Abstract
Mice exposed to aerosolized ovalbumin (OVA) develop increased airway responsiveness when deficient in γδ T cells. This finding suggests that γδ T cells function as negative regulators. The regulatory influence of γδ T cells is evident after OVA-sensitization and -challenge, and after OVA-challenge alone, but not in untreated mice. With aerosolized Abs to target pulmonary T cells, we now demonstrate that negative regulation of airway responsiveness is mediated by a small subpopulation of pulmonary γδ T cells. These cells express Vγ4 and depend in their function on the presence of IFN-γ and MHC class I. Moreover, their effect can be demonstrated in the absence of αβ T cells. This novel type of negative regulation seems to precede the development of the adaptive, antigen-specific allergic response.
Bronchial hyperreactivity or airway hyperresponsiveness (AHR) is a feature of asthma and chronic obstructive pulmonary disease and is the result of pathophysiological changes of the airways (1). The mechanisms leading to AHR are complex, and although environmental and genetic factors have been identified in predisposing the host to developing AHR in earlier studies (2–4), we and others demonstrated that γδ T cells are important modulators of airway function and allergic inflammation (5–8). In addition to their role in modulating allergic inflammation and AHR, we found that γδ T cells were already required in maintaining normal airway responsiveness in mice that were exposed to aerosolized ovalbumin (OVA) alone without prior sensitization to this antigen (5). Whereas in normal mice three consecutive daily exposures to nebulized OVA (3N) had no detectable effect on airway responsiveness to a cholinergic agonist, the same treatment did elicit substantial AHR in mice genetically deficient in or transiently depleted of γδ T cells. However, unlike AHR associated with allergic, antigen-specific immunity, AHR after 3N exposure did not depend on specific αβ T cell or Ab responses and there was no evidence of an inflammatory response. This observation prompted us to propose that γδ T cells can function as negative regulators of airway responsiveness even before the development of antigen-specific, allergic immune responses and airway damaging inflammation (5).
The adult murine lung harbors several subsets of γδ T cells distinguished by the expression of different Vγ genes, including in descending order of relative frequency Vγ6+, Vγ4+, and Vγ1+ T cells (9–12). Here, we show that pulmonary Vγ4+ T cells are responsible for maintaining normal airway responsiveness in mice exposed to aerosolized OVA alone. During the exposure to OVA a fraction of Vγ4+ T cells expressing CD8αβ accumulated in the lung. This increase as well as the negative regulation of airway responsiveness by Vγ4+ T cells depended on normal MHC class I expression.
Materials and Methods
Animals.
C57BL/6, B6.IFN-γ−/−, B6.TCR-β−/− (TCR, T cell antigen receptor), and 129/B6.TAP-1−/− were purchased from The Jackson Laboratory; TCR-Vγ4/6−/− mice (13) were a kind gift from K. Ikuta (Kyoto, Japan); and B6.β2M−/− mice were purchased from Taconic Farms. TCR-Vγ4/6−/− mice were backcrossed to a C57BL/6 background and were used after five backcrosses. B6.IFN-γ−/− and B6.TCR-β−/− were interbred to obtain B6.TCR-β−/−/IFN-γ−/− mice. Mice of the F2 and the subsequent generation were screened for absence of αβ T cells by flow cytometry and assessed for IFN-γ gene knockout by using primer sequences provided by The Jackson Laboratory [primers: IMR126 (5′-AGA AGT AAG TGG AAG GGC CCA GAA G-3′) and IMR127 (5′-AGG GAA ACT GGG AGA GGA GAA ATA T-3′) to amplify a 220-bp product from the endogenous Ifg allele; IMR128 (5′-TCA GCG CAG GGG CGC CCG GTT CTT T-3′) and IMR129 (5′-ATC GAC AAG ACC GGC TTC CAT CCG A-3′) to amplify a 375-bp product from the targeted Ifg allele]. All mice were cared for in the animal facility at National Jewish Medical and Research Center following guidelines for immunodeficient animals. All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Airway Challenge With OVA With and Without Aerosolized mAbs.
Mice (C57BL/6, TCR-β−/−, TAP-1−/−, β2M−/−, and TCR-Vγ4/6−/− mice—sham or anti-TCR mAb-treated) received the following treatments: (i) airway exposure to nebulized OVA (1% in saline, total volume 10 ml per exposure) alone, using ultrasonic nebulization for 20 min on 3 consecutive days (denoted “3N” in figures); (ii) airway exposure to nebulized OVA alone with addition of a dose of 10 μg/ml of the designated mAb to aerosol solution on the third exposure day (see below); and (iii) i.v. injection of mAbs as described (5). Airway responsiveness was assessed 48 h after the last 3N exposure for all 3N-treated mice. For each of these treatments, groups of at least 4 mice of each type were analyzed in every independent experiment, as detailed in the text and figure legends. Controls comprising OVA nontreated (NT) mice and OVA NT mice exposed to aerosolized mAb against TCR-δ or TCR-Vγ4 showed no alteration of airway responsiveness defining baseline responses.
Determination of Airway Responsiveness.
Airway responsiveness was assessed as a change in airway function after challenge with aerosolized methacholine (MCh) via the airways. Anesthetized, tracheostomized mice were mechanically ventilated and lung function was assessed as a modification to described procedures (5, 14, 15). Lung resistance (RL) and dynamic compliance (Cdyn) were continuously computed (labview, National Instruments, Austin, TX) by fitting flow, volume, and pressure to an equation of motion. After each aerosol MCh challenge, the data were continuously collected for 1–5 min, and maximum values of RL and minimum values of Cdyn were taken to express changes in murine airway function. Baseline and saline controls were similar among all examined mice, and results were reported as percentages of saline control.
Targeting T Cells with Anti-TCR mAb.
Systemic depletion of γδ T cells and subsets was accomplished with mAb as described (5) and used to determine the quality of mAbs for depletion and usage as aerosols. mAbs (200 μg) against TCRs, (i) anti-TCR-δ [1:1 mixture of mAbs GL3 (16) and 403A10 (17)], (ii) anti-TCR-Vγ1 (clone 2.11), and (iii) anti-TCR-Vγ4 (clone UC3-10A6) and anti-TCR-β (clone H57-597) were injected into the tail vein. Mice were then exposed to 3N treatment 3 days later and after a total of 7 days, depletion of pulmonary and splenic γδ T cells was assessed at the time of airway function measurements. On the third and last day of the 3N treatment, mAbs against TCR-δ, TCR-β, TCR-Vγ1, or TCR-Vγ4 were added to the OVA solution, and airway responsiveness was assessed 48 h later. The success of this treatment was assessed by staining for CD3+TCR-δ+ or CD3+TCR-β+ T cells after lung digestion and T cell enrichment with nylon-wool columns. Purity of CD3+ cells after nylon-wool enrichment was 70–80%.
Lung Digest and Cell Counts.
After airway function measurements, mice were exsanguinated by cutting the thoracic aorta and vena cava, and lungs were excised. Lungs were placed in Hanks' balanced salt solution, minced, and exposed to an enzymatic digestion mixture containing 0.125% dispase II (Roche Diagnostics)/0.2% collagenase II (Sigma)/0.2% collagenase IV (Sigma) for 90 min. After the enzymatic digestion, lung tissue was treated with Gey's red cell lysis solution and then passed through a 70-μm diameter (Falcon) mesh to remove fibrotic tissue. This pulmonary cell suspension was passed over a nylon-wool column to obtain lymphocyte-enriched cells (18). Total cell counts were obtained with a Coulter Counter Hemocytometer. Subsequent flow cytometry measurements with an XL2 (Coulter) were carried out to analyze live cells by using forward and side scatter profile and direct staining for respective T cell populations. These population counts were used to calculate total cell counts of the original lymphocyte-enriched pulmonary cell suspension.
Staining Reagents.
For cytofluorographic analysis, mAbs were conjugated with N-hydroxysuccinimido-biotin (Sigma) and/or fluorescein isothiocyanate isomer I on Celite (Sigma). Then, 0.5–1 × 106 cells per well in 96-well plates (Falcon–Becton Dickinson) were stained by using one-, two-, or three-color techniques and analyzed cytofluorographically on XL2 (Coulter) counting a minimum of 150,000 events per gated region. The following mAbs against murine TCR structures and all generated in Armenian hamster were grown as described (19): TCR-β [clone H57-597 (20)], TCR-δ [clone GL3 (16)]; clone 403A10 (ref. 17; a gift from S. Tonegawa, MIT, Cambridge, MA), TCR-Vγ1 [clone 2.11 (21); a gift from P. Pereira, Institute Pasteur, Paris], and TCR-Vγ4 [clone UC3-10A6 (22); a gift from J. Bluestone, Univ. of California, San Francisco]. FITC-conjugated anti-murine TCR-Vγ4 mAb (clone UC3-10A6), phycoerythrin (PE)-conjugated mAb anti-murine CD4 (clone GK1.5), PE- or FITC-conjugated mAb anti-murine CD8α (clone 53-6.7) and PE- or FITC-conjugated mAb anti-murine CD8β.2 (clone 53-5.8) were purchased from PharMingen. Intracellular staining was performed by following their guidelines on intracellular staining and using PE-conjugated anti-murine IFN-γ (clone XMG1.2) (PharMingen). Appropriate isotype-matched controls were used for all staining procedures.
Statistical Analysis.
All results are expressed as the mean and SD. ANOVA was used to determine the levels of difference among all groups. Pairs of groups were compared by unpaired two-tailed Student's t test. The P values for significance were set to 0.05.
Results
Airway Responsiveness Is Regulated by Pulmonary γδ T Cells.
In an earlier study we found that γδ T cells regulate airway responsiveness even in the absence of αβ T cells (5). Mice genetically deficient in γδ T cells (B6.TCR-δ−/−) or systemically depleted of γδ T cells by i.v. injection with anti-TCR-δ mAbs were exposed to aerosolized OVA on 3 consecutive days. Forty-eight hours later, airway responsiveness was assessed as changes in RL and Cdyn in response to inhaled MCh (14, 15). In both—genetically deficient and Ab-depleted mice—airway responsiveness was similarly increased, ruling out as a cause for increased airway reactivity long-term developmental changes caused by the absence of γδ T cells. However, it remained unclear whether pulmonary or systemic γδ T cells mediated this negative regulatory effect.
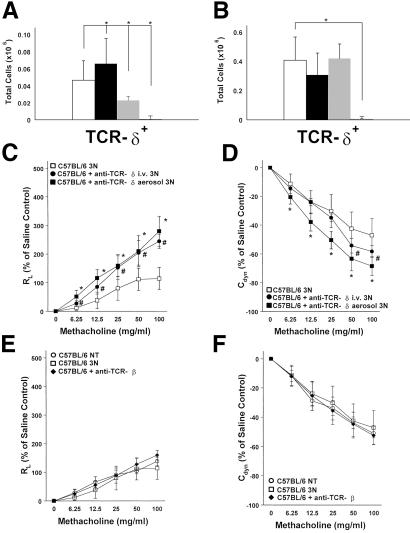
As an approach to answering this question, we used aerosolized instead of systemic anti-TCR mAbs to target pulmonary γδ T cells directly. Treatment with aerosolized mAb (10 μg/ml of anti-TCR-δ mAb) reduced detectable pulmonary γδ T cells (Fig. 1A) but had no discernible effect on splenic γδ T cells, both at 48 h (Fig. 1B) and 1 week after the last OVA aerosol exposure (not shown). As expected, the systemic Ab treatment by i.v. injection of anti-TCR-δ mAbs depleted pulmonary as well as splenic γδ T cells (Fig. 1 A and B). Thus, the aerosolized mAbs preferentially targeted pulmonary γδ T cells.
Figure 1.
Pulmonary γδ T cells and AHR in C57BL/6 mice after 3 days of airway challenge with OVA and treatment with mAbs against TCR-δ or TCR-β. (A and B) Total cell counts for γδ T cells after nylon-wool enrichment of pulmonary lymphocytes (A) and of splenic lymphocytes (B). No treatment (open bar), 3N treatment (black solid bars), 3N + anti-TCR-δ aerosol (gray bar), 3N + anti-TCR-δ i.v. (hatched bar). Each bar represents data from at least three independent experiments with a total of 9–12 mice. Significant differences (P < 0.05) are indicated by an asterisk. (C–F) After 3N treatment, changes in airway resistance (RL) (C and E) and Cdyn (D and F) are compared in C57BL/6 mice receiving the following treatments (see Materials and Methods): aerosolized OVA alone (□); aerosolized OVA after i.v. injection of mAbs against TCR-δ (●); aerosolized OVA combined with aerosolized mAbs against TCR-δ (■) (C and D) or with mAb against TCR-β (⧫) (E and F). Nontreated (NT) mice are shown in ○ (E and F). No significant differences in baseline responses to saline were observed in any of these groups; RL baseline values (in cm H2O/ml per second) were 0.53 ± 0.03 (NT C57BL/6), 0.55 ± 0.06 (3N C57BL/6), 0.62 ± 0.08 (C57BL/6 + anti-TCR-δ i.v.), 0.56 ± 0.02 (C57BL/6 + anti-TCR-δ aerosol), and 0.62 ± 0.08 (C57BL/6 + anti-TCR-β aerosol). Each curve represents data from at least three independent experiments with a total of 9–12 mice. Significant differences (P < 0.05) are indicated by an asterisk or #, and error bars indicate SD.
Unlike nonchallenged control mice, in which the absence of γδ T cells had no effect on airway responsiveness (5) (see Materials and Methods), depletion of γδ T cells in OVA-challenged C57BL/6 mice resulted in increased RL and decreased Cdyn in response to inhaled MCh (Fig. 1 C and D). Treatment with aerosolized anti-TCR-β mAb had no effect (Fig. 1 E and F). Treatment with aerosolized anti-TCR-δ mAbs administered only 48 h before MCh provocation elicited similar or larger changes in airway responsiveness when compared with the systemic administration of mAbs, even though the Ab dose necessary to achieve the same effects was much smaller. Moreover, systemic treatment required more time (about 1 week) to take effect (Fig. 1 C and D).
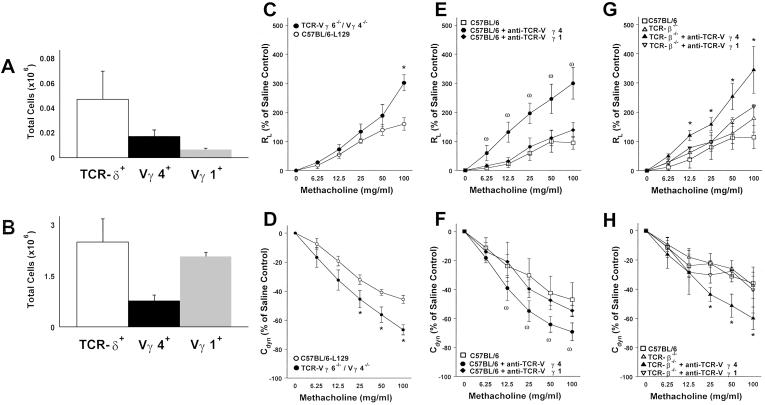
Vγ4+ T Cells Negatively Regulate Airway Responsiveness.
In normal C57BL/6 mice, the retrievable pulmonary γδ T cell population consisted of about 5 × 104 lymphocytes. At 48 h after the airway challenges with OVA, only minor increases in total γδ T cell numbers were detected. The majority of these cells express Vγ6 at the mRNA level (12), although Abs for the specific detection of such cells are not yet available. About one-third expressed Vγ4 and even fewer expressed Vγ1 (Fig. 2A). We first examined mice genetically deficient in both of the two major pulmonary γδ T cells subsets, Vγ6+ and Vγ4+ T cells (13). In comparison with either C57BL/6 mice or their littermates of similar genetic background (see Materials and Methods), these mice showed increased airway responsiveness after airway challenge with OVA (Fig. 2 C and D), consistent with a negative regulatory function of either one or of both of the deficient γδ T cell subsets, but not of Vγ1+ T cells.
Figure 2.
Pulmonary γδ T cell subsets in adult C57BL/6 and B6.TCR-β−/− mice and their effect on airway responsiveness after 3N treatment. (A and B) Total retrievable pulmonary γδ T cell numbers in untreated C57BL/6 are about 40-fold lower than in B6.TCR-β−/− (open bars A and B). Although the ratio of Vγ4+ to total pulmonary γδ T cells is similar in C57BL/6 and B6.TCR-β−/− mice (solid bar), the rate of Vγ1+ T cells is increased in B6.TCR-β−/− compared with C57BL/6 mice (gray bars). The data are representative of three independent experiments and each bar represents 4–6 mice. (C–H) After 3N treatment, changes in RL (C, E, and G) and Cdyn (D, F, and H) are compared in mice receiving the following treatments (see Materials and Methods): aerosolized OVA alone (○ C57BL/6–129; □ C57BL/6; ● TCR-Vγ4/6−/− mice; ▵ TCR-β−/−); aerosolized OVA + mAb against TCR-Vγ4 ( ); aerosolized OVA + mAb against TCR-Vγ1 (⧫); aerosolized OVA + mAb against TCR-Vγ4 in B6.TCR-β−/− (▴); and aerosolized OVA + mAb against TCR-Vγ1 in B6.TCR-β−/− (▿). No significant differences in baseline responses to saline were observed in any of these groups; RL baseline values (in cm H2O/ml per second) were 0.62 ± 0.03 (3N C57BL/6–129), 0.55 ± 0.06 (3N TCR-Vγ4/6−/−), 0.53 ± 0.07 (3N C57BL/6), 0.56 ± 0.03 (C57BL/6 + anti-TCR-Vγ1 aerosol), 0.54 ± 0.06 (C57BL/6 + anti-TCR-Vγ4 aerosol), 0.59 ± 0.08 (TCR-β−/−), 0.56 ± 0.02 (TCR-β−/− + anti-TCR-Vγ1 aerosol), and 0.53 ± 0.08 (TCR-β−/− + anti-TCR-Vγ4 aerosol). Each curve represents data from at least three independent experiments with a total of 9–12 mice. Significant differences (P < 0.05) are indicated by an asterisk or ω.
Next, we used available Vγ-specific mAbs and the aerosolized Ab-treatment protocol to assess and compare functional roles of the Vγ4+ and the Vγ1+ pulmonary subsets in normal C57BL/6 mice. Treatment with anti-TCR-Vγ4 mAb resulted in increased airway responsiveness (Fig. 2 E and F), equivalent to the increases obtained with either aerosolized or systemic pan-specific anti-TCR-δ mAbs (Fig. 1), or those observed in genetically γδ T cell-deficient mice (5). In contrast, anti-TCR-Vγ1 mAb had no effect (Fig. 2 E and F), although this Ab efficiently depletes Vγ1+ T cells as we have demonstrated (23). These results established the Vγ4+ T cell subset as a negative regulator of airway responsiveness in C57BL/6 mice.
Because of our earlier findings that γδ T cell-dependent negative regulation of airway responsiveness targets an αβ T cell-independent mechanism of airway stimulation (5), we examined whether the now more narrowly defined regulator population of pulmonary Vγ4+ T cells also shared this independence from αβ T cells. With B6.TCR-β−/− instead of C57BL/6 mice, we found this to be the case (Fig. 2 G and H). Moreover, this result confirmed the distinctive role of Vγ4+ T cells as negative regulators of airway responsiveness. B6.TCR-β−/− mice not only harbor more pulmonary γδ T cells than C57BL/6 mice, but also the relative sizes of the pulmonary subsets are skewed such that Vγ1+ T cells emerge as the predominant population (Fig. 2B). Despite this difference, there was no indication that Vγ1+ T cells contributed to the negative regulation of airway responsiveness in these mice.
Vγ4+ T Cells Require MHC Class I and IFN-γ for Negative Regulation of Airway Responsiveness.
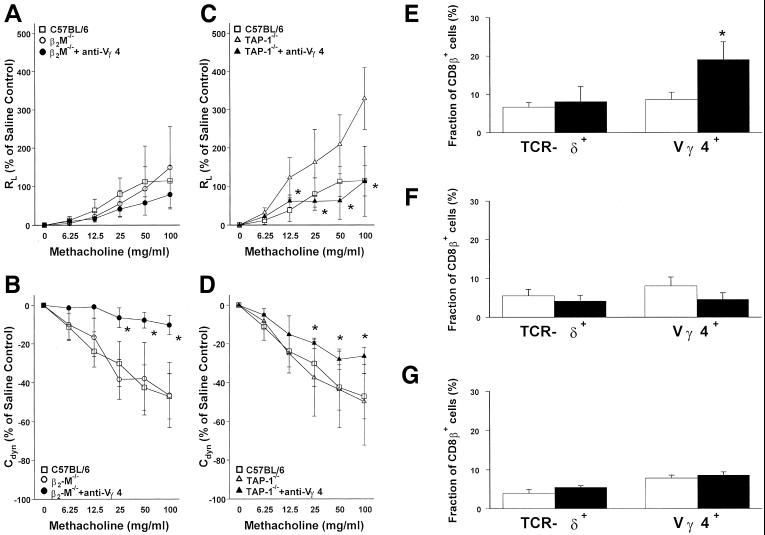
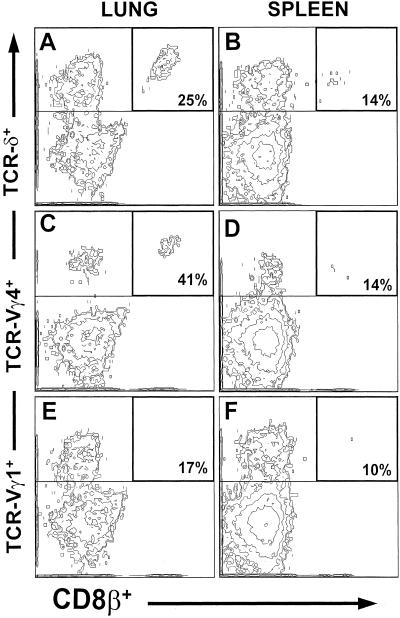
We analyzed the surface phenotype of the entire pulmonary γδ T cell population and Vγ4+ T cells in particular. In untreated C57BL/6 mice, about one-tenth of both total pulmonary γδ T cells and Vγ4+ T cells expressed CD8 (not shown). Of these, about one-quarter expressed CD8αα homodimers, whereas about three-quarters carried the CD8αβ heterodimers (Fig. 4E). After the airway challenge with OVA, there was some increase of CD8αα-expressing cells in all pulmonary γδ T cells (not shown), whereas CD8αβ+ cells increased preferentially within the Vγ4+ T cell population (Fig. 4E). We carried out a similar phenotypic analysis with pulmonary γδ T cells in untreated B6.TCR-β−/− mice, in which γδ T cells can be more easily examined because of their larger numbers in these mice (Fig. 3 A–F). In B6.TCR-β−/− mice, the relative frequency of CD8+ γδ T cells is higher than in C57BL/6 mice. Nevertheless, the pulmonary Vγ4+ T cells again contained the highest relative frequencies of CD8αβ+ T cells (about 41% of pulmonary γδ T cells; Fig. 3C) whereas Vγ1+ T cells contained CD8αβ+ cells at reduced frequencies (about 17%; Fig. 3E) compared with total pulmonary γδ T cells (about 25%; Fig. 3A). Moreover, CD8αβ expression among splenic γδ T cells (about 14%; Fig. 3B) and in particular among splenic Vγ4+ T cells (about 14%; Fig. 3D) was lower than among pulmonary γδ T cells. Taken together, these findings define a CD8αβ+ subset of pulmonary Vγ4+ T cells and show a distinctive response of this subset to airway stimulation. In contrast, none of the pulmonary γδ T cells in normal or OVA-challenged C57BL/6 mice expressed CD4 (not shown).
Figure 4.
MHC class I is required for the Vγ4+ T cell-mediated negative regulation of AHR and the pulmonary Vγ4+CD8β+ T cell expansion after 3N treatment. (A–D) After 3N treatment, changes in RL (A and C) and Cdyn (B and D) of B6.β2-M−/− and TAP-1−/− are compared with C57BL/6 mice receiving the following treatments (see Materials and Methods): aerosolized OVA alone (□ C57BL/6; ○ B6.β2-M−/−; ▵ TAP-1−/−); aerosolized OVA + mAb against TCR-Vγ4 in B6.β2-M−/− (●) and in TAP-1−/− (▴). No significant differences in baseline responses to saline were observed in any of these groups; RL baseline values (in cm H2O/ml per second) were 0.53 ± 0.07 (3N C57BL/6), 0.68 ± 0.01 (3N B6.β2-M−/−), 0.59 ± 0.03 (3N B6.β2-M−/− + anti-TCR-Vγ4 aerosol), 0.49 ± 0.03 (3N TAP-1−/−), and 0.53 ± 0.02 (TAP-1−/− + anti-TCR-Vγ4 aerosol). Each curve represents data from at least three independent experiments with a total of 8 mice. Significant differences (P < 0.05) are indicated by an asterisk. (E–G) Although Vγ4+CD8β+ T cells increase in C57BL/6 mice (E, black bars) after 3N treatment compared with no OVA exposure (open bars in E–G), this increase does not occur in B6.β2-M−/− mice (F, black bars) or TAP-1−/− mice (G, black bar). Each bar represents at least four mice of two independent experiments.
Figure 3.
Preferential CD8β expression in pulmonary Vγ4+ T cells of TCR-β−/− mice. Staining of CD8β in pulmonary (A, C, and E) and splenic (B, D, and F) γδ T cells (A and B) and subsets, Vγ4 (C and D) and Vγ1 (E and F), in untreated B6.TCR-β−/− mice. Gates are set to include cells staining positively with Abs against TCR-δ, TCR-Vγ4, or TCR-Vγ1. Double-positive cells staining for TCRs and CD8β are enclosed by thicker lines. Percentages indicate the frequency of double-positive cells relative to all gated TCR-positive cells. Results represent analysis of B6.TCR-β−/− mice from three independent experiments analyzing 6–8 B6.TCR-β−/− mice.
The preferential expression of CD8 among a significant portion of pulmonary Vγ4+ T cells suggested that these cells recognize MHC class I or related molecules with binding sites for the CD8 coreceptor (24), and further, that MHC class I expression may be required for their function. Therefore, we tested two types of mice with broad deficiencies in MHC class I expression: mice genetically deficient in the expression of β2-microglobulin (B6.β2M−/−, on C57BL/6 genetic background; see Materials and Methods) (Fig. 4 A and B) and mice genetically deficient in the peptide transporter protein TAP-1 (TAP-1−/− mice on mixed C57BL/6–129 genetic background; see Materials and Methods) (Fig. 4 C and D). In either case, after treatment with anti-Vγ4 Ab, airway responsiveness was not increased compared with the increases observed in C57BL/6 or other previously tested mouse strains with normal MHC class I expression. In fact, treatment with aerosolized mAb against TCR-Vγ4 caused a decrease in airway responsiveness, particularly in TAP-1−/− mice. Moreover, in either MHC class I-deficient strain, challenge with aerosolized OVA did not lead to an increase of CD8αβ+ T cells within the pulmonary Vγ4+ T cell subset in contrast to the increase seen in OVA-challenged C57BL/6 mice (Fig. 4 E–G). Taken together, these data suggest that β2M- and TAP-1-dependent MHC class I expression is required for the negative regulation of airway responsiveness by pulmonary Vγ4+ T cells as well as for the challenge-induced accumulation of CD8+ T cells within this subset. Thus, pulmonary Vγ4+CD8+ T cells may be the primary mediators of γδ T cell-dependent negative regulation of airway responsiveness.
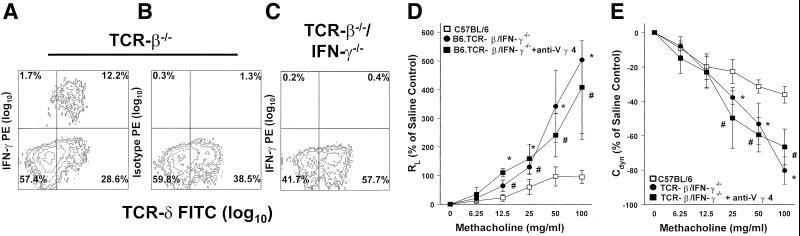
Next, we tested whether IFN-γ is required for negative regulation of airway responsiveness by Vγ4+ T cells. We crossed B6.TCR-β−/− mice with B6.IFN-γ−/− mice (same genetic background) and interbred mice of the F2 generation to derive a new double-deficient mutant strain (B6.TCR-β−/−/IFN-γ−/−). Like B6.TCR-β−/− mice, these animals contain an increased number of γδ T cells in the unstimulated lung, but these cells as well as other pulmonary leukocytes no longer produce IFN-γ (Fig. 5 A–C). After challenge with aerosolized OVA (3N), these mice showed increased airway responsiveness when compared with B6.TCR-β−/− mice (Fig. 2 G and H) or C57BL/6 mice (Fig. 5 D and E). This response was not increased further by treatment with aerosolized anti-Vγ4 mAb, indicating that IFN-γ is required for the regulatory effect of Vγ4+ T cells (Fig. 5 D and E). Last, we compared capabilities of different pulmonary lymphocyte subsets to produce IFN-γ after stimulation with phorbol 12-myristate 13-acetate/ionomycin with intracellular staining. Vγ4+ T cells isolated from untreated lungs of B6.TCR-β−/− mice produced about two-fold-higher levels of IFN-γ (MFI:103; MFI, mean fluorescence intensity) compared with all gated lymphocytes (MFI:40), and CD8+Vγ4+ T cells expressed IFN-γ at higher levels (MFI:122) than all CD8+ lymphocytes (MFI:75). This pattern was not significantly changed after 3N airway stimulation.
Figure 5.
IFN-γ is required for the Vγ4+ T cell-mediated negative regulation of AHR after 3N treatments. (A–C) After lung digest and nylon-wool purification, pulmonary lymphocytes of TCR-β−/− and B6.TCR-β−/−/IFN-γ−/− mice were stained for intracellular IFN-γ levels and for TCR-δ+ surface expression (A and C). A PE-conjugated isotype-matched Ab was used as specificity control (B). No IFN-γ production was evident in TCR-β−/−/IFN-γ−/− mice (C). (D and E) RL (D) and Cdyn (E) of B6.TCR-β−/−/IFN-γ−/− and C57BL/6 mice receiving the following treatments (see Materials and Methods): aerosolized OVA alone (□ C57BL/6; ● B6.TCR-β−/−/IFN-γ−/−) and aerosolized OVA + mAb against TCR-Vγ4 in B6.TCR-β−/−/IFN-γ−/− mice (■). No significant differences in baseline responses to saline were observed in any of these groups; RL baseline values (in cm H2O/ml per second) were 0.52 ± 0.07 (3N C57BL/6), 0.62 ± 0.02 (3N TCR-β−/−/IFN-γ−/−), and 0.58 ± 0.05 (B6.TCR-β−/−/IFN-γ−/− 3N + anti-TCR-Vγ4 aerosol). Each curve represents data from at least three independent experiments with a total of 8 mice. Significant differences (P < 0.05) are indicated for TCR-β−/−/IFN-γ−/− treated with 3N alone (*) and TCR-β−/−/IFN-γ−/− treated with 3N + anti-TCR-Vγ4 aerosol (#) compared with the genetic background C57BL/6.
Discussion
The data reported here indicate that under conditions of short-time exposure to aerosolized OVA a particular TCR-defined subset of γδ T cells prevents airway hyperreactivity. This finding is consistent with other recent studies associating TCR-defined subsets of γδ T cells with distinct functional roles (23, 25–28). The requirement for a TCR-defined population also suggests that TCR-ligand recognition and the particular specific ligand of the regulatory γδ T cell subset are important in maintaining normal airway function (29).
The regulatory cells express TCR-Vγ4. Other γδ T cells also present in the lung, and even in larger numbers, do not seem to be involved in this regulatory function. We do not rule out the possibility that Vγ6+ T cells have regulatory activity as well, but the Vγ4+ T cell subset alone is sufficient to account for the changes in airway responsiveness observed after γδ T cell depletion. In fact, because the treatments with pan-specific mAbs against TCR-δ and with subset-specific mAb against TCR-Vγ4 have comparable effects, no other subset seems to influence airway responsiveness under these defined conditions.
Our data imply that pulmonary γδ T cells are the major target of the aerosolized mAb treatment and therefore represent the regulatory γδ T cell population. However, the regulatory pulmonary population targeted by the aerosolized mAbs may consist of resident (11) and/or recently recruited γδ T cells. It is possible that the increase in pulmonary Vγ4+ T cells after aerosolized OVA exposure alone is the result of selective recruitment of these cells to the irritated airways or the result of their local expansion. In either case, our observations are best reconciled with the notion that in the 3N stimulation, γδ T cell-dependent regulation of airway responsiveness occurs locally, within the airways themselves.
The potency of the γδ T cell-dependent regulatory effect on airway responsiveness is emphasized by the small size of this regulatory cell population. In C57BL/6 mice the pulmonary subset of Vγ4+ T cells consisted of about 1–2 × 104 retrievable cells, and the CD8αβ+ subset of these (about 2–5 × 103) may represent the functional regulatory subset. This observation is reminiscent of an earlier study in which very small numbers of CD8+ γδ T cells, retrieved from the spleen of C57BL/6 mice that were repeatedly challenged with aerosolized OVA (>10 times), efficiently suppressed IgE Ab production in an adoptive cell transfer system (7). These regulatory γδ T cells produced IFN-γ and were thought to change the functional bias of αβ T helper cells developing in response to the immunization with OVA. Similarly, we have found that regulation of airway responsiveness by Vγ4+ T cells depends on the presence of IFN-γ. Moreover, the pulmonary Vγ4+CD8αβ+ T cell subset produces IFN-γ at higher levels than other pulmonary cell populations. In the 3N stimulation model αβ T cells are not the targets of regulation. IFN-γ may instead alter the innate response to airway stimulation. However, it is possible that the γδ T cells, which in our experimental model locally prevent airway hyperreactivity, are in fact related or identical to those modulating allergic αβ T cell-dependent responses in the adoptive transfer model.
We have described that γδ T cell deficiency in mice not previously exposed to aerosolized OVA did not result in increased airway responsiveness to MCh (5). The same was found in mice treated with aerosolized mAb against TCR-Vγ4. In fact, the regulatory function of γδ T cells became evident only after airway stimulation with OVA on 3 consecutive days. Exposure on 1 day alone was insufficient to induce γδ T cell-regulated airway responsiveness, and exposure on 5 consecutive days did not further increase the effect seen after 3 exposures (M.L., unpublished data). This finding suggested that the airways must be first stimulated for γδ T cell regulatory effects to occur. This stimulation could activate regulatory γδ T cells or induce their targets or both. The present data provide support for the first possibility while not ruling out the second. Specifically, we found that airway stimulation with aerosolized OVA induces a change in the regulatory subset that is likely to affect its function, namely a relative increase in the fraction of γδ T cells expressing CD8αβ. The increase required β2M and TAP-1 expression, implying that the affected Vγ4+ T cells respond to MHC class I or related molecules. With the possible exception of Qa-1b-reactive cells (30), all MHC class I-specific murine γδ T cells characterized thus far express Vγ4 (31–34). This survey suggests that the Vγ4+ T cell subset is biased toward MHC class I recognition. Moreover, Vγ4+ T cells recognize nonclassical class I molecules of the H2-T region, some of which are known to be expressed only after cellular activation or stress (28). Although we have not directly demonstrated that the CD8β+ fraction of the Vγ4 subset is responsible for regulation, this possibility is supported by our finding that the negative regulatory function was abrogated in mice deficient in MHC class I expression. In fact, the decrease in airway responsiveness after treatment of these mice with anti-TCR-Vγ4 mAb suggested a functional inversion in the Vγ4+ T cell subset toward positive regulation of airway responsiveness. Thus, the Vγ4+ T cell population may be further subdivided functionally. Only the MHC class I-dependent subset may be equipped for negative regulation. Whether CD8β expression indeed coincides with negative regulation remains to be determined as both untreated β2M−/− and TAP-1−/− mice contain Vγ4+CD8β+ T cells at normal frequencies. Thus far, only the expansion of these cells in 3N-stimulated C57BL/6 mice and the absence of their expansion in 3N-stimulated MHC class I-deficient strains are indicators that this may be the case, however.
In sum, our data reveal the capability of a small pulmonary lymphocyte population to preserve normal lung function after airway stimulation. This protective function seems to be triggered independently of the adaptive antigen-specific immune response and before the development of allergic airway hyperreactivity
Acknowledgments
Salary support to M.L. was provided by a Postdoctoral Fellowship award from the Arthritis Foundation and by the Melvin Garb Endowed Fellowship in Basic Immunology. This work was supported by National Institutes of Health Grants RO1 HL-65410 and AI-40611 (to W.K.B.), HL-36557 and HL61005 (to E.W.G.), and R01 AI-44920 (to R.L.O.), and by Environmental Protection Agency Grant R825702 (to E.W.G.).
Abbreviations
- AHR
airway hyperresponsiveness
- OVA
ovalbumin
- 3N
three exposures to nebulized OVA
- MCh
methacholine
- TCR
T cell antigen receptor
- PE
phycoerythrin
- RL
lung resistance
- Cdyn
dynamic compliance
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Woolcock A J. In: Textbook of Respiratory Medicine. Murray J F, Nadel J A, editors. Vol. 2. Philadelphia: Saunders; 1994. pp. 1288–1330. [Google Scholar]
- 2.De Sanctis G T, Itoh A, Green F H, Qin S, Kimura T, Grobholz J K, Martin T R, Maki T, Drazen J M. Nat Med. 1997;3:460–462. doi: 10.1038/nm0497-460. [DOI] [PubMed] [Google Scholar]
- 3.Gereda J E, Leung D Y, Thatayatikom A, Streib J E, Price M R, Klinnert M D, Liu A H. Lancet. 2000;355:1680–1683. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 4.Shirakawa T, Enomoto T, Shimazu S, Hopkin J M. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 5.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Kohler G, O'Brien R, Gelfand E W, Born W. Nat Med. 1999;5:1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 6.Yiamouyiannis C A, Schramm C M, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec W W, Whiteley H E, Thrall R S. Am J Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMenamin C, Pimm C, McKersey M, Holt P. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 8.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig B B, Pereira P, Pretolani M. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 9.Haas W, Pereira P, Tonegawa S. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 10.Lahn M. J Mol Med. 2000;78:409–425. doi: 10.1007/s001090000123. [DOI] [PubMed] [Google Scholar]
- 11.Augustin A, Kubo R T, Sim G K. Nature (London) 1989;340:239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 12.Sim G K, Rajaserkar R, Dessing M, Augustin A. Int Immunol. 1994;6:1287–1295. doi: 10.1093/intimm/6.9.1287. [DOI] [PubMed] [Google Scholar]
- 13.Sunaga S, Maki K, Komagata Y, Miyazaki J-i, Ikuta K. J Immunol. 1997;158:4223–4228. [PubMed] [Google Scholar]
- 14.Martin T R, Gerard N P, Galli S J, Drazen J M. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Hamelmann E, Joetham A, Shultz L D, Larsen G L, Irvin C G, Gelfand E W. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman T, Lefrancois L. J Exp Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itohara S, Nakanishi N, Kanagawa O, Kubo R, Tonegawa S. Proc Natl Acad Sci USA. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julius M H, Simpson E, Herzenberg L A. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 19.Lahn M, Kalataradi H, Mittelstadt P, Pflum E, Vollmer M, Cady C, Mukasa A, Vella A T, Ikle D, Harbeck R, et al. J Immunol. 1998;160:5221–5230. [PubMed] [Google Scholar]
- 20.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 21.Pereira P, Gerber D, Huang S Y, Tonegawa S. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent A L, Matis L A, Hooshmand F, Widacki S M, Bluestone J A, Hedrick S M. Nature (London) 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 23.Huber S A, Graveline D, Newell M K, Born W K, O'Brien R L. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 24.Potter T A, Rajan T V, Dick R F, 2nd, Bluestone J A. Nature (London) 1989;337:73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien R L, Yin X, Huber S A, Ikuta K, Born W K. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 26.Mukasa A, Born W K, O'Brien R L. J Immunol. 1999;162:4910–4913. [PubMed] [Google Scholar]
- 27.Havran W L, Chien Y H, Allison J P. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 28.Crowley M P, Fahrer A M, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 29.Born W K, Lahn M, Takeda K, Kanehiro A, O'Brien R L, Gelfand E W. Respir Res. 2000;1:151–158. doi: 10.1186/rr26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidovic D, Roglic M, McKune K, Guerder S, MacKay C, Dembic Z. Nature (London) 1989;340:646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- 31.Tsujimura K, Takahashi T, Morita A, Hasegawa-Nishiwaki H, Iwase S, Obata Y. J Exp Med. 1996;184:2175–2184. doi: 10.1084/jem.184.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schild H, Mavaddat N, Litzenberger C, Ehrich E W, Davis M M, Bluestone J A, Matis L, Draper R K, Chien Y H. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 33.Bonneville M, Ito K, Krecko E G, Itohara S, Kappes D, Ishida I, Kanagawa O, Janeway C A, Jr, Murphy D B, Tonegawa S. Proc Natl Acad Sci USA. 1989;86:5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P, Page M J, Poritz L S, Koltun W A, Chorney M J. Cell Immunol. 1997;176:153–157. doi: 10.1006/cimm.1996.1076. [DOI] [PubMed] [Google Scholar]