Abstract
1. Unloaded shortening velocity, stiffness, and the effects of potentiators were studied to understand the basis for the shallow ascending limb (1.65-2.0 micrometers sarcomere length) of the sarcomere length-tension diagram of from single fibres. 2. The velocity of externally unloaded shortening was found to be constant over most of the range. It is therefore unlikely that this part of the sarcomere length-tension diagram results from an internal force opposing shortening. 3.Stiffness was found not to vary in proportion with tension between sarcomere lengths 1.65 and 2.0 micrometers, nor to be constant between 2.0 and 2.2 micrometers, where tension is constant. By assuming a small filament compliance, the observations could be adequately modelled on the hypothesis that the variation in tension in the range of sarcomere lengths 1.65-20 micrometers was caused by variations in the number of attached cross-bridges. 4. The twitch potentiators Zn2+, tetraethylammonium (TEA), nitrate and caffeine were found not to change the shape of the sarcomere length-tension diagram. Potentiation in a tetanus was less than 3% in all experiments. 5. Contractures induced by raised [K+] in the bathing solution were found to produce more tension than a tetanus beyond optimum length, insignificantly different tension near optimum length, and less tension at sarcomere lengths near 1.7 micrometer. An explanation is proposed for these results in terms of inhomogeneous activation and internal motion. 6. It is concluded that there is no evidence from this work that a tetanized fibre is other than maximally activated over the range of sarcomere lengths spanned by the shallow ascending limb.
Full text
PDF


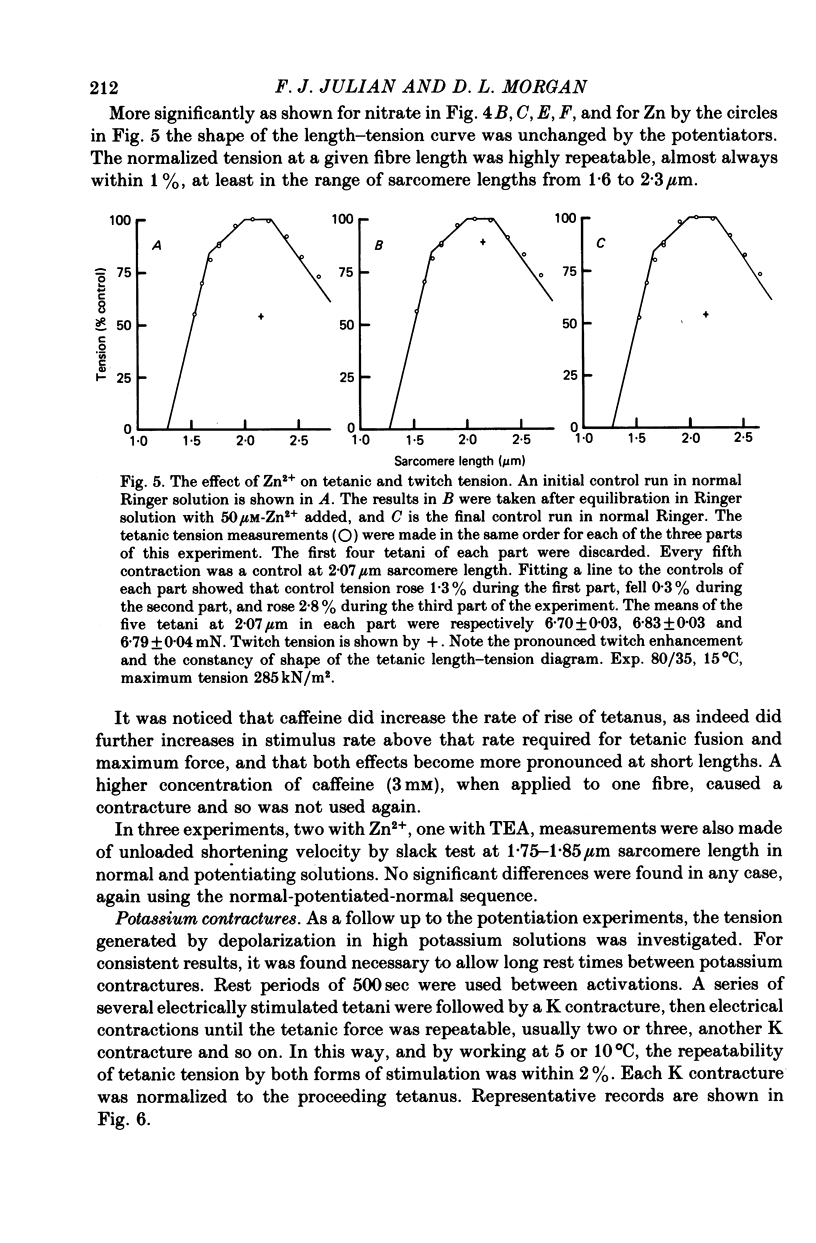
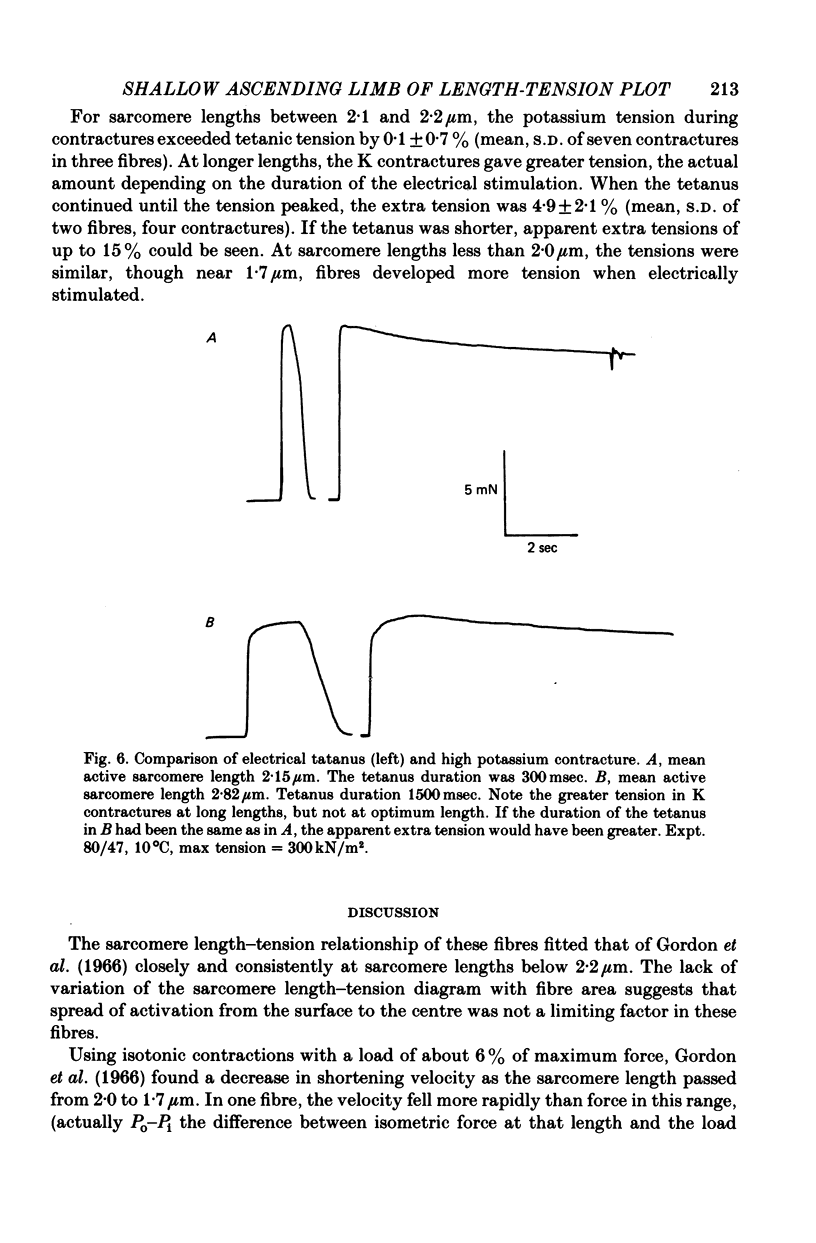




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler B. H., Clinch N. F. Cross bridges as the major source of compliance in contracting skeletal muscle. Nature. 1975 Jul 17;256(5514):221–222. doi: 10.1038/256221a0. [DOI] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J Physiol. 1978 Dec;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Intersarcomere dynamics during fixed-end tetanic contractions of frog muscle fibres. J Physiol. 1979 Aug;293:365–378. doi: 10.1113/jphysiol.1979.sp012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol. 1979 Aug;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R., Taylor S. R. Striated muscle fibers: facilitation of contraction at short lengths by caffeine. Science. 1971 Apr 23;172(3981):387–389. doi: 10.1126/science.172.3981.387. [DOI] [PubMed] [Google Scholar]
- SANDOW A. POTENTIATION OF MUSCULAR CONTRACTION. Arch Phys Med Rehabil. 1964 Feb;45:62–81. [PubMed] [Google Scholar]
- Taylor S. R. Vertebrate striated muscle: length dependence of calcium release during contraction. Eur J Cardiol. 1976 May;4 (Suppl):31–38. [PubMed] [Google Scholar]



