Abstract
1. The acetylcholine (ACh) content and spontaneous and evoked release of ACh in rat extensor digitorum longus (EDL) muscles were determined by pyrolysis-mass fragmentography. The determinations were made on muscles paralysed by local application of botulinum toxin (BoTx) type A, on unpoisoned muscles, surgically denervated or reinnervated muscles.
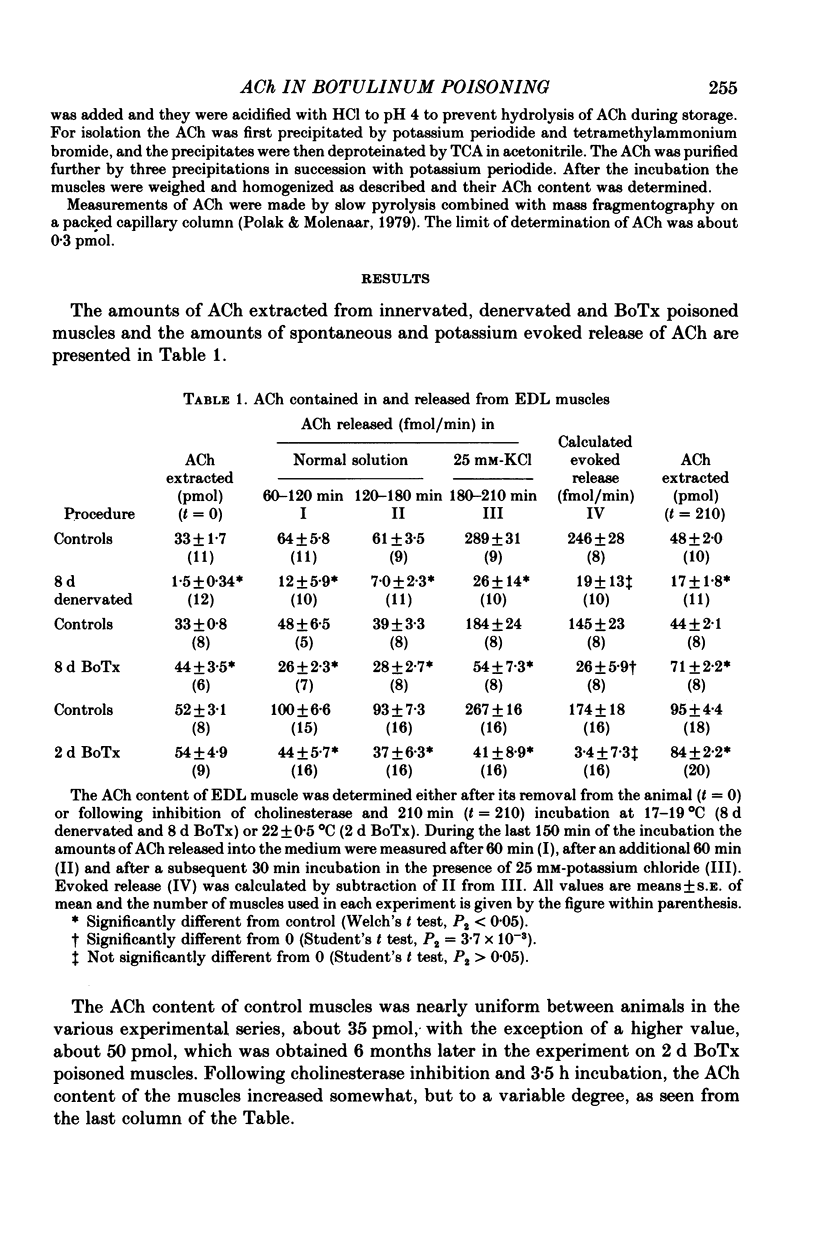
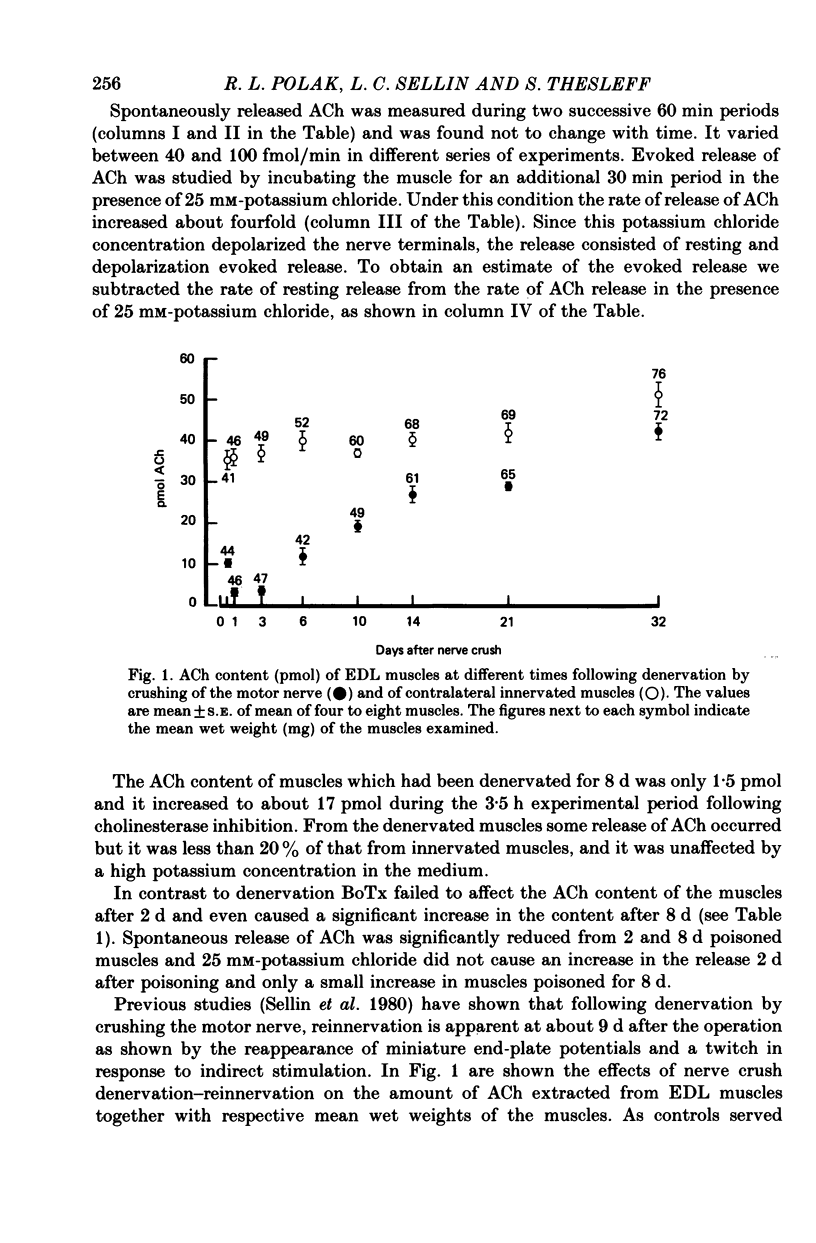
2. The ACh content of unpoisoned control muscles was nearly uniform between animals and varied in the experimental series between 36 and 50 pmol. BoTx failed to affect the ACh content after 2 d of poisoning and caused a slight increase in content after 8 d. Surgical denervation reduced the ACh content within 24 h to less than 10% of innervated muscles and upon reinnervation the ACh content was restored. Following cholinesterase inhibition the ACh content of innervated and denervated muscles increased somewhat, about equally with time.
3. Spontaneous release of ACh varied in normal innervated muscles between 40 and 100 fmol/min. In the presence of 25 mm-KCl the rate of release increased about fourfold. In BoTx poisoned muscles spontaneous release was reduced by up to 60% of control and high potassium failed to accelerate the release at 2 d after poisoning and caused only a small increase at 8 d. Denervated muscles released ACh at a rate which was less than 20% of control and it was not accelerated by high potassium.
4. The results show that more than 90% of total ACh in the innervated EDL muscle is present in the nerve and its terminals. The remaining ACh is apparently formed and stored in the muscle tissue. BoTx caused a larger reduction in ACh release than can be accounted for by assuming a selective blockade of quantal release of transmitter. It suggests that BoTx has an inhibitory effect also on non-quantal ACh release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W. An electron microscopic study of the changes induced by botulinum toxin in the motor end-plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 1971 Sep;14(1):47–60. doi: 10.1016/0022-510x(71)90129-8. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry. 1970 Feb;33(1):40–54. doi: 10.1136/jnnp.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P., Forrester T. The effect of curare on the release of acetylcholine from mammalian motor nerve terminals and an estimate of quantum content. J Physiol. 1975 Sep;251(1):131–144. doi: 10.1113/jphysiol.1975.sp011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Lennon A. M. Morphological evidence for exocytosis of acetylcholine during formation of synaptosomes from Torpedo electric organ. J Physiol. 1973 Aug;233(1):39P–41P. [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Motor units in the rat diaphragm. J Physiol. 1958 Mar 11;140(3):427–439. [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. The release of acetylcholine in the isolated rat diaphragm. J Physiol. 1961 Feb;155:246–262. doi: 10.1113/jphysiol.1961.sp006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. The effect of lanthanum ions on acetylcholine in frog muscle. J Physiol. 1980 Dec;309:199–214. doi: 10.1113/jphysiol.1980.sp013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol. 1963 Jan;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. A method for determination of acetylcholine by slow pyrolysis combined with mass fragmentography on a packed capillary column. J Neurochem. 1979 Feb;32(2):407–412. doi: 10.1111/j.1471-4159.1979.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. Pitfalls in determination of acetylcholine from brain by pyrolysis-gas chromatography/mass spectrometry. J Neurochem. 1974 Dec;23(6):1295–1297. doi: 10.1111/j.1471-4159.1974.tb12230.x. [DOI] [PubMed] [Google Scholar]
- RANDIC M., STRAUGHAN D. W. ANTIDROMIC ACTIVITY IN THE RAT PHRENIC NERVE-DIAPHRAGM PREPARATION. J Physiol. 1964 Sep;173:130–148. doi: 10.1113/jphysiol.1964.sp007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr, Mayer H. E., Schmid P. G. Choline acetyltransferase activity in guinea-pig heart in vitro. J Neurochem. 1974 Dec;23(6):1197–1200. doi: 10.1111/j.1471-4159.1974.tb12217.x. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucek S., Zelená J., Ge I., Vyskocil F. Choline acetyltransferase in transected nerves, denervated muscles and Schwann cells of the frog: correlation of biochemical electron microscopical and electrophysiological observations. Neuroscience. 1978;3(8):709–724. doi: 10.1016/0306-4522(78)90067-2. [DOI] [PubMed] [Google Scholar]
- Welsch F., Schmidt D. E., Dettbarn W. D. Acetylcholine, choline acetyltransferase and cholinesterases in motor and sensory nerves of the bull frog. Biochem Pharmacol. 1972 Mar 15;21(6):847–856. doi: 10.1016/0006-2952(72)90128-1. [DOI] [PubMed] [Google Scholar]
- White H. L., Wu J. C. Choline and carnitine acetyltransferases of heart. Biochemistry. 1973 Feb 27;12(5):841–846. doi: 10.1021/bi00729a009. [DOI] [PubMed] [Google Scholar]



