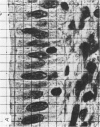
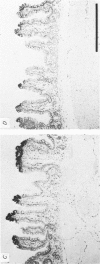
Abstract
1. An autoradiographic technique is described whereby the cellular location of tritiated amino acids can be determined following uptake by rabbit ileal mucosa. 2. Stirring solutions in contact with the intestinal mucosa during measurement of rapid influx changes the quantity, but not the distribution, of alanine taken up by the tissue. 3. Conditions predicted to favour either a high affinity system (Ly1) or a low affinity system (Ly2) were used to measure lysine distribution following uptake. Maximal uptake for both transport systems occurred in fully differentiated enterocytes at the tips of villi. Initial maturation of the Ly1 system, which was slow, was followed by a rapid phase of development. The Ly2 system lacked this rapid phase of late development. 4. The cellular distribution of alanine entering on a low affinity Na-independent neutral amino acid carrier closely resembles that determine for Ly1 system for lysine entry. 5. Arginine is a potent inhibitor of lysine uptake through the Ly2 system. Little or no diffusion of lysine appears to take place into rabbit ileal enterocytes. 6. The different distribution of the high and low affinity systems for lysine transport provides further support for their independent existence. It also suggests that more than one message exists fo the switching on of amino acid transport function in differentiating enterocytes.
Full text
PDF


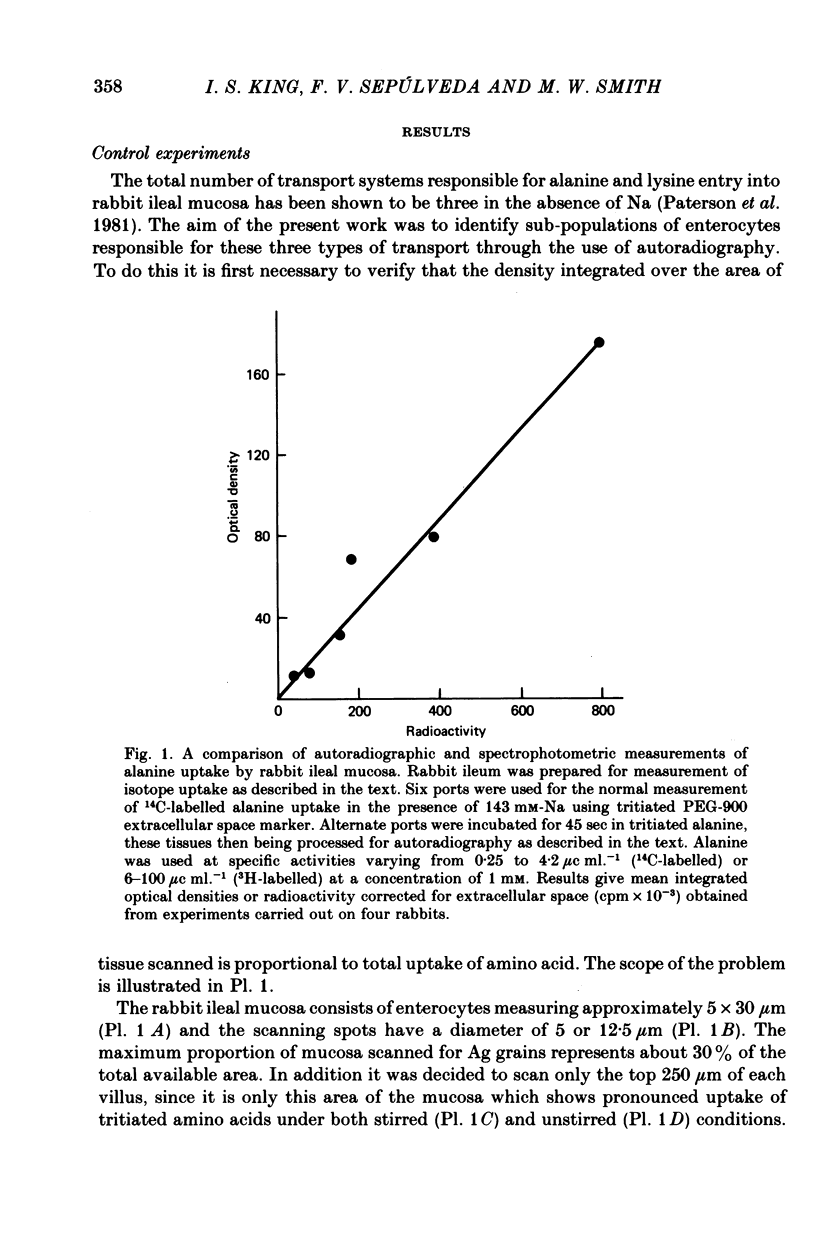
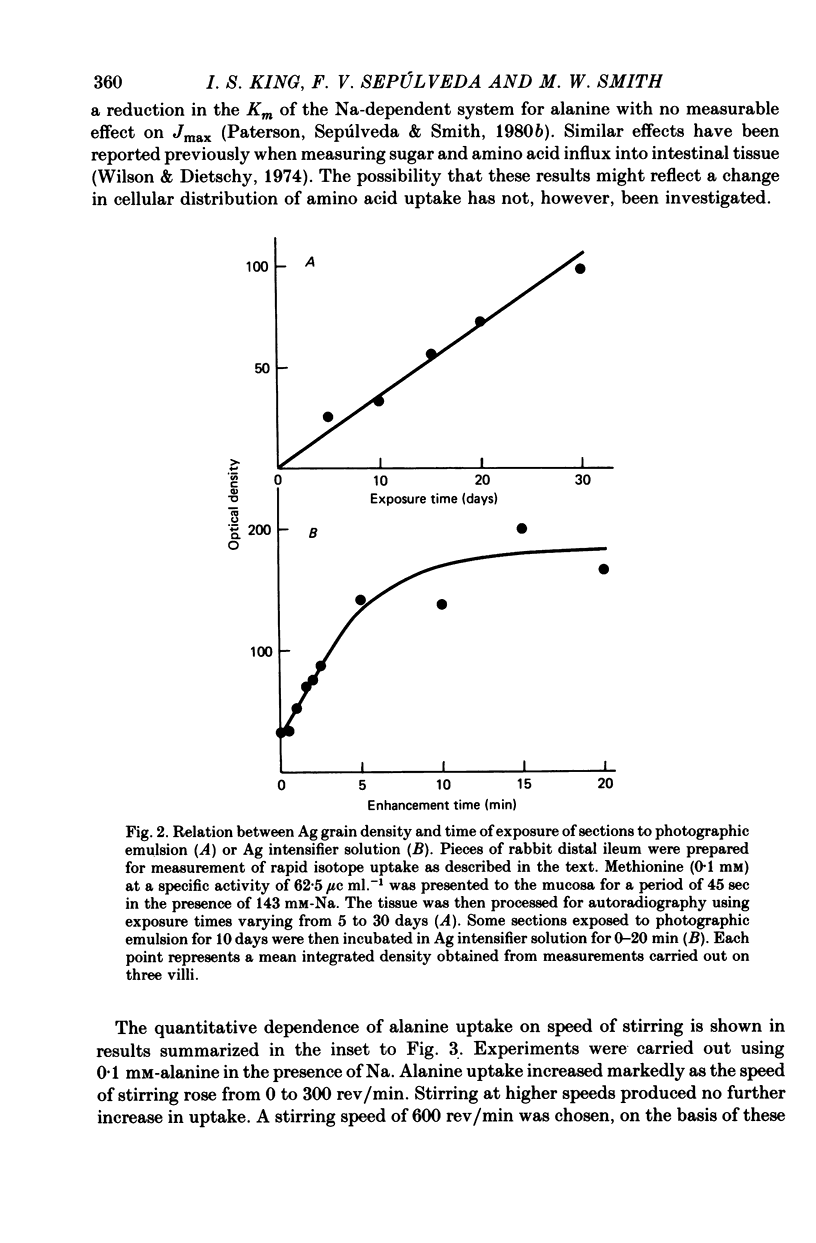
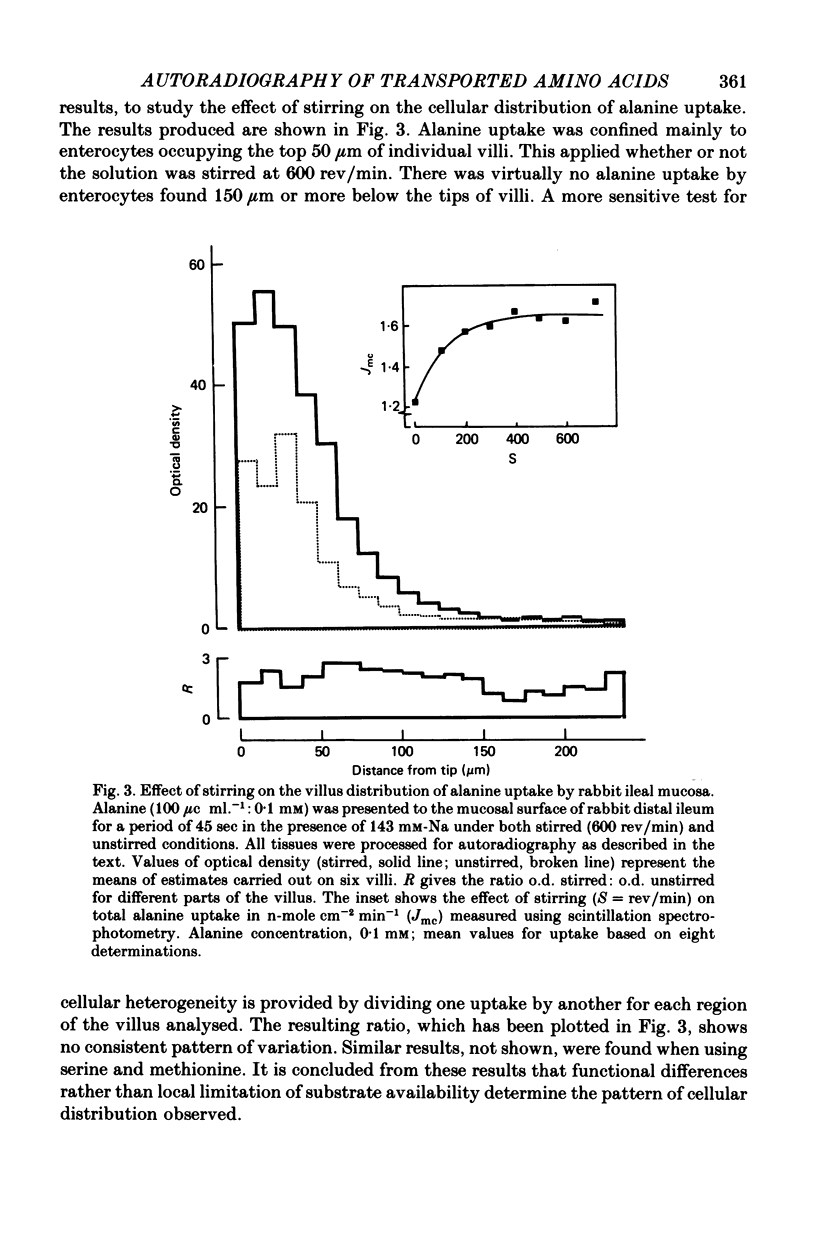

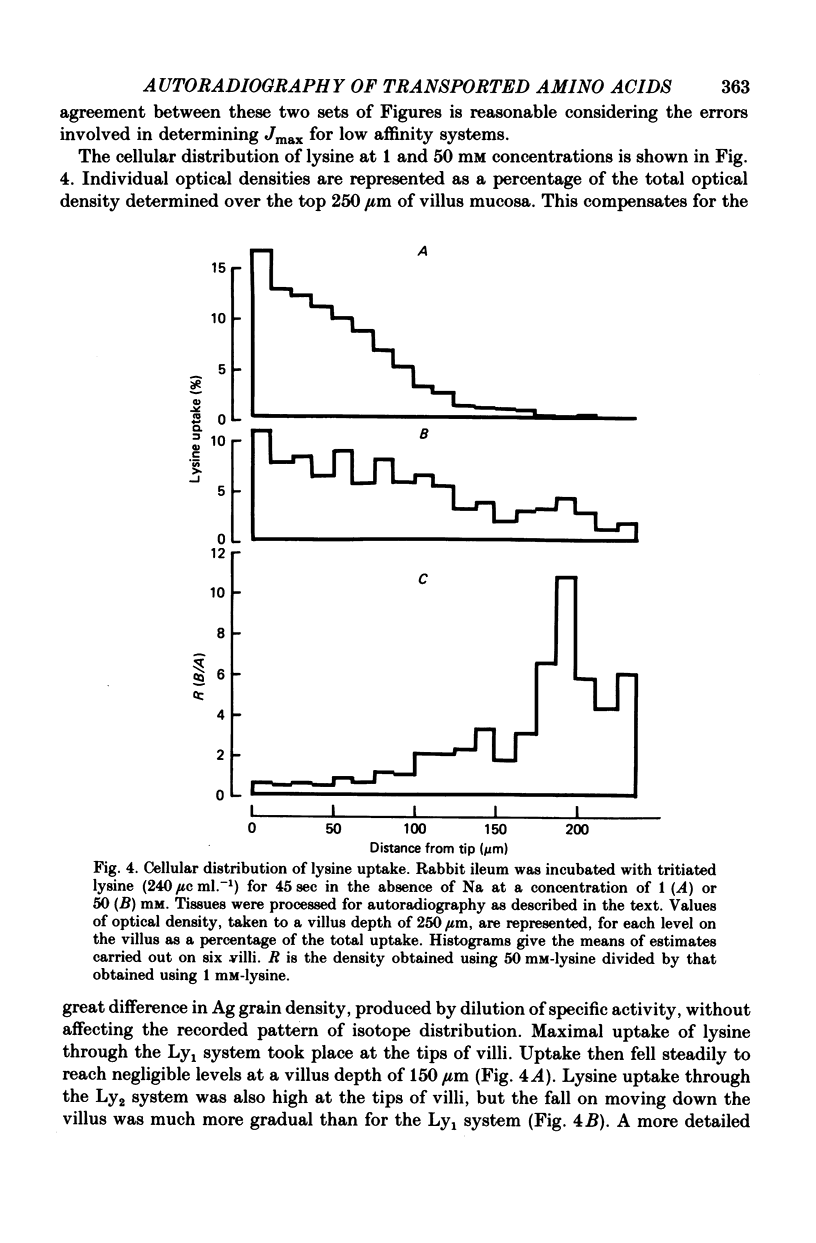
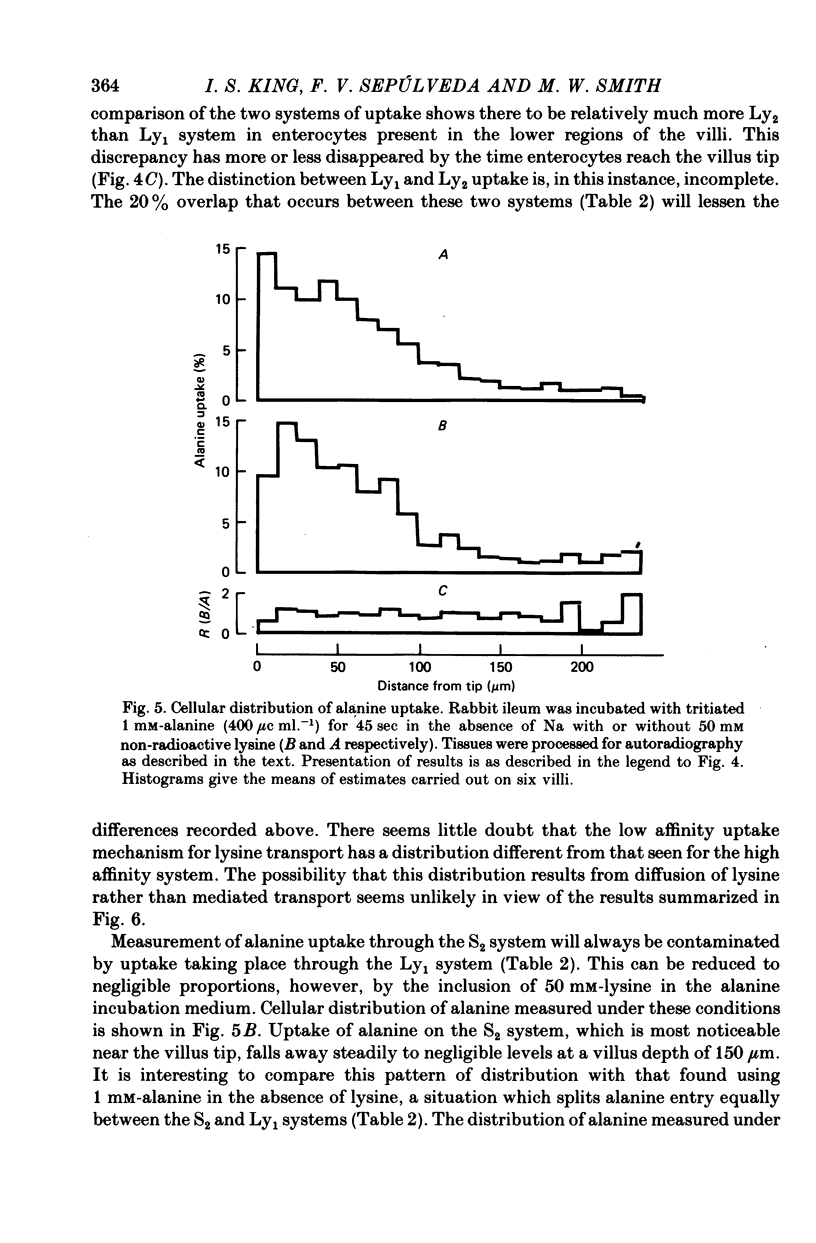
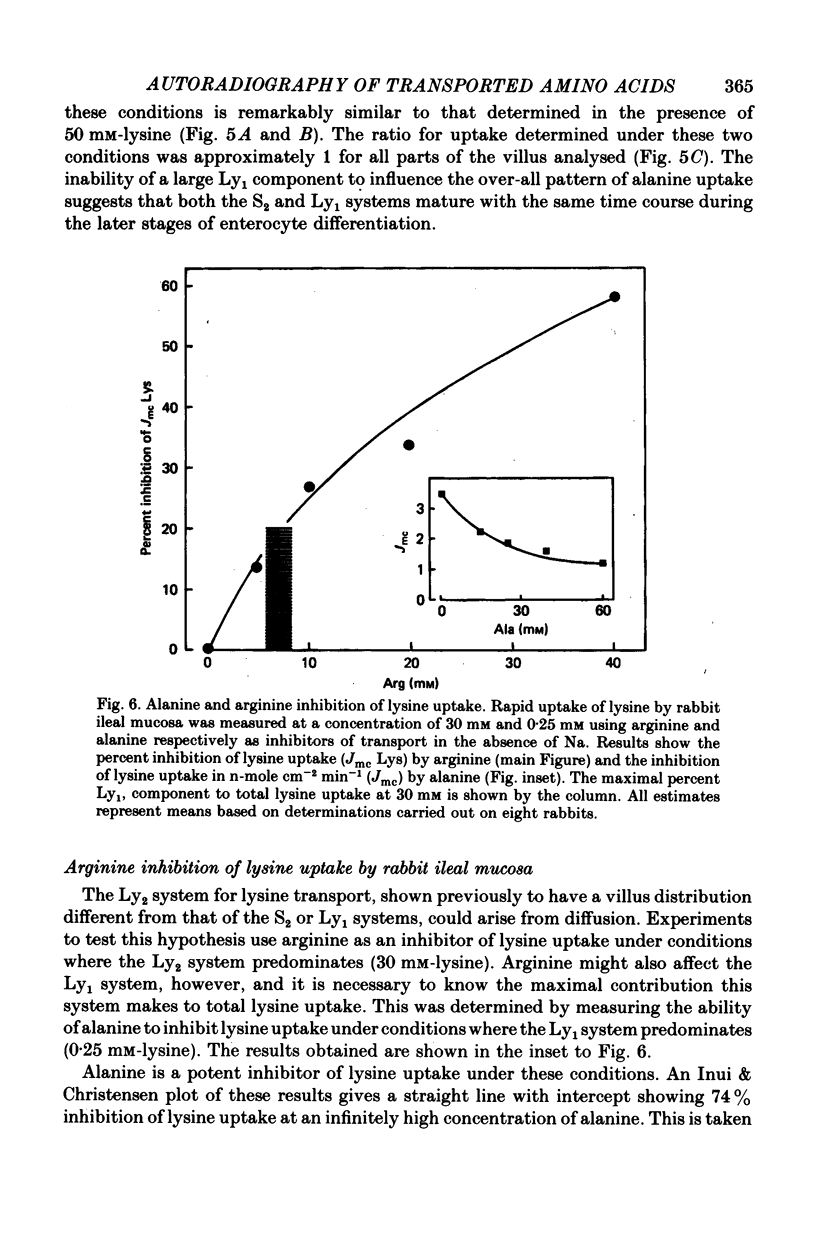



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curran P. F., Schultz S. G., Chez R. A., Fuisz R. E. Kinetic relations of the Na-amino acid interaction at the mucosal border of intestine. J Gen Physiol. 1967 May;50(5):1261–1286. doi: 10.1085/jgp.50.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. The development of spatial-frequency selectivity in kitten striate cortex. J Physiol. 1981 Jul;316:1–10. doi: 10.1113/jphysiol.1981.sp013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D., Knibiehler M., Benoit V., Sémériva M. Plasma membranes from rat intestinal epithelial cells at different stages of maturation. I. Preparation and characterization of plasma membrane subfractions originating from crypt cells and from villous cells. Biochim Biophys Acta. 1978 Oct 4;512(3):508–524. doi: 10.1016/0005-2736(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Inui Y., Christensen H. N. Discrimination of single transport systems. The Na plus-sensitive transport of neutral amino acids in the Ehrlich cell. J Gen Physiol. 1966 Sep;50(1):203–224. doi: 10.1085/jgp.50.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter W. B., Wilson T. H. AUTORADIOGRAPHIC STUDY OF SUGAR AND AMINO ACID ABSORPTION BY EVERTED SACS OF HAMSTER INTESTINE. J Cell Biol. 1965 May 1;25(2):19–39. doi: 10.1083/jcb.25.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Trier J. S., Neutra M. R. Structural changes in the plasma membrane accompanying differentiation of epithelial cells in human and monkey small intestine. Gastroenterology. 1980 May;78(5 Pt 1):963–975. [PubMed] [Google Scholar]
- Mircheff A. K., van Os C. H., Wright E. M. Pathways for alanine transport in intestinal basal lateral membrane vesicles. J Membr Biol. 1980 Jan 31;52(1):83–92. doi: 10.1007/BF01869009. [DOI] [PubMed] [Google Scholar]
- Morrison A., Porteous J. W. Changes in the synthesis of ribosomal ribonucleic acid and of poly(A)-containing ribonucleic acid during the differentiation of intestinal epithelial cells in the rat and in the chick. Biochem J. 1980 Jun 15;188(3):609–618. doi: 10.1042/bj1880609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck B. G., Schultz S. G. Lysine transport across isolated rabbit ileum. J Gen Physiol. 1969 Feb;53(2):157–182. doi: 10.1085/jgp.53.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd L. B. The preparation of thin sections from glycol methacrylate embedded tissue using a standard rotary microtome. Med Lab Sci. 1976 Jan;33(1):67–71. [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. A sodium-indpendent low affinity transport system for neutral amino acids in rabbit ileal mucosa. J Physiol. 1980 Jan;298:333–346. doi: 10.1113/jphysiol.1980.sp013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. Distinguishing transport systems having overlapping specificities for neutral and basic amino acids in the rabbit ileum. J Physiol. 1981;319:345–354. doi: 10.1113/jphysiol.1981.sp013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. Two-carrier influx of neutral amino acids into rabbit ileal mucosa. J Physiol. 1979 Jul;292:339–350. doi: 10.1113/jphysiol.1979.sp012854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisam M., Ripoche P. Redistribution of surface macromolecules in dissociated epithelial cells. J Cell Biol. 1976 Dec;71(3):907–920. doi: 10.1083/jcb.71.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda F. V., Smith M. W. Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J Physiol. 1978 Sep;282:73–90. doi: 10.1113/jphysiol.1978.sp012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice L. W., Carter R. L., Cahill M. B. Changes in tight junctions of rat intestinal crypt cells associated with changes in their mitotic activity. Tissue Cell. 1979;11(2):293–316. doi: 10.1016/0040-8166(79)90043-0. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. The intestinal unstirred layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta. 1974 Aug 21;363(1):112–126. doi: 10.1016/0005-2736(74)90010-8. [DOI] [PubMed] [Google Scholar]