Abstract
Vascular endothelial growth factor receptor-3 (VEGFR-3) is a major mediator of lymphangiogenesis. Recently, VEGFR-3 ligands, VEGF-C, and VEGF-D were reported to promote tumor lymphangiogenesis and lymphatic metastasis, and these processes were inhibited by blocking of the VEGFR-3-signaling pathway. Here, we have adapted the mouse corneal angiogenesis assay to study potential lymphangiogenic factors and inhibitors. Immunohistochemical analysis with lymphatic endothelial markers showed that VEGF-C induces lymphatic as well as blood vessel growth in the cornea. By contrast, VEGF induced angiogenesis but not lymphangiogenesis. Fibroblast growth factor-2 (FGF-2) stimulated both lymphangiogenesis and angiogenesis. FGF-2 up-regulated VEGF-C expression in vascular endothelial and perivascular cells. Furthermore, administration of blocking anti-VEGFR-3 antibodies inhibited the FGF-2-induced lymphangiogenesis. These findings show that VEGFR-3 can mediate lymphangiogenesis induced by other growth factors. Because increased expression of FGF-2 and VEGF-C has been associated with lymphatic metastasis, our results provide a potential strategy for the inhibition of lymphatic metastasis in cancer therapy.
Metastasis to the regional lymph nodes by the lymphatic vessels is a common step in the progression of cancer, an important prognostic factor in many types of cancer, and the basis for surgical and radiation treatment of local lymph nodes. Recent evidence suggests that tumor lymphangiogenesis, the growth of tumor-associated lymphatic vessels, promotes lymphatic metastasis (1–4) and that the inhibition of lymphangiogenesis may provide a new strategy to block lymph node metastasis in cancer therapy (5).
Vascular endothelial growth factor (VEGF)-C (6) and VEGF-D (7), which are related to the major angiogenic factor VEGF (8, 9), are distinguished by their capacity to stimulate the growth of lymphatic vessels in vivo. VEGF-C stimulates lymphangiogenesis in the mature avian chorioallantoic membrane and in the skin of transgenic mice (10, 11). In adult tissues, its receptor VEGFR-3 is expressed almost exclusively by the lymphatic endothelium and is thus considered to be a major regulator of lymphangiogenesis (12, 13). VEGF-D, another ligand for VEGFR-3, also induces lymphangiogenesis in transgenic mice (14). Recently, three cancer cell lines transfected with VEGF-C or VEGF-D were reported to promote increased tumor lymphangiogenesis and to undergo lymphatic metastasis (1, 3, 4). Furthermore, the blockade of VEGF-C by using a soluble VEGFR-3 extracellular domain inhibited tumor lymphangiogenesis (1). Increased expression of VEGF-C has also been associated with lymph node metastasis in a variety of cancers in humans (15–17).
Besides VEGF-C and VEGF-D, other factors may be involved in lymphangiogenesis. So far, no simple test system has been developed that permits a critical assessment of the various contributors to lymphangiogenesis. The corneal micropocket assay has been used to study the effects of potential angiogenic or antiangiogenic factors in vivo (18, 19). In the corneal assay, blood vessels must penetrate an avascular space to reach the angiogenesis-inducing stimulus. Dye injection and electron microscopic examination have shown that lymphatic vessels can grow into the cornea after injury, although no lymphatic vessels exist in the normal mammalian cornea (20, 21). Lymphatic vessels currently can be identified in tissues by using VEGFR-3 and two other markers for the lymphatic endothelium. These are LYVE-1 (22, 23), a new homologue of the CD44 glycoprotein, a lymphatic endothelial receptor for hyaluronan, and a transmembrane glycoprotein of the mucin class, podoplanin (24). We reasoned that the cornea would be a suitable site to study the effects of lymphangiogenic factors by using these markers.
Fibroblast growth factor-2 (FGF-2) is a heparin-binding protein that induces cell proliferation or differentiation in a variety of cell types of mesodermal and neuroectodermal origin (25). It is also one of the first factors shown to play an important role in physiological and pathological angiogenesis (26). In vitro, cultured blood vascular and lymphatic endothelial cells form capillary-like tubes in response to FGF-2 (27–29). Various tumors and tumor cell lines express FGF-2 (30–32), and significant correlations have been reported between the expression of FGF-2 and tumor spread (33).
Here we have adapted the mouse corneal assay to investigate pro- and antilymphangiogenic factors. We show that VEGF-C, but not VEGF, can induce LYVE-1-positive lymphatic vessels in the cornea, which grow together with the blood vessels. FGF-2 can also stimulate lymphangiogenesis in the cornea. FGF-2 up-regulates VEGF-C in vascular endothelial and perivascular cells. Furthermore, FGF2-induced lymphangiogenesis is inhibited by neutralizing antibodies against VEGFR-3.
Materials and Methods
Animals and Reagents.
Male 5- to 6-week-old C57BL6/J mice were caged in groups of four or fewer. For all procedures, the mice were anesthetized by using a mixture of dormicum and hypnorm (1:1), and killed with a lethal dose of CO2. All animal studies were reviewed and approved by the animal care and use committee of the Stockholm Animal Board. A recombinant, mature form of human VEGF-C was expressed in Pichia pastoris and purified as described (34). Recombinant human VEGF165 was obtained from R&D Systems, and FGF-2 from Pharmacia UpJohn (Milan, Italy). Rat monoclonal antibodies against mouse VEGFR-3 and rabbit polyclonal antibodies against mouse LYVE-1 were used as described (23, 35).
Mouse Corneal Micropocket Assay.
The mouse corneal assay was performed according to procedures described (19). Corneal micropockets were created with a modified von Graefe cataract knife in both eyes of each mouse. A micropellet (0.35 × 0.35 mm) of sucrose aluminum sulfate (Bukh Meditec, Copenhagen, Denmark) coated with hydron polymer type NCC (IFN Science, New Brunswick, NJ) containing 160 ng of VEGF-C or VEGF, or 80 ng of FGF-2, was implanted into each pocket. The pellet was positioned 0.6–0.8 mm from the limbus. After implantation, erythromycin ophthalmic ointment was applied to the eyes. The eyes were examined by a slit-lamp biomicroscope at the indicated days. Vessel length and clock hours of circumferential neovascularization were measured. For the inhibition studies, mice that received corneal implants containing FGF-2 were randomized into two groups and given i.p. injections of neutralizing anti-VEGFR-3 antibodies or nonblocking anti-VEGFR-2 antibodies (35) (600 μg per mouse) on postoperative days 0, 2, and 4. The corneas were photographed on day 5 by a slit-lamp biomicroscope, and the immunohistochemical analysis was performed as described below.
Immunohistochemistry.
Mice were killed between days 5 and 13 after the implantation of the pellets. Enucleated eyes were fixed in 3% paraformaldehyde, dehydrated, and embedded in paraffin and sectioned radially in parallel to the growing limbal vessels (see Fig. 2 c–e). Sections (8 μm) were immunostained by using monoclonal antibodies against CD31 (1:800, PharMingen) and VEGFR-3 (1 μg/ml, clone AFL4) (35), as well as polyclonal antibodies against mouse LYVE-1 (23), FGF receptor-1 (FGFR-1) (1:200, Santa Cruz Biotechnology) and VEGF-C (1:400, R&D Systems; 1:400, Santa Cruz Biotechnology, A-18). Peroxidase activity was developed with 3-amino-9-ethyl carbazole (Sigma) and the sections were counterstained with hematoxylin. The tyramide signal amplification (NEN Life Science) was used to enhance staining. In negative-control stainings the primary antibodies were omitted. The specificity of anti-VEGF-C antibodies was checked by blocking with a 10-fold molar excess of recombinant VEGF-C.
Figure 2.
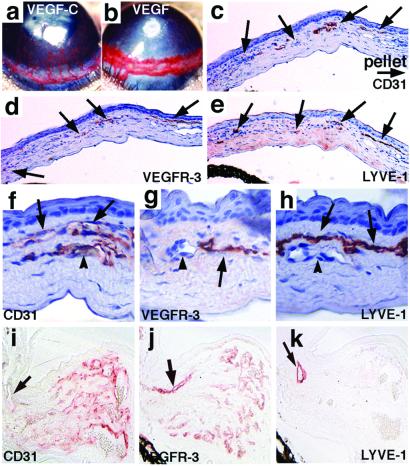
Corneal neovascularization stimulated by VEGF-C (a, c–h) or VEGF (b, i–k). Immunostaining of CD31 (c, f), VEGFR-3 (d, g), and LYVE-1 (e, h) in adjacent sections of the corneal neovasculature induced by VEGF-C shows the presence of corneal lymphatic vessels (arrows). The blood vessels indicated by the arrowheads (f–h) are positive only for CD31. The corneal neovasculature induced by VEGF is stained for CD31 (i), VEGFR-3 (j), and LYVE-1 (k) in adjacent, noncounterstained sections of the limbal (arrow) and corneal tissue. Note that several CD31-postive but LYVE-1-negative blood vessels are also stained for VEGFR-3 in the corneal area. A few LYVE-1-positive limbal lymphatic vessels (arrows) are present close to the induced capillaries. (Magnifications: c–e, ×100; f–h, ×400; i–k, ×200.)
Quantification of Corneal Neovasculature.
The angiogenic or lymphangiogenic grading scores were based on immunohistochemical analysis of the distance of vessel growth from the limbus. By staining several 8-μm sections with hematoxylin and eosin, sections including corneal vessels or pellets were found. At least five series of three sequential sections through the eyes were immunostained for CD31, LYVE-1, and VEGFR-3. The grading was: 0, no corneal lymphatic vessels, including the cases with growth of lymphatic vessels in the limbus only; 5, corneal lymphatic vessels penetrating into the pellet; 1–4, the corneal area between limbus and the pellet was divided into equal segments and scored from 1 to 4 in the order of distance from the limbus.
Cell Stimulation Assay.
Isolation of primary lymphatic and blood vascular endothelial cells from cultures of human dermal microvascular endothelial cells was performed as described (36). The endothelial cells, and smooth muscle cells derived from human coronary artery (HCASMC, Promo Cell, Heidelberg) were grown to subconfluence, starved for 48 h in serum- and growth factor-free medium, and then incubated with or without FGF-2 (5 ng/ml) for 24 h. RNA was isolated with RNeasy Mini (Qiagen, Hilden, Germany), electrophoresed, blotted, hybridized with the VEGF-C and glyceraldehyde3-phosphate dehydrogenase cDNA probes, and subjected to phosphoimaging.
Results
Lymphatic Vessels in the Eye.
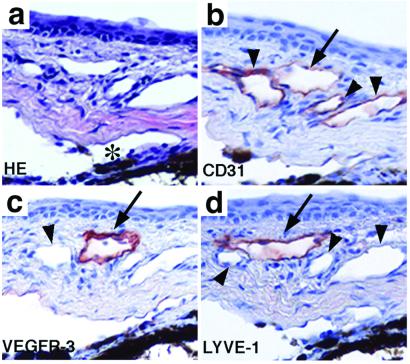
The distribution of lymphatic vessels in the mouse eye was analyzed by immunostaining for the lymphatic-specific markers LYVE-1 and VEGFR-3 and by comparison with the pan-endothelial marker CD31. In the limbal region, several vessels were positive for CD31 (Fig. 1 a and b). The vessels that stained weakly for CD31 (Fig. 1b, arrow) were also immunoreactive for VEGFR-3 (Fig. 1c) and LYVE-1 (Fig. 1d). These vessels had thin walls lined by endothelial cells with an attenuated cytoplasm and a protruding nucleus, and they were thus identified as lymphatic vessels. No immunostaining occurred in the cornea, consistent with the fact that the cornea represents an avascular tissue (data not shown).
Figure 1.
The distribution of blood and lymphatic vessels in adjacent sections of the limbal area of the eye. Hematoxylin and eosin-stained sections (a) show several vessels around the Schlemm's canal (*). These are positive for CD31 (b). One weakly CD31-positive lymphatic vessel indicated by the arrow is stained for VEGFR-3 (c) and LYVE-1 (d). Arrowheads point to the blood vessels. (Magnification, ×200.)
Induction of Corneal Lymphangiogenesis by VEGF-C.
Implantation of micropellets of aluminum sulfate coated with a slow-release polymer hydron containing VEGF-C induced an angiogenic response in the corneas, which was intensive on day 5 after implantation but started to regress on day 13 (Fig. 2 a; data not shown). Because the lymphatic vessels were not visible by intravital observation, histologic examination was performed. Because lymphatic vessel growth has been reported to follow that of blood vessels, and the newly formed lymphatic vessels also regress rapidly after wound healing (20, 37), the mice were killed and their eyes were analyzed between days 5 and 13 after implantation. Light microscopic studies of sections stained with hematoxylin and eosin disclosed no infiltration of inflammatory cells into the corneal stroma, suggesting that an inflammatory response is not a prerequisite for neovascularization in this model. Immunohistochemical analysis revealed that CD31-, VEGFR-3-, and LYVE-1-positive lymphatic vessels penetrated into the cornea and reached the pellet (Fig. 2 c–e). Blood vessels could be clearly distinguished from the lymphatic vessels by their thick endothelial cells, strong CD31 staining, and blood cells within the vessels, and by the lack of VEGFR-3 and LYVE-1 staining (Fig. 2 f–h). The lymphatic vessels were located adjacent to the blood vessels in the corneal stroma, generally within the anterior region immediately beneath the epithelium. Almost all corneal lymphatic vessels seemed collapsed in histologic sections.
VEGF Induces Angiogenesis but No Lymphangiogenesis.
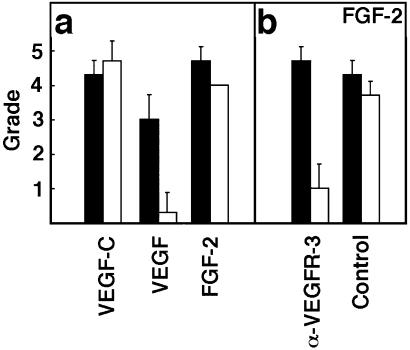
VEGF has been considered to induce angiogenesis without affecting the lymphatic vessels (10). To confirm that the corneal assay is suitable for the differential assessment of angiogenic and lymphangiogenic factors, pellets containing VEGF165 were implanted. The capillary vessel length in VEGF-implanted corneas was significantly shorter than that found in the VEGF-C-implanted corneas (Fig. 2b). The capillaries in both limbal and corneal areas were dilated and the vessel density was remarkably high. Immunostaining showed that CD31-positive vessels infiltrated into the VEGF-implanted corneas, whereas LYVE-1-positive vessels could not be observed in the corneas (Fig. 2 i and k). The limbus exhibited marked swelling with a very high density of CD31+/LYVE-1− blood vessels. The new capillaries induced by VEGF expressed VEGFR-3 (Fig. 2j). In quantitative analysis, VEGF-C induced about the same sized vessels of angiogenesis and lymphangiogenesis, whereas VEGF-induced angiogenic vessels were generally shorter in length (Fig. 3a).
Figure 3.
Quantification of the corneal angiogenesis and lymphangiogenesis. (a) Quantification of the angiogenesis and lymphangiogenesis induced by VEGF, VEGF-C, and FGF-2 (n = 3 each) analyzed by immunostaining as described in Materials and Methods. (b) Quantification of the angiogenesis and lymphangiogenesis in FGF-2-implanted corneas treated with anti-VEGFR3 (α-VEGFR-3) in comparison with the corneas of animals treated with nonblocking anti-VEGFR-2 control antibodies (Control). White bars, angiogenic score; black bars, lymphangiogenic score. The graphs represent mean values ± SEM.
FGF-2 Induces both Angiogenesis and Lymphangiogenesis in the Corneal Assay.
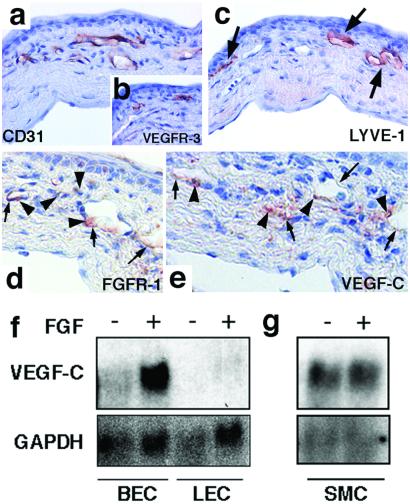
To investigate the ability of FGF-2 to induce lymphangiogenesis in vivo, hydron pellets containing FGF-2 were implanted into the corneas. Strong angiogenesis and lymphangiogenesis was stimulated by FGF-2 (Fig. 3a). CD31-, LYVE-1-, and VEGFR-3-positive lymphatic vessels were distributed in the same manner as in the VEGF-C-induced corneas (Fig. 4 a–c, arrows). The FGF2-induced corneal blood vessels were negative for VEGFR-3, except at the tips of the vessel sprouts (Fig. 4b; data not shown). FGFR-1 (23), the major receptor for FGF-2 in the vascular endothelium, was expressed by vascular endothelial cells and perivascular cells (Fig. 4d, small arrows and arrowheads).
Figure 4.
Induction of VEGF-C and corneal lymphangiogenesis by FGF-2. Immunostaining of CD31 (a), VEGFR-3 (b), LYVE-1 (c), FGFR-1 (d), and VEGF-C (e) in corneas containing FGF-2 implants. LYVE-1-positive lymphatic vessels indicated by arrows (c) penetrate into the corneas. (d and e) The small arrows indicate staining in vascular endothelial cells and the small arrowheads point to stained perivascular cells. (Magnification: a–c, ×400; d and e, ×450.) (f and g) Northern blot analysis of VEGF-C mRNA in FGF-2-treated primary blood and lymphatic vascular endothelial cells (BEC and LEC, respectively) and in vascular smooth muscle cells (SMC) (g). Glyceraldehyde dehydrogenase cDNA was used as a control for equal loading of the lanes.
FGF-2-Induced Lymphangiogenesis Is Mediated by VEGFR-3 Ligand.
To test the possibility that VEGF-C was induced in the corneas stimulated by FGF-2, immunostaining for VEGF-C was performed. No immunoreactivity for VEGF-C was detected in the normal corneas or limbus, or in the VEGF-implanted corneas (data not shown). In sections of the eyes containing FGF-2 pellets, VEGF-C staining showed a pattern similar to the staining for FGFR-1. The endothelium and perivascular cells of some newly formed capillaries in the corneas and pre-existing limbal vessels were stained with the anti-VEGF-C antibodies (Fig. 4e). The VEGF-C staining also decorated the endothelial cells in the dense conjunctival lymphatic capillary network, whereas no staining was observed on the side opposite to the pellet (data not shown).
To test the effect of FGF-2 on VEGF-C expression by endothelial cells and smooth muscle cells in vitro, isolated human primary blood vascular and lymphatic endothelial cells and smooth muscle cells were stimulated with 5 ng/ml of FGF-2 in serum-free medium for 24 h. Northern blot analysis revealed that VEGF-C mRNA expression was up-regulated by FGF-2 in the blood vascular endothelial cells (Fig. 4f). The FGF-2-treated lymphatic endothelial cells showed essentially no VEGF-C mRNA. Both endothelial cell types expressed FGFR-1 (data not shown). In contrast, FGF-2 stimulation did not affect the relatively high endogenous levels of VEGF-C mRNA in the smooth muscle cells (Fig. 4g).
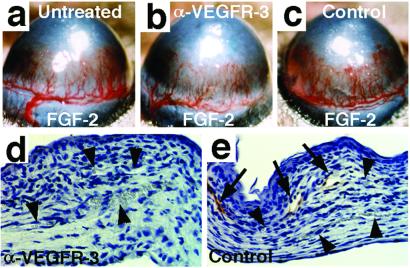
To investigate the functional significance of the up-regulation of VEGF-C during FGF-2-induced lymphangiogenesis, the mice that received corneal implants of pellets with FGF-2 were randomized into two groups that were given i.p. injections of the neutralizing anti-VEGFR-3 antibodies, or nonblocking anti-VEGFR-2 antibodies as a negative control. After the injections on postoperative days 0, 2, and 4, the corneas were evaluated and photographed on day 5. The slit-lamp observation showed no remarkable effect on angiogenesis in either group, whereas the capillaries in the anti-VEGFR-3-treated eyes were less dilated than in controls (Fig. 5 a–c). Histologic analysis with use of the LYVE-1 as a marker demonstrated that the extent of lymphangiogenesis was significantly reduced (Figs. 3b and 5 d and e), whereas almost equal numbers of blood vessels were present in the anti-VEGFR-3-treated eyes. The corneal thickness and cellularity were unexpectedly increased in the anti-VEGFR-3-treated eyes (Fig. 5d; data not shown).
Figure 5.
The effects of blocking anti-VEGFR-3 antibodies on FGF2-induced corneal neovasculature. Shown are macroscopic and immunohistochemical views of FGF-2-implanted corneas from (a) untreated, (b and d) anti-VEGFR-3-treated, and (c and e) control antibody-treated mice. The arrows point to LYVE-1-positive lymphatic vessels and arrowheads to blood vessels. (Magnification in d and e, ×200.)
Discussion
In this study, we show that VEGF-C induces not only angiogenesis but also lymphangiogenesis in the cornea. The corneal assay revealed that the angiogenic growth factor FGF-2 can also induce lymphangiogenesis and that inhibition of VEGFR-3 signaling by anti-VEGFR-3 antibodies suppresses the growth of corneal lymphatic vessels induced by FGF-2. These results demonstrate that FGF-2 stimulates lymphangiogenesis indirectly and that, in general, the mouse corneal assay is valuable for the investigation of pro- and antilymphangiogenic factors.
Lymphangiogenesis has been reported in vascularized corneas in several clinical and pathological states (21). Here, we applied purified exogenous factors in the mouse cornea to examine their lymphangiogenic potential by immunohistochemical analysis by using the newly identified lymphatic marker, LYVE-1. Although VEGF-C induced both corneal lymphangiogenesis and angiogenesis, VEGF induced only angiogenesis. VEGF caused the previously noted perilimbal edematous swelling, which may be due in part to the stimulation of vascular permeability by VEGF and in part to the lack of associated lymphangiogenesis. In VEGF-C-implanted corneas the lymphatic vessels were present along with the blood vessels. Because the mature form of VEGF-C used in the experiments binds to and activates both VEGFR-2 and VEGFR-3, VEGF-C should directly stimulate the endothelium of both the blood and lymphatic vessels in these experiments (34).
To investigate whether lymphatic vessels can penetrate into the cornea without a concurrent blood vessel growth, the VEGFR-3-specific mutant factor VEGF-C156S (38) was used in the corneal assay. However, VEGF-C156S could induce neither lymphangiogenesis nor angiogenesis (H.K., R.C., E.B., Y.C., and K.A., unpublished data). Our work has shown that transgenic mice overexpressing VEGF-C156S in the skin have hyperplastic cutaneous lymphatic vessels but normal blood vessels (14). The lack of lymphangiogenesis by VEGF-C156S suggests that factors produced during angiogenesis, such as proteolytic enzymes, may be essential to support lymphatic vessel sprouting into the avascular cornea. In fact, the corneal assay could be especially advantageous for further studies of the mechanisms involved, given the possibility to observe lymphatic vessels intravitally by way of dye injection or fluorescent imaging.
To date, numerous activators of blood vessel growth have been identified (39), and it is possible that various factors are also involved in lymphangiogenesis. Our results raise the possibility that FGF-2 is also involved in the regulation of physiological and pathological lymphatic vessel growth. The results of immunostaining showed that FGF-2 increases the amount of VEGF-C in vascular endothelial cells. VEGF-C was also up-regulated perivascularly in the corneal stroma near the FGF-2 pellets, but not around the VEGF pellets. Thus, VEGF-C can be an important effector of FGF-2-induced lymphangiogenesis. Some VEGF-C staining occurred in the limbal lymphatic vessels, possibly as a result of VEGF-C binding to its receptors in these cells. The fact that anti-VEGFR-3 antibodies inhibited the FGF-2-induced lymphangiogenesis indicates that VEGF-C (and/or VEGF-D) is a major downstream mediator of the lymphangiogenic activity of FGF-2.
From the physiological and rheological point of view, lymphatic vessels function as a drainage system and they are thus very different from blood vessels in vivo. In contrast to the in vitro assays, in vivo models permit the assessment of systemic host factors that influence the growth of the lymphatic vessels. For example, sprouting of lymphatic vessels into tumor stroma may not occur because these vessels lack sufficient pressure to penetrate into the stroma, which has a high interstitial pressure (40). Results with isolated lymphatic endothelial cells could also be misleading because many cultured endothelial cells and pericytes/smooth muscle cells located close to the lymphatic vessels in vivo express VEGF-C constitutively (13, 41). Endogenous VEGF-C mRNA was not down-regulated in the cultured vascular smooth muscle cells by serum starvation, nor was it stimulated by FGF-2. However, VEGF-C mRNA expression increased after FGF-2 stimulation in blood vascular, but not lymphatic endothelial cells. This finding might explain, in part, the phenomenon of concurrent blood vessel growth during lymphangiogenesis in vivo.
In addition to being expressed in the lymphatics, VEGFR-3 is also up-regulated in angiogenic blood vessels in many types of cancers (42, 43). The present result that VEGF induced VEGFR-3 in blood vessels may partly explain such a phenomenon. In a previous report, anti-VEGFR-3 antibodies destabilized blood vessels in C6 glioblastomas in nude mice, suggesting that VEGFR-3 could be involved in tumor angiogenesis (35). In the MCF-7 breast carcinoma model, angiogenesis was only slightly affected by the provision of soluble VEGFR-3 by an adenovirus (1), but tumor lymphangiogenesis was completely inhibited. Indeed, anti-VEGFR-3 therapy could influence angiogenesis in some tumors, as well as lymphangiogenesis, but one also needs to determine whether the inhibition of VEGFR-3 signaling has effects on normal tissues. Transgenic mice expressing soluble VEGFR-3 were largely devoid of lymphatic vessels for the first 4 weeks postnatal (44). Nevertheless, the lymphatics regenerated after this point despite continuous VEGFR-3 inhibition (44). So far, anti-VEGFR-3 antibodies and adenoviral administration of soluble VEGFR-3 have had no detectable side effects on adult mice in our studies (unpublished data). However, in the present experiments tissue swelling was observed in the VEGF-implanted eyes and in FGF-2-implanted eyes treated with anti-VEGFR-3, where lymphangiogenesis was significantly reduced, suggesting that an imbalance between blood vessel and lymphatic vessel regeneration influences the interstitial fluid balance during neovascularization. This aspect should be addressed in future studies.
In conclusion, we have adapted the mouse corneal assay for simultaneous studies of pro- and antilymphangiogenic factors. This assay may be useful to address several questions about lymphatic growth, such as what is the origin of the lymphatic endothelial cells in lymphangiogenesis? Do they sprout out from preexisting lymphatic vessels? Do they originate from bone marrow-derived lymphatic endothelial precursors or from endothelial cells of angiogenic blood vessels? Furthermore, FGF-2 was a pro-lymphangiogenic factor in this assay, and blockade of VEGFR-3 signaling by neutralizing antibodies inhibited FGF-2-induced lymphangiogenesis. Because tumor lymphangiogenesis has been associated with lymphatic metastasis, these results suggest that antilymphangiogenic therapy by inhibition of VEGFR-3 function may be attempted for the inhibition of lymphatic metastasis in various cancers.
Acknowledgments
We thank Dr. Steven A. Stacker and Dr. Marc G. Achen for their critical comments on this manuscript, David Jackson and Erkki Ruorlshti for anti-LYVE-1 antibodies, and T. Tainola, R. Kivirikko, and A. Parsons for excellent technical assistance. This study was supported by grants from the Finnish Academy of Sciences, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, the Human Frontier Science Program, and Swedish Cancer Foundation.
Abbreviations
- VEGF-C
vascular endothelial growth factor-C
- VEGFR
VEGF receptor
- FGF-2
fibroblast growth factor-2
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor-1
Note Added in Proof.
FGF-2-induced lymphangiogenesis via the VEGF-C/D–VEGFR-3 pathway in the corneal model has been independently discovered by L. K. Chang, G. Garcia-Cardena, F. Farnebo, R. C. Mulligan, J. Folkman, and A. Kaipainen (personal communication).
References
- 1.Karpanen T, Egeblad M, Karkkainen M, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 2.Mandriota S J, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson D G, et al. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacker S, Caesar C, Baldwin M, Thornton G, Williams R, Prevo R, Jackson D, Nishikawa S, Kubo H, Achen M. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 4.Skobe M, Hawighorst T, Jackson D G, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 5.Plate K. Nat Med. 2001;7:151–152. doi: 10.1038/84579. [DOI] [PubMed] [Google Scholar]
- 6.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 7.Achen M, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks A, Alitalo K. Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya M. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 10.Oh S, Jeltsch M, Birkenhager R, McCarthy J, Weich H, Christ B, Alitalo K, Wilting J. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 11.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain R, Alitalo K. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 12.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh V, Fang G, Dumont D, Breitman M, Alitalo K. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partanen T, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker S, Achen M, Alitalo K. FASEB J. 2000;14:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 14.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova T V, Kubo H, Thurston G, McDonald D M, Achen M G, et al. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta Y, Shridhar V, Bright R, Kalemkerian G, Du W, Carbone M, Watanabe Y, Pass H. Br J Cancer. 1999;81:54–61. doi: 10.1038/sj.bjc.6690650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T. Br J Cancer. 1999;80:309–313. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, Sasaki T. Clin Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 18.Folkman J, Klagsbrun M. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J, Claesson-Welsh L, Alitalo K. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collin H. Exp Eye Res. 1974;18:171–180. doi: 10.1016/0014-4835(74)90104-3. [DOI] [PubMed] [Google Scholar]
- 21.Mimura T, Amano S, Usui T, Kaji Y, Oshika T, Ishii Y. Exp Eye Res. 2001;72:71–78. doi: 10.1006/exer.2000.0925. [DOI] [PubMed] [Google Scholar]
- 22.Banerji S, Ni J, Wang S, Clasper S, Su J, Tammi R, Jones M, Jackson D. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevo R, Banerji S, Ferguson D, Clasper S, Jackson D. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 24.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikfalvi A, Klein S, Pintucci G, Rifkin D. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 26.Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M. Science. 1984;223:1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 27.Pepper M, Wasi S, Ferrara N. Exp Cell Res. 1994;210:298–305. doi: 10.1006/excr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 28.Pepper M, Mandriota S, Jeltsch M, Kumar V, Alitalo K. J Cell Physiol. 1998;177:439–452. doi: 10.1002/(SICI)1097-4652(199812)177:3<439::AID-JCP7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Tan Y. Jpn J Physiol. 1998;48:133–141. doi: 10.2170/jjphysiol.48.133. [DOI] [PubMed] [Google Scholar]
- 30.Moscatelli D, Presta M, Joseph-Silverstein J, Rifkin D. J Cell Physiol. 1986;129:273–276. doi: 10.1002/jcp.1041290220. [DOI] [PubMed] [Google Scholar]
- 31.Nakamoto T, Chang C, Li A, Chodak G. Cancer Res. 1992;52:571–577. [PubMed] [Google Scholar]
- 32.Yamanaka Y, Friess H, Buchler M, Beger H, Uchida E, Onda M, Kobrin M, Korc M. Cancer Res. 1993;53:5289–5296. [PubMed] [Google Scholar]
- 33.Takahashi Y, Cleary K, Mai M, Kitadai Y, Bucana C, Ellis L. Clin Cancer Res. 1996;2:1679–1684. [PubMed] [Google Scholar]
- 34.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, et al. Blood. 2000;96:546–553. [PubMed] [Google Scholar]
- 36.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Castimel B, Nice E, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Am J Pathol. 2000;156:1499–1504. doi: 10.1016/S0002-9440(10)65021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K. J Biol Chem. 1998;273:6599–6602. doi: 10.1074/jbc.273.12.6599. [DOI] [PubMed] [Google Scholar]
- 39.Kerbel R. Carcinogenesis. 2001;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 40.Leu A, Berk D, Lymboussaki A, Alitalo K, Jain R. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 41.Yonekura H, Sakurai S, Liu X, Migita H, Wang H, Yamagishi S, Nomura M, Abedin M, Unoki H, Yamamoto Y, Yamamoto H. J Biol Chem. 1999;274:35172–35178. doi: 10.1074/jbc.274.49.35172. [DOI] [PubMed] [Google Scholar]
- 42.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saaristo A, Karpanen T, Alitalo K. Oncogene. 2000;19:6122–6129. doi: 10.1038/sj.onc.1203969. [DOI] [PubMed] [Google Scholar]
- 44.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen M I, Pulkkanen K J, Kauppinen R, Jackson D G, Kubo H, Nishikawa S, et al. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]