Abstract
1. The spread of activation and background desensitization in rods was studied by recording membrane current from single outer segments in pieces of isolated toad retina.
2. Flash sensitivity changed slightly along the outer segment, falling by about 30% from base to tip.
3. When only the distal half of an outer segment was in the recording pipette, illumination of the unrecorded part elicited little or no photocurrent at the recorded part, indicating that a photoisomerization does not cause activation of the entire outer segment.
4. With diffuse illumination of an outer segment fully drawn into the pipette, the intensity—response relations at fixed times were invariant in form for most of the rising phase of the flash response and were considerably steeper than the Michaelis relation. The observed relation was consistent with a model in which a photoisomerization blocks all channels over a short region of the outer segment.
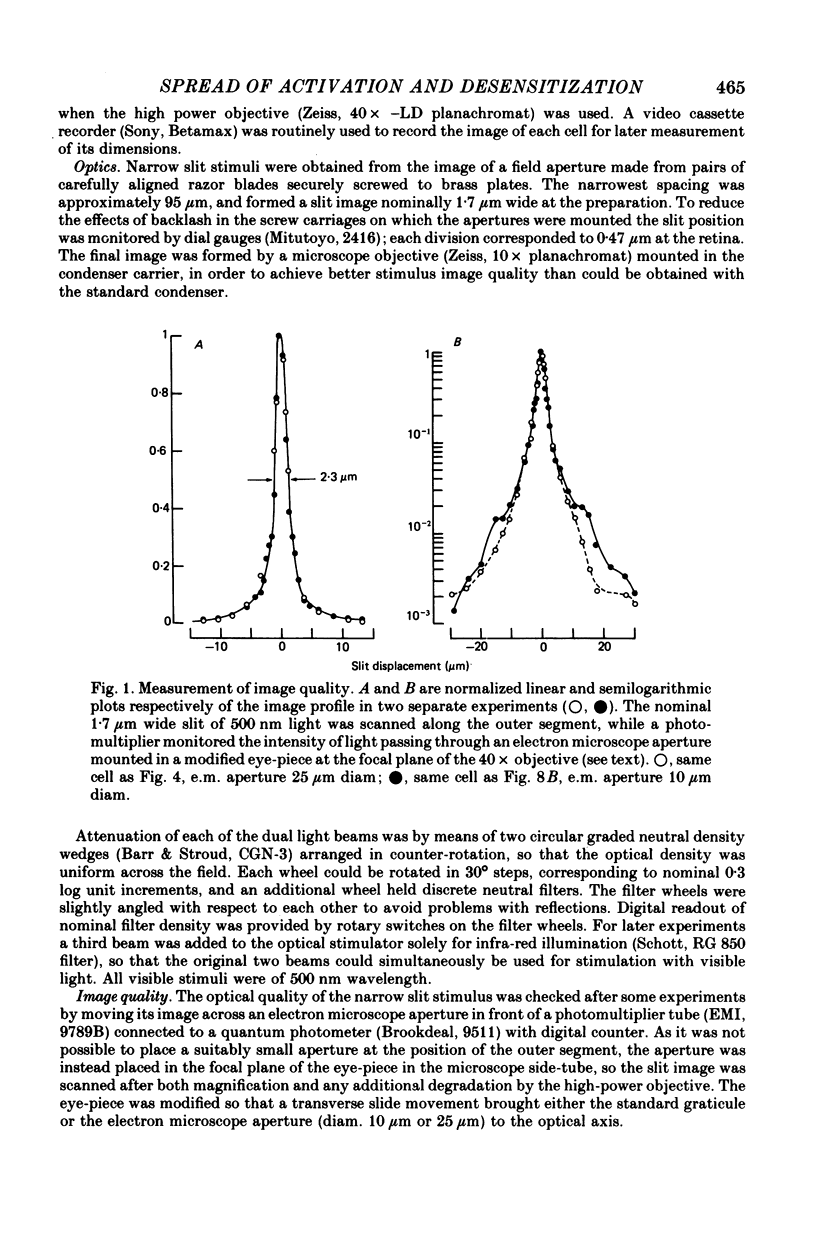
5. With illumination restricted to a narrow transverse slit, the intensity—response relations at fixed times were much less steep, as would be expected for a very limited longitudinal spread of activation. An upper limit for the effective longitudinal diffusion coefficient of the internal transmitter was estimated to be about 3 × 10-7 cm2 sec-1. This corresponds to a space constant for longitudinal spread of transmitter of about 3 μm at the time of the dim response peak.
6. The time course of flash responses elicited with light positioned either on the edge or on the centre of the outer segment was very similar.
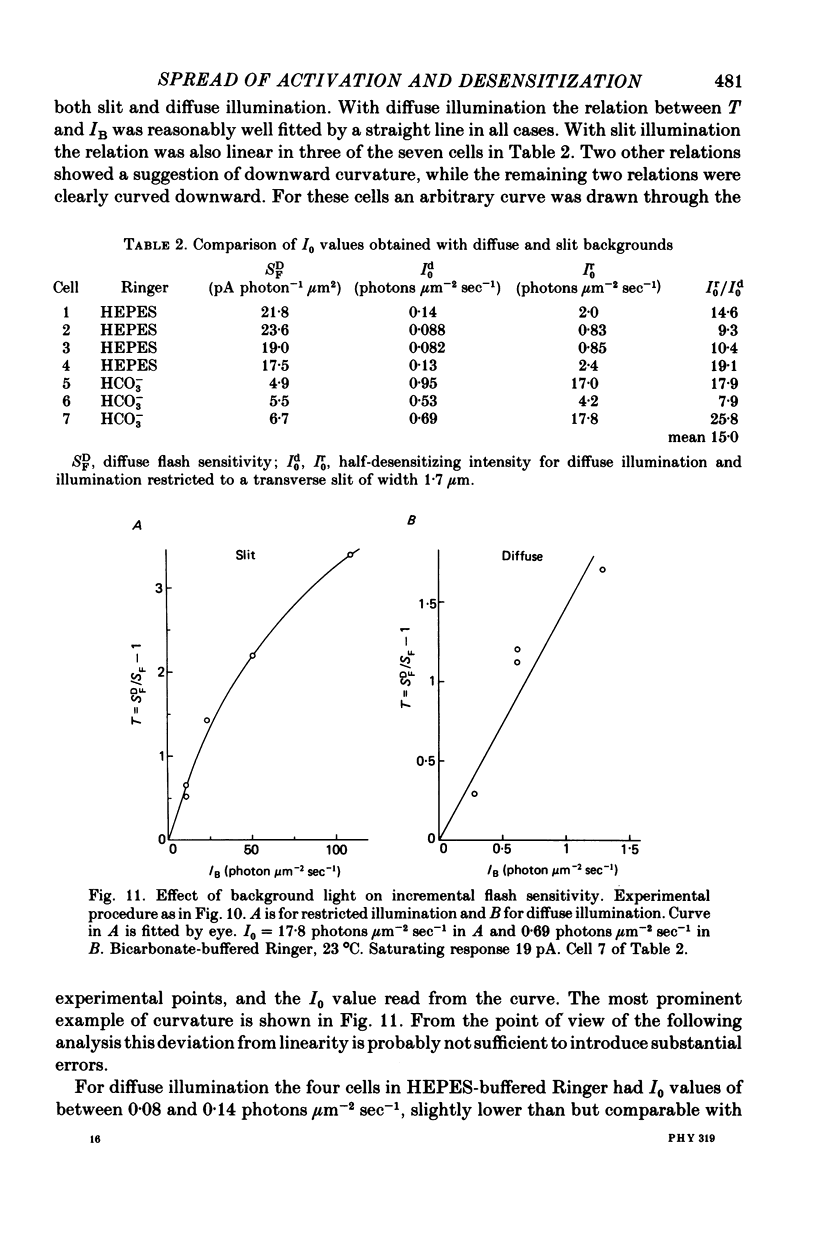
7. Desensitization resulting from steady illumination by a transverse slit was also localized longitudinally. A linear desensitization parameter T, defined in Results, decayed approximately exponentially along the outer segment, on either side of the site of photoisomerization, with a space constant of about 6 μm.
8. Transverse spread of desensitization was more effective than longitudinal spread.
9. After turning off a dim diffuse background light, the decay of T was roughly exponential with a time constant of several seconds. From this, and the steady state space constant of 6 μm, it is estimated that the effective longitudinal diffusion coefficient for a `desensitizing substance' would also be about 10-7 cm2 sec-1.
10. The restricted longitudinal spread of activation and desensitization may be explained by the barrier to diffusion presented by the stacked membranous disks in the outer segment. This baffling reduces the effective longitudinal diffusion coefficient to about 1/50 that of ordinary aqueous diffusion, but it does not significantly affect transverse spread.
Full text
PDF































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN P. K., GIBBONS I. R., WALD G. THE VISUAL CELLS AND VISUAL PIGMENT OF THE MUDPUPPY, NECTURUS. J Cell Biol. 1963 Oct;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. Excitation and adaptation in the vertebrate rod photoreceptor. Med Biol. 1978 Apr;56(2):52–63. [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaming D. G., Brown K. T. Effects of calcium on the intensity-response curve of toad rods. Nature. 1979 Apr 26;278(5707):852–853. doi: 10.1038/278852a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hemilä S., Reuter T. Longitudinal spread of adaptation in the rods of the frog's retina. J Physiol. 1981 Jan;310:501–528. doi: 10.1113/jphysiol.1981.sp013564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Obryan P. M. Internal recording of the early receptor potential in turtle cones. J Physiol. 1977 Jun;267(3):737–766. doi: 10.1113/jphysiol.1977.sp011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., Bownds M. D. Visual transduction in vertebrate photoreceptors. Annu Rev Neurosci. 1979;2:17–34. doi: 10.1146/annurev.ne.02.030179.000313. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A. Spread of activation along the toad rod outer segment [proceedings]. J Physiol. 1979 Oct;295:14P–15P. [PubMed] [Google Scholar]
- McLaughlin S., Brown J. Diffusion of calcium ions in retinal rods. A theoretical calculation. J Gen Physiol. 1981 Apr;77(4):475–487. doi: 10.1085/jgp.77.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A., Yau K. W., Lamb T. D. Spread of activation and desensitisation in rod outer segments. Nature. 1980 Jan 3;283(5742):85–87. doi: 10.1038/283085a0. [DOI] [PubMed] [Google Scholar]
- NILSSON S. E. THE ULTRASTRUCTURE OF THE RECEPTOR OUTER SEGMENTS IN THE RETINA OF THE LEOPARD FROG (RANA PIPIENS). J Ultrastruct Res. 1965 Feb;12:207–231. doi: 10.1016/s0022-5320(65)80016-8. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Warner A. E. Free calcium in Xenopus embryos measured with ion-selective microelectrodes. Nature. 1980 Feb 14;283(5748):658–660. doi: 10.1038/283658a0. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds M. D. Amplitude, kinetics, and reversibility of a light-induced decrease in guanosine 3',5'-cyclic monophosphate in frog photoreceptor membranes. J Gen Physiol. 1979 May;73(5):629–653. doi: 10.1085/jgp.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]


