Abstract
1. The isotonic velocity transients following quick changes in load were studied on tetanized frog skeletal muscle fibres with special reference to those following quick increases in load. 2. When the load was increased quickly from the maximum isometric force P0 to 1.05-1.3 P0, the fibres exhibited markedly oscillatory length changes with distinct reversal in the direction of movement before starting to lengthen with a nearly constant velocity. 3. The period of the oscillatory length changes increased with increasing magnitude of the load step, and decreased with increasing temperature. The amplitude of oscillatory length changes never exceeded 0.5% of the slack length L0, i.e. about 50 A per half-sarcomere. 4. If the load was increased quickly from P0 to 1.3-1.6 P0, the fibres lengthened continuously with velocities decreasing with time. 5. The response of fibres, shortening isotonically under a large load (about 0.8 P0), to quick increases in load was qualitatively similar to that of isometrically contracting fibres. 6. When quick increases in load were applied during isotonic shortening under a moderate or small load (0.1-0.6 P0), the fibres showed initial transient lengthening before starting to shorten against a new load, indicating a decrease in the ability of the fibres to sustain a load after a period of isotonic shortening and its restoration during the transient lengthening. 7. The extent of decrease in load-sustaining ability as well as its subsequent restoration process was dependent on both the amount of load and the duration of preceding isotonic shortening. 8. The decrease in the load-sustaining ability during the course of isotonic shortening appeared to be complete within 30-50 msec after the beginning of shortening. 9. These results are discussed in relation to the kinetic properties of the crossbridges responsible for muscle contraction.
Full text
PDF



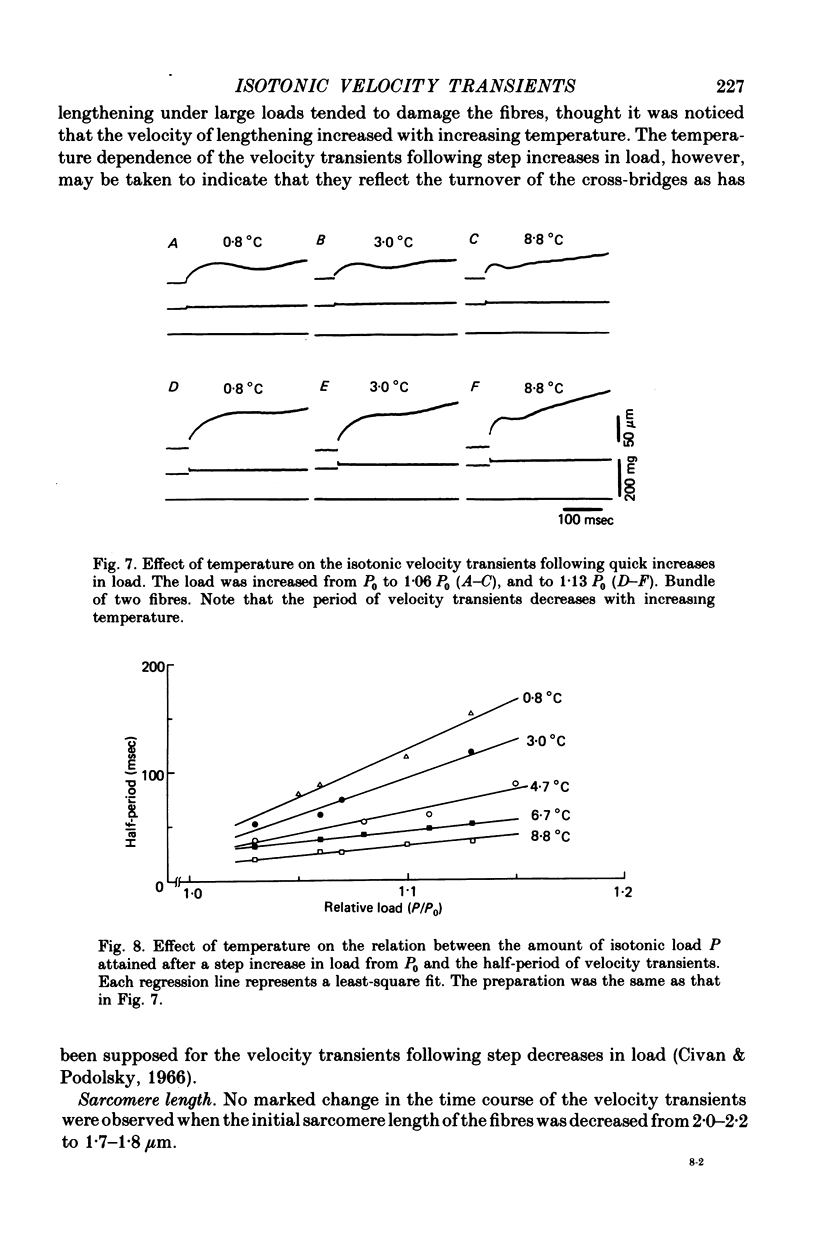
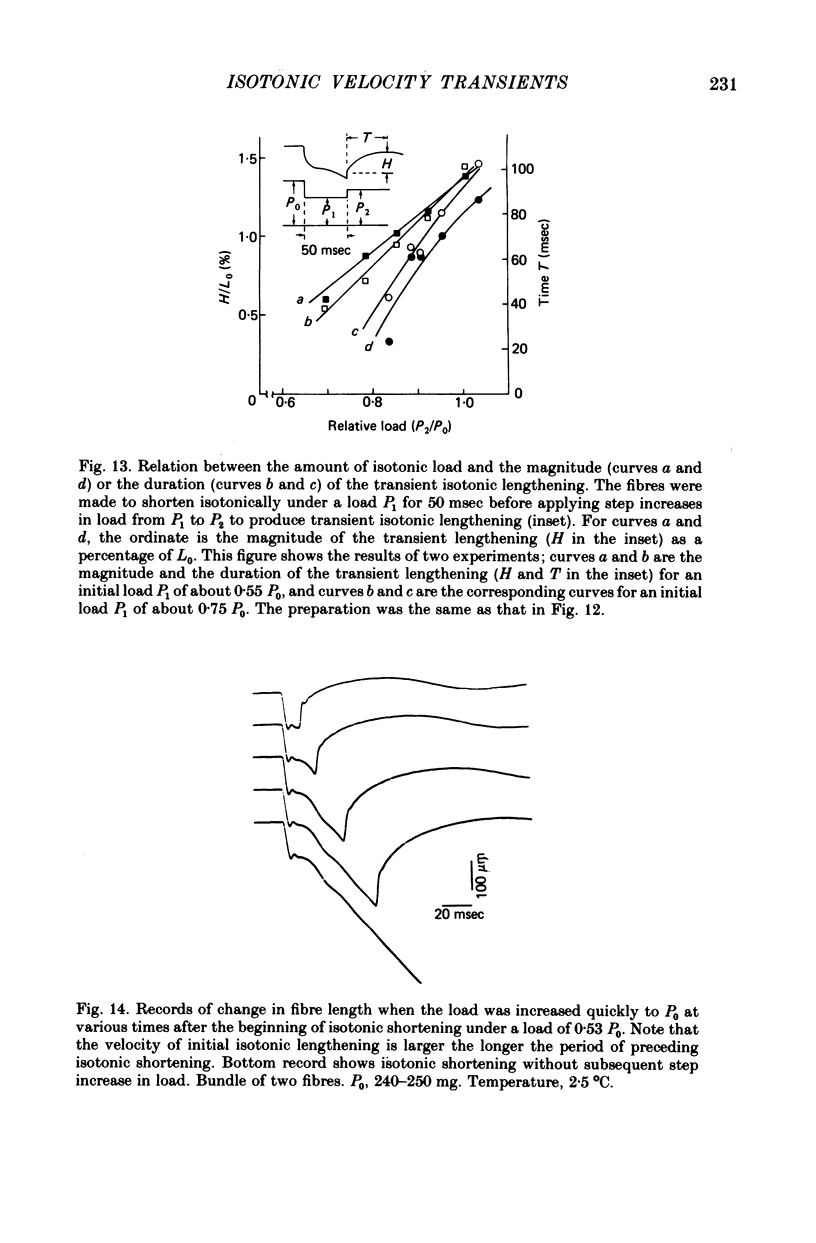
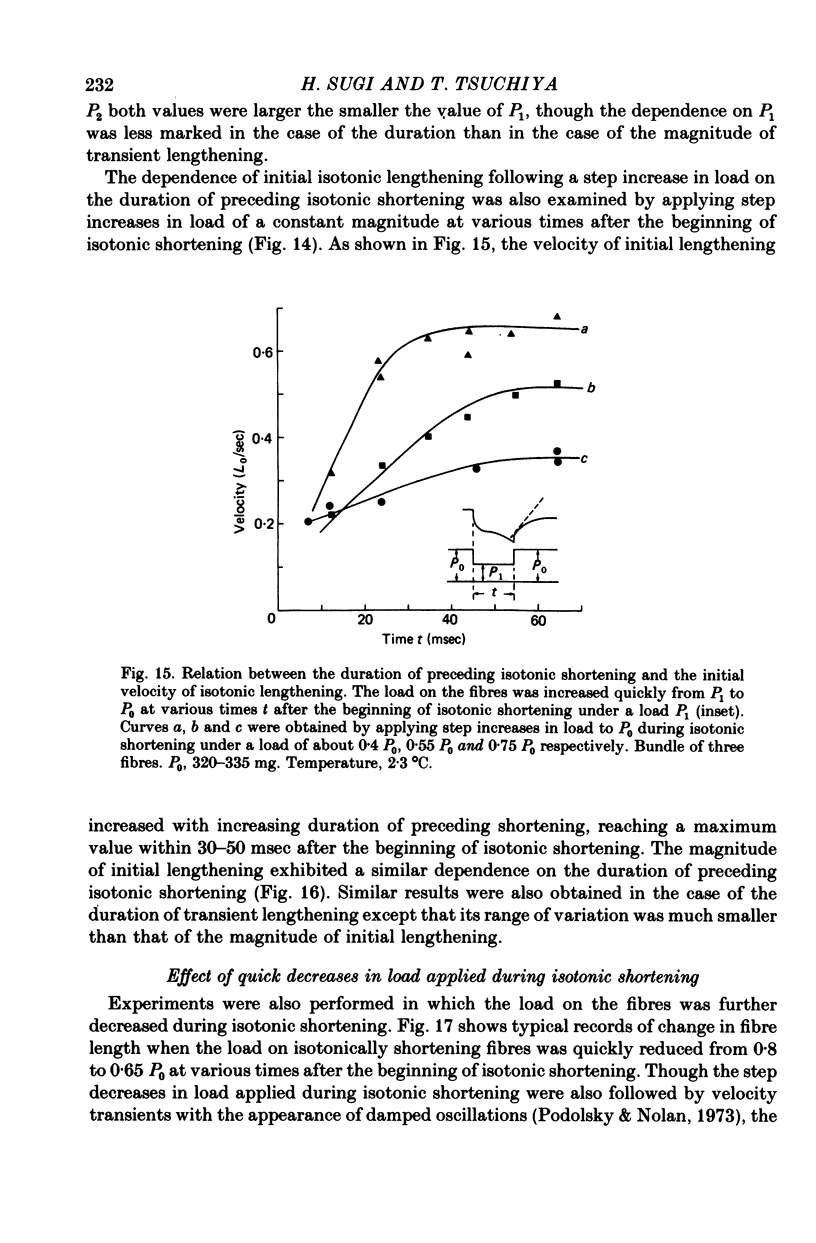
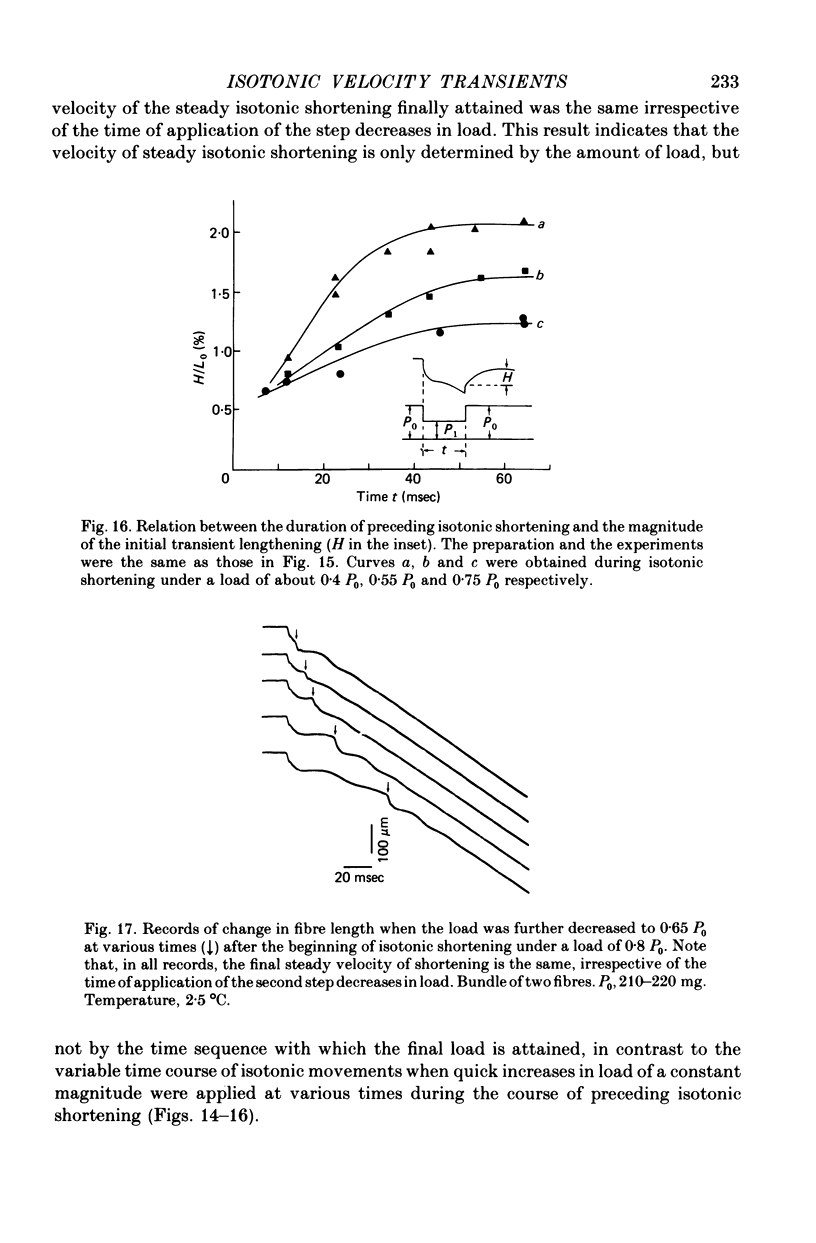

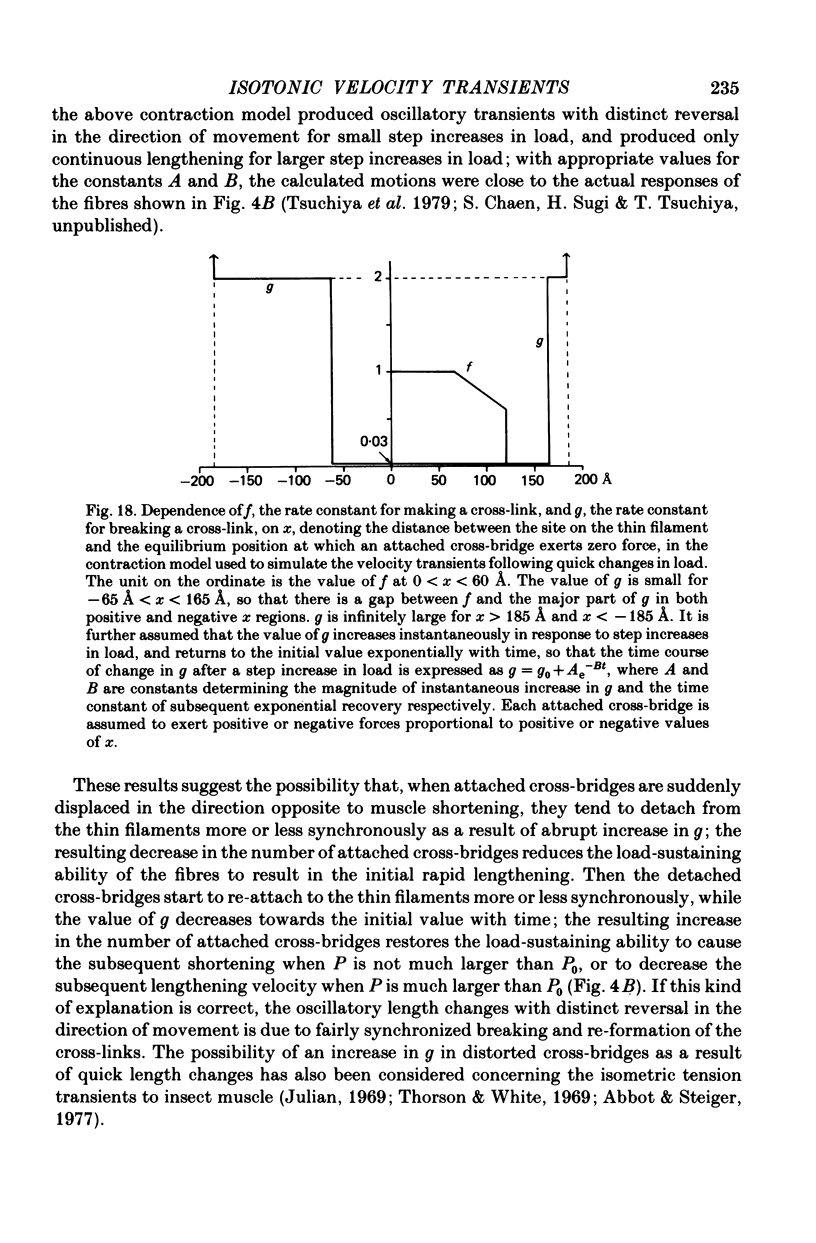



Selected References
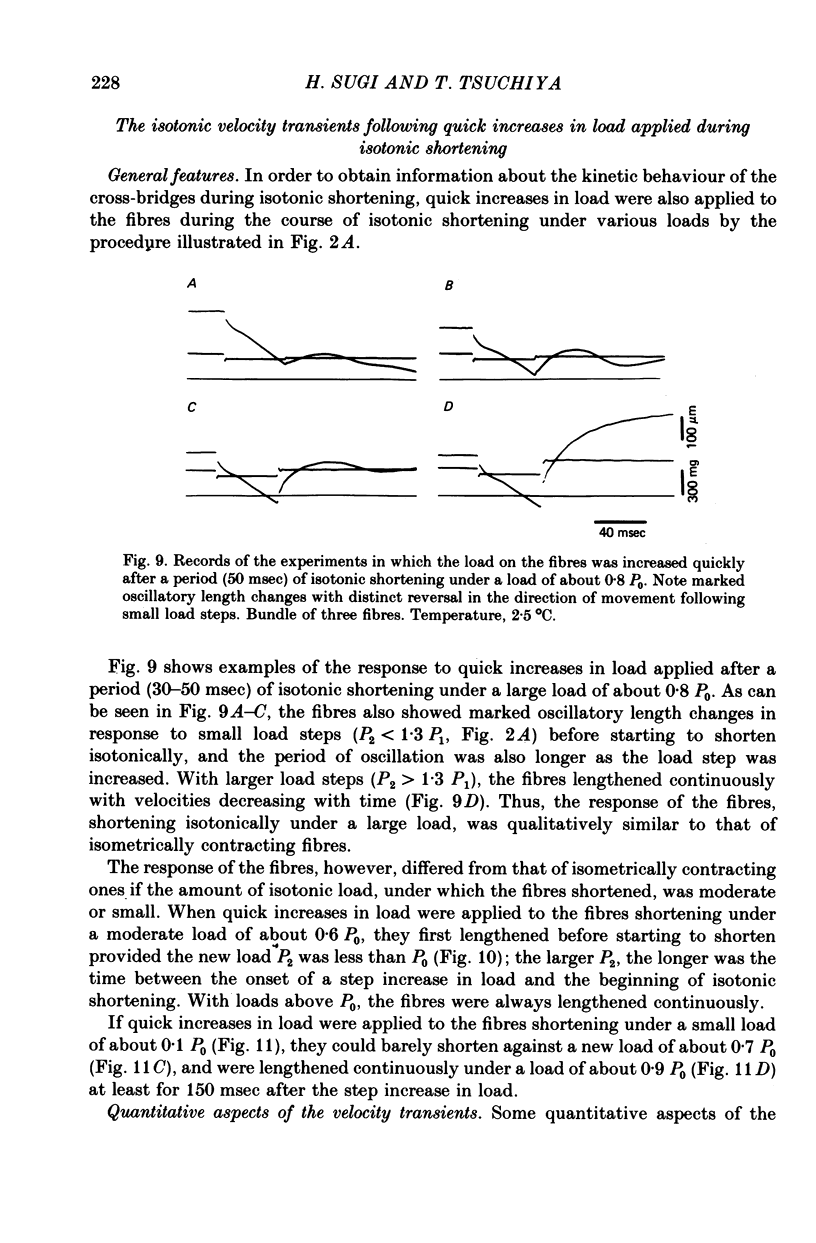
These references are in PubMed. This may not be the complete list of references from this article.
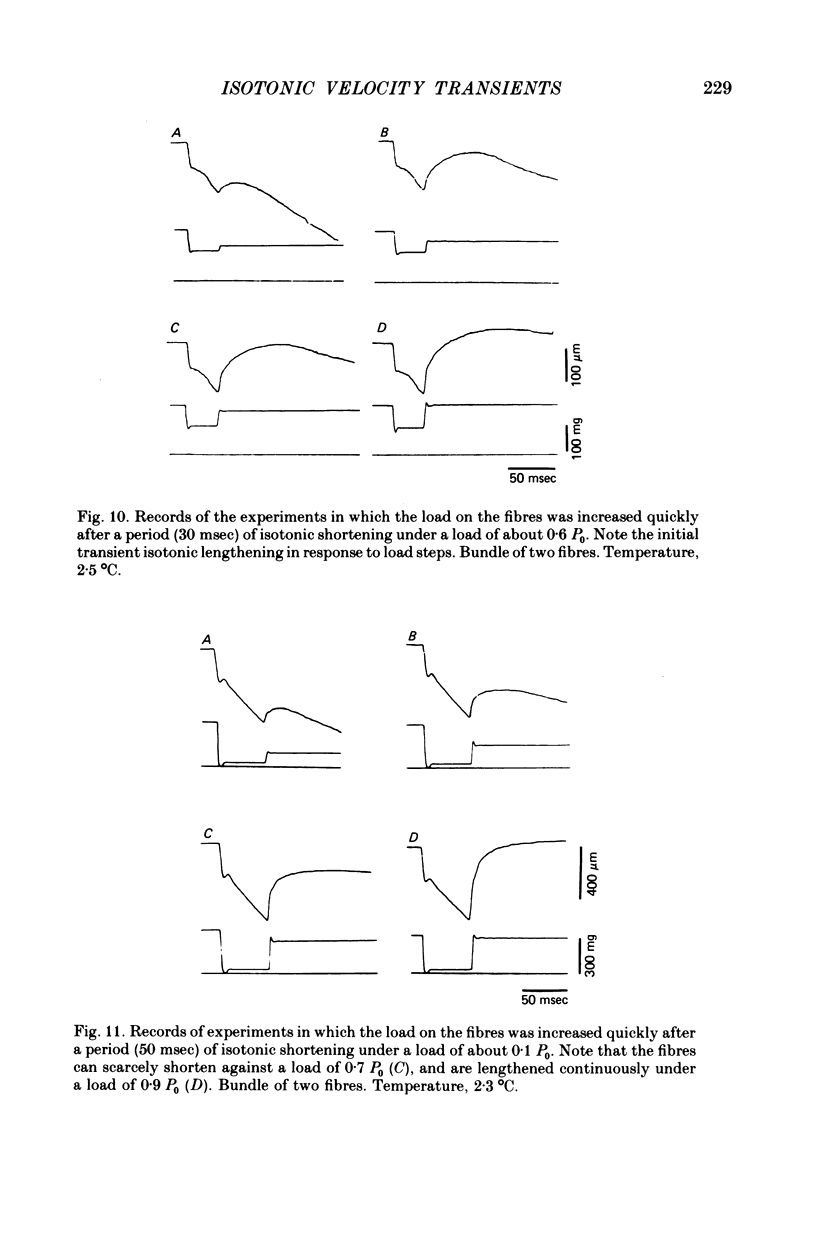
- Abbott R. H., Steiger G. J. Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol. 1977 Mar;266(1):13–42. doi: 10.1113/jphysiol.1977.sp011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
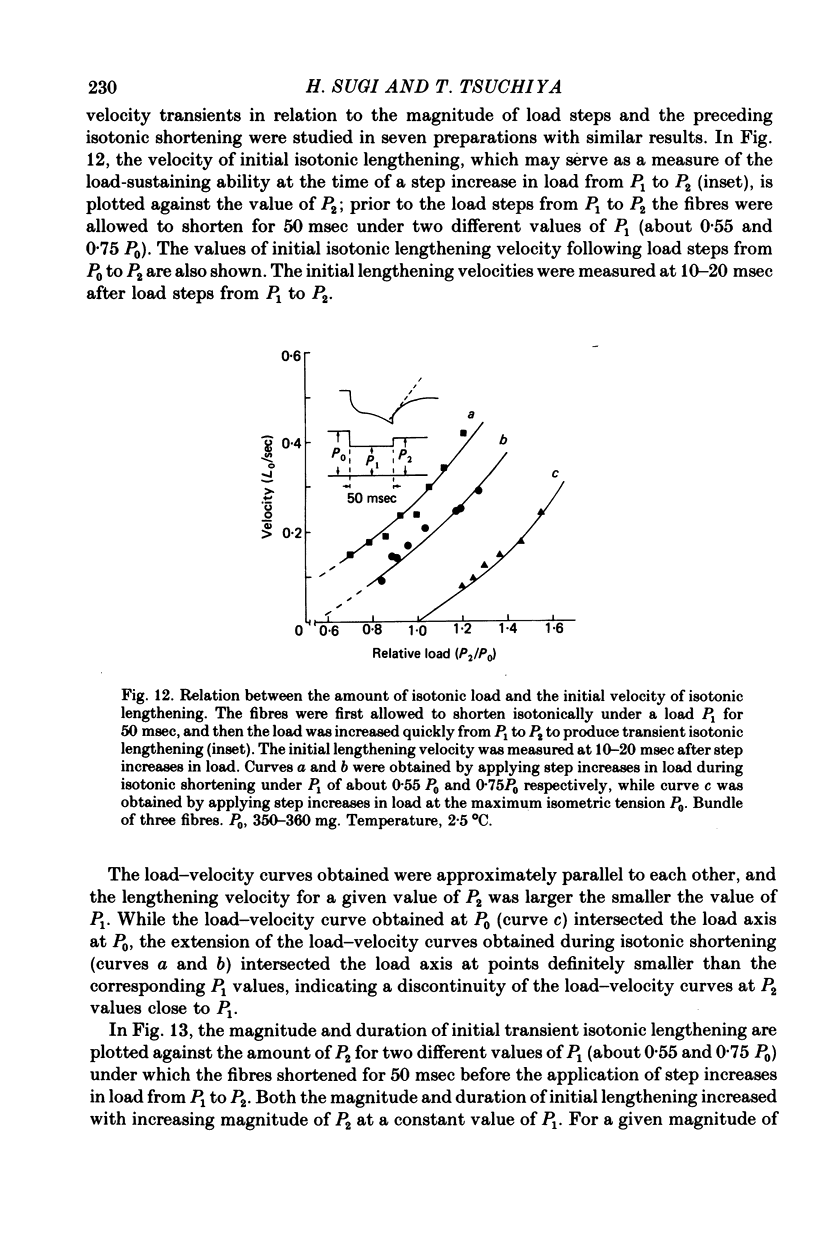
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Mechanical properties of the cross-bridges of frog striated muscle. J Physiol. 1971 Oct;218 (Suppl):59P–60P. [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J. 1969 Apr;9(4):547–570. doi: 10.1016/S0006-3495(69)86403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J. Kinetics of muscular contraction: the approach to the steady state. Nature. 1960 Nov 19;188:666–668. doi: 10.1038/188666a0. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Nolan A. C., Zaveler S. A. Cross-bridge properties derived from muscle isotonic velocity transients. Proc Natl Acad Sci U S A. 1969 Oct;64(2):504–511. doi: 10.1073/pnas.64.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H. Tension changes during and after stretch in frog muscle fibres. J Physiol. 1972 Aug;225(1):237–253. doi: 10.1113/jphysiol.1972.sp009935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H., Tsuchiya T. Enhancement of mechanical performance in frog muscle fibres after quick increases in load. J Physiol. 1981;319:239–252. doi: 10.1113/jphysiol.1981.sp013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J., White D. C. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



