J. W. THE ACTION OF BRIEF POLARIZING CURRENTS ON THE CEREBRAL CORTEX OF THE RAT (1) DURING CURRENT FLOW AND (2) IN THE PRODUCTION OF LONG-LASTING AFTER-EFFECTS. J Physiol. 1964 Aug;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURES J. The ontogenetic development of steady potential differences in the cerebral cortex in animals. Electroencephalogr Clin Neurophysiol. 1957 Feb;9(1):121–130. doi: 10.1016/0013-4694(57)90116-5. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Richards C. D. Some experiments with in vitro hippocampal slices. J Physiol. 1971;214 (Suppl):7P–9P. [PubMed] [Google Scholar]
- CREUTZFELDT O. D., FROMM G. H., KAPP H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962 Jun;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- GLOOR P., VERA C. L., SPERTI L. ELECTROPHYSIOLOGICAL STUDIES OF HIPPOCAMPAL NEURONS. I. CONFIGURATION AND LAMINAR ANALYSIS OF THE "RESTING" POTENTIAL GRADIENT, OF THE MAIN-TRANSIENT RESPONSE TO PERFORANT PATH, FIMBRIAL AND MOSSY FIBER VOLLEYS AND OF "SPONTANEOUS" ACTIVITY. Electroencephalogr Clin Neurophysiol. 1963 Jun;15:353–378. doi: 10.1016/0013-4694(63)90060-9. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R. Re-analysis of the antidromic cortical response. I. Potentials evoked by stimulation of the isolated pyramidal tract. Electroencephalogr Clin Neurophysiol. 1968 Feb;24(2):116–129. doi: 10.1016/0013-4694(68)90118-1. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Initiation and spread of action potentials in granule cells maintained in vitro in slices of guinea-pig hippocampus. J Physiol. 1979 Apr;289:375–388. doi: 10.1113/jphysiol.1979.sp012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatsch R. H., Cowan W. M. Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J Comp Neurol. 1966 Nov;128(3):359–395. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- Purpura D. P., Malliani A. Spike generation and propagation initiated in dendrites by transhippocampal polarization. Brain Res. 1966 May-Jun;1(4):403–406. doi: 10.1016/0006-8993(66)90132-6. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Westgaard R. H. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971 Dec 24;35(2):589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]
- Terzuolo C. A., Bullock T. H. MEASUREMENT OF IMPOSED VOLTAGE GRADIENT ADEQUATE TO MODULATE NEURONAL FIRING. Proc Natl Acad Sci U S A. 1956 Sep;42(9):687–694. doi: 10.1073/pnas.42.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalogr Clin Neurophysiol. 1974 Mar;36(3):291–301. doi: 10.1016/0013-4694(74)90171-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Kawai N. Presynaptic action of acetylcholine in thin sections from the guinea pig dentate gyrus in vitro. Exp Neurol. 1967 Oct;19(2):176–187. doi: 10.1016/0014-4886(67)90016-7. [DOI] [PubMed] [Google Scholar]


