Abstract
1. Sucrose and choline were utilized as NaCl substitutes in order to investigate Na—Ca interactions in the smooth muscle of the guinea-pig taenia coli.
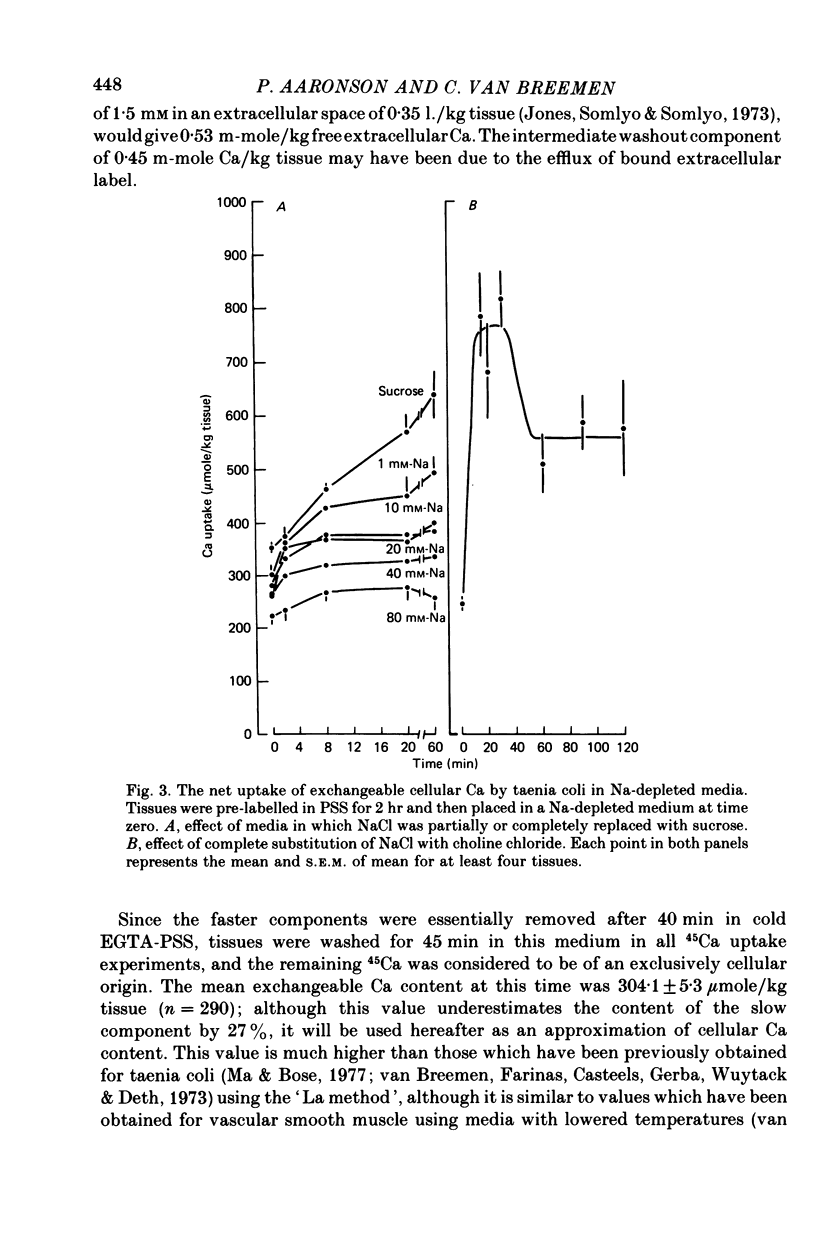
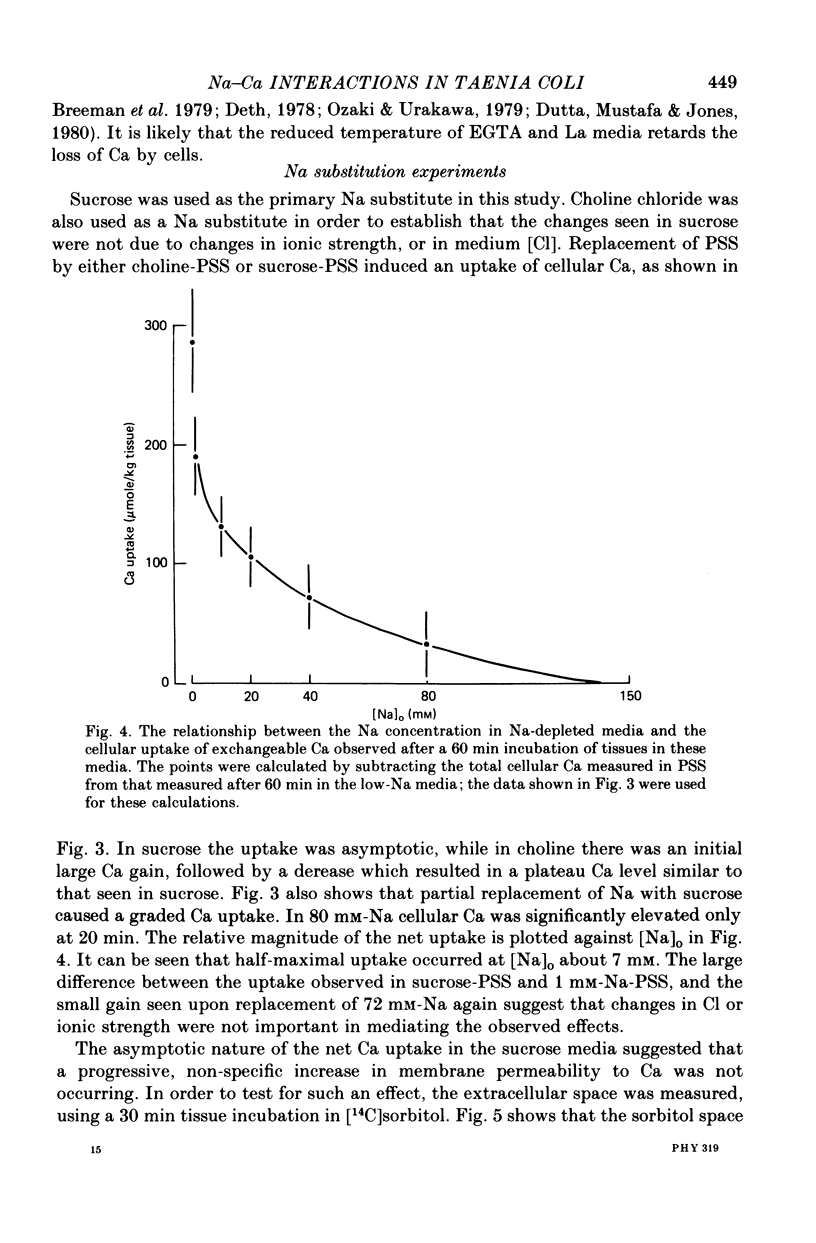
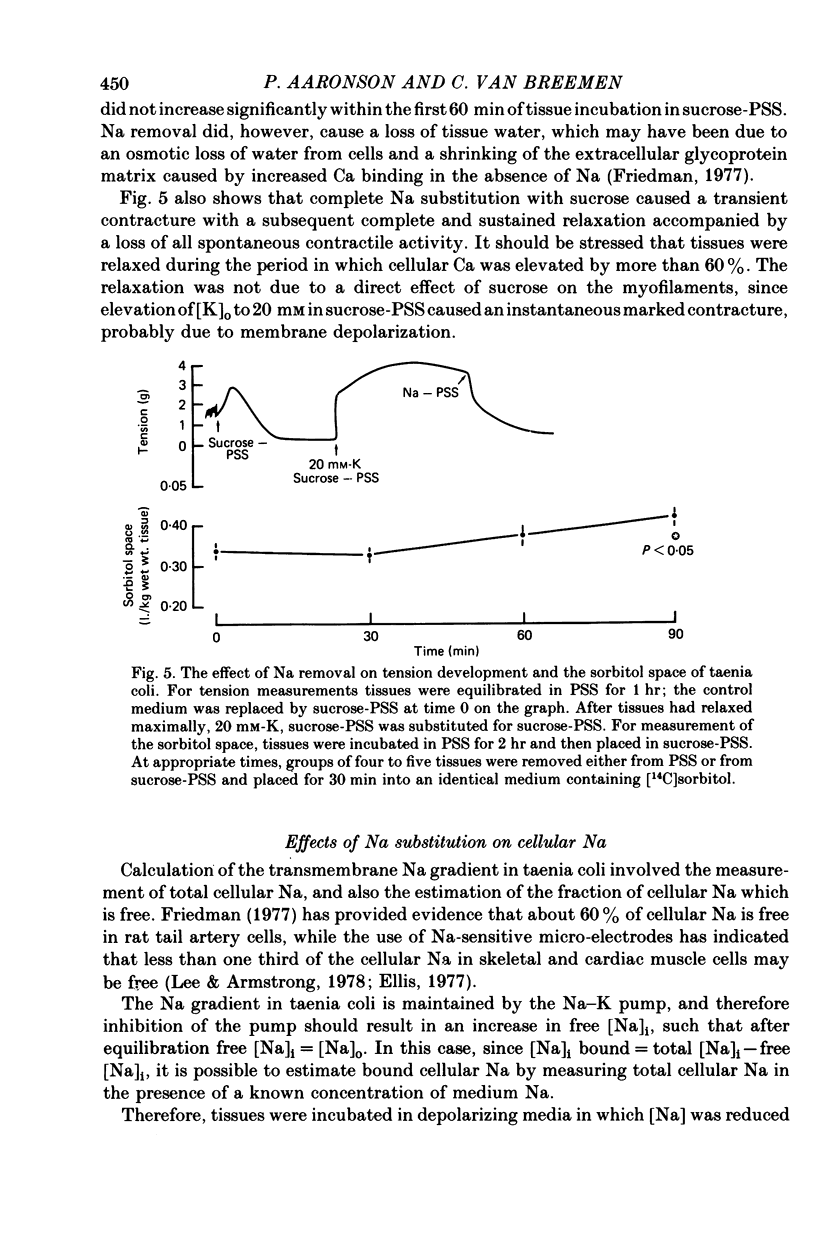
2. Progressive substitution of NaCl by sucrose caused a progressive increase in cellular exchangeable Ca. This uptake, which amounted to about 300 μmole Ca/kg tissue upon total Na replacement, reached a plateau within 20 min. Complete substitution of NaCl by choline chloride caused cellular Ca to increase rapidly to an initial peak, and then decrease to a stable plateau which was also about 300 μmole/kg above control.
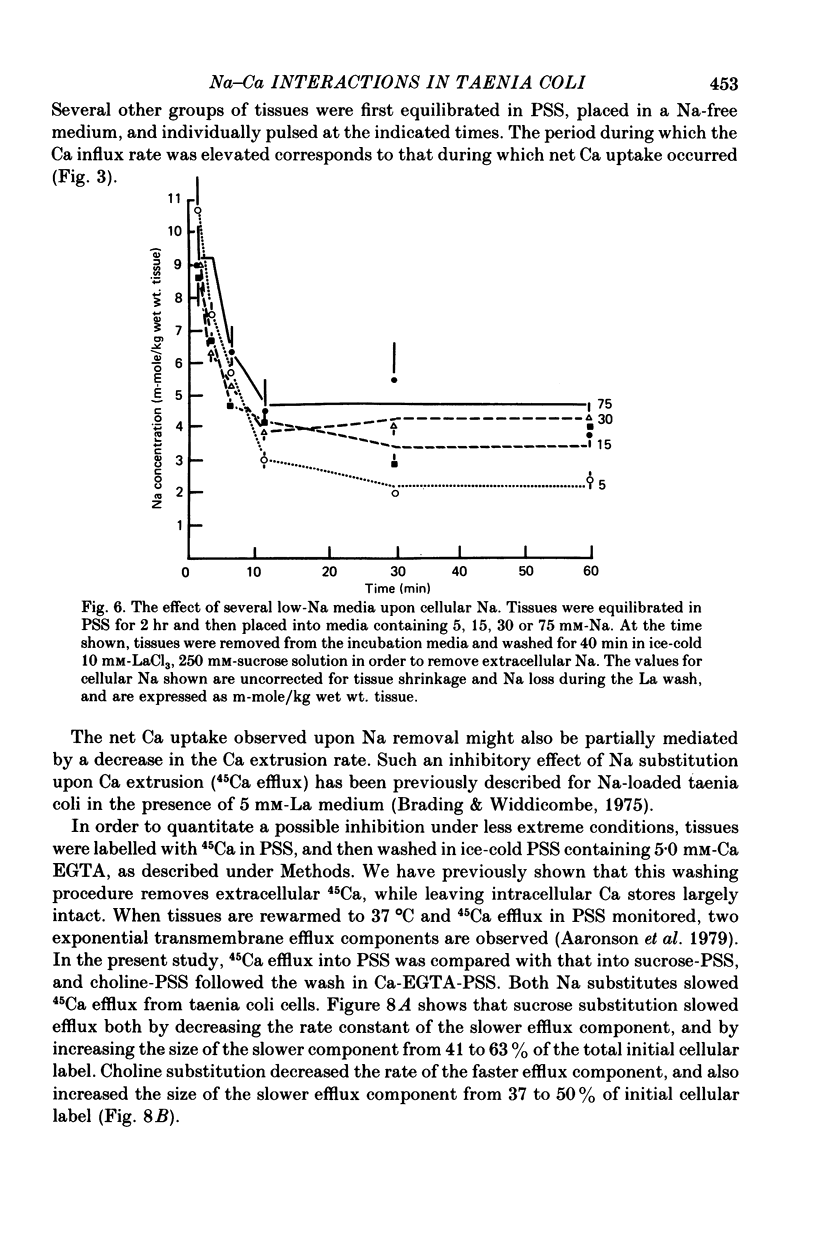
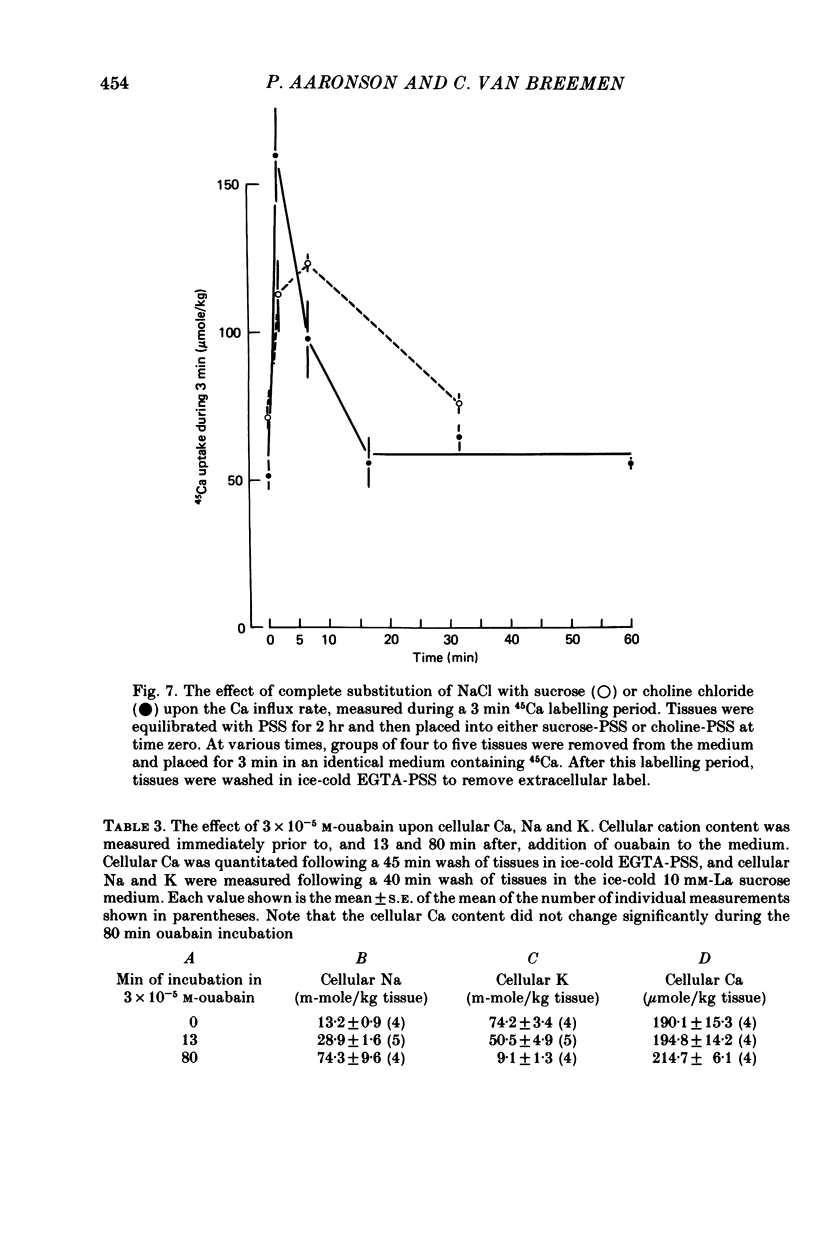
3. Replacement of NaCl by either sucrose or choline chloride caused a transient increase in the Ca influx rate, which was measured using a 3 min pulse labelling with 45Ca. This increase was more pronounced in choline chloride.
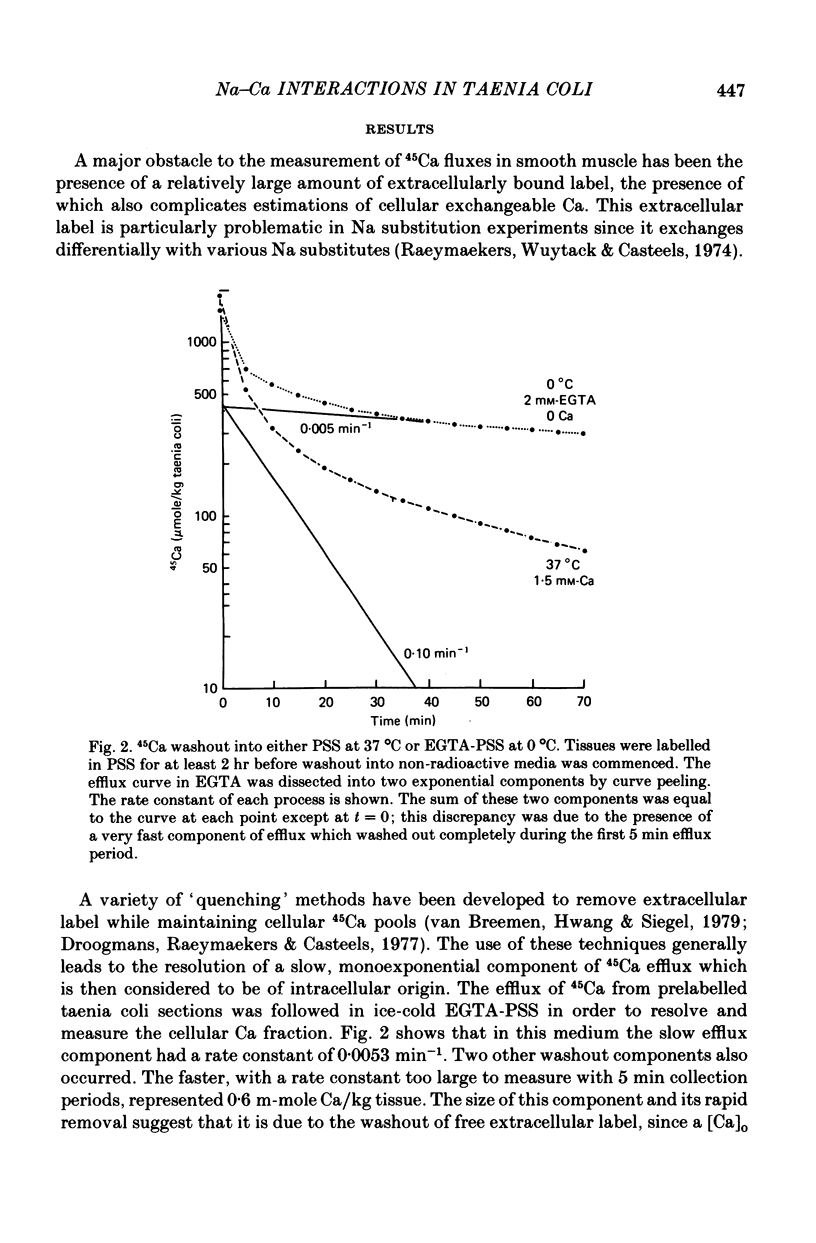
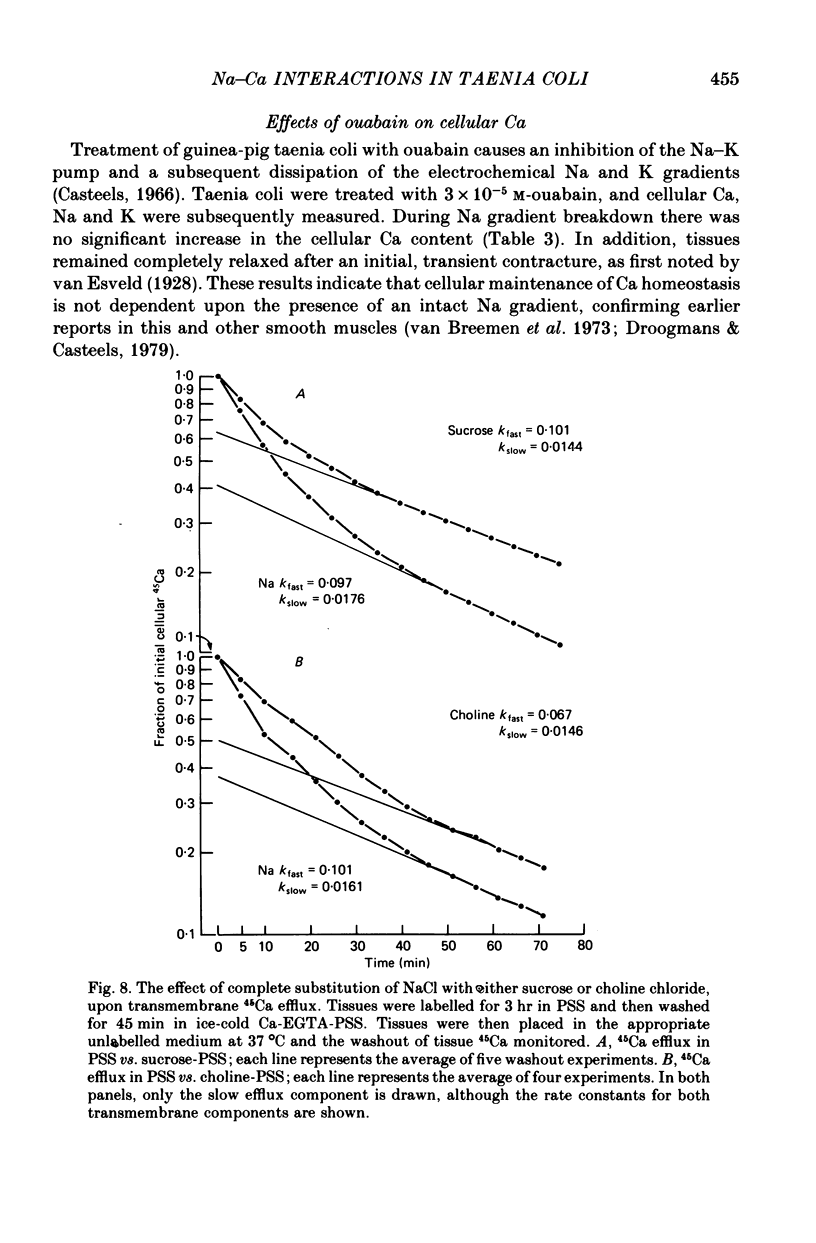
4. NaCl substitution by either sucrose or choline chloride caused a decrease in the 45Ca efflux rate. Two exponential components of transmembrane 45Ca efflux were found in control and Na-free media.
5. Treatment of tissues with 3 × 10-5 m-ouabain did not significantly affect the cellular Ca content after 80 min, at which time the Na and K gradients were largely dissipated.
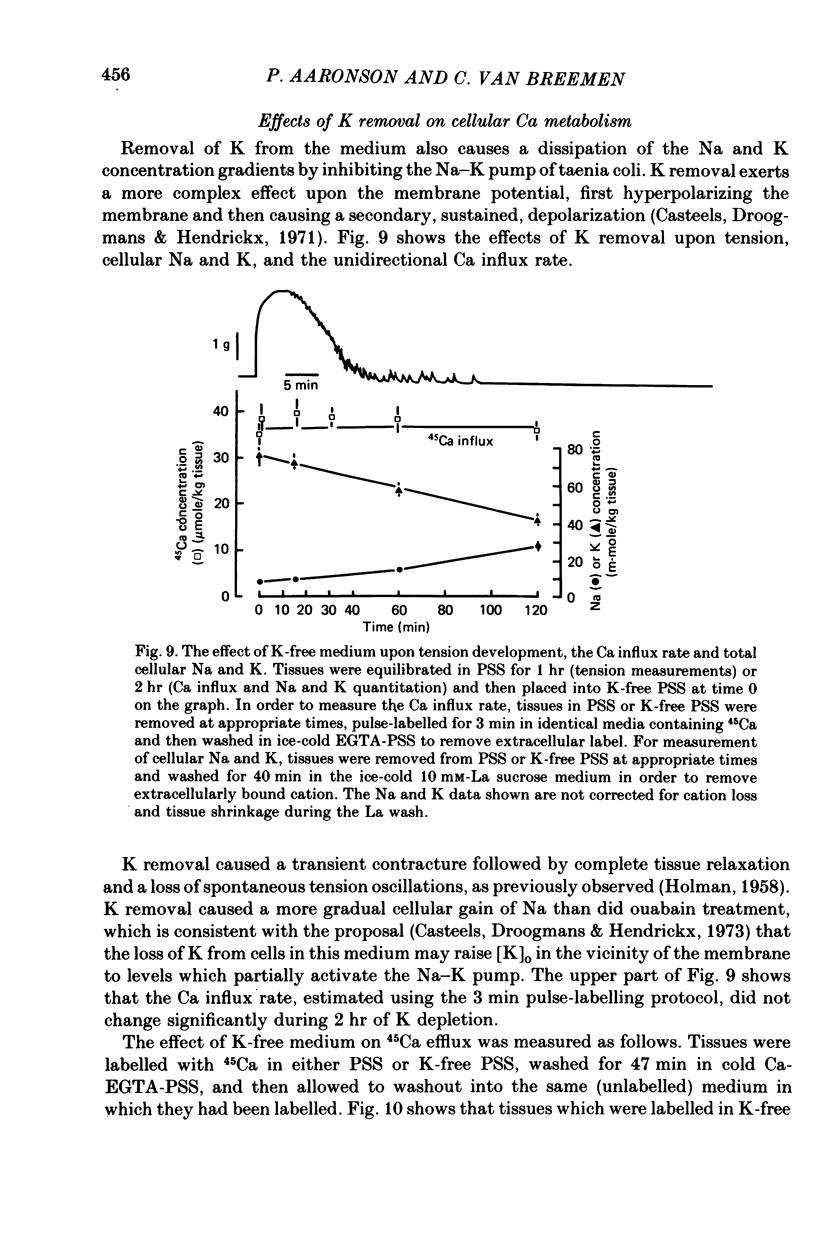
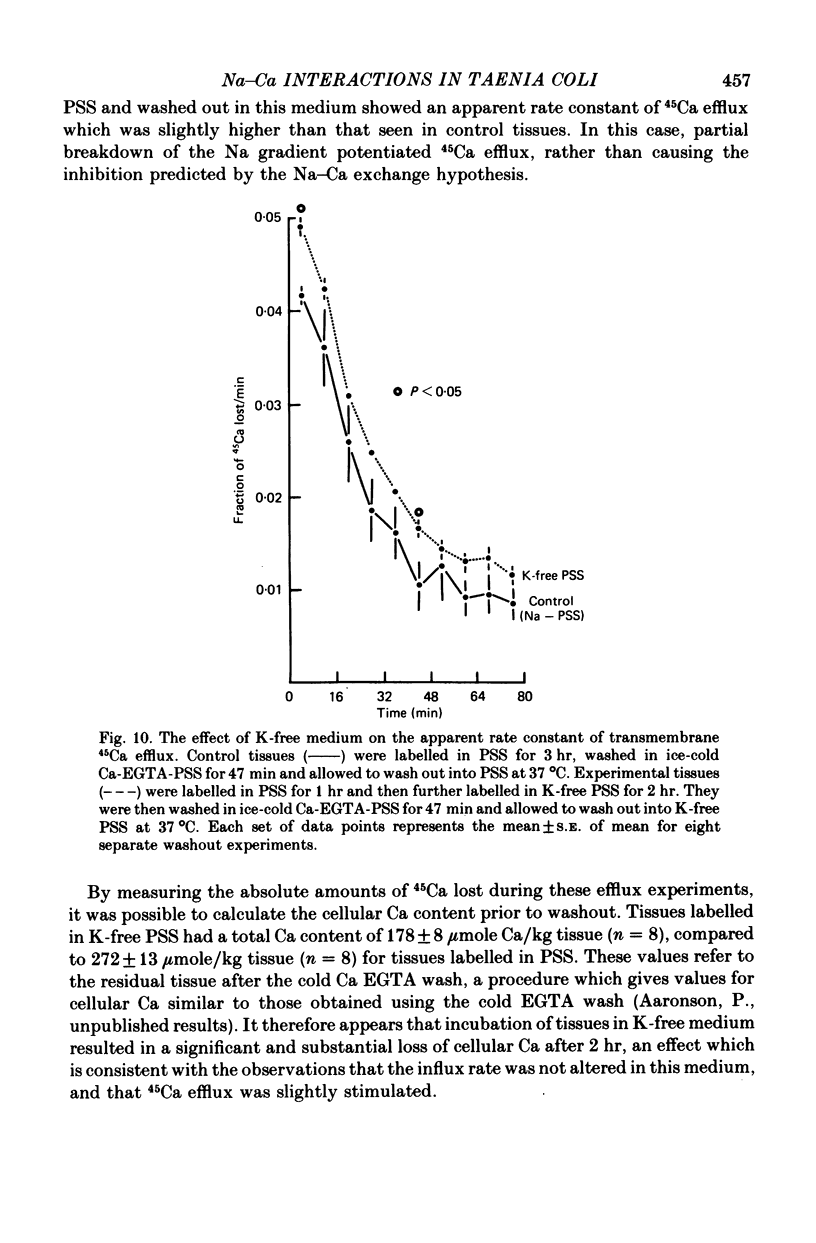
6. Removal of medium K caused a slower dissipation of the Na and K gradients. This treatment decreased cellular Ca, did not affect the Ca influx rate, and increased the 45Ca efflux rate.
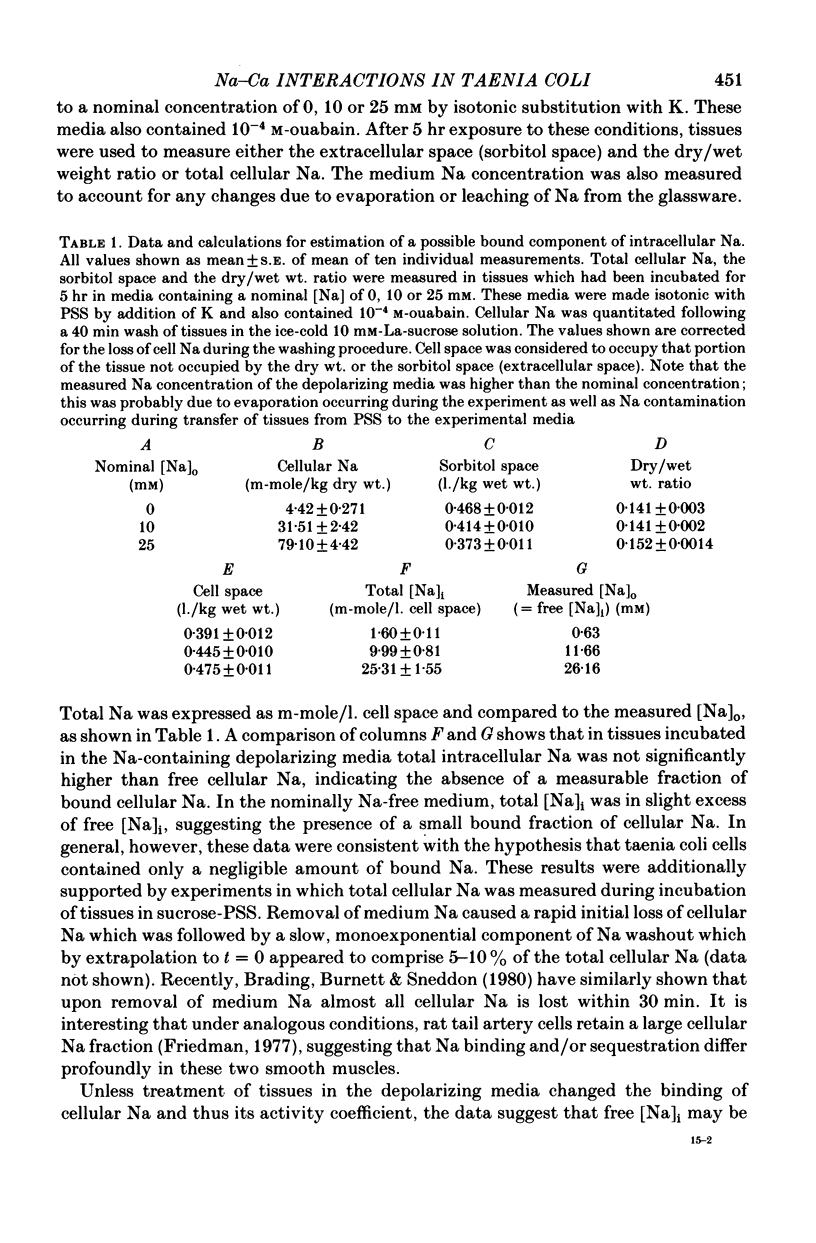
7. Tissues were incubated in depolarizing media containing 10-4 m-ouabain in order to remove the Na gradient. Subsequent measurement of cellular Na indicated the absence of a significant fraction of bound Na.
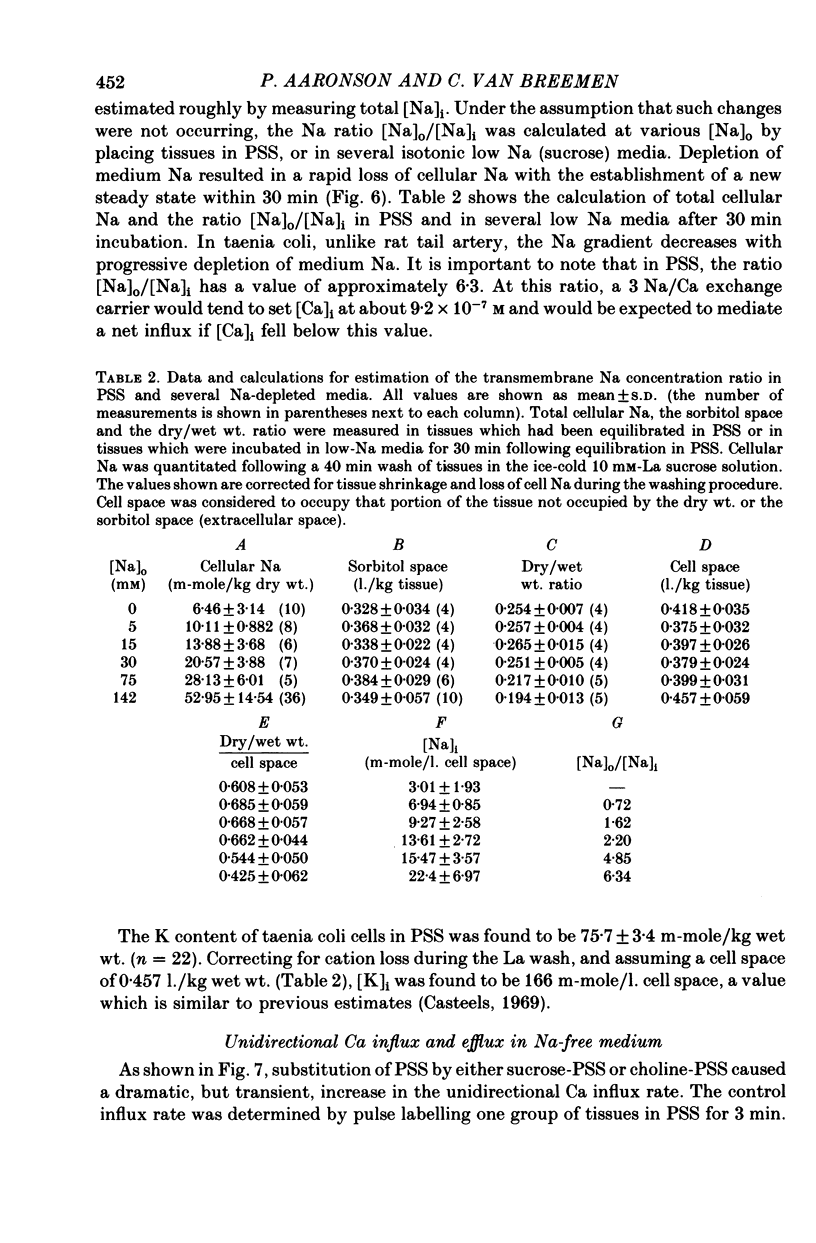
8. The ratio [Na]o/[Na]i had a value of 6.3 in control medium, and decreased as [Na]o was progressively lowered by sucrose substitution, reaching a value of < 1 in a medium containing 5 mm-Na.
9. These experiments provide evidence that a Na—Ca exchange carrier does not play an important role in regulation of tension in this muscle, and also indicate that the Ca gradient is not solely dependent on the Na gradient in guinea-pig taenia coli.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P., van Breemen C., Loutzenhiser R., Kolber M. A. A new method for measuring the kinetics of transmembrane calcium-45 efflux in the smooth muscle of the guinea pig tanea coli. Life Sci. 1979 Nov 19;25(21):1781–1789. doi: 10.1016/0024-3205(79)90424-7. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977 May;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Active ion transport and resting potential in smooth muscle cells. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):47–56. doi: 10.1098/rstb.1973.0008. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The action of ouabain on the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1966 May;184(1):131–142. doi: 10.1113/jphysiol.1966.sp007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Van Breemen C. Active and passive Ca2+ fluxes across cell membranes of the guinea-pig taenia coli. Pflugers Arch. 1975 Sep 9;359(3):197–207. doi: 10.1007/BF00587379. [DOI] [PubMed] [Google Scholar]
- Chiu V. C., Haynes D. H. Rapid kinetic study of the passive permeability of a Ca2+-ATPase rich fraction of the sarcoplasmic reticulum. J Membr Biol. 1980 Oct 31;56(3):203–218. doi: 10.1007/BF01869477. [DOI] [PubMed] [Google Scholar]
- Deth R. C. Effect of lanthanum and reduced temperature on 45Ca efflux from rabbit aorta. Am J Physiol. 1978 May;234(5):C139–C145. doi: 10.1152/ajpcell.1978.234.5.C139. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Casteels R. Sodium and calcium interactions in vascular smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1979 Jul;74(1):57–70. doi: 10.1085/jgp.74.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan P. F. Caclium uptake and associated adenosine triphosphatase activity in fragmented sarcoplasmic reticulum. Requirement for potassium ions. J Biol Chem. 1977 Mar 10;252(5):1620–1627. [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN S. M., JAMIESON J. D., FRIEDMAN C. L. Sodium gradient, smooth muscle tone, and blood pressure regulation. Circ Res. 1959 Jan;7(1):44–53. doi: 10.1161/01.res.7.1.44. [DOI] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. W., Somlyo A. P., Somlyo A. V. Potassium accumulation in smooth muscle and associated ultrastructural changes. J Physiol. 1973 Jul;232(2):247–273. doi: 10.1113/jphysiol.1973.sp010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katase T., Tomita T. Influences of sodium and calcium on the recovery process from potassium contracture in the guinea-pig taenia coli. J Physiol. 1972 Jul;224(2):489–500. doi: 10.1113/jphysiol.1972.sp009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T. S., Bose D. Sodium in smooth muscle relaxation. Am J Physiol. 1977 Jan;232(1):C59–C66. doi: 10.1152/ajpcell.1977.232.1.C59. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Urakawa N. Na-Ca exchange and tension development in guinea-pig aorta. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):171–178. doi: 10.1007/BF00501226. [DOI] [PubMed] [Google Scholar]
- Perry S. V., Grand R. J. Mechanisms of contraction and the specialized protein components of smooth muscle. Br Med Bull. 1979 Sep;35(3):219–226. doi: 10.1093/oxfordjournals.bmb.a071581. [DOI] [PubMed] [Google Scholar]
- Preiss R., Banaschak H. Na,K-ATPase in excitation-contraction coupling of vascular smooth muscle from cattle. Acta Biol Med Ger. 1979;38(1):83–96. [PubMed] [Google Scholar]
- Raeymaekers L., Wuytack F., Casteels R. Na-Ca exchange in Taenia coli of the guinea-pig. Pflugers Arch. 1974 Mar 25;347(4):329–340. doi: 10.1007/BF00587173. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


