Abstract
Previously we demonstrated that IL-15 and IL-2 control the number of memory CD8+ T cells in mice. IL-15 induces, and IL-2 suppresses the division of these cells. Here we show that CD25+CD4+ regulatory T cells play an important role in the IL-2-mediated control of memory phenotype CD8+ T cell number. In animals, the numbers of CD25+CD4+ T cells were inversely correlated with the numbers of memory phenotype CD8+ T cells with age. Treatment with anti-IL-2 caused CD25+CD4+ T cells to disappear and, concurrently, increased the numbers of memory phenotype CD8+ T cells. This increase in the numbers of CD8+ memory phenotype T cells was not manifest in animals lacking CD4+ cells. Importantly, adoptive transfer of CD25+CD4+ T cells significantly reduced division of memory phenotype CD8+ T cells. Thus, we conclude that CD25+CD4+ T cells are involved in the IL-2-mediated inhibition of memory CD8+ T cell division and that IL-2 controls memory phenotype CD8+ T cell numbers at least in part through maintenance of the CD25+CD4+ T cell population.
The problem of immunological memory has occupied investigators for many years. There is now evidence that immune memory takes a number of forms, ranging from the existence of long-lived antibody and plasma cells, to expansions of antigen-specific T cells that remain after infections have disappeared (1–3). It is thought that, for both CD4+ and CD8+ T cells, some survive the activation-induced cell death that occurs as antigen wanes, and these cells form the basis for T cell memory in the body. CD4+ and CD8+ memory T cells may not be maintained in the same way, however.
Recent reports have described some of the factors that control CD8+ memory T cell survival in animals. Studies showed that these cells do not persist as long-lived resting cells. On the contrary, these cells divide slowly in animals (4, 5). Their slow proliferation does not require the continued presence of antigen, rather it is driven by IL-15, a cytokine that is produced constitutively in animals (3, 6–9). This slow division allows memory CD8+ T cells to maintain their numbers despite slow attrition due to cell death. The requirement for IL-15 may be one of the characteristics of CD8+ memory T cells, amongst others, which controls their numbers (10, 11).
Our experiments on this subject revealed another feature of CD8+ memory T cells, the fact that their slow proliferation and accumulation was dramatically affected by IL-2 (7). Injection of anti-IL-2 or anti-IL-2 receptor α chain (anti-IL-2Rα) into mice dramatically increased the apparent rate of division of CD8+ memory T cells and also increased their numbers. Thus, IL-15 and IL-2 have opposite effects on the size of the CD8+ memory T cell population. This is a surprising finding because IL-2 and IL-15 act via receptors that are very closely related, both including the IL-2Rβ chain and γc and differing only in their α chains, IL-2Rα and IL-15Rα for IL-2 and IL-15, respectively. Moreover, the α chains of both these receptors are thought to act only to create high-affinity binding sites for the two cytokines and are not supposed to participate in signal transduction (12–14). Hence, cells bearing receptors for both cytokines are not thought to respond differently to IL-2 and IL-15. However, several facts suggest that IL-2 and IL-15 have different effects on the immune system. For example, mice lacking IL-2 or the IL-2Rα chain are susceptible to lymphoproliferative and autoimmune disease (15, 16), whereas animals lacking IL-15 or the IL-15Rα chain are not (17, 18). These ideas suggest that the effects of IL-2 and IL-15 on CD8+ memory T cells must be mediated by action of the cytokine on different cell populations bearing high-affinity receptors for IL-2 or IL-15 only.
The cells that have received the most attention in this context are those that bear CD4 and CD25 (IL-2Rα) and inhibit the responses of other T cells (19–24). These regulatory T cells inhibit T cell proliferation in vitro and autoimmune disease in vivo.
We therefore tested the possibility that the CD4+CD25+ regulatory T cells might be involved in the inhibitory effects of IL-2 on CD8+ memory T cells in vivo. Here we show that IL-2 does not affect CD8+ memory T cells directly in vitro. However, under two circumstances in vivo, the numbers of CD8+ memory T cells and CD4+CD25+ T cells are inversely correlated. First, the numbers of the memory T cells increase and the numbers of the CD4+CD25+ regulatory cells fall as animals age. Secondly, inhibition of the action of IL-2 causes an increase in the numbers of CD8+ memory T cells and a loss of the regulatory population. Also, the inhibitory effects of IL-2 on CD8+ T cell proliferation are abrogated in animals lacking CD4+ T cells. Finally, the rate of proliferation and recovery of transferred CD8+ memory T cells is usually reduced in animals given, in addition, CD4+CD25+ T cells. Thus we conclude that IL-2 inhibits division of CD8+ memory T cells at least in part by stimulating the survival and perhaps function of CD4+CD25+ regulatory T cells.
Methods
Mice.
C57BL/6J (B6) and C57BL/6.PLJ (B6.PL) mice were purchased from The Jackson Laboratory. Rag2- and CD25-deficient mice [RagKO (25) and CD25KO (16)] were bred from pairs obtained from same source. In some experiments, mice were sublethally irradiated (450Rad) to destroy lymphocytes including the CD4+CD25+ T cells. All mice were maintained in a pathogen-free environment in accordance with institutional guidelines.
T Cell Purification, Isolation, and Staining.
T cells were purified from lymph nodes and spleens on nylon wool columns and stained with CFSE (Molecular Probes) and/or antibodies (BD PharMingen) as described (26, 27). Analysis was performed on a FACScaliber or a FACScan Instrument (Becton Dickinson) and cell sorting was done on a MoFlo instrument (Cytomation, Fort Collins, CO). Sorting was performed as described (7). In brief, T cells were purified from lymph nodes and spleens on nylon wool columns and stained with APC-anti-CD8, Cyc-anti-CD4, PE-anti-CD122, and Oregon-green-anti-CD25 to sort memory CD8+ T cells, CD25+CD4+ T cells, and filler T cells (CD25+CD4+ T cell-depleted). The purity of the sorted T cells was confirmed by reanalysis on a FACScaliber or FACScan instrument (Becton Dickinson) and the purities of memory CD8+ T cells, CD25+CD4+ T cells, and filler T cells sorted were over 98%, 96.5%, and 98%, respectively. T cells were washed twice with BSS (balanced salts solution) buffer and injected intravenously into mice.
Cell Culture.
Cells were cultured as described (27). Mouse IL-2 and human IL-15 were purchased from R & D Systems. CFSE labeling and analysis was performed as described (7, 26).
Monoclonal Antibodies.
Monoclonal antibody cell lines used here have been described (7). The anti-CD4 antibody, GK-1.5 (28), was used to deplete CD4+ T cells in animals and the anti-CD4 antibody, RM4–5 (BD Biosciences PharMingen), was used to stain CD4+ cells. Anti-CD4 and anti-IL-2 were purified on protein G columns as described (7).
Results
Except where otherwise stated, the experiments in this paper were performed on T cells bearing CD8 and CD122, the IL-2Rβ chain, isolated from normal aging animals. These cells accumulate in mice as they age (see below) and also bear high levels of CD44. They therefore have the characteristics of CD8+ memory T cells generated after priming animals with antigen. They have the advantage over antigen induced memory T cells in that they are more easily isolated and are available in larger numbers. Where possible we have confirmed results with these CD8+ T cells of memory phenotype with cells induced by antigen. The CD8+ T cells of memory phenotype found in normal animals will, for convenience, be referred to as CD8+ memory T cells in this paper.
IL-2 Does Not Inhibit the Effects of IL-15 on CD8+ Memory T Cells in Vitro.
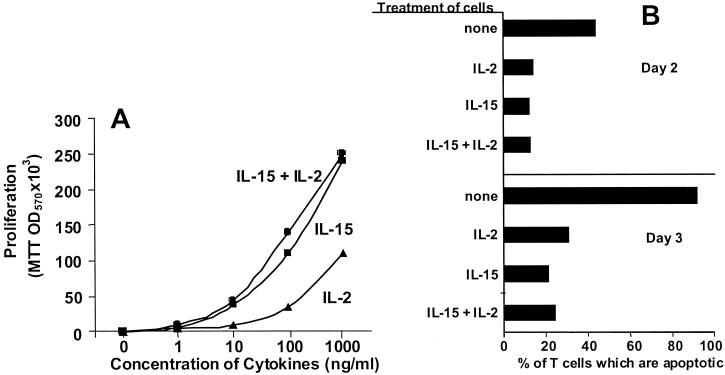
We previously reported that removal of IL-2 from animals allowed a rapid increase in the numbers of CD8+ memory T cells (7). This result suggested either that IL-2 acts directly on CD8+ memory T cells or via some other cell. We tested the former of these ideas by measuring the effects of IL-2 on the in vitro response of memory T cells to IL-15, the cytokine that supports their growth in vivo. CD8+ memory T cells were isolated from the spleens and lymph nodes of normal animals by high-speed cell sorting. The cells were cultured with various concentrations of IL-15 and/or IL-2. The cells proliferated vigorously in response to IL-15. They also divided in response to IL-2; however, in this assay the cells were about an order of magnitude less sensitive to IL-2 than to IL-15, probably because the cells lack high-affinity receptors for IL-15 (Fig. 1A).
Figure 1.
IL-2 does not inhibit the proliferation or survival of CD8+ memory T cells in response to IL-15 in vitro. T cells were isolated from C57BL/6 mice, stained with anti-CD8 and anti-IL-2Rβ or anti-CD44, and sorted to yield memory CD8+ T cell populations that were CD8+IL-2Rβhigh or CD8+CD44high. (A) Memory CD8+ T cells (5 × 105 per ml) were cultured with IL-2 and/or IL-15 (R & D Systems). Their proliferation was assayed on day 2 by incorporation of MTT (Sigma) measured by OD570. (B) Memory CD8+ T cells (2 × 105 per ml) were cultured with 10 ng/ml IL-2 and/or IL-15 and the percentages of apoptotic cells were measured by their light-scattering properties on a flow cytometer 2 and 3 days later.
To determine whether IL-2 selectively inhibited division of a subset of the CD8+ memory T cells, T cells were labeled with CFSE and then cultured for 3 days with various concentrations of IL-15 and/or IL-2. In the presence of 100 ng/ml IL-15, 89% of the CD8+CD44high (memory) T cells divided at least once during the culture period. If 100 ng/ml IL-2 were also present, 92% of the CD8+CD44high cells divided. Thus, IL-2 did not inhibit division of a subset of the cells.
IL-2 induces T cells to become sensitive to Fas-mediated apoptosis (29). Because activated CD8+ T cells bear also Fas ligand (30, 31), it was possible that IL-2 was acting via this pathway, although the facts that the CD8+ memory T cells do not bear high-affinity IL-2 receptors (7) and that IL-2 did not inhibit CD8+ memory T cell responses to IL-15 suggested otherwise. To check this directly, however, purified CD8+ memory T cells were cultured alone or with IL-15 and/or IL-2 and the percentage of apoptotic T cells measured 2 or 3 days later. A fairly large percentage of the cells were dead if they were cultured in the absence of cytokines. IL-15, IL-2, and the combination of IL-15 and IL-2 all protected the cells against death (Fig. 1B).
Together these results demonstrate that the inhibitory effects of IL-2 are probably not due to direct action of the cytokine on the CD8+ memory T cells themselves.
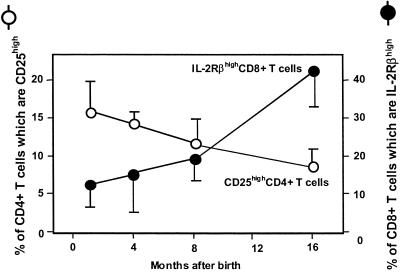
The Numbers of CD8+ Memory T Cells and CD4+CD25+ T Cells Increase and Decrease, Respectively, with Age.
B6 mice of various ages were killed and the percentages of their CD4+ T cells bearing CD25 and CD8+ T cells bearing CD122 were assessed. As shown in Fig. 2, the percentages of CD4+ T cells bearing CD25 were higher in young than in 1-year-old animals, and vice versa the percentages of CD8+ T cells of memory phenotype increased.
Figure 2.
Age-dependent decrease of CD25+CD4+ and increase of CD8+ memory T cells in mice. Peripheral blood lymphocytes were isolated from C57BL/6 mice (1, 3, and 12 months old; n = 6). The cells were stained with anti-CD4, anti-CD8, anti-CD25, and anti-IL-2Rβ and analyzed. Data shown are the means and SDs.
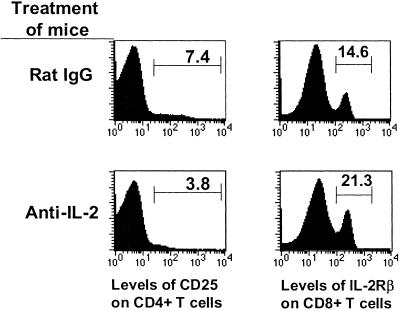
Inhibition of IL-2 in Mice Increases the Numbers of CD8+ Memory T Cells and Decreases the Numbers of CD4+CD25+ T Cells.
To determine whether treatment with anti-IL-2 affects the numbers of CD4+CD25+ T cells in animals, mice were treated with a blocking anti-IL-2 antibody for 5 days and the numbers of these cells then measured. As before, inhibition of IL-2 increased the percentage of CD8+ T cells bearing CD122—i.e., of memory phenotype (Fig. 3).
Figure 3.
CD25+CD4+ T cells require IL-2 for survival in mice. Lymph node and spleen T cells were isolated from C57BL/6 mice that have been injected with 1 mg/day of anti-IL-2 or control rat IgG for 5 days. The cells were stained with anti-CD4, anti-CD8, anti-CD25, and anti-IL-2Rβ and analyzed. The numbers of CD25highCD4+ T cells in spleens and lymph nodes were 2.2 × 105 ± 1.0 × 105 in mice treated with rIgG (n = 4) and 0.65 × 105 ± 1.1 × 105 in mice treated with anti-IL-2 (n = 3). Data were analyzed by Student's t test; P < 0.011.
Concomitantly, the treatment reduced the percentages of T cells bearing CD25. The effects on CD4+ T cells bearing high levels of CD25 were particularly dramatic. The results were similar if calculated in terms of the total numbers of these cells (data not shown). IL-2 is know to affect expression of its receptor, so the blocking anti-IL-2 antibody could have caused the disappearance of CD25+ cells either because lack of IL-2 led to death of the CD4+CD25+ T cells, or because it led to lower expression of CD25 by these same cells. To determine which of these ideas was correct, we isolated CD4+CD25+ cells from BL/6 mice by high-speed sorting and transferred them into normal B6.PL animals. These mice were then treated with anti-IL-2 antibody or control rat IgG. Six days later, lymph node and spleen cells were isolated from the mice and analyzed for their numbers of CD4+Thy1.2+ T cells with or without concomitant expression of CD25. Treatment with anti-IL-2 resulted in a lower yield of CD4+Thy1.2+ T cells from the recipients' spleens. This loss was due entirely to the fact that the yield of CD4+CD25+Thy1.2+ cells from anti-IL-2-treated animals was less than 50% of that obtained from the controls (7,300 vs. 15,600). In lymph nodes the situation was less clear. There was a lower yield of CD4+CD25+Thy1.2+ cells (2,400 vs. 4,800), but the total numbers of CD4+Thy1.2+ cells harvested from lymph nodes were unaffected by anti-IL-2 treatment. Hence it seems that blockage of IL-2 does reduce the numbers of CD4+CD25+ cells in spleen because of their death. However, in lymph nodes, CD4+CD25+ cell numbers may drop with anti-IL-2 treatment because they no longer express CD25+, not because they die. Whether this is accompanied by loss of their regulatory function remains to be determined.
Thus, IL-2 reduces the numbers of CD8+ memory T cells in mice. Conversely, lack of IL-2 leads to a fall in the numbers of CD4+CD25+ T cells in the animals, both because of cell death and because of loss of CD25.
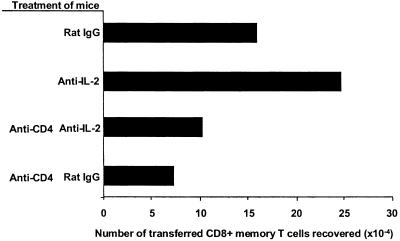
CD4+ T Cells Are Required for the Stimulatory Effects of Anti-IL-2 on CD8+ Memory T Cells.
If the effects of IL-2 on CD8+ memory T cells are mediated by CD4+CD25+ T cells, then removal of CD4+ T cells from animals should reduce the efficacy of anti-IL-2. To check this, purified T cells from relatively old B6.PL (Thy1.1+) mice were transferred into normal B6 (Thy1.2+) animals. Some of the mice were then treated with a depleting anti-CD4 antibody and overlapping groups of mice were treated with anti-IL-2 or control rat antibody. Six days later, T cells from the animals were analyzed for their percentages of CD4+ T cells by using an anti-CD4 antibody against a determinant that differed from the target of the depleting antibody. The results showed that CD4+ T cells had indeed been removed from the appropriate animals (data not shown). As expected, animals treated with anti-IL-2 alone contained more of the transferred CD8+ memory T cells than animals given rat IgG alone (Fig. 4). This was also true for the endogenous CD8+ T cells (data not shown). Animals from which CD4+ T cells had been removed contained fewer of the transferred CD8+ memory T cells than the did normal recipients, indicating a partial dependence of the CD8+ memory T cells on CD4+ T cells. However, anti-IL-2 treatment did not increase the numbers of CD8+ memory T cells in mice that contained no CD4+ T cells. These results show that CD8+ memory T cells require CD4+ T cells for their existence in mice. Moreover, they strongly suggest that the effects of IL-2 on CD8+ memory T cells are mediated by some type of CD4+ cell.
Figure 4.
Increase in the numbers of CD8+ memory T cells induced by removal of IL-2 depends on the presence of CD4+ T cells. C57BL/6, Thy1.2+ mice were depleted of CD4+ T cells by treatment with a depleting anti-CD4 antibody. These animals, and control mice, were injected with Thy1.1+ T cells and anti-IL-2 or control rat IgG. Six days later mice were killed and the numbers of Thy1.1+CD8+ T cells in the animals counted. The experiments included two mice in each group; the results shown are representative of three experiments.
CD4+CD25+ T Cells Inhibit the Growth and Recovery of CD8+ Memory T Cells from Mice.
To test directly whether CD4+CD25+ T cells were involved in the inhibitory effects of IL-2 on CD8+ memory T cells, we compared the fate of CD8+ memory T cells in animals that did or did not contain CD4+CD25+ T cells. This is not easy to achieve, because it is difficult to produce animals that lack CD4+CD25+ T cells but contain enough T cells to block the homeostatic expansion that occurs when T cells are transferred into animals lacking T cells (32–36). It has been shown that CD4+CD25+ T cells inhibit to some extent homeostatic expansion of other T cells (37). The slow division of memory T cells in normal mice may be related to T cell homeostatic expansion, although TCR-mediated signaling is important for homeostatic proliferation but not for the slow division of CD8+ memory T cells, so the relationship between the two phenomena is not clear. Therefore, we were anxious to establish conditions under which our results might not be confused by the effects of lack of T cells, and under which the slow, IL-15-driven proliferation of CD8+ T cells could be studied in the absence of other effects.
To accomplish this, animals lacking T cells were given large numbers of “filler” T cells lacking the CD4+CD25+ population. It is not easy to prepare large numbers of CD25− “filler” cells, so in different experiments they were prepared in different ways. In the first two experiments, the cells were prepared from animals lacking CD25. CD4+ cells from such animals by definition are not CD25+. It is likely that the animals also lack the CD4+ cells that would otherwise bear CD25 because these mice become autoimmune. However, this last idea has not been definitively established (38). In experiments 3 and 4, CD25− “filler” cells were prepared by sorting. In experiments 5 and 6, donors of the CD25− filler cells were cleared of CD4+CD25+ cells by pretreatment with anti-CD25 or anti-CD4, respectively.
The T cell-deficient mice used in these experiments were usually RagKO. However, in experiments 4 and 6 animals were rendered T cell-deficient by sublethal irradiation. Once the T cell-deficient mice had been injected with filler cells they were divided into two groups, one receiving T cells that included CD4+CD25+ regulatory T cells and the other, control T cells lacking the regulatory population. Both groups of mice were then also given indicator T cells, labeled with CFSE and including CD8+ memory T cells. Four days later, T cells were isolated from the animals and CFSE label was used to count the numbers of transferred CD8+ CFSE-labeled cells that had or had not divided. During the time of this experiment none of the CFSE-labeled cells had divided so many times that they had lost detectable CFSE labeling, suggesting that proliferation induced by absence of T cells (homeostatic expansion) was not a problem in these experiments.
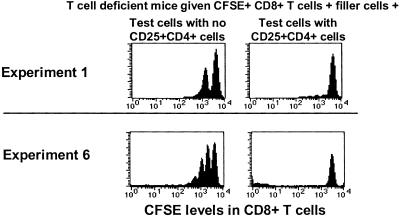
Examples of the results of these experiments are shown in Fig. 5. The results of six such experiments are summarized in Table 1. Table 1 shows the numbers and sources of the different cells transferred in each experiment. It also shows the numbers of CFSE-labeled CD8+ memory cells recovered that had or had not divided. Finally, Table 1 shows the ratios of the numbers of CFSE-labeled CD8+ cells that recovered from hosts that did or did not contain CD4+CD25+ cells.
Figure 5.
CD25+CD4+ T cells reduce the number of dividing memory phenotype CD8+ T cells in animals. T cells were purified from C57BL/6 (experiment 1) or C57BL/6.PL (experiment 2) mice, stained with anti-CD4, anti-CD8, and anti-CD25, and sorted to yield populations that lacked (CD25−CD4+) or included (CD25+CD4+) CD25+CD4+ T cells. The cells were labeled with CFSE and transferred into RagKO (experiment 1) or sublethally irradiated (450Rad, experiment 6) recipients. Other details of the experiments are listed in Table 1. Four days after transfer, T cells were isolated, stained with anti-CD8, anti-IL-2Rβ, or anti-CD44 and anti-Thy1.1 (experiment 2), and analyzed. In experiment 1, the CFSE-negative peak, consisting almost entirely of filler T cells, is not shown. In experiment 2, cells analyzed were gated to include only Thy1.1+ T cells. Data from these and four other similar experiments (Table 1) were analyzed by Wilcoxon and Student's t tests. Both analyses revealed statistically predictable decreases in the numbers of dividing memory phenotype CD8+ T cells in mice containing CD25+CD4+ T cells versus mice without CD25+CD4+ T cells (P < 0.05).
Table 1.
CD4+CD25+ T cells reduce the number of CD8+ memory cells in mice mainly by acting on dividing cells
| Experiment | Recipient | Cells transferred into T cell-deficient recipients
|
Number (×10−4) of CFSE+CD8+ memory T cells recovered from animals that received
|
Change in yield of CFSE+CD8+ memory T cells in the absence/presence of CD4+CD25+ T cells
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Filler cells lacking CD4+CD25+ cells
|
Number (×10−7) CFSE+ indicator cells | Number (×10−6) CD25+ or CD25− test cells | CD4+CD25− test T cells
|
CD4+CD25+ test T cells
|
|||||||
| Origin | Number (×10−7) | Nondividing | Dividing | Nondividing | Dividing | Nondividing | Dividing | ||||
| 1 | RagKO | CD25KO | 7 | 3 | 1.7 | 3.1 | 37 | 2.6 | 8.5 | 1.19 | 4.35 |
| 2 | RagKO | CD25KO | 7.5 | 2.5 | 2 | 4.9 | 7.4 | 3.2 | 2.7 | 1.53 | 2.74 |
| 3 | RagKO | Sorted | 1 | 8 | 0.5 | 5.2 | 64 | 4.2 | 8.1 | 1.24 | 7.90 |
| 4 | Irradiated | Sorted | 2.7 | 6.3 | 2 | 4.3 | 6.9 | 10 | 8.2 | 0.43 | 0.84 |
| 5 | RagKO | Anti-CD25 | 13 | 6 | 1.3 | 9.6 | 12.7 | 2.4 | 3.1 | 4.00 | 4.10 |
| 6 | Irradiated | Anti-CD4 | 7.5 | 2.3 | 1 | 9.9 | 22.5 | 5.4 | 5.5 | 1.83 | 4.09 |
Recovered memory cell numbers are those of the CD44high or IL-2Rβhigh CD8+CFSE+ cells in the spleen and inguinal, brachial, and mesenteric lymph nodes of recipients.
In five of the experiments, the total numbers of CFSE-labeled CD8+ T cells recovered from mice that lacked CD4+CD25+ T cells were somewhat greater than the numbers recovered from mice that contained the CD4+CD25+ population. The average ratio over all experiments ± standard deviation was 1.70 ± 1.22. The CD4+CD25+ T cells had a dramatic effect, however, on the average numbers of dividing CD8+ T cells recovered, with an average ratio in the presence versus absence of CD4+CD25+ cells of 4.00 ± 2.32. These results were quite erratic from experiment to experiment, however. In five of the experiments the CD4+CD25+ T cells had a clear inhibitory effect. However, in experiment 4 no effect of these cells was observed (Table 1). There was a concomitant reduction in nondividing cells recovered from the hosts that contained no CD4+CD25+ cells suggesting some overall failure in the experimental protocol for unknown reasons.
Overall in these experiments, few of the CD8+ CFSE-labeled cells divided after transfer into animals given control rat Ig and CD4+CD25+ test cells. This finding suggested that the use of filler cells had successfully overcome the problem of homeostatic proliferation that might otherwise have been induced in the CFSE-labeled cells by transfer into the T cell deficient hosts.
In toto these experiments suggested that at least part of the inhibitory effects of IL-2 on the slow proliferation of CD8+ memory T cells was mediated by CD4+CD25+ cells, cells that require IL-2 for their survival and that may inhibit directly the proliferation of CD8+ memory T cells.
Discussion
Immunologists have reached agreement that, at least in part, CD8+ memory T cells are maintained by slow division in response to IL-15 (3–9). Other factors, such as exposure to antigen, the presence of class I proteins and presence or absence of interferons may also help to keep the numbers of these cells up in animals (11, 39–41), but IL-15 clearly contributes. Less well understood are the factors that limit the numbers of CD8+ memory T cells. Presumably to some extent they are limited by competition between themselves and other IL-15 binding cells, such as natural killer cells, for IL-15 itself. However, type 1 interferons and competition for antigen and class I-presenting cells may also contribute to control of their numbers.
In tests of the effects of IL-2-related cytokines on memory T cells, the most dramatic results were obtained with IL-2 itself (7). Depletion of animals of IL-2 revealed that lack of this cytokine allowed a rapid increase in the size of the CD8+ memory T cell population. Our experiments investigating this phenomenon are described here. Although IL-2 might have limited the numbers of CD8+ memory cells by acting directly on them, we could find no evidence that this was so. On the contrary, experiments showed that IL-2 was a proliferative and survival factor for CD8+ memory T cells in vitro and did not reduce similar effects on the cells induced by IL-15. Moreover, there was no evidence that the numbers of CD8+ memory T cells were controlled by IL-2-induced death receptors such as Fas (data not shown). The fact that CD8+ memory T cells do not bear high-affinity receptors for IL-2 (7) serves as an additional clue that IL-2 is not acting directly on the CD8+ memory T cells themselves.
If IL-2 does not act on the memory cells, its effects must be mediated by some other cell that itself must bear receptors for IL-2. Few types of cells fall into this category, but those that do include recently activated T cells, dendritic cells (42), and CD4+CD25+ regulatory T cells. Of these, the last (CD4+CD25+ T cells) are attractive candidates because they are already well known to inhibit T cell proliferation, albeit in response to antigen, in many other systems (19–24).
The data in this paper suggest that these regulatory cells may indeed play a role in the effects of IL-2 on CD8+ memory T cells. Correlative evidence for the idea was provided by the observation that under circumstances in which CD8+ memory T cells were high in percentage, CD4+CD25+ T cells were reduced in number. Direct evidence came from two sets of experiments. The first of these showed that the inhibitory effects of IL-2 on CD8+ memory T cells required the presence of CD4+ T cells in animals. The second demonstrated that the proliferative rate of CD8+ memory T cells was high in animals lacking CD4+CD25+ T cells, and decreased when these regulatory cells were present.
These last experiments were hard to perform, because of the difficulty in producing animals that lacked the CD4+CD25+ population but still contained enough T cells to prevent homeostatic proliferation of all T cells in the recipient mice. Evidence suggested that this was accomplished, but it is possible that some residual expansion due to lack of T cells occurred. If so, this might be the reason why the experiments did not always demonstrate effects of the CD4+CD25+ cells. Alternatively, these experiments may have led to variable results because proliferation and survival of CD8+ memory T cells may be inhibited in more than one way. For example, their proliferation may be affected by regulatory cells that lack CD25 and that have been described in some experiments (43). Also, IL-2 may act via other cells in addition to the CD4+ population that bears high-affinity receptors for this cytokine.
The mode of action of CD4+CD25+ regulatory T cells is not well understood. Various experiments have suggested that they act via antiinflammatory cytokines such as IL-10 or transforming growth factor (TGF)-β (37). Other experiments indicate that they act by affecting the function of antigen-presenting cells (44). Other experiments failed to demonstrate requirements for any of these effects, but still showed that the regulatory cells could inhibit production of IL-2 by other T cells via some unknown pathway (45). We do not know how the CD4+CD25+ T cells inhibit proliferation of CD8+ memory T cells. Preliminary experiments suggest that neither IL-10 nor TGF-β is involved.
The experiments described here revealed another phenomenon, the fact that the numbers of CD8+ memory T cells in mice depended on the presence of CD4+ T cells (Fig. 4). In the absence of CD4+ cells, fewer CD8+ memory cells could be recovered from mice. CD4+ T cells could contribute to CD8+ T cell survival in a number of ways. For example, CD4+ T cells are known to induce CD40 on dendritic cells, and CD40 acts positively on CD8+ cells; however, CD40 is not thought to be required for maintenance of CD8+ memory (46). Alternatively, CD4+ T cells may act to promote production of IL-15 by other cells in the absence of antigen, although there is currently no evidence for this idea. Whatever the cause of this phenomenon, it does appear that, paradoxically, CD4+ T cells stimulate survival and/or proliferation of CD8+ memory T cells while a subpopulation of CD4+ cells, those bearing CD25, simultaneously inhibit the same cells.
CD8+ memory T cells do divide to some extent in normal mice that contain some CD4+CD25+ cells and also some IL-2. Thus the effects of these regulatory cells in particular and IL-2 in general are not absolute. In vivo, a balance between the inhibitory effects of IL-2 and/or regulatory T cells and the stimulatory consequences of IL-15 control the total numbers of CD8+ memory T cells.
Acknowledgments
We thank Bill Townend and Shirley Sobus for their help with cell sorting and analysis and Ella Kushnir and Tibor Vass for help with breeding mice. This work was supported by U.S. Public Health Service Grants AI-17134, AI-18785, and AI-22295.
References
- 1.Manz R A, Lohning M, Cassese G, Thiel A, Radbruch A. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams M G, Ahmed R. Curr Opin Immunol. 1999;11:172–179. doi: 10.1016/s0952-7915(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J, Tough D F. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 4.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 5.Swain S L, Hu H, Huston G. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 6.Tough D F, Sun S, Zhang X, Sprent J. Immunol Rev. 1999;170:39–47. doi: 10.1111/j.1600-065x.1999.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 7.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 8.Lodolce J P, Burkett P R, Boone D L, Chien M, Ma A. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azimi N, Nagai M, Jacobson S, Waldmann T A. Proc Natl Acad Sci USA. 2001;98:14559–14564. doi: 10.1073/pnas.251540598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selin L K, Lin M Y, Kraemer K A, Pardoll D M, Schneck J P, Varga S M, Santolucito P A, Pinto A K, Welsh R M. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 11.McNally J M, Zarozinski C C, Lin M Y, Brehm M A, Chen H D, Welsh R M. J Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson D M, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, Giri J, Copeland N G, Gilbert D J, Jenkins N A, et al. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 14.Johnston J A, Bacon C M, Riedy M C, O'Shea J J. J Leukocyte Biol. 1996;60:441–452. doi: 10.1002/jlb.60.4.441. [DOI] [PubMed] [Google Scholar]
- 15.Kundig T M, Schorle H, Bachmann M F, Hengartner H, Zinkernagel R M, Horak I. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 16.Willerford D M, Chen J, Ferry J A, Davidson L, Ma A, Alt F W. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 17.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith H, Sakamoto Y, Kasai K, Tung K S. J Immunol. 1991;147:2928–2933. [PubMed] [Google Scholar]
- 20.Sakaguchi S, Toda M, Asano M, Itoh M, Morse S S, Sakaguchi N. J Autoimmun. 1996;9:211–220. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 21.Read S, Mauze S, Asseman C, Bean A, Coffman R, Powrie F. Eur J Immunol. 1998;28:3435–3447. doi: 10.1002/(SICI)1521-4141(199811)28:11<3435::AID-IMMU3435>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Suri-Payer E, Amar A Z, Thornton A M, Shevach E M. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 23.Papiernik M, de Moraes M L, Pontoux C, Vasseur F, Penit C. Int Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 24.Stephens L A, Mason D. J Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 25.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M, et al. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 26.Weston S A, Parish C R. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 27.Teague T K, Marrack P, Kappler J W, Vella A T. J Immunol. 1997;158:5791–5796. [PubMed] [Google Scholar]
- 28.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 29.Lenardo M J. Nature (London) 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 30.Hanabuchi S, Koyanagi M, Kawasaki A, Shinohara N, Matsuzawa A, Nishimura Y, Kobayashi Y, Yonehara S, Yagita H, Okumura K. Proc Natl Acad Sci USA. 1994;91:4930–4934. doi: 10.1073/pnas.91.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anel A, Buferne M, Boyer C, Schmitt-Verhulst A M, Golstein P. Eur J Immunol. 1994;24:2469–2476. doi: 10.1002/eji.1830241032. [DOI] [PubMed] [Google Scholar]
- 32.Rocha B, Dautigny N, Pereira P. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 33.Ernst B, Lee D S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 34.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldrath A W, Bogatzki L Y, Bevan M J. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa T C, Cumano A, Bandeira A. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 38.Wolf M, Schimpl A, Hunig T. Eur J Immunol. 2001;31:1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 39.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanchot C, Lemonnier F A, Perarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 41.Tough D F, Zhang X, Sprent J. J Immunol. 2001;166:6007–6011. doi: 10.4049/jimmunol.166.10.6007. [DOI] [PubMed] [Google Scholar]
- 42.Kronin V, Vremec D, Shortman K. Int Immunol. 1998;10:237–240. doi: 10.1093/intimm/10.2.237. [DOI] [PubMed] [Google Scholar]
- 43.de Camargo Furtado G, Olivares-Villagomez D, Curotto De Lafaille M A, Wensky A K, Latkowski J A, Lafaille J J. Immunol Rev. 2001;182:122–134. doi: 10.1034/j.1600-065x.2001.1820110.x. [DOI] [PubMed] [Google Scholar]
- 44.Chang C C, Ciubotariu R, Manavalan J S, Yuan J, Colovai A I, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 45.Thornton A M, Shevach E M. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 46.Borrow P, Tough D F, Eto D, Tishon A, Grewal I S, Sprent J, Flavell R A, Oldstone M B. J Virol. 1998;72:7440–7449. doi: 10.1128/jvi.72.9.7440-7449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]