Abstract
Neuro-effector transmission in the smooth muscle layer of the dog trachea was studied in vitro using the micro-electrode and double sucrose gap methods.
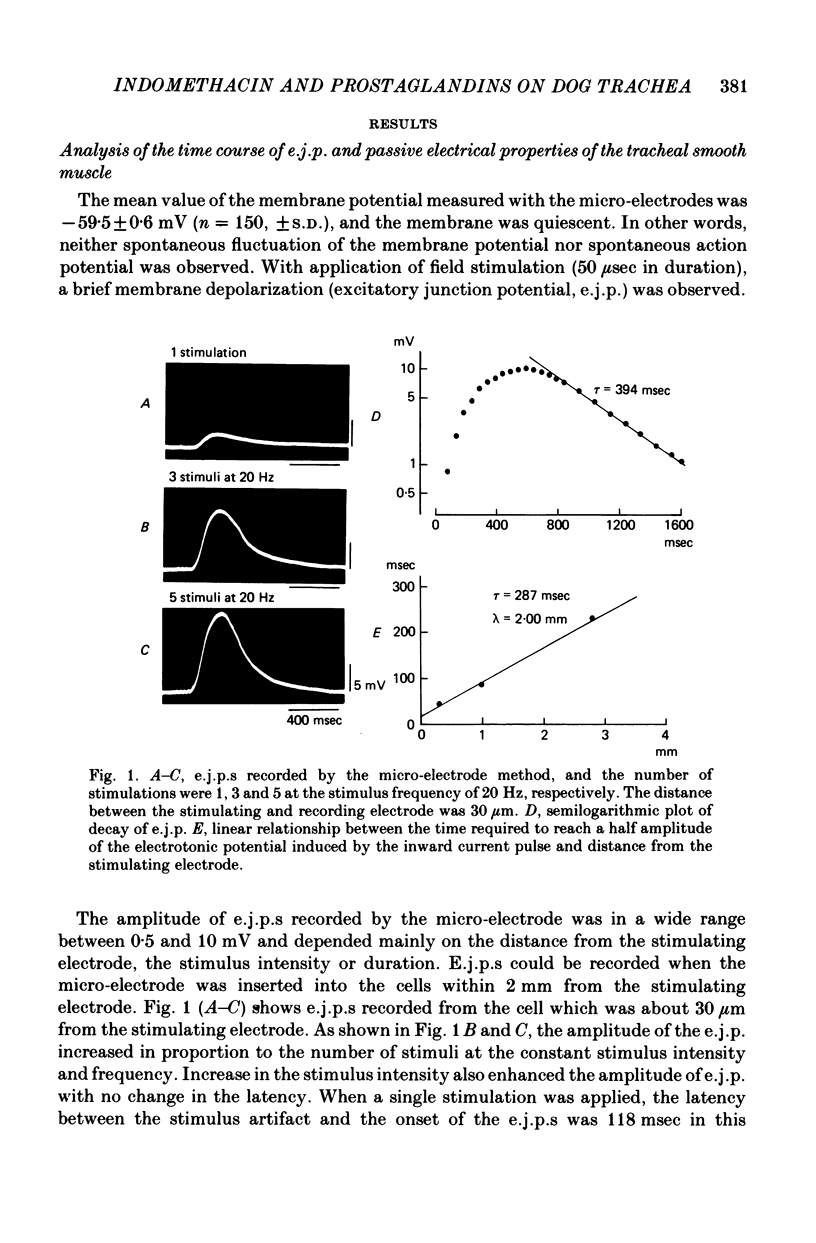
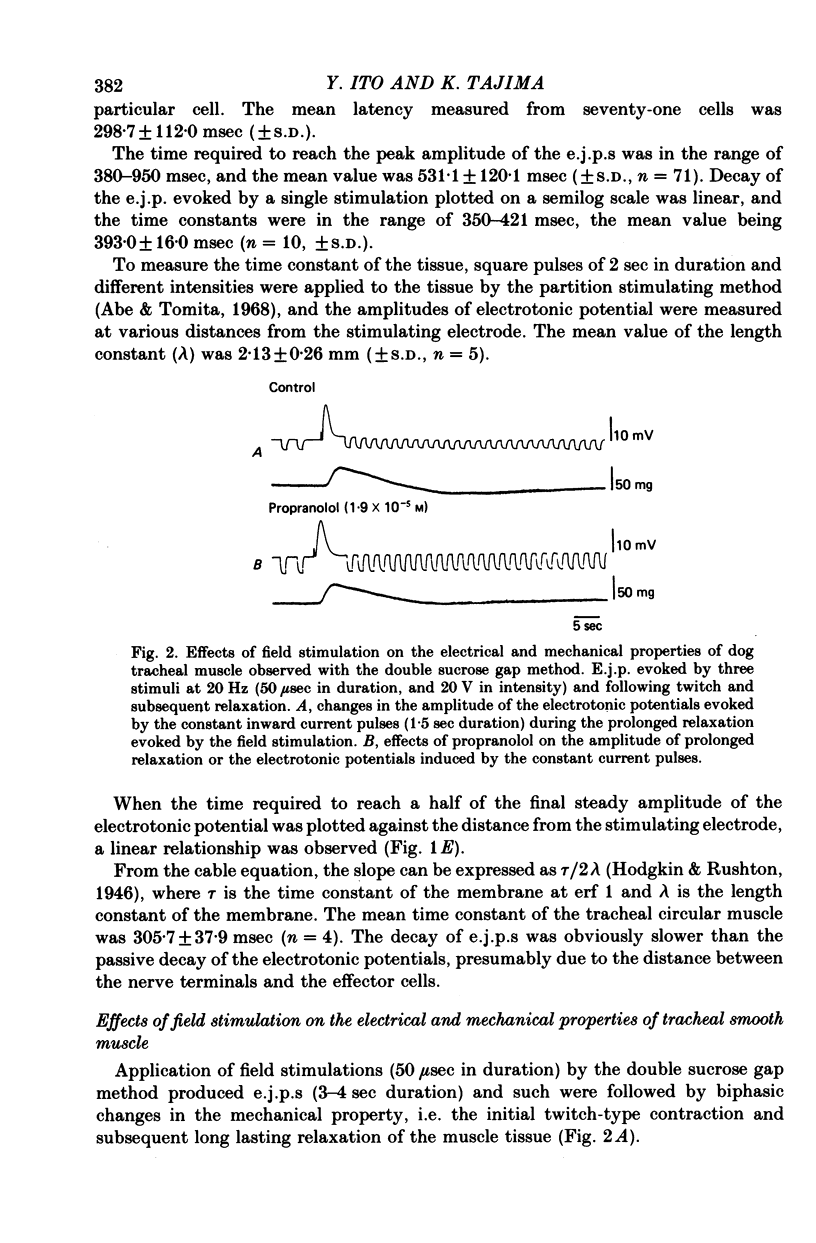
1. Electrical field stimulations with short duration (50-100 μsec) applied to the whole tissue produced an excitation of the intrinsic nerves, and evoked excitatory junction potentials (e.j.p.s) followed by twitch tension development and subsequent long lasting relaxation of the smooth muscle tissue.
2. The effects of field stimulations were abolished by tetrodotoxin (2 × 10-7 m), and atropine (1·7 × 10-5 m) selectively blocked both the e.j.p. and twitch tension. On the other hand, propranolol (1·9 × 10-5 m) suppressed the generation of the prolonged relaxation evoked by the field stimulations.
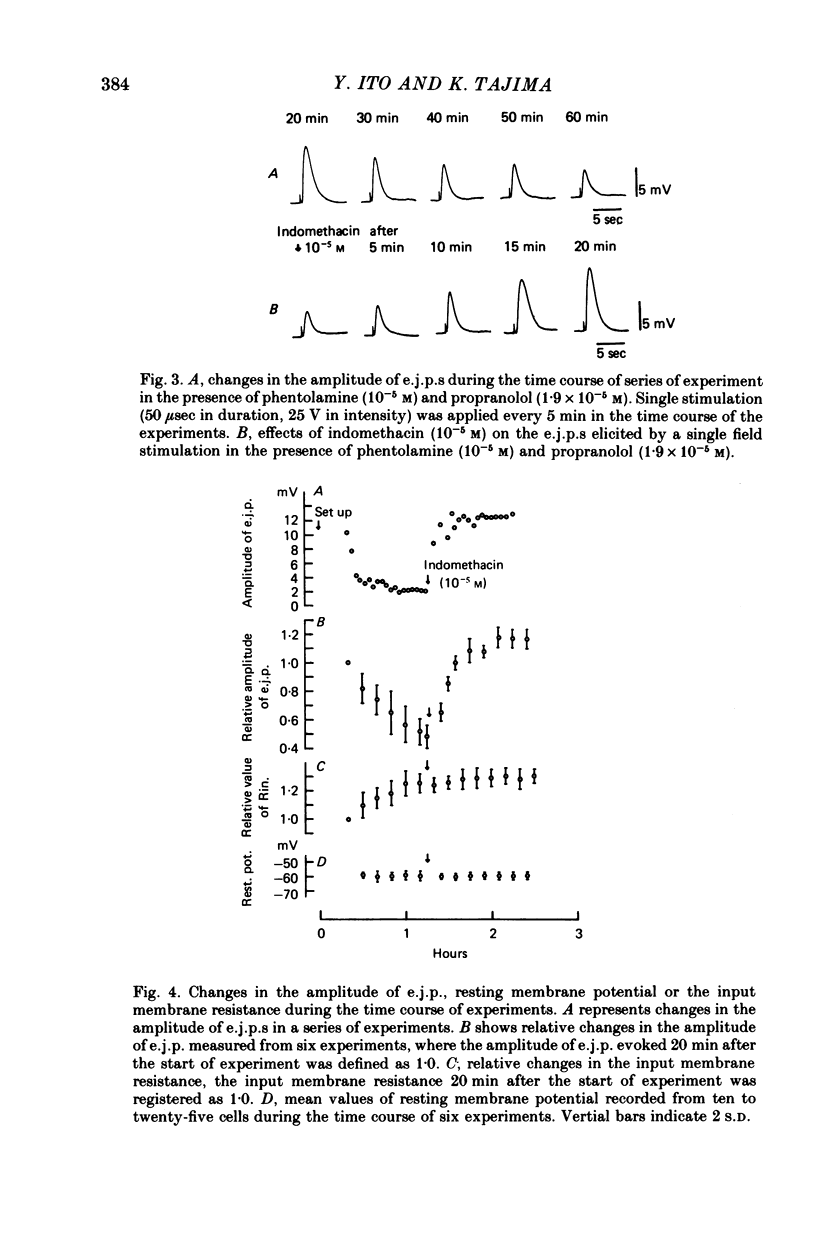
3. E.j.p.s recorded by the double sucrose gap method showed gradual and continuous reduction in amplitude during prolonged exposure in Krebs solution (1-2 hr), and there were no changes in the membrane potential or in the input membrane resistance.
4. With application of indomethacin (10-5 m), a gradual and continuous reduction in the amplitude of e.j.p. was no longer observed, and (after the initial increase in the amplitude) e.j.p.s with a constant amplitude were obtained during 1-1·5 hr. Indomethacin (10-5 m) modified neither the resting membrane potential nor the input membrane resistance of smooth muscle cells.
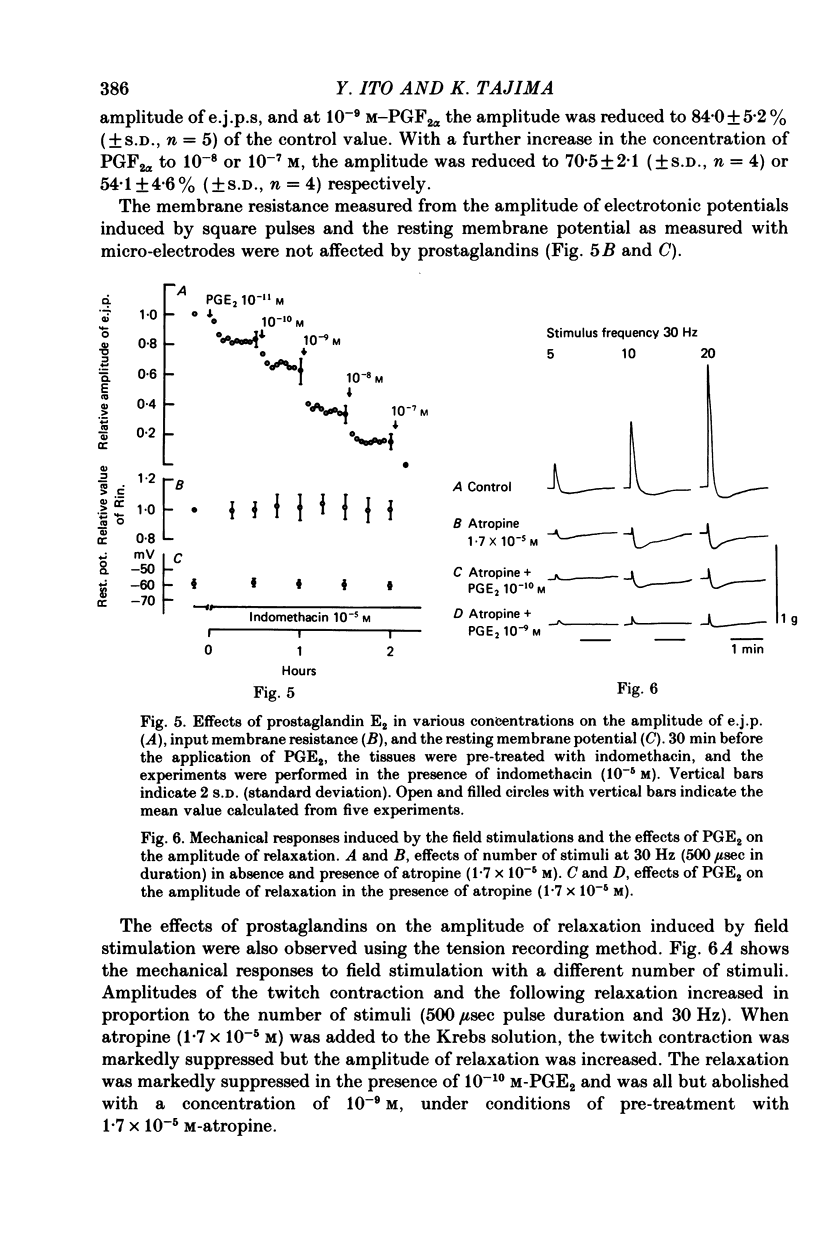
5. After pre-treatment with indomethacin, low concentrations (10-11-10-8 m) of prostaglandin E1 or E2 (PGE series) markedly suppressed the amplitude of e.j.p. with no changes in the resting membrane potential or in the input membrane resistance.
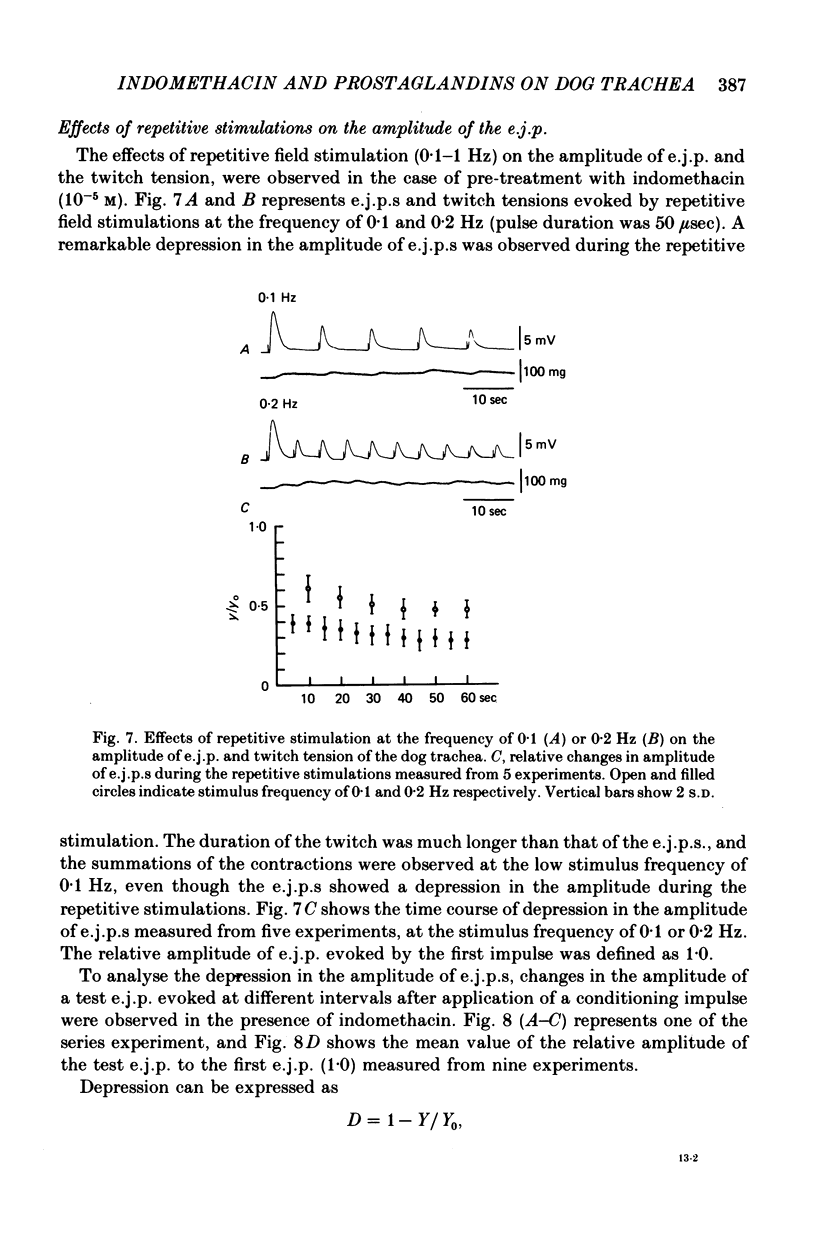
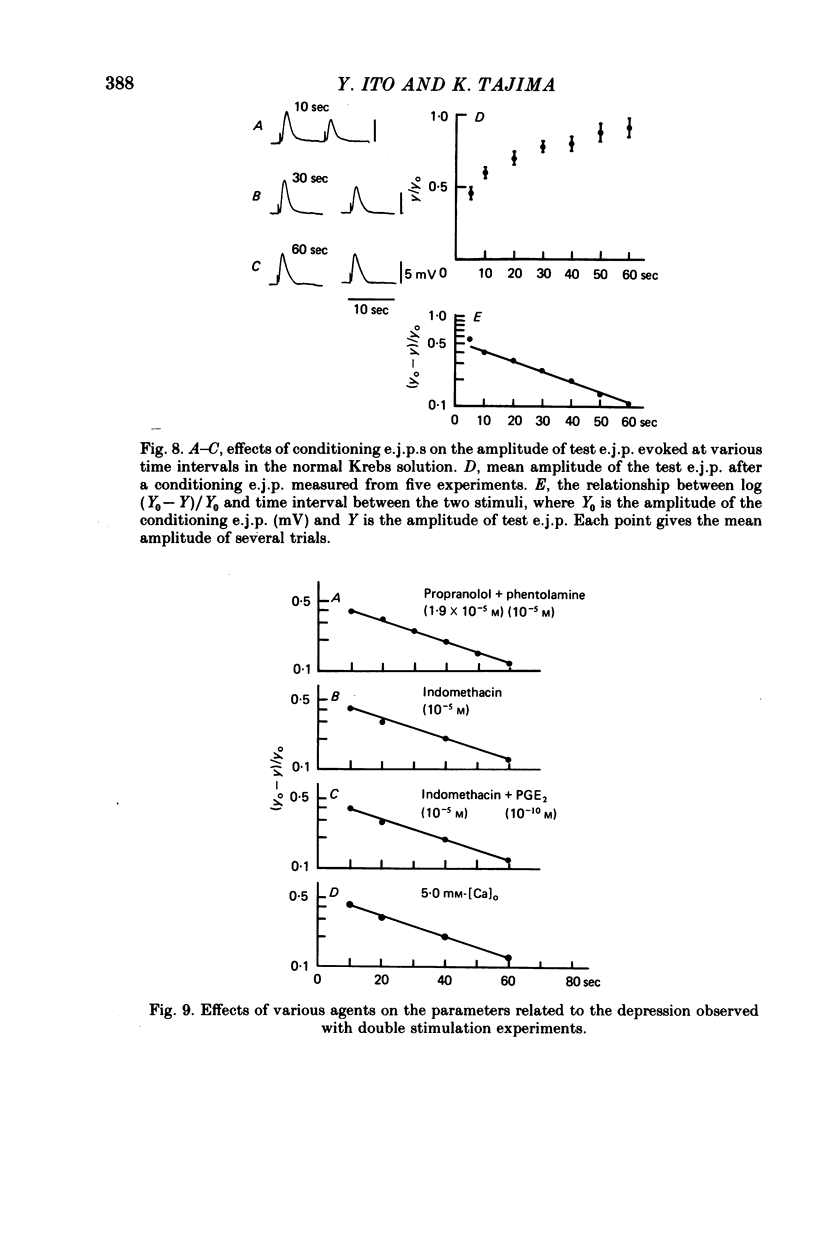
6. During the repetitive field stimulation at the stimulus frequency of 0·1-1 Hz, the amplitude of the e.j.p.s was gradually reduced (the depression process). The depression was not affected by applications of prostaglandins, indomethacin or α- and β-adrenoceptor blockers.
7. These results indicate that in the dog tracheal smooth muscles, the endogenous PGE series may play an important role in feed-back inhibitory mechanisms, at the nerve terminals related to acetylcholine release.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGGARD E., BERGSTROM S. Biological effects of an unsaturated trihydroxy acid (PGF2alfa) from normal swine lung. Acta Physiol Scand. 1963 May;58:1–12. doi: 10.1111/j.1748-1716.1963.tb02622.x. [DOI] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggård E. The isolation and determination of prostaglandins in lungs of sheep, guinea pig, monkey and man. Biochem Pharmacol. 1965 Nov;14(11):1507–1516. doi: 10.1016/0006-2952(65)90004-3. [DOI] [PubMed] [Google Scholar]
- Cameron A. R., Kirkpatrick C. T. A study of excitatory neuromuscular transmission in the bovine trachea. J Physiol. 1977 Sep;270(3):733–745. doi: 10.1113/jphysiol.1977.sp011979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973 May;224(5):1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Levy G. P. A non-adrenergic inhibitory nervous pathway in guinea-pig trachea. Br J Pharmacol. 1974 Oct;52(2):167–174. doi: 10.1111/j.1476-5381.1974.tb09697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- HORTON E. W., MAIN I. H. A COMPARISON OF THE ACTIONS OF PROSTAGLANDINS F2-ALPHA AND E1 ON SMOOTH MUSCLE. Br J Pharmacol Chemother. 1965 Apr;24:470–476. doi: 10.1111/j.1476-5381.1965.tb01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Kuriyama H. Effects of catecholamines on the cholinergic neuromuscular transmission in fish red muscle. J Physiol. 1969 Mar;201(1):61–71. doi: 10.1113/jphysiol.1969.sp008742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. On the roles of calcium ion during potassium induced contracture in the smooth muscle cells of the rabbit main pulmonary artery. Jpn J Physiol. 1977;27(6):755–770. doi: 10.2170/jjphysiol.27.755. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Action of morphine on the neuro-effector transmission in the guinea-pig ileum and in the mouse vas deferens. J Physiol. 1980 Oct;307:367–383. doi: 10.1113/jphysiol.1980.sp013440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tajima K. An electrophysiological analysis of the actions of prostaglandin on neuromuscular transmission in the guinea-pig vas deferens. J Physiol. 1979 Dec;297(0):521–537. doi: 10.1113/jphysiol.1979.sp013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Some effects produced by adrenaline upon neuromuscular propagation in rats. J Physiol. 1958 Apr 30;141(2):291–304. doi: 10.1113/jphysiol.1958.sp005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. EFFECT OF CALCIUM AND MAGNESIUM ON NEUROMUSCULAR TRANSMISSION IN THE HYPOGASTRIC NERVE-VAS DEFERENS PREPARATION OF THE GUINEA-PIG. J Physiol. 1964 Dec;175:211–230. doi: 10.1113/jphysiol.1964.sp007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S. M., Hillier K., Devlin J. Distribution of prostaglandins E1,E2, F1-alpha and F2-alpha in some animal tissues. J Pharm Pharmacol. 1968 Oct;20(10):749–753. doi: 10.1111/j.2042-7158.1968.tb09633.x. [DOI] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Tomita T. Noradrenaline action on nerve terminal in the rat diaphragm. J Physiol. 1971 Aug;217(1):19–31. doi: 10.1113/jphysiol.1971.sp009557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIN I. H. THE INHIBITORY ACTIONS OF PROSTAGLANDINS ON RESPIRATORY SMOOTH MUSCLE. Br J Pharmacol Chemother. 1964 Jun;22:511–519. doi: 10.1111/j.1476-5381.1964.tb01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathé A. A., Strandberg K., Aström A. Blockade by polyphloretin phosphate of the prostaglandin F2a action on isolated human bronchi. Nat New Biol. 1971 Apr 14;230(15):215–216. doi: 10.1038/newbio230215a0. [DOI] [PubMed] [Google Scholar]
- Mathé A. A., Yen S. S., Sohn R., Hedqvist P. Release of prostaglandins and histamine from sensitized and anaphylactic guinea pig lungs--changes in cyclic AMP levels. Biochem Pharmacol. 1977 Feb 1;26(3):181–188. doi: 10.1016/0006-2952(77)90300-8. [DOI] [PubMed] [Google Scholar]
- Purves R. D. Function of muscarinic and nicotinic acetylcholine receptors. Nature. 1976 May 13;261(5556):149–151. doi: 10.1038/261149a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. B., Bouchard T. Demonstration of a nonadrenergic inhibitory nervous system in the trachea of the guinea pig. J Allergy Clin Immunol. 1975 Dec;56(6):473–480. doi: 10.1016/0091-6749(75)90065-2. [DOI] [PubMed] [Google Scholar]
- Richardson J. B., Ferguson C. C. Neuromuscular structure and function in the airways. Fed Proc. 1979 Feb;38(2):202–208. [PubMed] [Google Scholar]
- Richardson J., Béland J. Nonadrenergic inhibitory nervous system in human airways. J Appl Physiol. 1976 Nov;41(5 Pt 1):764–771. doi: 10.1152/jappl.1976.41.5.764. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Morita K., Kuriyama H. Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol. 1976;26(3):303–320. doi: 10.2170/jjphysiol.26.303. [DOI] [PubMed] [Google Scholar]
- Sweatman W. J., Collier H. O. Effects of prostaglandins on human bronchial muscle. Nature. 1968 Jan 6;217(5123):69–69. doi: 10.1038/217069a0. [DOI] [PubMed] [Google Scholar]
- Vane J. R. The mode of action of aspirin and similar compounds. J Allergy Clin Immunol. 1976 Dec;58(6):691–712. doi: 10.1016/0091-6749(76)90181-0. [DOI] [PubMed] [Google Scholar]
- Vermeire P. A., Vanhoutte P. M. Inhibitory effects of catecholamine in isolated canine bronchial smooth muscle. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):787–791. doi: 10.1152/jappl.1979.46.4.787. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Regulation of tracheobronchial smooth muscle. Physiol Rev. 1963 Jan;43:1–37. doi: 10.1152/physrev.1963.43.1.1. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Mathé A. A., Dugan J. J. Release of prostaglandins from healthy and sensitized guinea-pig lung and trachea by histamine. Prostaglandins. 1976 Feb;11(2):227–239. doi: 10.1016/0090-6980(76)90146-5. [DOI] [PubMed] [Google Scholar]



